Abstract
Torpor is thought to slow age-related processes and to sustain growth and fattening of young individuals. Energy allocation into these processes represents a challenge for juveniles, especially for those born late in the season. We tested the hypothesis that late-born juvenile garden dormice (Eliomys quercinus) fed ad libitum (‘AL’, n = 9) or intermittently fasted (‘IF’, n = 9) use short torpor bouts to enhance growth and fat accumulation to survive winter. IF juveniles displayed more frequent and longer torpor bouts, compared with AL individuals before hibernation. Torpor frequency correlated negatively with energy expenditure and water turnover. Hence, IF juveniles gained mass at the same rate, reached similar pre-hibernation fattening and displayed identical hibernating patterns and mass losses as AL animals. We found no group differences in relative telomere length (RTL), an indicator of ageing, during the period of highest summer mass gain, despite greater torpor use by IF juveniles. Percentage change in RTL was negatively associated with mean and total euthermic durations among all individuals during hibernation. We conclude that torpor use promotes fattening in late-born juvenile dormice prior to hibernation. Furthermore, we provided the first evidence for a functional link between time spent in euthermy and ageing processes over winter.
Keywords: Eliomys quercinus, intermittent fasting, pre-hibernation fattening, winter survival, doubly labelled water, telomere length
1. Introduction
In seasonal environments, animals have to optimize the differential allocation of limited resources to processes of maintenance, growth and reproduction. To maximize individual fitness, animals in seasonal habitats employ diverse coping strategies, such as migration, food and/or energy storage or the use of hypometabolic states. In particular, many small mammals and birds have developed specific mechanisms of energy and water savings, achieved by active and controlled reductions of metabolic rate (MR) and hence body temperature (Tb), i.e. daily torpor or hibernation [1,2].
Before hibernation, most species consume large amounts of food and store energy laid down as white adipose tissue to survive several months of the cold season [3]. Most hibernators do not eat during the winter but rely entirely on body fat stores [3]. Therefore, gaining sufficient fat in autumn to last throughout hibernation is an important process and is essential for the individual's winter survival. Obtaining sufficient fat stores is particularly difficult for juveniles, especially for those born late in the season, due to the limited time available for growth and fattening prior to their first hibernation season. Hence, in juveniles pre-hibernation, body mass is a strong predictor of overwintering survival [4,5].
Beside the traditional view of torpor and hibernation as mechanisms that provide energy and water savings, other major functions of torpor have been discovered over the past decade [6]. For instance, torpor use can sustain and promote growth and pre-hibernation fattening in young individuals [7,8]. We have recently demonstrated that late-born juvenile garden dormice use short bouts of torpor to counteract intermittent periods of fasting (twice a week), sustaining a faster body mass gain compared with ad libitum (AL)-fed juveniles prior to hibernation [8]. Torpor and hibernation have also been associated with the slowing of ageing processes and the increase of longevity [9,10]. For instance, frequency of torpor use was positively associated with changes in relative telomere length (RTL), an indicator for biological ageing strongly impacted by oxidative stress [11], in the Djungarian hamster (Phodopus sungorus), a daily heterotherm [10]. In the hibernating edible dormouse (Glis glis), the rate of decrease in RTL was positively correlated with overwinter body mass loss, which represents an index of the number of periodic arousals, i.e. the regular phases of rewarming that interrupt prolonged torpor in all hibernators, or the time spent in euthermy, in hibernating edible dormice [12]. Hence, ageing processes are expected to occur mainly during torpor arousals, i.e. processes of rewarming from torpid to euthermic Tb, and the subsequent inter-bout euthermy (IBE), i.e. the time spent euthermic between two torpor bouts (starting at the end of the rewarming process and ending at the onset of the entrance into the next torpor bout), phases of high levels of MR and therefore potential for oxidative stress [10,12]. Opportunistic use of torpor bouts, interspaced by daylong active phases, is associated with the slowing of age-related processes [10]. Conversely, periodically rewarming from deep torpor and staying euthermic for only several hours during months of winter hibernation seems to impact negatively on physiological ageing mechanisms [12].
Prior to their first winter, juvenile hibernators have not only to accumulate sufficient fat reserves, but also to sustain the high energy-demanding process of structural growth. Somatic or structural growth is one of the major challenges occurring in the early life of an individual and sub-optimal or delayed growth can be deleterious to health at adulthood and individual fitness [13]. The situation becomes even more challenging for juveniles born late in the reproductive season when the time available before winter for both growth and fattening is minimal and when they might already have to cope with periods of reduced food availability [14,15], which is particularly true for insectivorous species [16,17].
Garden dormice (Eliomys quercinus, Gliridae) are small insectivorous and omnivorous hibernators endemic to Europe [18]. They are known for accumulating large fat reserves and for their long hibernation periods (October to March). Both in the field and in captivity, a second breeding event late in the season can occur [8,19,20].
Here, we aimed to extend our previous work on torpor use and pre-hibernation body mass gain in juvenile garden dormice born late in the season [8]. In this study, we therefore determined whether late-born juvenile garden dormice intermittently fasted (IF) (i) are able to efficiently compensate a higher frequency (three times a week) of fasting, (ii) gain similar pre-hibernation fat reserves and (iii) show similar subsequent hibernating patterns and overwintering mass losses, compared with AL-fed individuals. Specifically, we hypothesized that late-born IF juveniles will rely on short torpor bouts, saving energy and water, to promote accumulation of fat reserves, reaching similar pre-hibernation content as animals fed AL. Furthermore, we aimed to investigate how torpor use and hibernating patterns are associated with ageing processes during the period of highest summer mass gain and over winter hibernation. We hypothesized that (i) telomere shortening will be attenuated or absent among juveniles that use torpor frequently during the period of highest summer mass gain, and (ii) over winter hibernation, individuals spending more time in torpor and rewarming less often will show less telomere shortening compared with individuals spending more time in euthermy and showing a higher frequency of arousals.
2. Material and methods
(a). Animals
Eighteen juvenile garden dormice (seven males and 11 females) were used in these experiments. All individuals were born late in the reproductive season (11–15 August) and maintained under captive conditions at the Research Institute of Wildlife Ecology (University of Veterinary Medicine, Vienna, Austria; latitude 48°15′ N, longitude 16°22′ E). Each dormouse was housed in an individual cage (60 × 40 × 40 cm) equipped with branches and a nest-box filled with hay. Juveniles were kept indoors under natural variations of photoperiod and ambient temperature (Ta). The experimental room was poorly insulated and no heating source was provided inside the room and Ta of the room passively followed external temperature fluctuations. Each dormouse was marked with a miniature subcutaneous PIT tag (Destron Fearing, Life Chip Biotherm, Hvidovre, Denmark, http://www.destronfearing.com; temperature range: more than 25°C) for identification and measurement of subcutaneous Tb (i.e. whether or not animals were torpid). Food consisted of cat pellets, which contained 30% protein, 10% fat, 60% carbohydrate and sufficient vitamins and minerals (Topix, Saturn Petfood, GmbH, Bremen, Germany). Water was provided AL.
(b). Protocol overview
All animals were weaned at an age of five weeks and entered the experiments after one week of habituation to cages and nests. Then, juveniles were followed during the period of highest summer mass gain (weeks 0–4) and their subsequent winter hibernation (three months, from 8 November to 3 February). Ta was continuously recorded during the entire experiment using a temperature logger (EL-USB-2, Lascar Electronics, UK; resolution: 0.5°C, accuracy: ±0.5°C) placed next to the animal cages. During the pre-hibernation period, mean Ta gradually decreased from 20°C down to approximately 10°C, in steps of approximately 1°C per day (electronic supplementary material, figure S1). Then, Ta remained low (less than 10°C) during the entire winter, with values below 5°C during cold months.
During the period of highest summer mass gain, nine animals had access to food AL (‘AL group’) and nine were IF for 24 h three times a week (‘IF group’). Animals were never fasted for 2 days in a row. Torpor use was monitored continuously using a small temperature logger (see below for further details on logger specifications) in each nest. When juveniles started to display prolonged (more than 24 h) torpor bouts (i.e. after four weeks of experiment), the body composition and metabolic (energy and water) turnover of each individual were determined by doubly labelled water (DLW). Then, hibernation was induced, on 8 November (after six weeks of experiment), in all individuals maintained individually, by removing their food supply. Water was still provided AL.
As most juveniles had lost a significant percentage of body mass after one month of hibernation (IF: −12.8 ± 0.7, AL: −11.8 ± 0.7%), individuals were then grouped to allow huddling, in order to reduce mass loss and to ensure their survival. For each condition (AL or IF), two groups of four and five juveniles, respectively, were housed in two separate nests. Animals were hibernating under these conditions for two additional months. In the wild, garden dormice hibernate from one to seven months, depending on the latitude and/or altitude of their habitat. In Mediterranean climates, hibernation lasts only one to three months [20]. During the first winter month, hibernating patterns from single individuals were continuously recorded, except in four individuals, from which the logger data could not be retrieved (due to battery failures). After three months of hibernation, all animals were sacrificed and stored at −80°C for subsequent determination of fat contents.
(c). Body mass and food intake
Once a week, each individual was captured and weighed to the nearest of 0.1 g (Mettler Toledo, PM34, Delta Range). For each individual, body mass gain was calculated as the percentage of mass change relative to its initial body mass (week 0).
For each individual, weekly food intake was measured by weighing initial and remaining food masses once a week, always on the same day. Spilled food was also collected and re-weighed, in order to correct food intake calculations. Food intake in grams for each individual was converted into kilojoules, by using equivalents of 16.7, 37.2 and 16.7 kJ g−1 of protein, fat and carbohydrate, respectively [21]. Food composition with a coefficient of hydration of 9% was provided by the manufacturer, and water content was verified by drying samples.
(d). Measurements of torpor use and hibernating patterns
For each individual, torpor use during the study period was assessed by using a custom-made nest equipped with a temperature logger (build at the Research Institute of Wildlife Ecology, Vienna, Austria; resolution: 0.2°C, accuracy: ±0.06°C), on which the animal was sitting while resting and possibly entering torpor. This technique, previously described by Willis et al. [22], allowed us to record torpor patterns for each juvenile, during single housing. From torpor patterns (electronic supplementary material, figure S2), we then determined two parameters (torpor frequency and torpor duration) during the period of highest summer mass gain and six additional parameters (euthermic time before hibernation onset, number of arousals, mean torpor duration, total and mean IBE durations and total euthermic duration) during the winter hibernation period (for details, see the electronic supplementary material).
(e). Determination of body composition, total energy expenditure and water turnover
Pre-hibernation body composition, total energy expenditure (TEE) and water turnover (rH2O) were determined (for practical reasons) by the multi-point DLW methodology during a 6-day period after the first four weeks of the experiment [23]. Post-hibernation body fat levels were assessed by the Soxhlet method [24] (for details, see the electronic supplementary material).
(f). Determination of relative telomere lengths
RTL were determined in all individuals at the start of the experiments and after five weeks of treatments (i.e. one week before the hibernation start), and after three months of winter hibernation. We sampled from each individual epithelium tissue by gently scrapping inside the animal's mouth with a sterile mouth swab (Gynobrush, Heinz Herenz, Hamburg, Germany). Samples were collected individually in a re-suspension buffer (Sigma-Aldrich, Austria) and stored at −20°C. DNA was then extracted by using a DNA extraction kit (GenElute Mammalian Genomic DNA Minipre kit, Sigma, Austria) and stored at −80°C for subsequent telomere length analyses, by using a well-validated qPCR method [25,26] (for details, see the electronic supplementary material).
(g). Statistical analyses
Data analyses were carried out using R v. 2.15.1 [27]. The normality of residuals from statistical models was assessed by inspecting quantile–quantile-plots and histograms of distributions and, if necessary, response variables were Box-Cox transformed to achieve normality. For the period of highest summer mass gain, we used analyses of variance (R-package ‘car’ [28]) or linear mixed-effects models to test effects of group and time (week as a factor; subsequently called ‘time category’) on body mass gain, food intake, torpor variables (with food intake as explanatory variable) and RTL. Furthermore, we also used analysis of variance to assess effects of food intake and torpor frequency on body mass gain across both feeding groups. For the first month of winter hibernation, we employed linear mixed-effects models to assess group effect on torpor variables. Finally, ranged major axis models (R package ‘lmodel2’ [29,30]) were used for the different regression analyses. Owing to the limited sample size, and in order to avoid over-fitting, we restricted all statistical models to a maximum three predictor variables plus, in certain cases, one two-way interaction that was determined a priori to test specific hypotheses. p < 0.05 was considered as significant and all values are means ± s.e.m. (for details, see the electronic supplementary material).
3. Results
(a). Period of highest summer mass gain
(i). Food intake
During the period of highest summer mass gain, food intake increased significantly. As imposed by the protocol, IF individuals ate significantly less than AL individuals (1086.1 ± 304.8 versus 1269.9 ± 350.8 kJ/week; table 1). Because food intake was limited to 4 days in the IF group, mean daily energy intake was, however, higher in IF than in AL individuals (271.5 ± 12.7 versus 181.4 ± 8.4 kJ d−1, F = 35.1, p < 0.001).
Table 1.
Parameters of analyses of variance for the effects of time category and group on body mass gain, food intake, torpor frequency and total torpor duration, as well as for the effects of food intake and torpor frequency on body mass gain. Food intake was also added as an explanatory variable in the model for torpor frequency and total torpor duration. p-values shown in italic correspond to statistically significant and interpretable values.
| parameter | term | χ2 | p-value |
|---|---|---|---|
| food intake | time category | 14.96 | <0.001 |
| group | 8.21 | 0.023 | |
| torpor frequency | time category | 19.54 | <0.001 |
| group | 2.80 | 0.09 | |
| food intake | 17.63 | <0.001 | |
| time category : group | 31.97 | <0.001 | |
| total torpor duration | time category | 18.20 | <0.001 |
| group | 6.74 | <0.001 | |
| food intake | 17.54 | <0.001 | |
| time category : group | 14.10 | 0.003 | |
| body mass gain | time category | 140.87 | <0.001 |
| group | 0.42 | 0.29 | |
| time category : group | 0.93 | 0.82 | |
| body mass gain | food intake | 95.91 | <0.001 |
| torpor frequency | 15.42 | <0.001 | |
| food intake : torpor frequency | 5.24 | 0.016 |
(ii). Torpor parameters
The time courses of torpor frequency and total torpor duration differed significantly between IF and AL animals, as suggested by the highly significant time category–group interaction (table 1 and figure 1a,b). IF juveniles started to use torpor already in the first week, and subsequently increased torpor use (both frequency and duration) until week 4, whereas AL individuals displayed bouts of torpor only starting in the third week of the experiments (figure 1a,b). Food intake was also negatively correlated with both torpor frequency and total torpor duration (table 1). Dormice with low food intake used torpor more often and showed longer torpor bouts. As expected, minimal Tb during torpor was closely related to decreasing Ta over the period of highest summer mass gain, from 15.9 ± 0.2°C in week 1 down to 8.3 ± 0.1°C in week 4.
Figure 1.
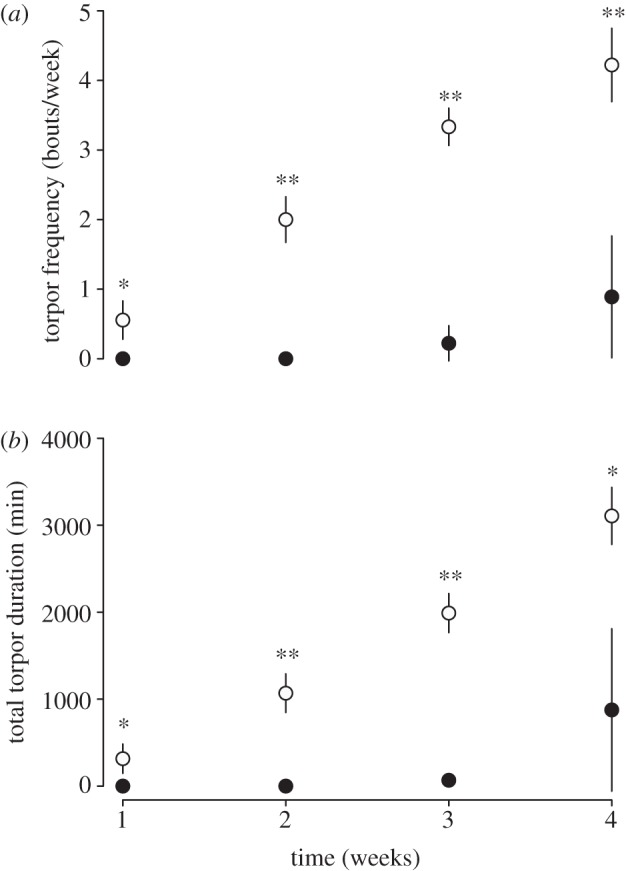
Torpor frequency (a) and total torpor duration (b) of AL (filled circles) or IF (open circles) juvenile garden dormice during the period of highest summer mass gain. There was a highly significant difference between groups over the four weeks (table 1). Tukey's post hoc comparisons: *p < 0.05, **p < 0.01.
TEE and rH2O did not significantly differ between feeding groups (AL versus IF: 106.7 ± 20.5 versus 98.9 ± 14.8, F = 1.62, p = 0.22). However, TEE and rH2O were negatively correlated with torpor frequency (TEE: intercept = 119.5, slope = −5.2, adjusted R2 = 0.50, p = 0.001; electronic supplementary material, figure S3A; rH2O: intercept = 26.5, slope = −1.2, adjusted R2 = 0.30, p = 0.013; electronic supplementary material, figure S3B).
(iii). Body mass gain and body composition prior to hibernation
At the start of the experiment, body mass did not differ between groups (AL versus IF: 44.2 ± 1.3 versus 44.3 ± 1.6 g, t = −0.03, p = 1.0). During the period of highest summer mass gain, rates of body mass gain also did not differ significantly between AL and IF individuals (figure 2 and table 1).
Figure 2.
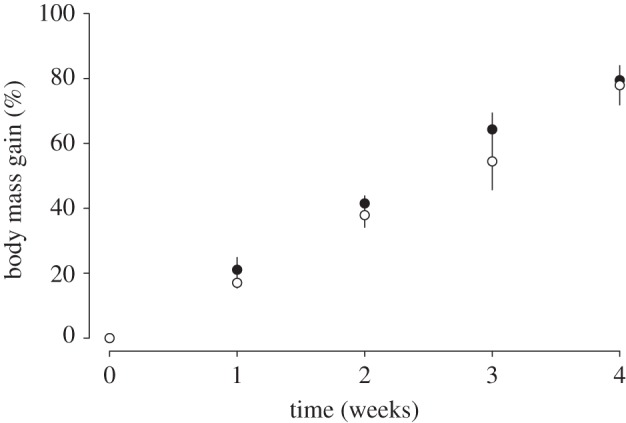
Body mass gain (% of the initial body mass) of late-born juvenile garden dormice fed AL (filled circles) or IF (open circles) during the period of highest summer mass gain. There was no significant difference in body mass gain between IF and AL group over the four weeks (table 1).
An analysis across both feeding groups showed that during the period of highest summer mass gain, both food intake and torpor frequency independently explained a significant amount of variation in body mass gain (table 2). Furthermore, the use of torpor had a much greater effect on body mass gain in individuals with high food intake, compared with animals with a low food intake, which was reflected by a significant food intake–torpor frequency interaction (table 2).
Table 2.
Parameters of winter hibernation for AL and IF juveniles. No significant differences were found between animal groups.
| parameter | AL juveniles | IF juveniles | F-value | p-value |
|---|---|---|---|---|
| euthermic time before hibernation onset (days) | 1.33 ± 0.59 | 1.39 ± 0.44 | 1.10 | 0.43 |
| number of arousals | 3.3 ± 0.8 | 3.0 ± 0.7 | 0.22 | 0.96 |
| mean torpor duration (days) | 6.6 ± 1.2 | 7.1 ± 0.8 | 0.93 | 0.52 |
| total IBE duration (days) | 0.96 ± 0.25 | 0.85 ± 0.28 | 0.23 | 0.96 |
| mean IBE duration (days) | 0.29 ± 0.04 | 0.28 ± 0.03 | 0.36 | 0.89 |
| total euthermic duration (days) | 2.3 ± 0.7 | 2.2 ± 0.4 | 0.29 | 0.93 |
| body mass post-hibernation (g) | 61.8 ± 3.1 | 61.7 ± 4.3 | 2.41 | 0.13 |
| fat mass post-hibernation (g) | 20.6 ± 1.5 | 20.6 ± 2.6 | 1.19 | 0.33 |
| fat-free mass post-hibernation (g) | 41.0 ± 1.7 | 41.1 ± 2.3 | 3.13 | 0.08 |
After the period of highest summer mass gain, fat mass and fat-free mass did not differ between AL and IF juveniles (fat mass: 28.8 ± 2.4 versus 27.5 ± 3.8 g, t = −0.3, p = 0.76; electronic supplementary material, figure S4A; fat-free mass: 53.8 ± 2.5 versus 54.5 ± 1.7 g, t = 0.21, p = 0.84; electronic supplementary material, figure S4B). Furthermore, no group difference in slope of the regressions of pre-hibernation body mass on fat mass was detected (group: t = −0.24, p = 0.81; body mass: t = 3.22, p = 0.007), suggesting that AL and IF juveniles allocated energy in the same way to growth and fattening.
(iv). Relative telomere lengths
At the start of the experiment, RTL did not differ between groups (AL versus IF: 0.86 ± 0.05 versus 0.99 ± 0.07 units, t = 1.33, p = 0.20; electronic supplementary material, figure S5). Furthermore, RTL did not significantly change over the period of highest summer mass gain (estimate = −0.004 ± 0.060, t = −0.07, p = 0.94; electronic supplementary material, figure S5), with no group difference (estimate = 0.089 ± 0.072, t = 1.23, p = 0.23; electronic supplementary material, figure S5). Furthermore, torpor frequency had no significant effects on RTL (estimate = 0.0001 ± 0.025, t = 0.004, p = 1.00).
(b). Winter hibernation
During the first month of winter hibernation (i.e. when individuals were hibernating singly in nests), both groups of juveniles showed similar patterns of hibernation; no significant differences were found between feeding groups (table 2).
Body mass, fat mass and fat-free mass did not significantly differ between animal groups after winter hibernation (table 2). As a consequence, AL and IF juveniles lost a similar percentage of body mass, fat mass and fat-free mass over hibernation (figure 3). Interestingly, the percentage loss of fat mass and fat-free mass over the three months of hibernation did not significantly differ from each other (figure 3, Tukey's post hoc test: p = 0.9). Moreover, we found no significant relations with pre-hibernation body mass, neither for fat mass loss (F = 1.71, p = 0.22), nor for fat-free mass loss (F = 0.93, p = 0.35).
Figure 3.
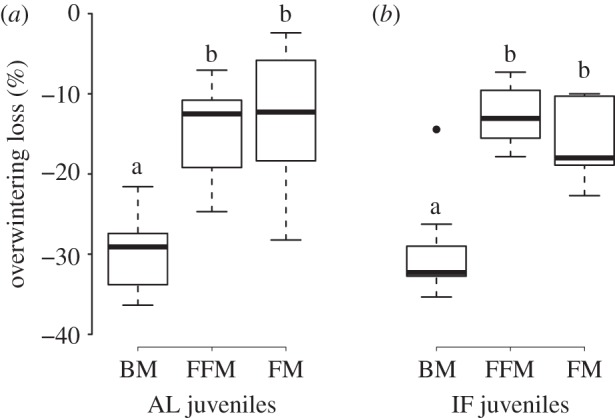
Box-plot graphs of overwintering loss of body mass (‘BM’), fat mass (‘FM’) and fat-free mass (‘FFM’) of (a) late-born juvenile garden dormice fed AL (‘AL juveniles') and (b) IF juveniles. Groups differing significantly (p < 0.01, Tukey's post hoc comparisons) are denoted by different superscripts.
Over the three months of winter hibernation, RTL did not significantly change (time effect: −0.079 ± 0.086, z = 0.92, p = 0.36), and no significant differences in RTL were detected between AL and IF individuals (group effect: −0.002 ± 0.092, z = 0.02, p = 0.99). However, changes in RTL over winter were negatively associated with the mean IBE duration across all juveniles of the first winter month (intercept = 343.39, slope = −37.5, adjusted R2 = 0.19, p = 0.02, figure 4a). Furthermore, RTL changes were also negatively correlated with the total time spent in euthermy during hibernation (i.e. total euthermic duration) across all individuals of the first winter month (intercept = 238.97, slope = −2.79, adjusted R2 = 0.18, p = 0.03, figure 4b).
Figure 4.
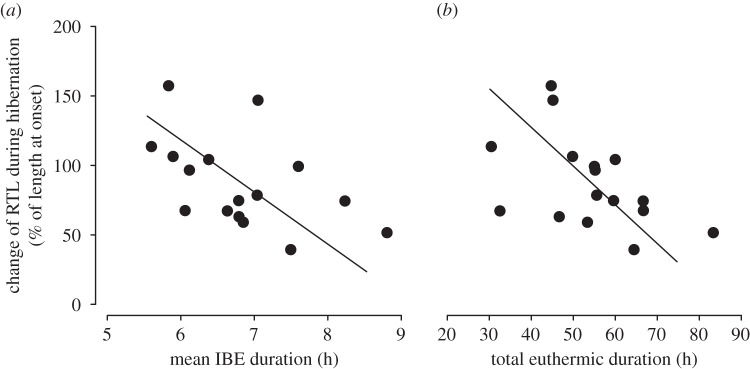
Change of RTL during hibernation (% of length at onset) as a function of (a) the mean IBE duration and (b) the total euthermic duration, across all animals.
4. Discussion
(a). Use of torpor compensates for food shortage during fattening in juvenile garden dormice
This study shows that torpor use promotes body mass gain and fat accumulation prior to hibernation and that the use of torpor can completely compensate periods of intermittent food shortage. Juvenile garden dormice experiencing intermittent starvation (IF) showed higher torpor frequency and total torpor duration prior to hibernation compared with individuals fed AL. As a consequence, IF juveniles were able to gain body mass at the same rate, and to ensure a growth level and a pre-hibernation fat content similar to AL-fed animals.
A similar energy-compensating role of torpor has been shown in insectivorous long-eared bats (Plecotus auritus) in preparation for hibernation [31], and also in both captive and wild hummingbirds at night prior to migration or at migratory stop-over. Captive hummingbirds (Selasphorus rufus) enter the longest and most frequent bouts of spontaneous torpor during the autumnal migration period [32].
We also found that torpor use had a much greater effect on body mass gain in individuals with high food intake, as reflected by the synergistic effect of the use of torpor and food intake on body mass gain (table 1). These results are in line with findings in food-storing hibernators, in which high energy assimilation is associated with increased use of torpor [33]. For instance, the proportion of time that different individuals of eastern chipmunks (Tamias striatus) spent in torpor was highly variable, positively correlated with dry matter digestibility and negatively correlated with energy consumption. Thus, by both increasing conversion efficiency and reducing energy requirements, torpor appears to provide a double benefit for energy conservation in food-storing hibernators [33]. It seems that, in late-born juvenile garden dormice, torpor use by well-fed individuals allows a greater amount of ‘excess’ energy to be diverted to enhancing growth and fattening, whereas torpor in low-fed individuals might principally compensate for the reduced food intake while still allowing some energy to be invested in growth.
(b). Potential limits and costs of torpor use prior to hibernation
IF juvenile dormice spent more time torpid compared with AL individuals, saving energy prior to hibernation. Despite lower energy intake by IF juveniles, we cannot exclude that the ingestion of similar amounts of food in a shorter time (4 days instead of 7) may have increased the passage rate of food through the gastrointestinal tract, leading to decreased energy assimilation coefficients [34]. However, the enhancing effect of torpor use on body mass gain is consistent with the hypothesis by Humphries et al. [33] that energy assimilation coefficients are not reduced, but rather increased, in animals using torpor frequently.
In our study, reducing energy availability resulted in increased torpor use. We also found that, as Ta decreased, torpor depth increased. Several studies have already shown that food restriction triggers the use of torpor [35–37]. In addition to food limitation, cold exposure can also exert a modulating effect on torpor use [38,39]. However, when food availability was sufficient, juveniles used torpor less frequently, which also indicates that torpor has costs [40–43]. Costs of torpor could be related to increased exposure to reactive oxygen species during rewarming phases [44,45], a view not supported by this study, as we did not find any relation between torpor use and telomere loss during pre-hibernation. Alternatively, torpor could also be associated with negative effects on immune competence, which is thought to be reduced at low Tb (approx. 15°C) while most pathogens remain active [46].
Interestingly, even if juveniles employed torpor more frequently when fasted intermittently, we found no difference in RTL between the two groups over the pre-hibernation period. This result contrasts with the study of Turbill et al. [10], showing that frequency of spontaneous torpor use was positively associated with changes in RTL in the Djungarian hamster (P. sungorus). This discrepancy could be due to the much longer period of torpor assessment in hamsters [10] and different control mechanisms of these two forms of hypothermia (fasting-induced versus spontaneous torpor) [47].
(c). Consequences on overwintering hibernating patterns and age-related processes
Our results show that juveniles in the IF group hibernated in the same way as the AL group, spending similar times torpid and euthermic, displaying similar number of arousals and losing similar amounts of fat reserves. This result was expected since IF juveniles had similar amounts of fat reserves compared with AL animals prior to hibernation.
Our data further suggest that individuals spending more time in euthermy during winter might have been negatively affected by high levels of oxidative stress associated with periodic arousals from torpor. Indeed, we found in this study the first evidence for a direct link between the time an individual spent euthermic and physiological processes assumed to affect ageing. More specifically, RTL on average, over three months of hibernation, did not change in juveniles with short IBE, but decreased by 50% in individuals with high IBE during the first hibernation month (figure 4). Telomeres shorten with each cell division at a rate strongly affected by oxidative stress [11]. During winter hibernation, oxidative stress is restricted to rewarming and euthermic phases [48] and mitosis is blocked in torpor, occurring exclusively during euthermy [49,50]. For instance, Kruman et al. [49] found that the rate of mitosis rose abruptly at least 2 h after arousal from torpor. Such a high mitotic activity, in combination with a drastic increase in oxidative stress produced during rewarming from torpor [44,48,51], is likely to explain the shortening of RTL found in some of the juveniles over winter.
Some of the animals, however, increased the length of their telomeres over the three months of winter hibernation, suggesting that processes of telomere lengthening occurred at some stages during winter. Indeed, changes in RTL result from a balance between the processes of telomere shortening and lengthening via telomerase activity [52]. Similar findings have been reported in edible dormice (G. glis) [12]. The authors found that sub-adult dormice increase their RTL during hibernation, contrary to adult individuals. It appears critical for the fitness of young dormice to maintain physiological functions over the first winter season, since notably the spring following birth is the first breeding opportunity for animals living in cold climate. Our study showed that the percentage change in RTL was negatively associated with the mean IBE duration or the total time spent in euthermy. It seems likely that during deep torpor itself, when mitosis is arrested [49], there is no change in telomere length at all, and that telomere shortening occurs during arousal periods. However, even though both our study and that of Turbill et al. [12] found a negative effect of increased euthermic time on changes in RTL, hibernation as a life-history trait might have an overall positive effect on the somatic maintenance of organisms, but this is thought to occur mainly during the summer time [12].
(d). Substrate utilization over winter hibernation in juveniles
Interestingly, we found that the overwintering body mass loss of the juvenile dormice was equally due to reductions of the fat mass and the fat-free (lean) mass. The direct consequence of a fat-free mass reduction for juveniles is a lowering of their metabolic costs, as the fat-free mass accounts for the major part of the resting MR of the individual [53]. Also, reductions of any tissue will lower the total mass that has to be rewarmed, at high energetic costs, during arousals. In our juveniles, the loss of fat-free mass represented a decrease of 10–15% of the body mass and can likely be explained by a reduction of the gastrointestinal mass and alimentary organs, as already reported in several hibernators during long-term fast [54–56]. Such a significant reduction of fat-free mass could also include loss of muscle mass. It remains to be determined to what extent a reduction of the gastrointestinal tract accounts for the loss of fat-free mass, and to which degree muscle mass is also affected, in hibernating juvenile dormice.
5. Conclusion
We conclude that torpor use promotes fat accumulation during periods of food shortage in late-born juvenile garden dormice prior to hibernation. This leads to pre-hibernation fat reserves that are indistinguishable from animals fed AL. During hibernation, juveniles lost as much lean body mass as fat mass. It remains to be seen if this phenomenon is more common among hibernators than previously thought. Moreover, our data indicate that the main effects of hibernation on ageing processes are linked to euthermic episodes, which are associated with the shortening of telomeres.
Supplementary Material
Supplementary Material
Acknowledgements
The authors thank Peter Steiger and Michaela Salaba for the animal care, and Ines Hofer for the practical work done in this study. We also thank Alexandre Zahariev for the analyses of stable isotopes and Steve Smith for his comments on the last version of the manuscript.
Ethics statement
All procedures were approved by the institutional ethic committee and the national authority according to §26 of Law for Animal Experiments, Tierversuchsgesetz 2012—TGV 2012 (BMF—68.205/0175-II/3b/2011).
Data accessibility
DNA sequences: GenBank accession nos.: AB253957 and AB253958.
Funding statement
S.G. and this research work were financially supported by a postdoctoral fellowship of the University of Veterinary Medicine Vienna (Austria).
References
- 1.Geiser F, Ruf T. 1995. Hibernation versus daily torpor in mammals and birds: physiological variables and classification of torpor patterns. Physiol. Zool. 68, 935–966. [Google Scholar]
- 2.Heldmaier G, Ruf T. 1992. Body temperature and metabolic rate during natural hypothermia in endotherms. J. Comp. Physiol. B 162, 696–706. ( 10.1007/BF00301619) [DOI] [PubMed] [Google Scholar]
- 3.Dark J. 2005. Annual lipid cycles in hibernators: integration of physiology and behavior. Annu. Rev. Nutr. 25, 469–497. ( 10.1146/annurev.nutr.25.050304.092514) [DOI] [PubMed] [Google Scholar]
- 4.Arnold W. 1990. The evolution of marmot sociality. I. Why disperse late? Behav. Ecol. Sociobiol. 27, 229–237. ( 10.1007/BF00164894) [DOI] [Google Scholar]
- 5.Pilastro A, Gomiero T, Marin G. 1994. Factors affecting body mass of young fat dormice (Glis glis) at weaning and by hibernation. J. Zool. 234, 13–23. ( 10.1111/j.1469-7998.1994.tb06053.x) [DOI] [Google Scholar]
- 6.Geiser F, Brigham RM. 2012. The other functions of torpor. In Living in a seasonal world thermoregulatory and metabolic adaptations (eds Ruf T, Bieber C, Arnold W, Millesi E.), pp. 109–121. Berlin, Germany: Springer. [Google Scholar]
- 7.Geiser F. 2008. Ontogeny and phylogeny of endothermy and torpor in mammals and birds. Comp. Biochem. Physiol. A 150, 176–180. ( 10.1016/j.cbpa.2007.02.041) [DOI] [PubMed] [Google Scholar]
- 8.Giroud S, Turbill C, Ruf T. 2012. Torpor use and body mass gain during pre-hibernation in late-born juvenile garden dormice exposed to food shortage. In Living in a seasonal world thermoregulatory and metabolic adaptations (eds Ruf T, Bieber C, Arnold W, Millesi E.), pp. 481–491. Berlin, Germany: Springer. [Google Scholar]
- 9.Turbill C, Bieber C, Ruf T. 2011. Hibernation is associated with increased survival and the evolution of slow life histories among mammals. Proc. R. Soc. B 278, 3355–3363. ( 10.1098/rspb.2011.0190) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Turbill C, Smith S, Deimel C, Ruf T. 2012. Daily torpor is associated with telomere length change over winter in Djungarian hamsters. Biol. Lett. 8, 304–307. ( 10.1098/rsbl.2011.0758) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.von Zglinicki T. 2002. Oxidative stress shortens telomeres. Trends Biochem. Sci. 27, 339–344. ( 10.1016/S0968-0004(02)02110-2) [DOI] [PubMed] [Google Scholar]
- 12.Turbill C, Ruf T, Smith S, Bieber C. 2013. Seasonal variation in telomere length of a hibernating rodent. Biol. Lett. 9, 20121095 ( 10.1098/rsbl.2012.1095) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Metcalfe NB, Monaghan P. 2003. Growth versus lifespan: perspectives from evolutionary ecology. Exp. Gerontol. 38, 935–940. ( 10.1016/S0531-5565(03)00159-1) [DOI] [PubMed] [Google Scholar]
- 14.Bieber C, Juškaitis R, Turbill C, Ruf T. 2012. High survival during hibernation affects onset and timing of reproduction. Oecologia 169, 155–166. ( 10.1007/s00442-011-2194-7) [DOI] [PubMed] [Google Scholar]
- 15.Bieber C, Ruf T. 2004. Seasonal timing of reproduction and hibernation in the edible dormouse (Glis glis). In Life in the cold V: evolution, mechanism, adaptation, and application twelfth international hibernation symposium (eds Barnes BM, Carey HV.), pp. 113–125. Fairbanks, AK: Institute of Arctic Biology, University of Alaska. [Google Scholar]
- 16.Kunz TH. 1974. Feeding ecology of a temperate insectivorous bat (Myotis velifer). Ecology 55, 693–711. ( 10.2307/1934408) [DOI] [Google Scholar]
- 17.Southwood TRE, Wint GRW, Kennedy CEJ, Greenwood SR. 2004. Seasonality, abundance, species richness and specificity of the phytophagous guild of insects on oak (Quercus) canopies. Eur. J. Entomol. 101, 43–50. ( 10.14411/eje.2004.011) [DOI] [Google Scholar]
- 18.Mrosovsky N, Lang K. 1980. Body weights of garden dormice, Eliomys quercinus, kept in constant conditions for two years. Comp. Biochem. Physiol. A 67, 667–669. ( 10.1016/0300-9629(80)90257-1) [DOI] [Google Scholar]
- 19.Bertolino S, Viano C, Currado I. 2001. Population dynamics, breeding patterns and spatial use of the garden dormouse (Eliomys quercinus) in an Alpine habitat. J. Zool. 253, 513–521. ( 10.1017/S0952836901000474) [DOI] [Google Scholar]
- 20.Moreno S. 1988. Reproduction of garden dormouse Eliomys quercinus lusitanicus, in southwest Spain. Mammalia 52, 401–408. ( 10.1515/mamm-1988-0310) [DOI] [Google Scholar]
- 21.Buchholz AC, Schoeller DA. 2004. Is a calorie a calorie? Am. J. Clin. Nutr. 79, 899S–906S. [DOI] [PubMed] [Google Scholar]
- 22.Willis CKR, Goldzieher A, Geiser F. 2005. A non-invasive method for quantifying patterns of torpor and activity under semi-natural conditions. J. Therm. Biol. 30, 551–556. ( 10.1016/j.jtherbio.2005.07.001) [DOI] [Google Scholar]
- 23.Blanc S, Géloën A, Pachiaudi C, Gharib C, Normand S. 2000. Validation of the doubly labeled water method in rats during isolation and simulated weightlessness. Am. J. Physiol. Reg. Int. Comp. Physiol. 279, R1964–R1979. [DOI] [PubMed] [Google Scholar]
- 24.Soxhlet FV. 1879. Die gewichtsanalytische Bestimmung des Milchfettes. Polytechn. J. 232, 461–465. [Google Scholar]
- 25.Callicott RJ, Womack JE. 2006. Real-time PCR assay for measurement of mouse telomeres. Comp. Med. 56, 17–22. See http://www.ingentaconnect.com/content/aalas/cm/2006/00000056/00000001/art00003. [PubMed] [Google Scholar]
- 26.Cawthon RM. 2002. Telomere measurement by quantitative PCR. Nucleic Acids Res. 30, E47 ( 10.1093/nar/30.10.e47) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.R Development Core Team. 2009. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 28.Fox J, Weisberg S. 2011. An R companion to applied regression, XXII, 449 p., 2nd edn Thousand Oaks, CA: Sage. [Google Scholar]
- 29.Legendre P, Legendre L. 1998. Numerical ecology: second English edition, 870 p Amsterdam, The Netherlands: Elsevier; Developments in environmental modelling, 20. [Google Scholar]
- 30.Sokal RR, Rohlf FJ. 1995. Biometry. The principles and practice of statistics in biological research, 887 p, 3rd edn New York, NY: WH. Freeman. [Google Scholar]
- 31.Speakman JR, Rowland A. 1999. Preparing for inactivity: how insectivorous bats deposit a fat store for hibernation. Proc. Nutr. Soc. 58, 123–131. ( 10.1079/Pns19990017) [DOI] [PubMed] [Google Scholar]
- 32.Hiebert SM. 1991. Seasonal differences in the response of rufous hummingbirds to food restriction: body mass and the use of torpor. Condor 93, 526–537. ( 10.2307/1368184) [DOI] [Google Scholar]
- 33.Humphries MM, Thomas DW, Kramer DL. 2001. Torpor and digestion in food-storing hibernators. Physiol. Biochem. Zool. 74, 283–292. ( 10.1086/319659) [DOI] [PubMed] [Google Scholar]
- 34.Clauss M, Streich WJ, Schwarm A, Ortmann S, Hummel J. 2007. The relationship of food intake and ingesta passage predicts feeding ecology in two different megaherbivore groups. Oikos 116, 209–216. ( 10.1111/j.2006.0030-1299.15461.x,) [DOI] [Google Scholar]
- 35.Bae H, Larkin JE, Zucker I. 2003. Juvenile siberian hamsters display torpor and modified locomotor activity and body temperature rhythms in response ro reduced food availability. Physiol. Biochem. Zool. 76, 858–867. ( 10.1086/381462) [DOI] [PubMed] [Google Scholar]
- 36.Overton JM, Williams TD. 2004. Behavioral and physiologic responses to caloric restriction in mice. Physiol. Behav. 81, 749–754. ( 10.1016/j.physbeh.2004.04.025) [DOI] [PubMed] [Google Scholar]
- 37.Ruf T, Klingenspor M, Preis H, Heldmaier G. 1991. Daily torpor in the Djungarian hamster (Phodopus sungorus): interactions with food intake, activity, and social behaviour. J. Comp. Physiol. B 160, 609–615. ( 10.1007/BF00571257) [DOI] [Google Scholar]
- 38.Ortmann S, Heldmaier G. 2000. Regulation of body temperatures and energy requirements of hibernating Alpine marmots (Marmota marmota). Am. J. Physiol. Reg. Int. Comp. Physiol. 278, 698–704. See http://ajpregu.physiology.org/content/278/3/R698. [DOI] [PubMed] [Google Scholar]
- 39.Ruf T, Stieglitz A, Steinlechner S, Blank JL, Heldmaier G. 1993. Cold exposure and food restriction facilitate physiological responses to short photoperiod in Djungarian hamsters (Phodopus sungorus). J. Exp. Zool. 267, 104–112. ( 10.1002/jez.1402670203) [DOI] [PubMed] [Google Scholar]
- 40.Humphries MM, Thomas DW, Kramer DL. 2003. The role of energy availability in mammalian hibernation: a cost–benefit approach. Physiol. Biochem. Zool. 76, 165–179. ( 10.1086/367950) [DOI] [PubMed] [Google Scholar]
- 41.Bieber C, Lebl K, Stalder G, Geiser F, Ruf T. 2014. Body mass dependent use of hibernation: why not prolong the active season, if they can? Funct. Ecol. 28, 167–177. ( 10.1111/1365-2435.12173) [DOI] [Google Scholar]
- 42.Zervanos SM, Maher CR, Waldvogel JA, Florant GL. 2010. Latitudinal differences in the hibernation characteristics of woodchucks (Marmota monax). Physiol. Biochem. Zool. 83, 135–141. ( 10.1086/648736) [DOI] [PubMed] [Google Scholar]
- 43.Giroud S, et al. 2009. Dietary palmitate and linoleate oxidations, oxidative stress, and DNA damage differ according to season in mouse lemurs exposed to a chronic food deprivation. Am. J. Physiol. Reg. Int. Comp. Physiol. 297, R950–R959. ( 10.1152/ajpregu.00214.2009) [DOI] [PubMed] [Google Scholar]
- 44.Carey HV, Frank CL, Seifert JP. 2000. Hibernation induces oxidative stress and activation of NF-κB in ground squirrel intestine. J. Comp. Physiol. B 170, 551–559. ( 10.1007/s003600000135) [DOI] [PubMed] [Google Scholar]
- 45.Harlow HJ, Frank CL. 2001. The role of dietary fatty acids in the evolution of spontaneous and facultative hibernation patterns in prairie dogs. J. Comp. Physiol. B 171, 77–84. ( 10.1007/s003600000148) [DOI] [PubMed] [Google Scholar]
- 46.Wiebe WJ, Sheldon WM, Pomeroy LR. 1992. Bacterial growth in the cold: evidence for an enhanced substrate requirement. Appl. Environ. Microbiol. 58, 359–364. See http://aem.asm.org/content/58/1/359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Diedrich V, Steinlechner S. 2012. Spontaneous daily torpor versus fasting-induced torpor in the Djungarian hamster (Phodopus sungorus): two sides of a medal or distinct phenomena? In Living in a seasonal world thermoregulatory and metabolic adaptations (eds Ruf T, Bieber C, Arnold W, Millesi E.), pp. 231–242. Berlin, Germany: Springer. [Google Scholar]
- 48.Tøien Ø, Drew KL, Chao ML, Rice ME. 2001. Ascorbate dynamics and oxygen consumption during arousal from hibernation in Arctic ground squirrels. Am. J. Physiol. Reg. Int. Comp. Physiol. 281, R572–R583. See http://ajpregu.physiology.org/content/281/2/R572. [DOI] [PubMed] [Google Scholar]
- 49.Kruman II, Ilyasova EN, Rudchenko SA, Khurkhulu ZS. 1988. The intestinal epithelial cells of ground squirrel (Citellus undulatus) accumulate at G2 phase of the cell cycle throughout a bout of hibernation. Comp. Biochem. Physiol. A 90, 233–236. ( 10.1016/0300-9629(88)91109-7) [DOI] [PubMed] [Google Scholar]
- 50.Vinogradova MS. 1988. Mitotic activity of stomach epithelium in the ground squirrel, Citellus erythrogenys Brandt. Comp. Biochem. Physiol. A 91, 235–239. ( 10.1016/0300-9629(88)90410-0) [DOI] [PubMed] [Google Scholar]
- 51.Carey HV, Andrews MT, Martin SL. 2003. Mammalian hibernation: cellular and molecular responses to depressed metabolism and low temperature. Physiol. Rev. 83, 1153–1181. ( 10.1152/physrev.00008.2003) [DOI] [PubMed] [Google Scholar]
- 52.Monaghan P. 2010. Telomeres and life histories: the long and the short of it. Ann. NY Acad. Sci. 1206, 130–142. ( 10.1111/j.1749-6632.2010.05705.x) [DOI] [PubMed] [Google Scholar]
- 53.Rolfe DF, Brown GC. 1997. Cellular energy utilization and molecular origin of standard metabolic rate in mammals. Physiol. Rev. 77, 731–758. See http://physrev.physiology.org/content/77/3/731. [DOI] [PubMed] [Google Scholar]
- 54.Carey HV. 1990. Seasonal changes in mucosal structure and function in ground squirrel intestine. Am. J. Physiol. Reg. Int. Comp. Physiol. 259, R385–R392. See http://ajpregu.physiology.org/content/259/2/R385. [DOI] [PubMed] [Google Scholar]
- 55.Hume D, Beiglböck C, Ruf T, Frey-Roos F, Bruns U, Arnold W. 2002. Seasonal changes in morphology and function of the gastrointestinal tract of free-living alpine marmots (Marmota marmota). J. Comp. Physiol. B 172, 197–207. ( 10.1007/s00360-001-0240-1) [DOI] [PubMed] [Google Scholar]
- 56.Bieber C, Außerlechner K, Skerget C, Walzer C, Ruf T. 2011. Seasonal changes in liver size in edible dormice (Glis glis): non-invasive measurements using ultrasonography. Eur. J. Wildl. Res. 57, 657–662. ( 10.1007/s10344-010-0476-8) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
DNA sequences: GenBank accession nos.: AB253957 and AB253958.


