Abstract
Physiological rhythms are sensitive to social interactions and could contribute to defining social rhythms. Nevertheless, our knowledge of the implications of breathing in conversational turn exchanges remains limited. In this paper, we addressed the idea that breathing may contribute to timing and coordination between dialogue partners. The relationships between turns and breathing were analysed in unconstrained face-to-face conversations involving female speakers. No overall relationship between breathing and turn-taking rates was observed, as breathing rate was specific to the subjects' activity in dialogue (listening versus taking the turn versus holding the turn). A general inter-personal coordination of breathing over the whole conversation was not evident. However, specific coordinative patterns were observed in shorter time-windows when participants engaged in taking turns. The type of turn-taking had an effect on the respective coordination in breathing. Most of the smooth and interrupted turns were taken just after an inhalation, with specific profiles of alignment to partner breathing. Unsuccessful attempts to take the turn were initiated late in the exhalation phase and with no clear inter-personal coordination. Finally, breathing profiles at turn-taking were different than those at turn-holding. The results support the idea that breathing is actively involved in turn-taking and turn-holding.
Keywords: breathing, dialogue, turn-taking, inter-personal coordination, respiration, adaptation
1. Introduction
When two people talk to each other, they regularly exchange roles from speaker to listener. In most cases, such exchanges occur smoothly, avoiding gaps and overlaps in conversation. This ‘apportioning of who is to speak next and when’ is ‘a fundamental part of the infrastructure of the conversation’, known as ‘turn-taking’ [1, p. 10 587]. Turn-taking constitutes the ‘oscillating rhythm of conversation’ [2, p. 9] and has been extensively investigated in previous research. The conception of conversation as a joint activity [3,4] has motivated the study of inter-personal behaviours involved in conversation, and their potential links to turn-taking. Physiological rhythms are involved in social interaction (e.g. [5–7]). However, few studies have empirically investigated the relationship between breathing and turn-taking in spontaneous conversation [8]. Building on previous research on breathing during oral communication and joint activities involving oral production, this study reports the results of an experiment investigating breathing in unconstrained face-to-face conversations involving female native German speakers.
(a). Breathing cycles at rest and during speech production
At rest, every 5 s on average, our respiratory muscles contract automatically to expand our chest cavity, decreasing pressure in the lungs and drawing air in (inhalation phase). Owing to elastic recoil forces, respiratory muscles then return to their resting position, progressively decreasing the volume of the chest cavity and moving air out of the lungs (exhalation phase) [9]. Successive inhalation and exhalation constitute the breathing cycle, a fundamental rhythmic unit of our organism. At rest, breathing cycles are relatively regular and symmetrical with respect to the duration of inhalation and exhalation phases and to the volume of the air inhaled and exhaled. This profile changes during speech production, based on the involvement of specific neural networks to control the respiratory muscles [10,11].
When we speak, we take short and fast inhalations (which last half a second, on average). We then exhale slowly, for several seconds, in synchrony with the speech flow. This gives speech breathing its well-known asymmetrical profile [12–14]. Breathing cycles in speech are also irregular: the depth and duration of inhalation and exhalation vary as a function of the utterance produced over the course of the exhalation phase. Longer utterances involve longer exhalation, and also tend to be preceded by longer and deeper inhalations [15–21]. In addition, inhalation pauses are coordinated with the linguistic structure of the text: speakers mainly take breaths at syntactic boundaries. Moreover, the volume and duration of inhalation are related to these syntactic constituents (e.g. inhalation is greater before a main clause than an embedded clause). These properties are observed in read speech [16,17,19,20,22] and, to a lesser extent, in spontaneous speech [18,19,21]. These results show that, at least during monologue, the respiratory system works in coordination with speech and language constraints, and that our oral communicative ability is closely linked to the respiratory function.
(b). Inter-personal coordination in breathing during listening, reading and singing
Research on breathing behaviour while listening to speech provides us with some preliminary indications as to inter-personal influences on breathing, with pioneering work on the topic having been carried out over 50 years ago. For example, Ainsworth [23] found that breathing is more variable when listening to stuttered than normal speech. In another study, Brown [24] characterized breathing while listening to speech as compared to vegetative breathing, via a task that involved listening to live speech produced by a female speaker sitting in front of listener participants. The analyses showed an increase in breathing rate during listening compared to vegetative breathing. The results also showed some correlation of the rate and depth of breathing between the listener and the speaker. However, this study did not provide any indication that the modification of breathing during listening supported a specific adaptation of listener breathing towards speaker breathing. Garssen [25] addressed respiratory synchronization between subjects watching videos of medical interviews and a model of patient involved in the interviews. The analyses mainly focused on a single ‘patient’ who demonstrated deep, slow and audible respiration. Synchronization between the subject's and patient's breathing occurred locally—e.g. it was limited to short time-windows and was globally weak. In a previous study [26], we observed that when listening to a female reader, the breathing rate of a female listener increased when the reader increased her vocal effort, and decreased when the reader spoke slower as compared with normal speech. Although changes in listener breathing often followed in the direction of speaker breathing, they did not completely mirror it. Moreover, analysis of the temporal alignment of the listener's breathing cycle to the reader's breathing cycle did not provide any evidence of overall synchronization.
In these studies of breathing during listening to speech, no overall imitation or coordination of breathing was found between listeners and speakers. This suggests that respiratory rhythm is sensitive to speech stimuli, and probably to attention or mental effort [25,26], but that listeners may not coordinate their breathing with speakers'. This result could be due to the fact that pure listening tasks require neither direct interaction nor joint action.
Few studies have characterized inter-personal coordination of breathing in joint activity involving vocal production. Bailly et al. [27] investigated synchronization in breathing in male and female dyads, during three reading conditions with increasing constraints on coordination: reading paragraphs in alternation, reading sentences in alternation and synchronous reading. The strongest in-phase coordination profile was found during synchronous reading. Reading sentences in alternation led to an anti-phase coordination of breathing, while reading paragraphs in alternation did not reveal consistent overall coordination. Müller & Lindenberger [28] studied inter-personal synchronization of breathing during choral singing. They found more synchronization when singing in unison than when singing with multiple voice parts, and a close coordination between the conductor and the choir singers, with the lead taken by the conductor.
Hence, people align their breathing when required to speak or sing synchronously. In both cases, respiratory rhythm becomes similar among different people because they have to fulfil the same task at the same time; with breathing behaviour being, to a large extent, determined by task.
(c). Turn-taking and inter-personal breathing during conversation
Turn-taking is one of the most salient features in unconstrained conversation and gives rhythm to the conversation [1,29]. During dialogue, tasks are organized by the exchange of turns between interlocutors. While conversational turns are classifiable into different categories, for this paper we considered the classification introduced by Beattie [29] (see also [30,31]). This classification distinguishes turn-taking types based on the success of taking a turn, the presence or absence of interruptions from the other person, and any overlap that occurs between turns. In general, turns are taken smoothly, without interruptions from conversational partners (smooth turns, [1,32]). In spontaneous dialogue, however, people regularly interrupt each other successfully (interruptions, [30,31]) or unsuccessfully (butting-in, [30,31]). Each kind of turn can potentially include overlaps or not (e.g. interruption may occur during a pause; smooth turns may start before the other person has totally finished). In this classification, a final category, backchannel, corresponds to speech events produced by the listener as feedback to the speaker, such as ‘yes’, ‘yeah’, ‘mhm’, etc. This category is not turn-taking in a strict sense, as the listener is not trying to take the turn but rather indicates to the speaker that she is expecting her to continue. As in McFarland [8], backchannels were therefore considered as part of the listening phase in the current study.
Some authors [8,33] suggest that breathing may contribute to the timing of conversation and, in particular, could be related to turn-taking [8]. According to Guaïtella [34], breathing may adapt to the rhythmic organization of dialogue, but dialogue may also be constrained by the limits of respiratory rhythm. She also hypothesized that dialogue rhythm may rest on an implicit knowledge of the duration of the exhalation phase. This implicit knowledge may indicate to the listener when the speaker will take an inhalation pause, providing an opportunity to take the turn. Experimental evidence for these suggestions is sparse, however, due to the small number of studies that have directly investigated breathing in dialogue.
Warner et al. [33] addressed the relationship between vocal activity and respiratory oscillations in speakers involved in spontaneous face-to-face conversation. For each speaker, the major low-frequency oscillation in vocal activity was related to the major low-frequency oscillation in respiration. Mutual entrainment of vocalization and respiration was found to be subject-specific and evident for one subject only. Further analyses of inter-personal behaviour demonstrated a close coordination of vocalizations between dialogue partners: when speaker 1 produced periods of high vocal activity (long utterances and short pauses), speaker 2 realized periods of low vocal activity (short utterances and long pauses). While the relationship between turn-taking and breathing was not addressed in this study, and analysis of inter-personal coordination of breathing was not provided, the authors did not find evidence of mutual entrainment in breathing oscillations between conversation partners.
Warner et al. reported an overall analysis of coordinative profiles, regardless of dialogue phases. Other studies have characterized breathing in dialogue more locally, according to dialogue events [8,34,35]. The results of these studies show different breathing profiles during both the speaking and listening phases of dialogue. Contrasting with monologue studies [18,19,21], they also reported a lack of relationship between the duration of speech utterances and inhalation pauses [34,35]. These studies [34,35], however, involved a single dyad. To our knowledge, McFarland [8] is the only study to have characterized the breathing cycle and the inter-personal coordination of breathing in relation to turn-taking. The study included 10 female dyads involved in scripted and spontaneous dialogues, with breathing cycles identified by rib-cage displacements and characterized by the duration of the inhalation and exhalation phases. The author found an anticipation of turn-taking in listener breathing, visible in the reduction of the duration of the inhalation phase (i.e. more speech-like inhalation) when turn-taking approached. This anticipation was more salient in scripted dialogue than in spontaneous conversation. This result was due to the lack of successive silent listening cycles in spontaneous conversation, as listeners often produced short utterances while listening in this last case (e.g. backchannels). Using cross-correlation applied to a restricted window around specific events (laughter, turn-taking), McFarland found some inter-personal coordination of breathing when individuals were laughing together or during turn-taking. Coordination of breathing in laughter turned out to be mainly in-phase, while breathing at turn-taking tended to be either in-phase or anti-phase. Although the author did not categorize turns along their pragmatic dimension, it may be that such differences in coordination patterns result from different turn types, such as interruptions and smooth turns.
In summary, breathing adapts to dialogue phases, and inter-personal coordination of breathing can be observed locally, during turn-taking [8]. By contrast, global analyses, regardless of dialogue events, do not show any clear coordination between partners' breathing in dialogue [33]. While the idea that the rhythm of breathing plays a role in the rhythm of dialogue is an old hypothesis [33,34], we still lack empirical studies of this phenomenon. This could be explained at least by methodological limits, as recording the breathing of two conversational partners simultaneously requires specific equipment. The aim of the current study was to improve our knowledge of breathing in dialogue by providing new individual and inter-individual analyses of breathing kinematics in spontaneous dialogue. In particular, we focused our analyses on the idea that breathing could be specifically involved in turn-taking and could constitute a coordinative unit for turn exchange.
(d). Research approach
Our approach was to analyse breathing kinematics and its relation to turn-taking in spontaneous conversations involving eleven female subjects, each talking successively with the same two female partners. This situation was used to evaluate changes in subjects' behaviour according to their partners (as in [26,27], while [8,33] involved dyads with different people). The analyses were based on the identification of the breathing cycle (e.g. [8,25–27]) and on the annotation of turns according to their pragmatic function (e.g. [30,31]). Two groups of analyses were used to better understand the implication of breathing in turn-taking:
(A) Global analyses, without distinction between the listening and speaking phases, which evaluated: the relationship between the breathing rate and the number of turn exchanges (Analysis A.a); the temporal alignment of a subject's breathing to the partner's breathing (Analysis A.b);
(B) Local analyses, with a distinction between the listening and speaking phases, and a specific investigation of breathing behaviour at turn-taking. As compared to McFarland [8], the original aspect of our approach was to distinguish turns according to their pragmatic function. We also analysed the breathing cycles occurring inside a turn, when the speaker needs to inhale but then continues her turn. We characterized: the relationship between the breathing cycle and the turn (Analysis B.a); the adaptation of the breathing cycle according to dialogue events (Analysis B.b); and the temporal alignment of a subject's breathing to their partner's breathing at turn-taking (Analysis B.c).
In general, the results provide evidence for an adaptation of breathing in dialogue, which is specific to the dialogue phase, and in particular to turn-taking. These results extend our knowledge of respiratory markers of conversational interaction [8] and support the idea that breathing is actively involved in turn-taking.
2. Material and methods
(a). Speakers
The study involved eleven subjects (average age: 31, range 25–46) and two conversation partners: Partner 1, who was a researcher (the second author of the paper) aged 42, and Partner 2, who was a PhD student aged 28. Subjects were undergraduate students or academics with a university degree. Participants and partners were all female native German speakers. Only female subjects were involved in this study to avoid possible confounds arising from complex effects of gender on dialogue organization [36,37]. The choice to include the same two partners for all subjects controlled for potential changes in subjects' behaviour according to partner (similar procedures have previously been used to investigate coordination between speakers and listeners from brain [38] and breathing [26,27] signals).
(b). Procedure
Each subject had five short conversations (2.5 min each) with each partner. The subject and partner together chose the topic of conversation, choosing either to continue with the topic or move on to a new topic from one conversation to the next. During the conversation, subject and partner were seated facing each other. They were asked to keep their hands on their knees in order to limit torso and arm movements, as these movements could strongly interfere with recording their breathing.
Breathing kinematics were recorded synchronously for the two speakers by means of two Inductotraces (formerly Respitrace). This system monitored the expansion (inhalation) and compression (exhalation) of the rib cage and abdomen, which result from changes in lung volume during breathing [39]. Inductotrace requires the use of two elastic bands positioned at the level of the axilla (rib cage) and of the umbilicus (abdomen) on the speakers' torsos. The acoustic signals were recorded with two directional microphones coupled with a pre-amplifier. Breathing kinematics and the acoustic signals for both interlocutors were simultaneously sampled at 11 030 Hz.
(c). Annotation of dialogue phases
Figure 1a illustrates the annotations of dialogue phases, which were carried out using acoustic recordings, regardless of respiratory signals, and then superimposed on the breathing signals to illustrate breathing behaviour during different dialogue phases.
Figure 1.
(a) Sample of breathing kinematics from a conversation for one subject and partner. The vertical displacements along the y-axis correspond to normalized changes (z-scores) in rib cage and abdominal volume with inhalation (upward displacement) and exhalation (downward displacement), and are plotted across time on the x-axis. Coloured rectangles represent speech inter-pausal units (IPUs). The different phases of the dialogue are indicated on the bottom of the graph (turn-taking, turn-holding and listening). (b) Schematic representation of a breathing cycle. The breathing cycle was defined from the inhalation onset (here, I_on[i] for the subject and I_on[j] for the partner) to the next inhalation onset (e.g. I_on[i+1]). In our study, exhalation onset (e.g. E_on[i])) corresponded to the offset of the inhalation phase. The vertical line indicates the localization of the subject's inhalation onset in relation to the partner's breathing cycle (see text for details). (Online version in colour.)
We first distinguished between speech and silence phases by detecting inter-pausal units (IPUs) ‘as a maximal sequence of words from one speaker surrounded by silence longer than 50 ms' [31, p. 3007]. IPUs are represented in figure 1a with background rectangles. These rectangles thus correspond to the spoken phases of the dialogue.
A student assistant orthographically transcribed the text associated with each IPU. The transcription was verified and corrected where required by a trained phonetician (the second author of the paper). The phonetician also identified IPUs corresponding to a backchannel, or to the onset of a new turn (turn-taking, see [31]). As indicated in figure 1a, backchannels were treated as part of the listening phase, as in McFarland [8]. A turn-taking IPU corresponded to an IPU by which the speaker tried or managed to take the turn. Turn-taking IPUs are indicated in figure 1a by continuous lines with a left-pointing arrow and are detailed below. IPUs that were neither turn-taking nor backchannel were annotated as turn-holding (dotted lines with a right-pointing arrow in figure 1a). A turn-holding IPU thus corresponded to a turn continuation after a silence greater than 50 ms.
On the basis of these annotations, a full turn was defined as the interval from a turn-taking IPU to the next turn-taking IPU by the other speaker. A turn always included at least one turn-taking IPU and one breathing cycle, but could also include one or several additional turn-holding IPU(s) and one or more additional breathing cycle(s) (see figure 1a). Turn-taking IPUs were classified into three main categories, adapted from previous work [30,31], that also defined the turn type:
— smooth turn, the speaker took the turn successfully without interrupting her interlocutor, with or without overlap;
— interruption, the speaker interrupted her interlocutor successfully;
— butting-in, the speaker tried, but failed, to interrupt her interlocutor.
Smooth turn-taking sometimes included overlaps in speech—for instance, when a speaker lengthened the final part of a word while another speaker had already started to speak. By comparison with Beattie and Beňuš et al. [30,31], we did not consider the ‘overlap’ dimension (see ‘Introduction’) to have a significant number of observations in each category. In particular, pause interruptions were grouped with overlapped interruptions, and smooth turns with and without overlaps were grouped together. Laughter, coughs and sighs were not included in the analysis as they specifically perturbed breathing kinematics.
(d). Annotation and processing of breathing cycles
Before the recording, the gain levels of the Inductotraces were set up to be the same for the rib cage and abdomen for all participants. Breathing events were detected using the weighted sum of two thorax measures for one abdominal measure [40]. To improve detection of the onset and offset of movements, and to limit data storage size, signals were band-pass filtered at 2–40 Hz (finite response filter) and resampled at 100 Hz. The onset and offset of inhalation movements were detected at 10% of the value of the velocity peak before and after the peak, respectively. This method is common in movement sciences, and allows automatic and reliable detection of the onset and offset of movement automatically. The labelling was then visualized and corrected when required.
A breathing cycle, cycle[n], with n representing the position of the breathing cycle in the breathing flow, was characterized based on the detection of the onset (I_on[n]) and offset of inhalation (figure 1b). The offset of inhalation was considered as the onset of the exhalation phase (E_on[n]), with the offset of the breathing cycle corresponding to the onset of the next inhalation (I_on[n+1]) (see [26] for similar annotation methods). The breathing cycle was defined based on successive inhalation onsets, since the onset and offset of the exhalation phase are difficult to detect reliably [13,26]. Breathing cycles were also categorized in relation to the speech events they included. The Listening cycle category included quiet cycles or cycles associated with backchannels, while the Speaking cycle category included breathing cycles associated with one or more IPU(s). These cycles were categorized as turn-taking cycles when the first IPU occurred during turn-taking, and as turn-holding cycles when the first IPU occurred during turn-holding (see figure 1a).
(e). Measurements
The dataset was then characterized according to the research approach detailed in §1d using the following parameters:
- (2) The cycle asymmetry index, measured for a given cycle[n] as the proportion of the inhalation duration relative to the cycle duration (see [25]):
The relation between inhalation and exhalation durations is generally used to characterize the shape of the breathing cycle and shows, for example, that the inhalation and exhalation phases during listening are more similar than during speech (e.g. [8,13] and §1).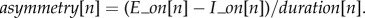
-
(3) The temporal alignment of the inhalation onset in relation to the other speaker's breathing cycle (coordination_index). To compute this index, the onset of each breathing cycle was associated with the breathing cycle of the other speaker that temporally included it. For a given cycle[i] of a subject starting during a cycle[j] of her partner, the coordination index was obtained as follows:
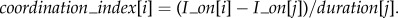
Values close to 0 or 1 mean that the participant began to inhale in synchrony with her partner (for similar methods see [26,27] for the study of inter-personal coordination of breathing or [41] for the study of coordination between the articulators in speech production).
- (4) The breathing rate, measured for a dialogue d as the number of breathing cycles produced during the dialogue (n_cycles[d]), divided by the sum of the duration of these cycles (duration_cycles[d]):

-
(5) The speech onset relative to the exhalation phase, computed for a given speaking breathing cycle[n], as the delay between the speech onset time (speech_onset[n]) and the exhalation onset, normalized by the duration of the exhalation phase:
Speech_position was expressed relative to the duration of the exhalation phase due to the large variability in the duration of exhalations (e.g. the same delay expressed in absolute value could mean the onset, middle or end of the exhalation phase, according to the duration of the exhalation). A value of speech_position close to 0 signifies that the speech onset is synchronized with the exhalation onset.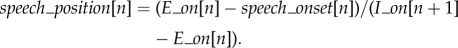
We then counted:
(6) The number of turns taken by the subject and partner in each dialogue.
(7) The number of breathing cycles involved in the turn (number_of_cycles), which corresponded to cycles whose onset or offset occurred during the turn, or to cycles that included the whole turn (when that turn was realized on a single breathing cycle).
Global analyses (point A in §1d) were based on measurements (4) and (6) to characterize the relationship between breathing and turn exchange rate (Analysis A.a), and on measurement (3) to address the overall coordination of breathing between subject and partner (Analysis A.b).
Local analyses (point B in §1d) were based on measurements (5) and (7) to characterize the relationship between the breathing cycle and the turn (Analysis B.a), on measurements (1) and (2) to characterize the breathing cycle according to the dialogue events (Analysis B.b), and on measurement (3) to characterize the coordination of breathing at turn-taking (Analysis B.c).
(f). Statistical analyses
The effects on the different variables were tested using linear mixed models in R 2.14.0 (lme4 library, [42]).
To obtain an overall description of the dialogues (Analysis A.a), we ran two models with breathing_rate as the dependent variable and the conversational partner (Partner 1 versus Partner 2) as the independent variable. In one model, we used the partner data and accounted for differences among the partners. In the other model, we used the subject data and accounted for subjects' behaviour according to the partners.
To test how much breathing kinematics (duration and asymmetry) depend on dialogue events, we ran a series of models (Analysis B.b). In the first model, we tested the following two factors—Dialogue Event (Speaking versus Listening) and Partner (Partner 1 versus Partner 2). In the second model, we tested whether duration and asymmetry differed with respect to general turn events (Turn-taking versus Turn-holding) and Partner (Partner 1 versus Partner 2). In a final model, we tested whether duration and asymmetry differed with respect to the three turn-taking types (Smooth turn versus Interruption versus Butting-in) and Partner (Partner 1 versus Partner 2). Duration and asymmetry variables were always transformed to a logarithmic scale to guarantee a linear distribution of the residuals. These were checked using diagnostic tools such as qqnorm in R. Subjects and trials (i.e. the five dialogues per dyad) were included as random factors. Comparisons between the different levels of factors were carried out by changing the reference level, and pMCMC values were obtained using the languageR library. In linear mixed models, a valid alternative to standard p-values is to calculate the p-value from MONTE CARLO sampling by Markov chain (pMCMC = Monte-Carlo Markov Chain [43]). Under these tests, no significant interactions were found, mainly because subjects did not systematically change their breathing kinematics with respect to the two different partners. We thus ran additive models.
Inter-personal coordination was evaluated by studying the distribution of the coordination_index over the course of the dialogue or at particular turn-taking events (Analysis A.b and B.c). The distributions of the index value for all observations were first compared to the uniform distribution (equal number of observations in each interval of values) in order to verify whether there was indeed a coordinative trend. Moreover, when two speakers breathe continuously, their inhalation onsets might at some point align randomly, without necessarily being coordinated [8,26]. This might induce peaky distributions due to random alignments that could still differ from the uniform distribution. We used the following methods in order to ensure that the effect was not random (see [8,26] for similar approaches): the subject's breathing in a given conversation was associated with the partner's breathing in four other conversations (surrogate associations). The coordination_index was computed for each of these surrogate associations. We then compared the distribution of the coordination_index for the original association with its distribution for the surrogate associations. If these two distributions were different, we considered that a coordinative pattern was present. All comparisons were carried out using the Kolmogorov–Smirnov test.
Number_of_cycles and speech_position were analysed by looking at the distribution of turns into different ranges of values for each variable (table 1, Analysis B.b). Chi-squared tests were used to test these distributions. The butting-in turns were not considered for the analysis of number_of_cycles, as these turns were always achieved within a single breathing cycle. Comparisons between the turn types were run regardless of the partner, and with smooth turns as a reference, due to the small number of interruption and butting-in turns in some categories. The Bonferroni correction was applied to p-values (significant if p < 0.05/5 for number_of_cycles and to p < 0.05/8 for speech_position).
Table 1.
Upper part: number of observations (and percentage) of turns that involved 1, 2, 3 or more breathing cycles (number_of_cycles, see text for details). Lower part: number of observations (and percentage) of turns in different ranges of the position of the turn onset relative to the exhalation phase (speech_position, see text for details). Data are split according to the type of turn (Sm, smooth turn; In, interruption; Bu, butting-in). S.P1, subjects talking with Partner 1; S.P2, subjects talking with Partner 2.
| S.P1 |
S.P2 |
|||||
|---|---|---|---|---|---|---|
| Sm | In | Bu | Sm | In | Bu | |
| number_of_cycles | ||||||
| 1 | 193 (52) | 19 (37) | 45 (100) | 173 (53) | 41 (48) | 74 (100) |
| 2 | 66 (18) | 9 (17) | 0 | 55 (17) | 22 (25) | 0 |
| 3 | 45 (12) | 8 (15) | 0 | 32 (10) | 11 (13) | 0 |
| >3 | 65 (18) | 16 (31) | 0 | 66 (20) | 12 (14) | 0 |
| speech_position | ||||||
| <0.25 | 241 (65) | 35 (67) | 26 (58) | 213 (65) | 57 (68) | 37 (50) |
| 0.25 to 0.5 | 66 (18) | 8 (15) | 11 (24) | 59 (18) | 14 (16) | 11 (15) |
| 0.5 to 0.75 | 46 (13) | 7 (14) | 3 (7) | 37 (12) | 10 (11) | 17 (23) |
| 0.75 to 1 | 16 (4) | 2 (4) | 5 (11) | 17 (5) | 4 (5) | 9 (12) |
3. Results
We first addressed whether breathing and turn-taking rates were related, and whether the conversation induced a global coordination of breathing over the whole exchange, as observed previously for dialogue [33] and in reading and singing [27,28] (§§3a,b). We then analysed the relationship between turns and breathing more locally and considering the type of turn (§§3c–e).
(a). Analysis A.a: the relationship between breathing rate and number of turns exchanged
Taking all the conversations together, we found a median of approximately nine turns for partners and subjects for the 2.5 min conversations (figure 2a). This number was similar for the conversations involving Partners 1 and 2, showing similar turn-taking rates regardless of the partner. Analysis of breathing_rate showed (figure 2b) that it was faster for Partner 2 than Partner 1 (t = 12.7, pMCMC < 0.001). However, subjects did not change their breathing rate accordingly, and realized a similar breathing rate when talking with Partner 1 and Partner 2.
Figure 2.

Number of turns (a) and breathing rate (b). Values are medians for a 2.5 min conversation and dispersion over conversations for Partner 1 (P1) and Partner 2 (P2), and for subjects speaking with Partner 1 (S.P1) and Partner 2 (S.P2).
(b). Analysis A.b: the temporal alignment of subject breathing to partner breathing
The distribution of the coordination_index values, which localize subject breathing relative to partner breathing over the whole dialogue, was analysed (figure 3a). The results are merged for the conversations with Partners 1 and 2, as no significant effect of partner was observed. The original distribution of the coordination_index was not significantly different from that of the surrogate analysis. In other words, when taking all phases of the conversation together, subjects' inhalations were not systematically coordinated with partners' inhalations.
Figure 3.

Distribution of coordination index for subjects' breathing cycles for all the phases of the dialogue (a) and at turn-taking according to the type of turn (b). The number of observations is indicated on each graph. The dotted curves represent the distributions for surrogate associations (see text for details). (Online version in colour.)
In summary, no clear overall relationship appeared between turn-taking and breathing rate, and no coordinative trend was observed between subjects' and partners' inhalations.
(c). Analysis B.a: the relationship between the breathing cycle and the turn
Despite this lack of coordination between breathing and turns at a global level, it is still possible that conversational turns and breathing cycles are related locally. In this case, we might expect that speakers would take a new breath before taking the turn and that they would not delay their speech onset considerably with respect to the exhalation phase. Moreover, we might also expect there to be a preferred number of inhalation events to achieve a turn. The following analyses were restricted to the subjects' data as they are more representative of general behaviour, while the partners were considered as a factor that might influence subjects' behaviour.
The upper part of table 1 displays the distribution of turns according to number_of_cycles. Butting-in attempts were always realized on a single breathing cycle. More than half of the subjects' smooth and interruption turns were also realized within a single breathing cycle. Approximately 80% of all smooth and interruption turns included less than four breathing cycles.
The bottom part of table 1 displays the distribution of turns according to speech_position. Note that it was possible for values of speech_position to be either positive or negative, with negative values corresponding to turns starting during the inhalation phase. However, these negative values in fact represented approximately 5% of the turns and were greater than −0.08 (except for a single value that was excluded from the analyses). Hence, the range of values smaller than 0.25 represents turns that started close to the onset of the exhalation phase. The results show that turns were mostly taken in this early range (more than 50% of the observations were in a range smaller than 0.25, regardless of the turn type, and more than 60% for the smooth and interruption turns). Butting-in occurred later in the exhalation phase (speech_position was greater than 0.5 for approximately 29% of butting-in turn-takings, but for only 17% and 16% for the smooth and interruption turn-takings).
Chi-squared tests showed that all distributions were significantly different than random for both measurements and all types of turn (χ2 > 26, d.f. = 3, p(Bonferroni) < 0.0001), except for number_of_cycles for subjects talking with Partner 1. The comparison of interruption with smooth turns was not reliable for either number_of_cycles or speech_position, while butting-in turns were significantly different than smooth turns for speech_position (χ2 = 16, d.f. = 3, p(Bonferroni) < 0.01).
These results suggest that speech onset at turn-taking was generally well timed with inhalation events, at least when turn-taking was successful. In addition, more than half of the turns were associated with a single breathing cycle, and a large proportion of turns included less than four breathing cycles.
(d). Analysis B.b: the adaptation of the breathing cycle according to dialogue events
In order to better understand how breathing adapts to different dialogue events, we then compared the properties of breathing cycles during listening and speaking as well as turn-taking and turn-holding phases.
We first contrasted the duration and asymmetry of the breathing cycle during listening with all cycles related to speaking events (figure 4). During listening phases, breathing cycles were shorter than during spoken phases (t = 21.8, pMCMC < 0.001). In addition, less asymmetry was observed in listening phases in comparison to spoken phases (t = 44.6, pMCMC < 0.001). This is visible in figure 4, which represents the position of inhalation onset relative to the whole cycle (horizontal lines superimposed on each bar).
Figure 4.
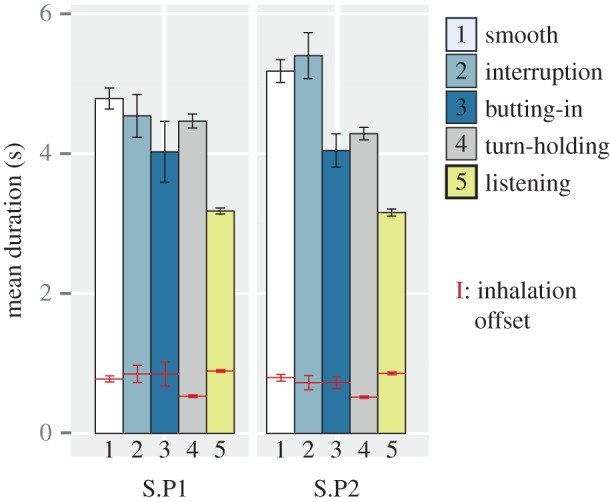
Average duration of subjects' breathing cycle (bars) when talking with Partner 1 (S.P1) and Partner 2 (S.P2), according to the type of cycle (see text for details). The position of inhalation offset relative to the whole cycle duration is superimposed on each bar. Vertical lines indicate standard errors. (Online version in colour.)
Breathing cycles were then split into those at turn-taking (smooth, interruption, butting-in) and those occurring inside a turn (turn-holding). Breathing cycles at turn-taking varied in duration according to the type of turn. When butting-in, breathing cycles were shorter than for smooth or interruption turns (all comparisons t > 2.8, pMCMC < 0.01). In other words, when subjects took a breath and tried to take the turn but failed, they shortened their breathing cycle. Consequently, breathing cycles for butting-in were also less asymmetrical than when taking the turn successfully (all comparisons t > 2.1, pMCMC < 0.05).
When turn-holding, breathing cycles were generally shorter than when turn-taking (t = 4.0, pMCMC = 0.001). Owing to a clear reduction of the inhalation phase, turn-holding cycles were also more asymmetrical than turn-taking ones (t = 9.8, pMCMC < 0.001). These results suggest that speakers reduced inhalation duration inside a turn, which could preserve their turn by indicating to their interlocutor that they have not finished speaking.
The properties of breathing cycles over the different events of the dialogues described above were in general similar when participants spoke with Partners 1 and 2. However, breathing cycles at smooth turn-taking or interruption tended to be longer and more asymmetrical when talking with Partner 2 than Partner 1 (only asymmetry reached significance: t = 2.1, pMCMC < 0.05).
In summary, breathing profiles differed not only between listening and speaking phases but also between turn-taking and turn-holding. To some extent, breathing cycles also changed with respect to the type of turn-taking. This suggests that the ability to coordinate breathing with turn-taking might contribute to the success of turn-taking.
(e). Analysis B.c: the temporal alignment of subject breathing to partner breathing at turn-taking
In conversation, inter-personal coordination may occur at turn-taking and may rest on breathing [8].
In our dataset, we observed some coordinative trends locally, at turn-taking, that were specific to the type of turn (figure 3b). The distribution of the coordination_index for smooth turns was more asymmetrical toward the right, suggesting that subjects tended to inhale during the last part of the partner's exhalation phase, while interruption turns showed the most consistent profile with a clear peak in the latter part of the partner's exhalation phase. For smooth and interruption turns, distributions were different from the uniform and surrogate distributions (p < 0.02 in all cases), showing that there was indeed a non-random pattern of alignment. The distribution for butting-in was sparser and not significantly different than the uniform distribution, which could be due to the smaller number of observations and also to the fact that butting-in turns were less prepared, resulting in failure to interrupt the partner.
In summary, subjects' breathing displayed specific temporal coordination with their conversation partners' breathing at turn-taking. This coordination depended on the type of turn, with more systematic coordination for interruption than smooth turns, suggesting that interruptions were more planned than smooth turns.
4. Discussion
The aim of the current work was to improve our knowledge of breathing in conversation by empirically addressing the idea that breathing could constitute a coordinative unit for turn exchange. Our analyses of a corpus of eleven subjects talking successively with two partners show that: (i) at a global level, when the overall conversation is considered regardless of the activity, turn-taking rate and breathing rate are not strongly related, and breathing cycles are not systematically coordinated between subject and partner; (ii) breathing is, nevertheless, actively involved in turn-taking. This is visible in the fact that most successful turns were taken just after a new inhalation. The relation between inhalation and turn-taking suggests that speakers coordinate breathing to turn-taking; (iii) the average duration of a turn can be expressed in terms of the number of inhalation pauses, with more than half of turns realized on a single breathing cycle and 80% of the turns occurring after less than three inhalation pauses. When occurring inside a turn, inhalations were shorter than when starting a new turn, suggesting that subjects also adapt their breathing to hold turns. These results shed new light on the relationship between turns and breathing in unconstrained dialogue and will be discussed here in relation to previous work.
Our analyses showed no overall inter-personal coordination of breathing when all the phases of dialogue were taken together, and no relationship between breathing and turn-taking rate. In their paper, analysing entrainment between vocal and respiratory activity, Warner et al. [33, p. 1332] mention in their discussion that ‘An additional set of analyses not reported here sought evidence of direct entrainment between the respiration cycles of social-interaction partners, and essentially no relationship was found’. This conclusion was probably driven by analyses similar to the analyses of coordination between vocal and breathing activity that was the focus of Warner et al.'s paper (e.g. cross-spectral analyses based on the extraction of the main low-frequency oscillation in the signal). This conclusion is hard to evaluate, however, as the authors did not provide more information. Yet, the present analyses seem consistent with the absence of entrainment of inter-personal breathing overall during dialogue. We assume that this is the result of different activities (listening and speaking) during conversation, which go hand in hand with switches in breathing frequency. These make a tight coordination of inter-personal breathing and a tight link between breathing and turn-taking rates rather unlikely. Moreover, our finding is consistent with the lack of breathing imitation or coordination observed between listeners and speakers in previous work [23–26].
McFarland [8] did not provide a global analysis of inter-personal coordination of breathing, nor of the relationship between breathing and turn-taking rates. However, using cross-correlation on a time-window around turn transitions, he found in-phase or anti-phase coordination between speakers. This coordination was particularly in evidence when laughing or speaking simultaneously. Our analyses used a finer categorization of turn-taking, based on pragmatic function. Even though our analyses of synchronization were limited to the labelling of the breathing cycle and were not continuous, they provide further evidence of inter-personal temporal coordination of breathing at turn-taking. In particular, we provided evidence that successful interruption rested on a clear anticipation of inhalation. This appeared as a clear peak in the coordination index, with inhalation onset starting at approximately 70% of the interlocutor's breathing cycle. The profile for the coordination index did not show a comparably high peak when a turn was taken smoothly, but a tendency to start inhalation in the last part of the exhalation phase of the interlocutor's breathing cycle. This suggests that interruptions could be planned more systematically than smooth turns and that frequent interruptions in dialogue [30] could also be related to issues in breathing control.
In this study, approximately half of the turns were realized on a single breathing cycle and most turns involved one to three breathing cycles. Moreover, most of the successful turns started at the onset of the exhalation phase: subjects tended to take a breath just before taking their turn. These findings suggest that interlocutors may use the breathing cycle as a unit to anticipate the next turn and that turn constructions are not independent of physiological processes. If Guaïtella [34] suggested that the dialogue rhythm could rest on an implicit knowledge of the duration of the exhalation phase, our observations suggest that turn-taking could also be negotiated implicitly in terms of the number of inhalation pauses inside the turn. This relation between the number of turns and the number of breathing cycles may also depend on the nature of the dialogue. For example, in uncooperative dialogues, one speaker may dominate over the other in taking the floor, and turns may include more breathing cycles than in cooperative dialogues.
Butting-in attempts were delayed relative to the onset of the exhalation phase as compared with smooth and interruption turns. Hence, when subjects tried to interrupt the other speaker without taking a new breath, this interruption tended to fail. Two complementary phenomena could explain this result. Firstly, as the speaker is not taking a new breath, she is not providing inhalation indices (acoustic noises, visual indices of changes rib-cage volume) to her interlocutor that could indicate she will start to speak and interrupt her. Secondly, the absence of inhalation before turn-taking may reduce the amount of air available to ‘fight’ for the turn. The first aim of our study was to describe the kinematics of breathing during conversation. Our set-up was not designed to analyse breathing noises, and the microphones were too far from the speaker's nose and mouth to allow a reliable post hoc analysis of these noises. It might be interesting to address the role of auditory and visual correlates of breathing in the organization of turn-taking, as these indices may contribute to inter-personal adaptation of breathing [25].
The analyses of the duration and asymmetry of the breathing cycles are consistent with previous work and bring new results. Similarly to McFarland and Guaïtella [8,34], breathing cycles during listening were different from speaking phases, with less asymmetrical and shorter breathing cycles in the former than in the latter. We extended these findings by showing that breathing cycles are also linked with the nature of speech events. Breathing cycles inside a turn were shorter and more asymmetrical than cycles at the start of a turn. This could be explained by the fact that when subjects needed to inhale but wanted to keep the turn, they took more rapid inhalations in order to reduce pauses and prevent the other speaker from taking the turn. When taking a breath and taking the turn successfully, breathing cycles were similar in duration and asymmetry for smooth turns and interruptions. When butting-in, however, the breathing cycle was shortened in comparison to successful interruptions, suggesting that subjects quickly reverted to a listener role when their attempt to take the turn failed.
The current study involved only female speakers conversing with two different partners. Subjects and partners exchanged dialogue in a cooperative fashion, both being actively involved in the task. Despite the fact that the two partners were members of the laboratory, and so were not totally naive with regard to the purpose of the experiment, the dialogues were globally fluid and balanced. Dialogues with Partner 2 were, however, characterized by more interruptions, or interruption attempts, as compared with dialogues with Partner 1 (table 1). This could be explained by differences in professional status and age (Partner 1 was a researcher and Partner 2 was a PhD student). Partner 2 also breathed faster than Partner 1, but this difference in partner breathing rate did not affect subject breathing rates. Further studies are required to investigate more systematically how inter-personal adaptation of breathing occurs during dialogue, and how it could sustain the variety of inter-personal alignment observed in conversation [4]. It will also be necessary to analyse the linguistic structure and the acoustic parameters of the spoken text together with the prosodic and nonverbal cues known to be involved in turn-taking [42].
Overall, the results are consistent with the idea that breathing profiles are good correlates of conversational events and timing [8]. Breathing cycles appear as possible organizational units of conversation: taking the turn requires that the speaker takes a breath appropriately with respect to the speech and breathing flow of her interlocutor. We found a relationship between turn-taking and inhalation, but no strong inter-personal coordination in breathing throughout the whole dialogue. Inter-personal coordination of breathing was rather local, related to turn-taking, however, the current dataset did not allow us to split turns into further categories, such as pause interruptions and overlaps [30,31], as the amount of data in these categories was limited. Moreover, the pauses every 2.5 min in our experimental procedure may have broken the dialogue rhythms, and longer time-windows may be required to observe more global coordination and mutual adaptation over time. More systematic investigation of breathing during dialogue is required to further understand how breathing determines inter-personal exchanges. In particular, larger corpora should include longer conversations, speakers with different physiology, subjects with respiratory pathologies, and cross-linguistic comparisons. The analyses of breathing in those corpora would have benefits for speech technology and speech rehabilitation and would further our understanding of how social behaviour could be embodied in physiological rhythms.
Acknowledgements
We thank Jörg Dreyer for technical support, Leonardo Lancia for support in methodological issues, Anna Sapronova for initial speech annotation, our subjects and Caroline Magister for support during the experiments.
Funding statement
This work was supported by a grant received from the Federal Ministry of Education and Research (BMBF) by the Laboratory Phonology group at ZAS (01UG1411).
References
- 1.Stivers T, et al. 2009. Universals and cultural variation in turn-taking in conversation. Proc. Natl Acad. Sci. USA 106, 10 587–10 592. ( 10.1073/pnas.0903616106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jaffe J, Feldstein S. 1970. Rhythms of dialogue. London, UK: Academic Press. [Google Scholar]
- 3.Clark HH. 1996. Using language. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 4.Garrod S, Pickering MJ. 2009. Joint action, interactive alignment, and dialog. Topics Cogn. Sci. 1, 292–304. ( 10.1111/j.1756-8765.2009.01020.x) [DOI] [PubMed] [Google Scholar]
- 5.Levenson RW, Gottman JM. 1983. Marital interaction: physiological linkage and affective exchange. J. Pers. Soc. Psychol. 45, 587–597. ( 10.1037/0022-3514.45.3.587) [DOI] [PubMed] [Google Scholar]
- 6.Helm JL, Sbarra D, Ferrer E. 2012. Assessing cross-partner associations in physiological responses via coupled oscillator models. Emotion 12, 748–762. ( 10.1037/a0025036) [DOI] [PubMed] [Google Scholar]
- 7.Konvalinka I, Xygalatas D, Bulbulia J, Schjødt U, Jegindø E-M, Wallot S, Van Orden G, Roepstorff A. 2011. Synchronized arousal between performers and related spectators in a fire-walking ritual. Proc. Natl Acad. Sci. USA 108, 8514–8519. ( 10.1073/pnas.1016955108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McFarland DH. 2001. Respiratory markers of conversational interaction. J Speech Lang. Hear. Res. 44, 128–143. ( 10.1044/1092-4388(2001/012)) [DOI] [PubMed] [Google Scholar]
- 9.Perkins WH, Kent RD. 1986. Functional anatomy of speech, language, and hearing. Boston, MA: Allyn and Bacon. [Google Scholar]
- 10.Shea SA. 1996. Behavioural and arousal-related influences on breathing in humans. Exp. Physiol. 81, 1–26. [DOI] [PubMed] [Google Scholar]
- 11.Aleksandrova NP, Breslav IS. 2009. Human respiratory muscles: three levels of control. Hum. Physiol. 35, 222–229. ( 10.1134/S0362119709020133) [DOI] [PubMed] [Google Scholar]
- 12.Hixon TJ, Goldman MD, Mead J. 1973. Kinematics of the chest wall during speech production: volume displacements of the rib cage, abdomen, and lung. J. Speech Hear. Res. 16, 78–115. ( 10.1044/jshr.1601.78) [DOI] [PubMed] [Google Scholar]
- 13.Conrad B, Schönle P. 1979. Speech and respiration. Arch. Psychiatr. Nervenkrankheiten 226, 251–268. ( 10.1007/BF00342238) [DOI] [PubMed] [Google Scholar]
- 14.Hoit JD, Lohmeier HL. 2000. Influence of continuous speaking on ventilation. J. Speech Lang. Hear. Res. 43, 1240–1251. ( 10.1044/jslhr.4305.1240) [DOI] [PubMed] [Google Scholar]
- 15.McFarland DH, Smith A. 1992. Effects of vocal task and respiratory phase on prephonatory chest wall movements. J. Speech Lang. Hear. Res. 35, 971–982. ( 10.1044/jshr.3505.971) [DOI] [PubMed] [Google Scholar]
- 16.Whalen DH, Kinsella-Shaw JM. 1997. Exploring the relationship of inspiration duration to utterance duration. Phonetica 54, 138–152. ( 10.1159/000262218) [DOI] [PubMed] [Google Scholar]
- 17.Winkworth AL, Davis PJ, Ellis E, Adams RD. 1994. Variability and consistency in speech breathing during reading: lung volumes, speech intensity, and linguistic factors. J. Speech Lang. Hear. Res. 37, 535–556. ( 10.1044/jshr.3703.535) [DOI] [PubMed] [Google Scholar]
- 18.Winkworth AL, Davis PJ, Adams RD, Ellis E. 1995. Breathing patterns during spontaneous speech. J. Speech Lang. Hear. Res. 38, 124–144. ( 10.1044/jshr.3801.124) [DOI] [PubMed] [Google Scholar]
- 19.Wang YT, Green JR, Nip IS, Kent RD, Kent JF. 2010. Breath group analysis for reading and spontaneous speech in healthy adults. Folia Phoniatr. Logopaedica 62, 297–302. ( 10.1159/000316976) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fuchs S, Petrone C, Krivokapić J, Hoole P. 2013. Acoustic and respiratory evidence for utterance planning in German. J. Phon. 41, 29–47. ( 10.1016/j.wocn.2012.08.007) [DOI] [Google Scholar]
- 21.Rochet-Capellan A, Fuchs S. 2013. The interplay of linguistic structure and breathing in German spontaneous speech. In Proc. 14th Annu. Conf. Int. Speech Commun. Assoc. Speech in Life Sciences and Human Societies (eds F Bimbot, C Cerisara, C Fougeron, G Gravier, L Lamel, F Pellegrino, P Perrier), pp. 2013-2016. Lyon, France: ISCA. [Google Scholar]
- 22.Conrad B, Thalacker S, Schönle P. 1983. Speech respiration as an indicator of integrative contextual processing. Folia Phoniatr. (Basel) 35, 220–225. ( 10.1159/000265766) [DOI] [PubMed] [Google Scholar]
- 23.Ainsworth S. 1939. Empathic breathing of auditors while listening to stuttering speech. J. Speech Lang. Hear. Res. IV, 139–156. [Google Scholar]
- 24.Brown CT. 1962. Introductory study of breathing as an index of listening. Speech Monogr. 29, 79–83. ( 10.1080/03637756209375340) [DOI] [Google Scholar]
- 25.Garssen B. 1979. Synchronization of respiration. Biol. Psychol. 8, 311–315. ( 10.1016/0301-0511(79)90013-9) [DOI] [PubMed] [Google Scholar]
- 26.Rochet-Capellan A, Fuchs S. 2013. Changes in breathing while listening to read speech: the effect of reader and speech mode. Front. Psychol. 4, 960 ( 10.3389/fpsyg.2013.00906) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bailly G, Rochet-Capellan A, Vilain C. 2013. Adaptation of respiratory patterns in collaborative reading. In Proc. 14th Annu. Conf. Int. Speech Commun. Assoc. Speech in Life Sciences and Human Societies (eds F Bimbot, C Cerisara, C Fougeron, G Gravier, L Lamel, F Pellegrino, P Perrier), pp. 1652–1655. Lyon, France: ISCA. [Google Scholar]
- 28.Müller V, Lindenberger U. 2011. Cardiac and respiratory patterns synchronize between persons during choir singing. PLoS ONE 6, e24893 ( 10.1371/journal.pone.0024893) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wilson M, Wilson TP. 2005. An oscillator model of the timing of turn-taking. Psychon. Bull. Rev. 12, 957–968. ( 10.3758/BF03206432) [DOI] [PubMed] [Google Scholar]
- 30.Beattie GW. 1982. Turn-taking and interruption in political interviews: Margaret Thatcher and Jim Callaghan compared and contrasted. Semiotica 39, 93–114. ( 10.1515/semi.1982.39.1-2.93) [DOI] [Google Scholar]
- 31.Beňuš Š, Gravano A, Hirschberg J. 2011. Pragmatic aspects of temporal accommodation in turn-taking. J. Pragmat. 43, 3001–3027. ( 10.1016/j.pragma.2011.05.011) [DOI] [Google Scholar]
- 32.Sacks H, Schleghoff EA, Jefferson G. 1974. A simplest systematics for the organization of turn-taking for conversation. Language 50, 696–735. ( 10.2307/412243) [DOI] [Google Scholar]
- 33.Warner RM, Waggener TB, Kronauer RE. 1983. Synchronized cycles in ventilation and vocal activity during spontaneous conversational speech. J. Appl. Physiol. 54, 1324–1334. [DOI] [PubMed] [Google Scholar]
- 34.Guaïtella I. 1992. Etude expérimentale de la respiration en dialogue spontané. [Experimental study of respiration in spontaneous dialogue]. Folia Phoniatr. 45, 273–279. ( 10.1159/000266275) [DOI] [PubMed] [Google Scholar]
- 35.Autesserre D, Nishinuma Y, Guaitella I. 1989. Breathing, pausing, and speaking in dialogue. In 1st Eur. Conf. Speech Communication and Technology (EUROSPEECH 1989) (eds J Tubach, JJ Mariani), pp. 2433–2436. Paris, France: ISCA. [Google Scholar]
- 36.Zimmermann DH, West C. 1996. Sex roles, interruptions and silences in conversation. In Towards a critical sociolinguistics (ed. R Singh), pp. 211–235. Amsterdam, The Netherlands: John Benjamin. [Google Scholar]
- 37.Bilous FR, Krauss RM. 1988. Dominance and accommodation in the conversational behaviours of same and mixed-gender dyads. Lang. Commun. 8, 183–194. ( 10.1016/0271-5309(88)90016-X) [DOI] [Google Scholar]
- 38.Stephens GJ, Silbert LJ, Hasson U. 2010. Speaker–listener neural coupling underlies successful communication. Proc. Natl Acad. Sci. 107, 14 425–14 430. ( 10.1073/pnas.1008662107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Konno K, Mead J. 1967. Measurement of the separate volume changes of rib cage and abdomen during breathing. J. Appl. Physiol. 22, 407–422. [DOI] [PubMed] [Google Scholar]
- 40.Banzett RB, Mahan ST, Garner DM, Brughera A, Loring SH. 1995. A simple and reliable method to calibrate respiratory magnetometers and respitrace. J. Appl. Physiol. 79, 2169. [DOI] [PubMed] [Google Scholar]
- 41.Rochet-Capellan A, Schwartz JL. 2007. An articulatory basis for the labial-to-coronal effect: /pata/ seems a more stable articulatory pattern than /tapa/. J. Acoust. Soc. Am. 121, 3740–3754. ( 10.1121/1.2734497) [DOI] [PubMed] [Google Scholar]
- 42.R Development Core Team. 2011. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 43.Baayen R. 2008. Analyzing linguistic data: a practical introduction to statistics using R. Cambridge, UK: Cambridge University Press. [Google Scholar]



