Abstract
Aims
To estimate 3-year risk for diabetic foot ulcer (DFU), lower extremity amputation (LEA) and death; determine predictive variables and assess derived models accuracy.
Material and Methods
Retrospective cohort study including all subjects with diabetes enrolled in our diabetic foot outpatient clinic from beginning 2002 until middle 2010. Data was collected from clinical records.
Results
644 subjects with mean age of 65.1 (±11.2) and diabetes duration of 16.1 (±10.8) years. Cumulative incidence was 26.6% for DFU, 5.8% for LEA and 14.0% for death. In multivariate analysis, physical impairment, peripheral arterial disease complication history, complication count and previous DFU were associated with DFU; complication count, foot pulses and previous DFU with LEA and age, complication count and previous DFU with death. Predictive models’ areas under the ROC curves from 0.80 to 0.83. A simplified model including previous DFU and complication count presented high accuracy. Previous DFU was associated with all outcomes, even when adjusted for complication count, in addition to more complex models.
Conclusions
DFU seems more than a marker of complication status, having independent impact on LEA and mortality risk. Proposed models may be applicable in healthcare settings to identify patients at higher risk of DFU, LEA and death.
Keywords: Diabetes, Diabetic Foot, Foot ulcer
INTRODUCTION
Diabetes mellitus (DM) is one of the most frequent metabolic disorders, with an estimate of 371 million people living with this condition worldwide [1]. Incidence and prevalence are rising, carrying high costs (more than 471 billion US dollars in 2012) and rates of morbid-mortality, with premature deaths [1,2]. Around 4.8 million people died in 2012 due to diabetes, half of them were under 60 years [1,2].
The diabetic foot is one of the major complications of this disease, with an estimated 10% to 25% of diabetic patients developing a diabetic foot ulcer (DFU) in their lifetimes [3], causing a considerable burden in health care and patient well-being [1, 4, 5].
The occurrence of a DFU bodes poorly for the clinical course of patients with diabetes, with higher rates of re-ulceration, LEA, contralateral LEA and death compared to persons with diabetes who have not experienced a DFU [3].
Given the limited health care resources, it is important to optimize their allocation. To do so, an adequate stratification of subjects with diabetes by their risk of morbidity, namely DFU and LEA, as well as mortality, is crucial. Thus, identification of variables associated with these outcomes is the first step in the pathway for the creation or optimization of preventive/therapeutic programmes.
Even though the cascade of diabetic foot complications-DFU-LEA has been linked to higher mortality risk [6], increasing number of DM complications is also associated with higher mortality [7]. DFU is usually considered a marker of diabetes complication status, i. e., a marker for neuropathy and associated vascular disease in the foot. Still, some authors hypothesized that DFU occurrence could be per se an independent predictive variable of LEA as well as mortality [8].
Nevertheless, adjustment for baseline complications was rarely conducted when assessing the impact of DFU on LEA, and of both on the mortality risk [8]. In addition, simple models for their prediction (specially using the same core variables) were seldom proposed.
Given the current state of knowledge, we considered it essential to 1) estimate the risk at 3 years for DFU, LEA and death in a cohort of patients with diabetes followed in our Diabetic Foot Outpatient Clinic, 2) determine factors that independently predict LEA and mortality using multivariate analysis and 3) determine the ability of the models to discriminate between those who did and did not experience the outcomes of interest.
SUBJECTS
A retrospective cohort study was conducted including all subjects with diabetes followed in Centro Hospitalar de Vila Nova de Gaia/Espinho, Entidade Pública Empresarial, Diabetic Foot Outpatient Clinic from the 1st of January 2002 until the 31st of May 2010. Subjects were excluded if they met any of the following criteria: active DFU at the moment of inclusion, inability to ambulate, communication or cognitive impairment (due to aphasia and/or dementia), missing data on any covariate (except for vibration sensation assessed using a tuning fork and HbA1c), follow-up period of less than 3 years, or outside our referral area.
The Diabetic Foot Clinic is a tertiary care unit, with a multidisciplinary team and specialized diabetic foot care, treating patients from primary care institutions (usually with high risk feet and/or unavailable appropriate care in their residence area) or from other departments and hospitals.
The study was approved by the Ethics Committee of our institution and no adverse event occurred in any subject due to participation in this research.
MATERIAL AND METHODS
Data collection
Clinical records were reviewed and data collected from 1st until the 30th of June 2013.
All variables were collected in the first podiatric appointment in the clinic, through a structured interview and detailed foot exam, apart from by one of the two department podiatrists who were experienced in the care of diabetic foot complications.
Demographic characteristics (age at the time of inclusion, gender, education level), DM type (classified according to the WHO definition [9]), duration and treatment (diet only, oral medication or insulin), metabolic control (through HbA1c), physical (inability to reach his/hers own feet [13]) and/or visual impairment and smoking habits (absent, current, former) were recorded.
DM complications [retinopathy, nephropathy, neuropathy, cerebrovascular, cardiovascular, peripheral arterial disease and metabolic (ketoacidosis, hyperosmolar coma or other coma)] were classified in accordance to the Diabetes Complications Severity Index created by Young et al [10], according to their protocol, using ICD-9 Codes, through clinical record review.
As Young et al concluded that the accuracy of the number of complications was similar to the Complication Severity Index and as the number of complications is easier to calculate we opted to use it. Nephropathy was also staged by the American Diabetes Association (ADA) classification [11], using the serum creatinine value closest to the date of the first appointment. Participants’ feet were characterized using the variables more frequently described in DFU development risk stratification systems [5] and with proved association with its occurrence [13], namely, the presence of deformities, onychomycosis, diabetic peripheral neuropathy (DPN) [using the Texas Verbal Questionnaire [12], Semmes-Weinstein monofilament (SWM) insensitivity and tuning fork vibration sensation], peripheral arterial disease (PAD) (characterized by total foot pulses and intermittent claudication), oedema and history of previous DFU or LEA. There is a lack of studies assessing the reliability of these measurements [13]. Previous DFU was collected through foot assessment, patient self-report and all were additionally confirmed by medical record review. All the above described variables in addition to visual and/or physical impairment, presence of onychomycosis and DFU occurrence were collected and defined according to the protocol previously described by Monteiro-Soares et al [14].
Vibration sensation test (VST) was assessed with a 128 Hz tuning fork applied, perpendicularly with a constant pressure, on a bony part on the dorsal side of the distal phalanx of the first toe. This procedure was repeated twice and two incorrect answers were classified as altered sensation [15]. This procedure was instituted in 2008 and therefore patients entered into the study prior to this time do not have this assessment.
Subjects were followed from the time of inclusion to death or completion of the 3-year follow-up.
Minor LEA was defined as the surgical removal of toe(s), ray(s) or forefoot. Major LEA was considered amputation of the entire foot by any level of the leg (including the ankle).
HbA1c value was not always available (n= 164) as several patients were followed for their metabolic control mainly by primary care physicians.
DFU and/or LEA occurrence and death dates were registered. Subjects were advised to contact the clinic if any lesion developed and during appointments they were asked if any DFU occurred. Furthermore, complete medical records from the hospital as well as primary care institutions were reviewed in order to detect missed events. LEA and death (date and cause) are automatically registered in the individuals’ computerized clinical file. Death causes were collected using the ICD-9 codes.
Statistical analysis
Association between variables and outcomes (DFU, LEA or death) was conducted using univariate logistic regression. Values of p ≤ 0.05 were considered as statistically significant and ≤ 0.1 as pertinent for initial inclusion into the predictive models. Multivariate analysis to estimate odds ratios for amputation and mortality in relation to DFU adjusted for covariates was performed using logistic regression analysis employing a backwards stepwise algorithm approach. In addition, all multivariable models included age, gender and diabetes duration.
After the model creation for each outcome, a multivariable score was computed for each subject using the β coefficient values and the actual values for the covariates for those subjects. The ability of the score to discriminate between patients who did and did not develop the outcomes of interest was assessed using the area under the receiver operating characteristic curve (AUC) with the 95% confidence interval (CI).
All statistical analyses were conducted using the programme IBM SPSS, version 20.0 (Chicago, IL, USA).
Missing and indeterminate results were excluded from analysis.
RESULTS
Participant characteristics
In this study, 644 subjects were included and followed for a median of 36 months (range 1–36). At baseline, patients had a mean age of 65.1 (±11.2) years; mean diabetes duration of 16.1 (±10.8) years; and mean HbA1c of 7.8% (±3.7). The majority had type 2 DM and less than half were on insulin. More than half were female; over 80% were undereducated (primary school level or less) and over a quarter had some form of impairment (visual and/or physical). The most frequent complications were PAD related (63.0%) and the least frequent was metabolic complication history (3.6%). Forty-one percent of our population had a history of previous DFU (See Table 1).
Table 1.
Participants’ baseline characteristics
| Variables | Values (n= 644) |
|---|---|
| Subject characterization | |
|
| |
| Age [mean (SD)] | 65.1 (11.2) |
|
| |
| Female gender [n (%)] | 339 (52.6) |
|
| |
| Analphabetic or primary school [n (%)] | 529 (82.2) |
|
| |
| Visual impairment [n (%)] | 248 (38.5) |
|
| |
| Physical impairment [n (%)] | 237 (36.8) |
|
| |
| Past or present smoker [n (%)] | 134 (20.8) |
|
| |
| DM and its complications | |
|
| |
| Type 2 [n (%)] | 629 (97.7) |
|
| |
| Duration (in years) [mean (SD)] | 16.1 (10.8) |
|
| |
| Insulin use [n (%)] | 260 (40.4) |
|
| |
| HbA1c (in %) [mean (SD)]a | 7.8 (3.7) |
|
| |
| Cardiovascular complications history [n (%)]b | 219 (34.0) |
|
| |
| Retinopathy [n (%)]b | 297 (46.1) |
| Laser photocoagulation [n (%)] | 211 (32.8) |
|
| |
| Nephropathy [n (%)]b | 98 (15.2) |
| 4–5 stage in ADA classification [n (%)] | 37 (5.8) |
|
| |
| PVD complications history [n (%)]b | 406 (63.0) |
|
| |
| Neuropathy complications history [n (%)]b | 340 (52.8) |
|
| |
| Metabolic complications history [n (%)]b | 23 (3.6) |
|
| |
| Complications count [mean (SD)]b | 1.7 (1.1) |
|
| |
| Foot characterization | |
|
| |
| Foot deformity [n (%)] | 503 (78.1) |
|
| |
| Oedema [n (%)] | 165 (25.6) |
|
| |
| Onychomycosis [n (%)] | 379 (58.9) |
|
| |
| Total foot pulses ≤ 2 [n (%)] | 241 (37.4) |
|
| |
| Intermittent claudication [n (%)]c | 180 (28.2) |
|
| |
| DPN symptoms [n (%)] | 395 (61.3) |
|
| |
| Altered SWM sensation [n (%)]d | 309 (49.6) |
|
| |
| Altered VST [n (%)]e | 134 (33.9) |
|
| |
| Previous DFU [n (%)] | 264 (41.0) |
|
| |
| Previous LEA [n (%)] | 74 (11.5) |
164 missing values,
Using the Young et al (2008) proposed complications’ classification,
7 indeterminate values,
21 indeterminate values,
249 indeterminate/missing values,
HbA1c: Glycated Haemoglobin, ADA: American Diabetes Association, DFU: Diabetic Foot Ulcer, DM: Diabetes Mellitus, DPN: Diabetic Peripheral Neuropathy, LEA: Lower Extremity Amputation, SD: Standard Deviation, SWM: Semmes-Weinstein Monofilament, VST: Vibration Sensation Test
Cumulative incidence at 3 years for DFU and the outcomes of interest was as follows: DFU 26.6% (95% CI 23.2–30.0), recurrent DFU 34.5% (95% CI 27.4–48.4), minor LEA 2.7% (95% CI 1.4–4.0), major LEA 3.1% (95% CI 1.8–4.4), total LEA 5.8% (95% CI 3.9–7.5) and death 14.0% (95% CI 11.3–16.7).
DFU development risk variables
In univariate analysis, variables associated with DFU occurrence were age, gender, visual impairment, physical impairment, DM duration, retinopathy, nephropathy, PAD complications history, neuropathy complications history, complication count, and all foot characteristic variables except oedema.
In multivariate analysis only physical impairment, PAD complications history, complications count and previous DFU remained statistically significant (See Table 2). Using these variables we were able to create a model that discriminated between those patients who did and did not develop a DFU with an Area Under the Receiver Operating Characteristic Curve (AUC) value of 0.80 (See Figure 1). Considering a simplified model that included complications count and previous DFU only, the AUC value was 0.79 (CI 95% 0.76–0.83) (See Figure 2).
Table 2.
Variables’ association with diabetic foot ulcer, lower extremity amputation and death occurrence
| Variables | DFU (n= 171) | LEA (n= 37) | Death (n= 90) | |||
|---|---|---|---|---|---|---|
|
| ||||||
| Univariate OR (95% CI) | Multivariate OR (95% CI) | Univariate OR (95% CI) | Multivariate OR (95% CI) | Univariate OR (95% CI) | Multivariate OR (95% CI) | |
| Subject characterization | ||||||
|
| ||||||
| Age | 1.02 (1.01–1.04) | NS | 1.03 (0.99–1.07) | - | 1.13 (1.09–1.16) | 1.12 (1.09–1.15) |
|
| ||||||
| Female gender | 0.49 (0.35–0.70) | NS | 0.47 (0.23–0.93) | NS | 0.65 (0.41–1.02) | - |
|
| ||||||
| Analphabetic or primary school | 1.55 (0.95–2.55) | - | 1.85 (0.64–5.32) | - | 1.10 (0.61–2.00) | - |
|
| ||||||
| Visual impairment | 1.60 (1.12–2.28) | NS | 2.20 (1.12–4.30) | NS | 1.73 (1.11–2.71) | NS |
|
| ||||||
| Physical impairment | 2.38 (1.67–3.41) | 1.73 (1.15–2.59) | 2.11 (1.09–4.12) | NS | 1.99 (1.27–3.11) | NS |
|
| ||||||
| Past or present smoker | 1.06 (0.78–1.44) | - | 1.05 (0.47–2.36) | - | 0.92 (0.61–1.39) | - |
|
| ||||||
| DM and its complications | ||||||
|
| ||||||
| Type 2 | 2.39 (0.53–10.69) | NS | NA a | - | NAa | - |
|
| ||||||
| Duration (in years) | 1.03 (1.01–1.05) | NS | 1.02 (0.99–1.05) | - | 1.03 (1.01–1.05) | NS |
|
| ||||||
| Insulin use | 0.99 (0.70–1.43) | - | 1.64 (0.80–3.39) | - | 0.94 (0.65–1.60) | - |
|
| ||||||
| HbA1c (in %) b | 1.05 (0.97–1.14) | - | 0.99 (0.84–1.16) | - | 1.02 (0.97–1.08) | - |
|
| ||||||
| Cardiovascular complications history c | 1.32 (0.91–1.89) | - | 1.91 (0.98–3.72) | NS | 2.43 (1.55–3.81) | NS |
|
| ||||||
| Retinopathy c | 1.62 (1.14–2.31) | NS | 1.57 (0.81–3.08) | - | 1.08 (0.69–1.69) | - |
| Laser photocoagulation | 1.63 (1.14–2.35) | NS | 1.61 (0.82–3.15) | - | 0.71 (0.43–1.18) | - |
|
| ||||||
| Nephropathy c | 1.71 (1.08–2.69) | NS | 2.56 (1.22–5.38) | NS | 1.63 (0.93–2.86) | - |
| 4–5 stage in ADA classification | 2.81 (1.44–5.50) | NS | 2.81 (1.03–7.69) | - | 2.44 (1.14–5.23) | NS |
|
| ||||||
| PAD complications history c | 11.03 (6.09–19.96) | 2.52 (1.17–5.45) | 23.06 (3.14–169.31) | NS | 3.69 (2.03–6.68) | NS |
|
| ||||||
| Neuropathy complications history c | 3.14 (2.14–4.60) | NS | 3.45 (1.55–7.67) | NS | 1.48 (0.94–2.34) | - |
|
| ||||||
| Metabolic complications history c | 0.57 (0.19–1.71) | - | NAd | - | 2.26 (0.87–5.89) | - |
|
| ||||||
| Complication count c | 2.03 (1.69–2.43) | 1.31 (1.03–1.67) | 2.17 (1.57–3.01) | 1.74 (1.15–2.62) | 1.76 (1.42–2.18) | 1.50 (1.17–1.94) |
|
| ||||||
| Foot characterization | ||||||
|
| ||||||
| Foot deformity | 2.03 (1.25–3.25) | NS | 0.74 (0.35–1.57) | - | 0.85 (0.50–1.43) | - |
|
| ||||||
| Edema | 1.10 (0.74–1.63) | - | 0.93 (0.43–2.01) | - | 1.55 (0.96–2.51) | - |
|
| ||||||
| Onychomycosis | 1.75 (1.21–2.53) | NS | 0.58 (0.30–1.12) | - | 1.99 (1.22–3.25) | NS |
|
| ||||||
| Total foot pulses ≤ 2 | 3.43 (2.39–4.94) | NS | 8.04 (3.47–18.62) | 4.17 (1.76–9.88) | 2.51 (1.59–3.94) | NS |
|
| ||||||
| Intermittent claudication e | 1.70 (1.12–2.47) | NS | 2.03 (1.03–3.98) | NS | 1.10 (0.67–1.80) | - |
|
| ||||||
| DPN symptoms | 1.52 (1.05–2.20) | NS | 1.53 (0.74–3.14) | - | 0.94 (0.59–1.48) | - |
|
| ||||||
| Altered SWM sensation f | 3.16 (2.16–4.64) | NS | 3.25 (1.50–7.02) | NS | 1.30 (0.82–2.04) | NS |
|
| ||||||
| Altered vibration sensation test g | 3.54 (2.21–5.68) | NS | 5.03 (1.74–14.61) | NS | 2.38 (1.31–4.33) | NS |
|
| ||||||
| Previous DFU | 8.74 (5.80–13.17) | 4.54 (2.79–7.38) | 10.35 (3.97–26.93) | 5.54 (2.09–14.72) | 2.46 (1.56–3.87) | 1.73 (1.04–2.88) |
|
| ||||||
| Previous LEA | 5.12 (3.09–8.47) | NS | 4.22 (2.02–8.82) | NS | 0.72 (0.33–1.56) | - |
-: Not included in the multivariate analysis,
Model extrapolated values due to reduced number of subjects with diabetes type 1 and no event occurrence in such group,
164 missing values,
Using the Young et al (2008) proposed complications’ classification,
Model extrapolated values due to reduced number of subjects with history of metabolic complications and no event occurrence in such group,
7 indeterminate values,
21 indeterminate values,
249 missing values,
HbA1c: Glycated Haemoglobin, ADA: American Diabetes Association, CI: Confidence Interval, DFU: Diabetic Foot Ulcer, DM: Diabetes Mellitus, DPN: Diabetic Peripheral Neuropathy, FU: Follow Up, LEA: Lower Extremity Amputation, NA: Not Applicable, NS: Not statistical Significant association observed, OR: Odds Ratio, SD: Standard Deviation, SWM: Semmes-Weinstein Monofilament
Figure 1.
Receiver operating characteristic curve of predictive models for diabetic foot ulcer (a), lower extremity amputation (b) and death (c) occurrence
Figure 2.
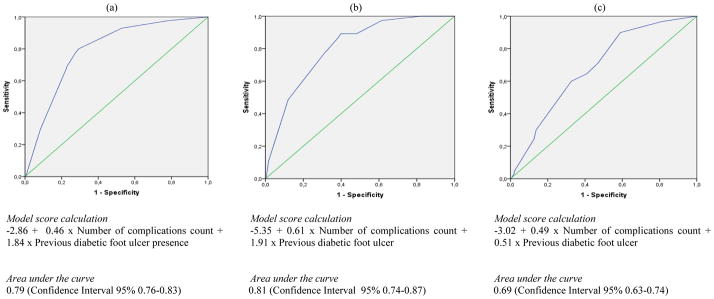
Receiver operating characteristic curve of predictive models for diabetic foot ulcer (a), lower extremity amputation (b) and death (c) occurrence using only complication count and previous diabetic foot ulcer
Previous DFU history remained associated with greater risk of incident DFU (p<0.001) even when adjusted for age, gender, visual and physical impairment, diabetes type and duration, PAD complications history, complication count and previous LEA.
LEA occurrence risk variables
In univariate analysis, variables associated with LEA were gender, visual and physical impairment, cardiovascular complications history, nephropathy, PAD complications history, neuropathy complications history, complication count, two or fewer of four foot pulses, intermittent claudication, altered SWMS and VST, and previous foot complications (DFU and/or LEA).
In multivariate analysis only complication count, two or fewer of four foot pulses and previous DFU maintained statistical significance (See Table 2), producing a score with an AUC value of 0.83 for the discrimination between those who did or did not experience an incident LEA (See Figure 1). When using a simplified model, including only complications count and previous DFU, the AUC value was 0.81 (CI 95% 0.74–0.87) (See Figure 2).
Once more, when adjusting for age, gender, physical impairment, diabetes duration, complication count, total foot pulses ≤2 and previous LEA, previous DFU maintained a statistically significant association with LEA risk (p=0.001).
Death occurrence risk variables
In univariate analysis, variables associated with death were age, visual and/or physical impairment, DM duration, cardiovascular complications history, end-stage renal disease, PAD complications history, complication count, onychomycosis, foot pulses, altered VST and previous DFU.
Age, complication count and previous DFU were the only variables that remained statistically significant in multivariate analysis (See Table 2). The resultant predictive model yielded an AUC value of 0.81 in the discrimination between patients who did and did not die during follow-up (See Figure 1). However, using the simplified model including complications count and previous DFU the AUC value dropped to 0.69 (CI 95% 0.63–0.74) (See Figure 2).
Once again, DFU history was associated with a higher mortality rate independent of age, gender, visual and physical impairment, diabetes duration, complication count and previous LEA (p<0.05) (data not shown).
We must highlight that patients developing a DFU during follow-up also had a significantly higher death rate (OR 1.75, CI 95% 1.09–2.79), although the same was not observed when adjusting for previous DFU (OR 1.18, CI 95% 0.70–1.99) or among those who had an LEA during follow-up (OR 2.09, CI 95% 0.95–4.58).
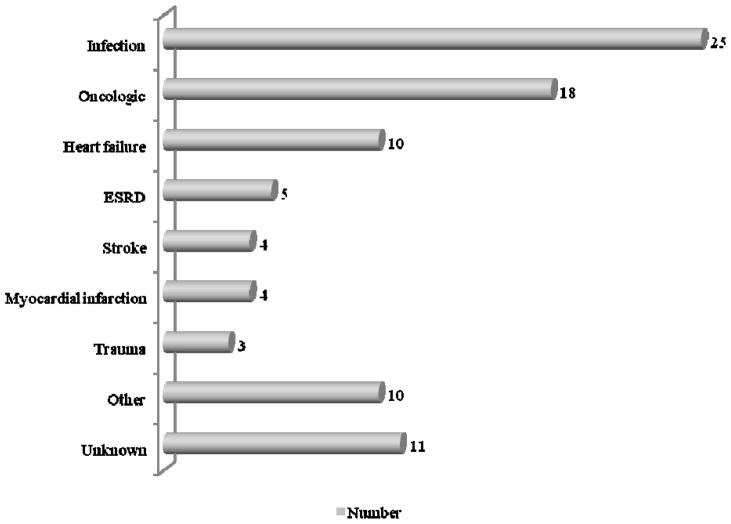
The most frequent causes of death were infections (27.8%), oncologic disease (20%), and heart failure (9%) (See Figure 3).
Figure 3.
Causes of death
ESRD: End-Stage Renal Disease
ICD-9 codes verified
Infection 52, 421, 464, 466, 480–488, 490–508, 519, 590, 595, 681, 682, 785
Oncologic 151, 153, 154, 157, 161, 162, 171–174, 185, 188, 189, 191, 203
Heart failure 428
ESRD 250.4, 585
Stroke 434, 436
Myocardial infarction 410
Trauma 800–804, 820–829
DISCUSSION
Several investigations have assessed all-cause mortality in type 2 DM with the derivation and validation of multivariate models [16,17]. However, and despite the proved impact of DFU on mortality risk [5,18], it was not included in such models.
On the other hand, DFU’s link with death occurrence has rarely been adjusted for other pertinent variables (such as age and baseline complications presence) [8].
Therefore, we have conducted this study assessing DFU impact on LEA and death risk, in a large cohort of consecutively enrolled subjects (n= 644), using the STROBE [19] and STARD [20] checklists as the basis for its development and reporting, and conducting adequate statistical adjustment. Moreover, due to the cohort design, observers were blind to outcome occurrence when collecting baseline data.
We observed that the different outcomes on which we focused shared several common predictive variables in univariate analysis, such as physical impairment; cardiovascular and PAD complications history, complications count; total foot pulses number, altered VST and previous DFU. However, few remained statistically associated in multivariate analysis, and different predictors of the outcomes of interest were seen across the three models for the outcomes DFU, LEA, and death, with the exception of complications count and previous DFU. On the other hand, the 3 derived models (using 3 to 5 variables) for each outcome were able to produce high AUC values (from 0.81 to 0.83). A simplified model that included complications count and previous DFU only retained high AUC values for DFU and LEA occurrence (0.79 and 0.81, respectively) but dropped to 0.69 in the case of death. This may be explained greatly by the fact that advancing age is highly and directly linked to death.
This 2 variable model is very simple, uses easily collected data from a clinical appointment, can be employed in every clinical setting, from primary to tertiary care, to identify subjects at higher risk of developing DFU and/or LEA. This may, consequently, lead to increased surveillance of such individuals in order to prevent these complications from occurring. The simplified model to predict death under performs compared to the full model that includes age, so the full model should be used for the prediction of this outcome because it is more accurate.
In the multivariate analysis, previous DFU maintained statistical significance for all the outcomes (even when using a broad group of variables for statistical adjustment).
Surprisingly, LEA only achieved statistical significance in the multivariate analysis for DFU occurrence prediction. It was not associated with higher risk of death (not even in the univariate analysis). During follow-up, 10.8% of subjects with a past history of LEA died, in comparison to 24.3% of those requiring any type of LEA during follow-up and 35% in the case of a major LEA.
In 1996, Boyko et al [8] assessed the relationship of DFU and mortality also adjusting the risk of death for some variables. However, 98% of the population were men and they tested a smaller range of variables. Cusick et al [21], in 2005, also conducted a multivariate analysis evaluating the association between mortality and several diabetes complications in patients with type 1 and type 2 diabetes, although all patients had retinopathy. Moreover, in both articles evaluation of complications was made by assessing the presence or absence of each one at baseline while we in contrast used the validated complication count proposed by Young et al [10] (its accuracy was considered similar to the Complication Severity Index).
There is substantial literature on mechanisms to explain many of the associations we describe between the outcomes of interest and predictors. Peripheral arterial disease (or diminished foot pulses as its correlate) has been independently associated with both LEA [22] and DFU [23], probably due to impairment in wound healing due to inadequate circulation. Diabetes complication count and physical impairment signal greater disease severity, which has also been shown to predict a higher risk of DFU, LEA, and death [10, 21, 23]. Previous DFU is an instance of a diabetes complication signaling high disease burden of specific importance in the development of foot complications such as future DFU and amputation [22,23]. In addition, and not surprisingly, the higher disease burden also predicts greater mortality [8, 21,24].
Limitations of our study include its retrospective nature, the exclusion of patients outside our direct referral area, the presence and exclusion from analysis of missing data of VST and HbA1c values, as well as indeterminate results for intermittent claudication and SWM sensation.
We must emphasize that, due to the selected design, all the assessed patient-related events (i.e. inclusion, follow-up and determination of outcomes) occurred prior to the research being undertaken.
The VST exam only started in the middle of 2008. Regarding HbA1c values, our hospital is a tertiary care centre for diabetic foot care, but nevertheless a recent HbA1c value (within less than 3 months) was not always available.
Even though there are works addressing the impact of depression in the mortality of patients with DFU [25,26], this variable is not collected in our daily practice and therefore was not available in the subjects’ clinical file for incorporation into prediction models.
We have observed several indeterminate results when assessing intermittent claudication due to the presence of patients that have extremely reduced ambulation and/or symptoms similar to DPN. SWM sensation test result in some patients was difficult to assess due to the presence of several callus/dry skin and patients’ automatic and constantly positive response (even when false positive test points were being conducted). In 23 patients, where hallux or transmetatarsal LEA was present in both feet VST was not possible to conduct.
We have decided to use the complication count proposed by Young et al [10], instead of the Complication Severity Index. This choice was due to the fact that both report equal accuracy and the first was easier to apply and interpret in our population.
Our data reveals a high rate of DFU development (> 8% annually) [4], consistent with our high risk referral practice from which we selected study participants, of whom 41.0% had previous DFU.
Our mortality rate is in accordance with the ones described in the literature, namely in the Eurodiale study [27]. In addition, our population has a high rate of comorbidities (13% cardiovascular disease and 63% PVD). Conversely, our LEA rate is inferior to Eurodiale results [27], as it would be expected, since we started with a population without active DFU while they included only patients with active DFU
The referral nature of the study setting, high prevalence of type 2 diabetes (97.7%), and low education level (82.2% primary school level or less) may limit the generalizability of these results to dissimilar populations.
As stated in the methods section, foot related variables were registered at the first podiatric appointment by one of 2 podiatrists with high experience in diabetic foot using a standardized form. We must highlight that both the professionals and form remained unchanged during the study period. Variables that were collected by clinical interview may present information bias. To overcome this limitation we have searched the clinical file and the national data platform in order to get access to the subjects’ most complete and accurate information. For all this and the long study period we believe that misclassification bias may have occurred. However, due to the selected type of study (a cohort) we believe that it was not differential.
Given the retrospective nature of the study we present several variables with missing data, as presented in the tables. However, we must emphasize that there was no missing data for the variables included in the models. Therefore, AUC values and respective 95% CI were calculated using the entire sample. On the other hand, we must highlight that we believe to have identified the great majority (if not all) the outcome events. We have conducted a broad search in the Hospitals’ and Health Data Platform (a program with access to data regarding all public healthcare institutions), in which is registered automatically all occurrences of LEA and death. We encouraged subjects to contact our service if any DFU occurred, thus enhancing our ability to capture this outcome.
We only used the ICD-9 codes when considering the cause of death and grouped them, acknowledging the potential limitations of the existing codes.
We conclude that DFU occurrence has a major and independent impact on LEA and death, even when adjusted for baseline complications. Thus the history of a DFU is a marker for poorer outcomes in patients with diabetes in this population. These findings also suggest that DFU prevention may be a potential path for better survival and diminished morbidity in persons with diabetes. New studies are needed in order to better understand this link. In our opinion, DFU presence implies a decrease of the subjects’ mobility and general well-being and, consequently, of the quality of life, higher infection risk and inflammatory, immune and physiologic changes. All of these most certainly lead to a higher mortality risk.
These models were obtained in a high risk context. So they should be tested in primary care to assess if they are clinically relevant and valid enough per se, or if they should be added to pre-existing models/classifications.
Acknowledgments
Sponsored by “Fundação para a Ciência e Tecnologia (FCT)”, Portugal; Grant number: SFRH/BD/86201/2
Dr Monteiro-Soares participation in this research was supported by the “Fundação para a Ciência e Tecnologia (FCT)”, Portugal; Grant number: SFRH/BD/86201/2.
Dr Boyko’s participation in this research was supported by the Diabetes Research Center, University of Washington P30DK017047 and VA Puget Sound.
Abbreviations
- ABI
Ankle–brachial Index
- ADA
American Diabetes Association
- AUC
Area Under the Receiver Operating Characteristic Curve
- ARR
Absolute Risk Reduction
- CI
Confidence Interval
- CRP
C-reactive Protein
- DFU
Diabetic Foot Ulcer
- DM
Diabetes mellitus
- DPN
Diabetic Peripheral Neuropathy
- ESR
Erythrocyte Sedimentation Rate
- HbA1c
Glycated Haemoglobin
- HR
Hazard Ratio
- Hz
Hertz
- ICD-9
The International Classification of Diseases, 9th Revision
- LEA
Lower Extremity Amputation
- LR
Likelihood Ratio
- mmHg
Millimetres of Mercury
- NNT
Number Needed to Treat
- PAD
Peripheral Arterial Disease
- RR
Relative Risk
- RRR
Relative Risk Reduction
- SD
Standard Deviation
- SWM
Semmes-Weinstein Monofilament
- TcPO2
Transcutaneous Partial Pressure of Oxygen
- VST
Vibration Sensation Test
- WHO
World Health Organization
Contributor Information
D. Martins-Mendes, Diabetic Foot Clinic; Endocrinology, Diabetes and Metabolism Department - Centro Hospitalar de Vila Nova de Gaia/Espinho EPE; Vila Nova de Gaia, Portugal. Internal Medicine Department - Centro Hospitalar de Vila Nova de Gaia/Espinho EPE; Vila Nova de Gaia, Portugal. Biochemistry Department (U38-FCT) - Faculty of Medicine of the University of Porto; Oporto, Portugal.
M. Monteiro-Soares, CIDES/CINTESIS (U753-FCT) – Health Information and Decision Sciences Department, Oporto Faculty of Medicine, Oporto, Portugal.
E. J. Boyko, Seattle Epidemiologic Research and Information Centre – Department of Veterans Affairs Puget Sound Health Care System and the University of Washington; Seattle, WA, USA
M. Ribeiro, Diabetic Foot Clinic; Endocrinology, Diabetes and Metabolism Department – Centro Hospitalar de Vila Nova de Gaia/Espinho EPE; Vila Nova de Gaia, Portugal
P. Barata, Health Sciences Faculty of the Fernando Pessoa’s University; Oporto, Portugal
J. Lima, Cancer Biology Group - Institute of Molecular Pathology and Immunology of the University of Porto; Oporto; Portugal; Faculty of Medicine of the University of Porto; Oporto, Portugal
R. Soares, Biochemistry Department (U38-FCT) - Faculty of Medicine of the University of Porto; Oporto, Portugal
References
- 1.IDF.org [internet] Fifth Edition Update 2012. Brussels: IDF Diabetes Atlas; [updated 2013; cited July 2013]. Available from: http://www.idf.org/sites/default/files/5E_IDFAtlasPoster_2012_EN.pdf. [Google Scholar]
- 2.WHO.org [internet] Geneva: Health for all database; [updated July 2013; cited October 2013] Online version. Available from: http://data.euro.who.int/hfadb/ [Google Scholar]
- 3.Frykberg RG, Zgonis T, Armstrong DG, et al. Diabetic foot disorders: a clinical practice guideline (2006 revision) J Foot Ankle Surg. 2006;45(S5):S1–S66. doi: 10.1016/S1067-2516(07)60001-5. [DOI] [PubMed] [Google Scholar]
- 4.Singh N, Armstrong DG, Lipsky BA. Preventing foot ulcers in patients with diabetes. JAMA. 2005;293(2):217–228. doi: 10.1001/jama.293.2.217. [DOI] [PubMed] [Google Scholar]
- 5.Monteiro-Soares M, Boyko EJ, Ribeiro J, Ribeiro I, Dinis-Ribeiro M. Risk stratification systems for diabetic foot ulcers: a systematic review. Diabetologia. 2011;54(5):1190–1199. doi: 10.1007/s00125-010-2030-3. Erratumin: Diabetologia 2011, 54 (6), 1585. [DOI] [PubMed] [Google Scholar]
- 6.Fortington LV, Geertzen JH, van Netten JJ, Postema K, Rommers GM, Dijkstra PU. Short and long term mortality rates after a lower limb amputation. Eur J Vasc Endovasc Surg. 2013;46(1):124–31. doi: 10.1016/j.ejvs.2013.03.024. [DOI] [PubMed] [Google Scholar]
- 7.Brownrigg JR, Davey J, Holt PJ, Davis WA, Thompson MM, Ray KK, Hinchliffe RJ. The association of ulceration of the foot with cardiovascular and all-cause mortality in patients with diabetes: a meta-analysis. Diabetologia. 2012;55(11):2906–12. doi: 10.1007/s00125-012-2673-3. [DOI] [PubMed] [Google Scholar]
- 8.Boyko EJ, Ahroni JH, Smith DG, Davignon D. Increased mortality associated with diabetic foot ulcer. Diabet Med. 1996;13(11):967–72. doi: 10.1002/(SICI)1096-9136(199611)13:11<967::AID-DIA266>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 9.WHO.org [internet] Definition and diagnosis of diabetes mellitus and intermediate hyperglycaemia. Geneva: [updated 2006; cited July 2013]. Available from: www.who.int/entity/diabetes/publications/diagnosis_diabetes2006/en. [Google Scholar]
- 10.Young BA, Lin E, Von Korff M, Simon G, Ciechanowski P, Ludman EJ, Everson-Stewart S, Kinder L, Oliver M, Boyko EJ, Katon WJ. Diabetes complications severity index and risk of mortality, hospitalization, and healthcare utilization. Am J Manag Care. 2008;14(1):15–23. [PMC free article] [PubMed] [Google Scholar]
- 11.American Diabetes Association. Standards of Medical Care in Diabetes – 2013. Diabetes Care. 2013;36(S1):S11–S66. doi: 10.2337/dc13-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Armstrong DG, Peters EJ, Athanasiou KA, Lavery LA. Is there a critical level of plantar foot pressure to identify patients at risk for neuropathic foot ulceration? J Foot Ankle Surg. 1998;37:303–307. doi: 10.1016/s1067-2516(98)80066-5. [DOI] [PubMed] [Google Scholar]
- 13.Monteiro-Soares M, Boyko EJ, Ribeiro J, Ribeiro I, Dinis-Ribeiro M. Predictive factors for diabetic foot ulceration: a systematic review. Diabetes Metab Res Ver. 2012;28:574–600. doi: 10.1002/dmrr.2319. [DOI] [PubMed] [Google Scholar]
- 14.Monteiro-Soares M, Dinis-Ribeiro M. External validation and optimisation of a model for predicting foot ulcers in patients with diabetes. Diabetologia. 2010;53(7):1525–1533. doi: 10.1007/s00125-010-1731-y. [DOI] [PubMed] [Google Scholar]
- 15.Bakker K, Apelqvist J, Schaper NC International Working Group on Diabetic Foot Editorial Board. Practical guidelines on the management and prevention of the diabetic foot 2011. Diabetes Metab Res Rev. 2012;28 (S1):225–31. doi: 10.1002/dmrr.2253. [DOI] [PubMed] [Google Scholar]
- 16.Hayes AJ, Leal J, Gray AM, Holman RR, Clarke PM. UKPDS Outcomes Model 2: a new version of a model to simulate lifetime health outcomes of patients with type 2 diabetes mellitus using data from the 30 year United Kingdom Prospective Diabetes Study: UKPDS 82. Diabetologia. 2013;56(9):1925–33. doi: 10.1007/s00125-013-2940-y. [DOI] [PubMed] [Google Scholar]
- 17.Yang X, Ma RC, So WY, Kong AP, Ko GT, Ho CS, Lam CW, Cockram CS, Tong PC, Chan JC. Development and validation of a risk score for hospitalization for heart failure in patients with Type 2 diabetes mellitus. Cardiovasc Diabetol. 2008;7:9. doi: 10.1186/1475-2840-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Robbins JM, Strauss G, Aron D, Long J, Kuba J, Kaplan Y. Mortality rates and diabetic foot ulcers. Is it time to communicate mortality risk to patients with diabetic foot ulceration? J Am Podiatr Med Assoc. 2008;98(6):489–493. doi: 10.7547/0980489. [DOI] [PubMed] [Google Scholar]
- 19.Vandenbroucke J, von Elm E, Altman D, Gøtzsche P, Mulrow C, Pocock S, Poole C, Schlesselman J, Egger M STROBE initiative. Strengthening the reporting of observational studies in epidemiology (STROBE): Explanation and elaboration. Ann Intern Med. 2007;147:W163–W194. doi: 10.7326/0003-4819-147-8-200710160-00010-w1. [DOI] [PubMed] [Google Scholar]
- 20.Bossuyt PM, Reitsma JB, Bruns DE, et al. The STARD statement for reporting studies of diagnostic accuracy: explanation and elaboration. Ann Intern Med. 2003;138(1):W1–W12. doi: 10.7326/0003-4819-138-1-200301070-00012-w1. [DOI] [PubMed] [Google Scholar]
- 21.Cusick M, Meleth A, Agrón E, et al. Associations of mortality and diabetes complications in patients with type 1 and type 2 diabetes. Diabetes Care. 2005;28(3):617–625. doi: 10.2337/diacare.28.3.617. [DOI] [PubMed] [Google Scholar]
- 22.Adler A, Boyko E, Ahroni J, Smith D. Lower-extremity amputation in diabetes. The independent effects of peripheral vascular disease, sensory neuropathy, and foot ulcers. Diabetes Care. 1999;22(7):1029–1035. doi: 10.2337/diacare.22.7.1029. [DOI] [PubMed] [Google Scholar]
- 23.Boyko E, Ahroni J, Stensel V, Forsberg R, Davignon D, Smith D. A prospective study of risk factors for diabetic foot ulcer. Diabetes Care. 1999;22(7):1036–1042. doi: 10.2337/diacare.22.7.1036. [DOI] [PubMed] [Google Scholar]
- 24.Ramsey S, Newton K, Blough D, et al. Incidence, outcomes, and cost of foot ulcers in patients with diabetes. Diabetes Care. 1999;22(3):382–387. doi: 10.2337/diacare.22.3.382. [DOI] [PubMed] [Google Scholar]
- 25.Ismail K, Winkley K, Stahl D, Chalder T, Edmonds M. A cohort study of people with diabetes and their first foot ulcer. Diabetes Care. 2007;30(6):1473–9. doi: 10.2337/dc06-2313. [DOI] [PubMed] [Google Scholar]
- 26.Winkley K, Sallis H, Kariyawasam D, et al. Five-year follow-up of a cohort of people with their first diabetic foot ulcer: the persistent effect of depression on mortality. Diabetologia. 2012;55(2):303–10. doi: 10.1007/s00125-011-2359-2. [DOI] [PubMed] [Google Scholar]
- 27.Schaper N. Lessons from Eurodiale. Diabetes Metab Res Rev. 2012;28(Suppl 1):21–26. doi: 10.1002/dmrr.2266. [DOI] [PubMed] [Google Scholar]