Abstract
Sexual selection of high-quality mates can conflict with species recognition if traits that govern intraspecific mate preferences also influence interspecific recognition. This conflict might be resolved by developmental plasticity and learned mate preferences, which could drive preference divergence in populations that differ in local species composition. We integrate field and laboratory experiments on two calopterygid damselfly species with population genetic data to investigate how sex differences in developmental plasticity affect population divergence in the face of gene flow. Whereas male species recognition is fixed at emergence, females instead learn to recognize heterospecifics. Females are therefore more plastic in their mate preferences than males. We suggest that this results from sex differences in the balance between sexual selection for high-quality mates and selection for species recognition. As a result of these sex differences, females develop more pronounced population divergence in their mate preferences compared with males. Local ecological community context and presence of heterospecifics in combination with sex differences in plasticity and canalization therefore shape population divergence in mate preferences. As ongoing environmental change and habitat fragmentation bring formerly allopatric species into secondary contact, developmental plasticity of mate preferences in either or both sexes might facilitate coexistence and prevent local species extinction.
Keywords: community ecology, evolutionary rescue, gene flow, learning, mate preferences, speciation
1. Introduction
Classical speciation theory assumes that mate preferences evolve as strict genetic traits by natural or sexual selection and that these preferences can become reinforced when incipient species come into secondary contact and unfit hybrids are formed [1–4]. Although there is accumulating empirical evidence for reproductive character displacement [5], and the underlying genetic architecture of mate preferences for some taxa [6,7], in most cases the genetic basis of mate preferences is unknown. However, there is increasing evidence for a learned component of mate preferences [8–10]. Mate preferences can hence be subject to phenotypic plasticity, which can both affect sexual selection and play a critical role in the early stages of speciation [11–13]. Moreover, sex differences in genetic or learned mate preferences might have profound evolutionary consequences for how sexual isolation and speciation can proceed, although the joint effect of learning and sex differences is unknown [14,15]. Until now, no studies have investigated how sex differences in the developmental plasticity in mate preference and learning could allow populations to diverge and become locally adapted.
In nature, local adaptation is often constrained by the homogenizing effects of gene flow [16]. Theory suggests that the outcome of selection when opposed by gene flow in temporally and spatially variable environments is either the evolution of phenotypic plasticity or local adaptation [16], or the establishment and local coexistence of plastic and canalized genotypes [17]. These general evolutionary theories were originally developed to understand whether local adaption or phenotypic plasticity would be expected to evolve in heterogeneous environments. Only recently these theories about plasticity and canalization have been related to sex differences [18] and incorporated in theoretical models of mate preferences and sexual selection [13].
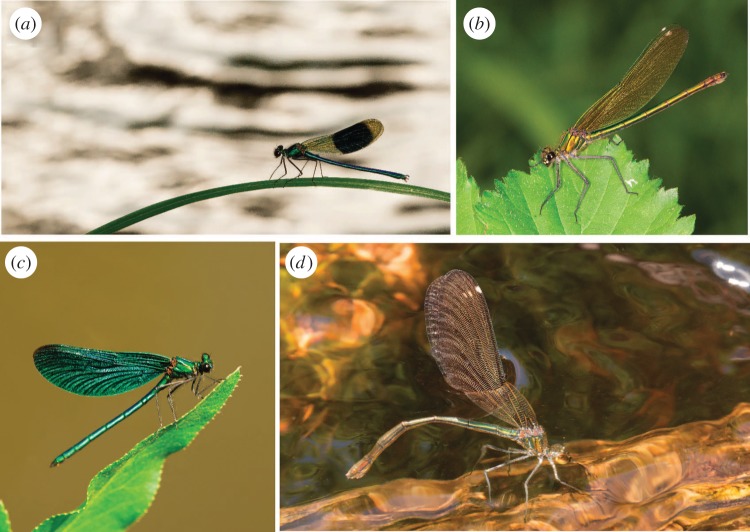
Here, we demonstrate striking sex differences in species discrimination propensity to learn mate preferences in an insect species (banded demoiselle, Calopteryx splendens) and discuss the evolutionary and ecological implications of such sex differences. In this sexually dimorphic insect (figure 1), both intraspecific mate preferences and species recognition (figure 1) are largely influenced by wing coloration in both sexes [10,15]. Our recent comparative phylogenetic study on demoiselles and their allies strongly suggested that coloration is causally involved in elevated speciation rates at the macroevolutionary level [19]. As wing coloration is a target of both natural and sexual selection [20–23] and also functions as a sexual isolation character [15], wing coloration fulfils several of the criteria for a so-called ‘magic trait’ in the speciation literature [24]. Moreover, female C. splendens exhibit learned mate preferences [10]. These features of the ecology and natural history of demoiselles make them excellent study organisms to investigate how sex differences in developmental plasticity and learning might affect local adaptation and population divergence in the presence or absence of conspecifics and in the face of gene flow. As these demoiselles and other odonates (dragonflies and damselflies) often show weak ecological differentiation between species [25,26], they challenge models of ecological speciation and point to non-ecological speciation mechanisms through social selection, sexual selection, sexual conflict and/or learning [27–30].
Figure 1.
Male Calopteryx splendens (a) have a melanized wing patch, unlike females (b), which have transparent wings. The wing patch is a target of intra- and intersexual selection and functions as a sexual isolation character against the sympatric congener, the beautiful demoiselle Calopteryx virgo (males: c, females: d). Although males (a,c) of both species have melanized wings, note the interspecific difference in the amount of melanization between both males (a,c) and females (b,d).
Our study organism C. splendens co-occurs with a closely related congener, the beautiful demoiselle (Calopteryx virgo), over most parts of Europe [10,31]. These two phenotypically and ecologically very similar species differ mainly in the amount of wing melanin in males and females (figure 1), and wing pigmentation functions as a species recognition character [15]. Although heterospecific matings occur, interspecific hybrids have very low fitness [15]. Sympatric populations of C. splendens show signs of local adaptation, as females in this species strongly discriminate against heterospecific C. virgo males, presumably to avoid gametic wastage through the production of low fitness hybrid offspring [10,15]. In southern Sweden, these two congeneric species occur in a mosaic of sympatric (C. splendens and C. virgo) and allopatric (C. splendens only) populations. Our previous work has revealed that these C. splendens populations were only weakly to moderately genetically differentiated from each other [20].
These differing allopatric and sympatric local environmental conditions in combination with the high costs of mating with heterospecifics through gametic wastage will create a mosaic of variable selection pressures across a small geographic scale for these damselflies. The local community context males and females will encounter will be either one or two of these calopterygid species. This local ecological context will influence the costs and benefits of male and female mate preferences, through the need to find high-quality males and the spatially varying relative risks of encountering nearly incompatible mates. We quantified the magnitude of gene flow between C. splendens populations that are either allopatric or sympatric populations with C. virgo and integrated these population genetic data with field and laboratory experiments aimed to investigate sex differences in learning and developmental plasticity of mate preferences. Our study has implications for the evolutionary question of how to develop local adaptation in the face of gene flow through means of plasticity or canalization, and for the more ecological question of local species coexistence through maintaining locally adaptive mate preferences.
2. Material and methods
(a). Ecology and population biology of Calopteryx demoiselles
Demoiselles (family Calopterygidae) are an ancient insect group known for strong sexual selection [32] (figure 1). Demoiselles are often sexually dimorphic in wing pigmentation [32] and in many species, the females have lost part of their wing pigmentation [19]. Field observations and experiments have revealed that wing pigmentation is a target of sexual selection [10], functions as a sexual isolation character between species [15] and affects predation risk in the field [22,23]. Demoiselles have an aquatic larval stage, lasting one to several years [33]. In southern Sweden where our study took place, the life cycle is usually 2 years [32,33]. All field and laboratory experiments in this study were performed in southern Sweden, where two calopterygid damselfly species occur in regional sympatry: the banded demoiselle (C. splendens) and the beautiful demoiselle (C. virgo) [32] (figure 1). Dispersal mainly takes place during the adult part of the life cycle, and C. splendens males have been shown to disperse up to 15 km from their natal streams in other parts of Fennoscandia [32]. Hence, we expect large to moderate gene flow between populations at the spatial scale in this study (electronic supplementary material, table S1).
(b). Study populations
We estimated molecular population differentiation and mate preferences from a series of ‘micro-allopatric’ (A1–A5) and sympatric (S1–S4) populations during the summers 2003–2006 and 2008–2009 (electronic supplementary material, table S1). For the majority of these populations, we had complete mate response data for both sexes as well as population genetic estimates (electronic supplementary material, table S1). We classified a population and site as sympatric when the percentage of males of the rare species (C. virgo) was at least 5% at its peak density during the season [10]. By contrast, we considered populations microallopatric if they contained only C. splendens or where (temporarily) fewer than 5% C. virgo males were present (electronic supplementary material, table S1). The ecological status (allopatry or sympatry) of each of these populations has been stable for over a decade, at least since 2000.
(c). Field experiments on population divergence in mate preferences
We visited our study populations during the reproductive season from June to July and quantified local mate preferences of adult male and female C. splendens towards both conspecific (C. splendens) and heterospecific (C. virgo) mates. These field experiments recorded population variation in adult male and female mate preferences, that is, the mate preferences that have developed during their recent past ontogeny. We obtained data on male responses towards con- and heterospecific females from populations A1–A4 (N = 90; 20–30 males per population) and S1 and S4 (N = 99; 39 and 60 males per population) during the summers of 2008 and 2009. Data on female responses were obtained from populations A1–A5 (N = 299; 35–80 females per population) and S1–S3 (N = 230; 65–91 females per population) during the summers of 2003–2006.
Field tethering experiments in both males and females were performed using the same general procedure. We used a 0.5 m-long thread to tie stimulus males and females at the thorax, without binding their legs, and tied the other end of the thread to a 1.5 m-long bamboo stick. Tethered individuals were thereafter used during presentation sessions that lasted for 10–30 min. During these sessions, the individuals were brought in close proximity to focal individuals of the opposite sex in the field. These free-flying males and females were sexually mature individuals that were resting on vegetation along the river when the presentations started. The tests were performed between 10.00 and 16.00 on sunny days with little wind when mating activity was high.
Female sexual responses were quantified using an 11-degree nominal scale ranging from 0 (female attacks the male) to 10 (tandem formation and/or successful copulation of the male) [10]. The scale (see the electronic supplementary material, Appendix 1, in [21]) is similar to the approach used by previous workers investigating sexual isolation in damselflies [34,35]. We modified and developed this behavioural scale of female sexual responses by including several additional steps to obtain a fine-grained scale that included all the discrete and well-defined pre-copulatory behaviours that have been described in the genus Calopteryx [36].
To avoid statistical pseudoreplication due to multiple presentations to the same individuals in the field, all responses to each stimulus individual were averaged. These mean response scores were approximately normally distributed [21], justifying the use of parametric tests. Each tethered female was presented three times to the same male. As with males (see below), the average female response scores were used as independent data points in the statistical analyses to avoid pseudoreplication. Although female responses could take any value between 0 and 10 (see above), the average female response towards males was quite low, because most male approaches were rejected by females, reflecting the general mating biology of this species. Male mating harassment of females is frequent in damselflies [36], and most male mating attempts in the field are rejected by females (E. I. Svensson 2003–2014, unpublished data). Hence, our female mating response scale [21] should be interpreted both as female propensity to mate and as indicating female resistance towards unwanted male mating attempts. Female response scores can also be viewed as a form of proximity score to a given male, as only males that were able to approach a female closely were subsequently able to achieve physical contact and form a tandem position with the female [10].
Male sexual responses were quantified using a four-degree scale, reflecting their more simple mating behaviours. The steps in this behavioural scale were 0: male escaped female, 1: male ignorance or lack of a response, 2: male courtship flight and positive mating signal, and 3: male clasped female [37,38]. At each site, at least 10 females of each species were presented to at least 10 C. splendens males. Male mate responses could therefore take any value between 0 and 4, although the average score was usually quite low and below 1 in most cases.
(d). Laboratory experiments on mate preferences of sexually naive females and males
To investigate the role of learning in the development of adult mate preferences, we quantified the mate preferences of sexually naive males and females in laboratory experiments. Teneral (newly emerged) males and females were caught in the field and kept isolated from the other sex in cages until sexual maturity. Females were allowed to mature in outdoor cages with several other females (ca 0.75 × 0.5 × 0.5 m3), but without any males for 2 days until sexually mature, i.e. until their exoskeleton had hardened [10]. Males, who unlike females are territorial, were kept individually in cylindrical cages (15 cm diameter and 30 cm height) within an outdoor tent (two-person dome-shaped tent), with grass, small insects and water provided, until they were sexually mature. After reaching sexual maturity, the mate responses towards con- and heterospecific members of the opposite sex were recorded in test cages (see below), using the same sex-specific mating response scales as described above). The aim of these experiments was to quantify the degree of plasticity and canalization in mate preferences of sexually naive males and females, respectively. The sex with more canalized mate preferences is expected to show significant negative discrimination against heterospecific mates when sexually naive. By contrast, the more plastic sex is expected to develop mate preferences by learning and should not discriminate between con- and heterospecific mates initially and when sexually naive [10].
We captured teneral (sexually naive) females from two populations (Klingavälsåns Naturreservat in 2008; a sympatric population; N = 63) and Höje Å (Värpinge in 2009; an allopatric population; N = 133). During the period in captivity, the females received live food in the form of small moths that we captured and released in the cages. Females also had access to water and plants to rest on, and the cages were kept in the shade. During periods of hot weather, we sprayed the females with water to avoid overheating and excessive water loss. These females are hereafter denoted ‘sexually naive females', to emphasize that they have had no, or at least very few previous sexual interactions with males in the field before reaching sexual maturity. Once mature, we quantified female mating responses to con- and heterospecific males, using the 11-degree sexual response scale (described above). This was done by presenting either a tethered C. splendens male or a tethered C. virgo male to a single female in the cage.
We caught teneral (sexually naive) males from the same sympatric population as where we caught females (Klingavälsåns Naturreservat) in 2012 (n = 18) and 2013 (n = 8). After reaching sexual maturity, we tested each naive male by placing him in a cage (ca 0.75 × 0.5 × 0.5 m3) and presenting him with tethered females of both species, alternating the species' presentation order between males. We used the same four-degree mating behavioural scale as in the field experiments and presented each male three times with a C. splendens female, and three times with a C. virgo female, averaging the three responses per female. The stimuli females used for these presentations were caught either the same day as the presentations or on the day the teneral males were caught and housed ‘free’ flying in an outdoor tent (two-person dome-shaped tent). Females had access to vegetation, water and small insects for food. Survival of both males and females in captivity was 100%.
(e). Statistical analysis of mating responses: comparisons between sexes and populations
Both female and male mate responses were analysed using general linear models. The use of parametric statistical tests was justified by the fact that average mate responses were roughly normally distributed (see the electronic supplementary material, appendix 1, in [21]). As we used different mate preference scales for males and females with a different number of steps (11 steps for females and four steps for males), we normalized the data within each sex to facilitate between-sex comparisons. This was achieved by dividing each individual mate response by the average mate response towards conspecifics within that sex. This normalization procedure meant that the average mate response towards conspecific members of the opposite sex was set to 1.0 in both males and females, allowing comparison of mate preference strength and direction between as well as within sexes and populations. A mate response below unity therefore reveals negative mate discrimination, whereas a mate response above unity means a positive response (i.e. an attractive mate).
To compare mate responses between allopatric (A1–A5) and sympatric (S1–S4) populations within males and females, respectively, we first used two-way analysis of variance (ANOVA) with mate response as dependent variable and population identity, species of prospective mate (conspecific or heterospecifics) and their interaction as factors. To compare male and female mate preferences in relation to local ecology, we performed a mixed model with ‘Sex’ (males and females) and ‘Population ecology’ (allopatry versus sympatry) and their interaction (‘Sex × Population ecology’) as independent fixed factors, and ‘Population identity’ as a random factor (nested within the fixed factor ‘Population ecology’), and using the ‘nlme’-package in R v. 2.15.2 [39] (electronic supplementary material, table S2). By including ‘Population identity’ as a random factor, we adjusted for the fact all our populations contributed with multiple individuals to the analysis and hence our data points (individual mate responses) are not entirely statistically independent.
(f). Molecular laboratory work
We obtained genetic data from 133 individuals from seven Swedish study populations and one outgroup consisting of 90 individuals inhabiting populations along the River Loire, France (breakdown of sample sizes per population are reported in the electronic supplementary material, table S1). DNA was extracted from the head of each individual by proteinase K digestion followed by a standard phenol/chloroform-isoamylalcohol extraction [40]. The purified DNA was re-suspended in 100 µl of sterile water. The genotypes of all damselflies were assayed at 13 microsatellite loci previously isolated for this species [41]. These loci were described as being polymorphic with high heterozygosity and none of them was found to deviate from Hardy–Weinberg equilibrium or be in linkage disequilibrium with each other.
The polymerase chain reaction (PCR) was done with four primer multiplexes. The PCR was run with 2 μl DNA sample (5 ng μl−1), 3 μl Qiagen Mastermix, 0.2 μl of each primer and ddH2O until the volume was 10 μl. The PCR cycling followed the recommended Qiagen protocol. Following precipitation, samples were diluted 1 : 10 and sequenced on an ABI PRISM 3730 Genetic Analyzer (Applied Biosystems). Resulting data were analysed with the program geneious v. 6.0. [42] for internal standard and fragment size determination and for allelic designations. We checked for presence of null alleles with the software micro-checker [43] and departure from Hardy–Weinberg expectations using fstat [44].
(g). Analysis of gene flow and molecular population differentiation
Genetic differentiation (FST) between populations was estimated with fstat [44]. The program structure v. 2.3.4 [45] was run on individual multilocus genotypes for a number of clusters K ranging from 1 to 12 using a burn-in length of 50 000 and a run length of 200 000 iterations. The likelihood for the data given each of K clusters was recorded, and structure harvester [46] was used to illustrate the results and apply the Evanno method to detect the most probable K [47].
We employed BayesAss 3 [48] to estimate migration rates between sampled populations, using one run with 10 million iterations (1 million discarded as burn-in) and 1000 iterations between MCMC sampling and 10 runs of 1 million iterations (100 000 discarded as burn-in) and 100 iterations between MCMC sampling. Mixing parameter for allele frequencies, inbreeding coefficients and migration rates were iteratively adjusted to accrue acceptance rates of 35, 42 and 44%, respectively.
3. Results
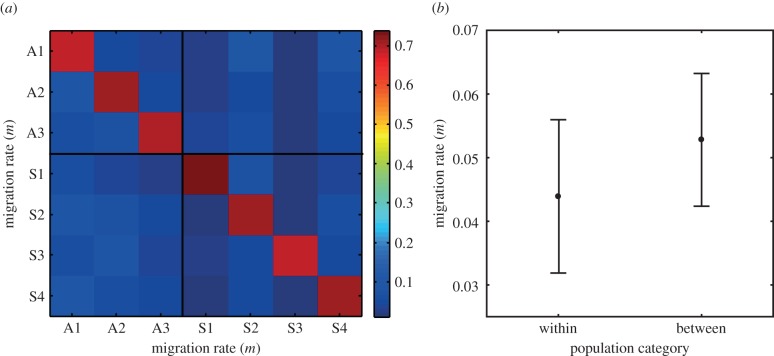
Our new population genetic estimates of migration rates (m) using microsatellites suggest that these closely located allopatric and sympatric C. splendens populations frequently exchange migrants at a high rate (figure 2), or alternatively recent divergence explains the genetic similarity of these populations (electronic supplementary material, figures S1–S4 and tables S3 and S4). We estimated that 26–31% of all individuals in these closely located allopatric and sympatric C. splendens populations were of immigrant origin (figure 2a; electronic supplementary material, table S4). This estimate was similar for migration within (i.e. sympatry–sympatry, allopatry–allopatry) and between ecological categories (i.e. allopatry–sympatry, sympatry–allopatry) (figure 2b; electronic supplementary material, figures S5 and S6). These molecular data therefore suggest that gene flow could readily counteract local genetic differentiation in mate preferences between these C. splendens populations.
Figure 2.
The graphs illustrate the magnitude of estimated of gene flow (migration rate: m) between C. splendens populations that were either allopatric (three populations designated ‘A’; containing only C. splendens) or sympatric (four populations designated ‘S'; containing both C. splendens and C. virgo). For explanations of population abbreviations, see electronic supplementary material, table S1. (a) Matrix of migration rates (m) between all seven populations, estimated using BayesAss 3 (electronic supplementary material). (b) Estimates of m within ecological categories (i.e. allopatric to allopatric and sympatric to sympatric versus allopatric to sympatric and sympatric to allopatric).
In spite of the estimated high gene flow between these allopatric and sympatric C. splendens populations, mate preferences of sexually experienced individuals towards con- and heterospecifics showed pronounced population differentiation and differed between males and females (figure 3). Female C. splendens responded differently to heterospecific (C. virgo) males and were influenced by local population ecology (sympatry or allopatry; figure 3a). In sympatric populations, female C. splendens discriminated strongly against heterospecific C. virgo males, generating a pattern of population divergence similar to reinforcement (figure 3a). By contrast, C. splendens females in allopatric populations showed a stronger mate preference response towards heterospecific C. virgo males compared with conspecific C. splendens males, consistent with sexual selection for large wing patches as a signal of high mate quality in allopatry (figure 3a). Therefore, female C. splendens mate responses towards heterospecific males did not change only in magnitude between sympatry and allopatry, but also in sign, showing negative discrimination in sympatry and positive response in allopatry (figure 3c).
Figure 3.
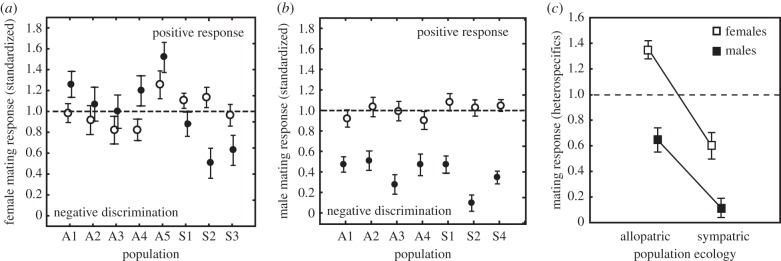
Population divergence in mate preferences towards con- and heterospecific mates in allopatric (A1–A4) and sympatric populations (S1–S4). Line at ‘1.0’ shows the standardized average response towards conspecific mates. Open symbols shows mean (±95% CIs) response towards conspecifics, and closed symbols mean response (±95% CIs) towards heterospecifics. (a) Female population divergence (two-way ANOVA: population (P): F7,395 = 35.475, p < 0.001; species (S): F1,395 = 4.217, p = 0.04; P × S: F7,395 = 24.828, p < 0.001). (b) Male population divergence (two-way ANOVA: population (P): F6,205 = 8.741, p < 0.001; species (S): F1,205 = 853.357, p < 0.001; P × S: F6,205 = 9.852, p < 0.001). (c) Elevated population divergence in female and male mate preferences towards heterospecifics in allopatric versus sympatric populations (nested mixed model analysis: sex × population ecology: F1,226 = 13.640; p = 0.003; electronic supplementary material, table S2: Model 2).
In contrast to females, C. splendens males discriminated against heterospecific C. virgo females in all populations (figure 3b), although male discrimination was stronger in magnitude in sympatry than in allopatry (figure 3c). Therefore, under both sympatric and allopatric ecological conditions, C. splendens males showed negative discrimination against heterospecific C. virgo females (figure 3). As a result of the sex differences in C. splendens, male and female population divergence in mate preferences were markedly different. Female C. splendens showed elevated population divergence and their mate preferences changed in both sign and magnitude between allopatry and sympatry, whereas male C. splendens had more constrained mate preferences which changed only in magnitude (figure 3c).
To investigate the developmental basis of these sex differences, we compared the mate responses of sexually naive (newly emerged, virgin) C. splendens males and females towards prospective con- and heterospecific mates (figure 4). Sexually naive C. splendens males discriminated against C. virgo females and responded less to these heterospecific females than they responded to conspecific C. splendens females (figure 4a). By contrast, sexually naive C. splendens females did not discriminate more strongly against heterospecific males, but showed equal preference towards C. splendens and C. virgo males (figure 4b). This difference between male and female C. splendens mate responses was highly significant (F1,60 = 12.903; p < 0.001; see figure 4c). These results suggest that C. splendens males have a more or less fixed species recognition ability at emergence, whereas in C. splendens females species recognition instead develops gradually upon exposure to con- and heterospecific males, through learning [10].
Figure 4.
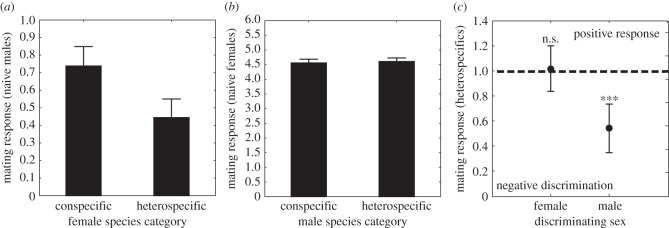
Mate responses of sexually naive C. splendens males and females towards con- and heterospecific mates of the opposite sex. (a) Mean (±s.e.) mate responses of sexually naive C. splendens males towards conspecific females and heterospecific females. These sexually naive males showed a significantly lower response towards heterospecific females, compared with their response towards conspecific females (paired t-test: t1,29 = 2.713; p = 0.011). (b) Mean (±s.e.) mate responses of sexually naive C. splendens females towards conspecific and heterospecific males. There was a significant effect of female population origin (Klingavälsån or Höje Å) on mean mate responses (F1,193 = 27.314; p < 0.001), but no there was no significant difference in the strength of naive female responses towards con- and heterospecific males (F1,193 = 0.067; p = 0.80). (c) Comparison between sexually naive female and male C. splendens in their mean response towards heterospecific putative mates (line at 1.0 indicates the average standardized response towards conspecifics). Means (±95 CIs) are shown (data only from Klingavälsån). Sexually naive female C. splendens do not discriminate against heterospecifics mates, unlike sexually naive male C. splendens (ANOVA for effect of sex: F1,60 = 12.903; p = 0.001).
4. Discussion
A recent theoretical model suggested that learned mate preferences can either enhance or constrain population divergence, depending on ecological conditions, the learning potential of males and females and the timing of when learning occurs [13]. Learned mate preferences have been predicted to enhance population divergence when females learn, whereas population divergence might be constrained when males learn [13]. Whereas male mate preferences are influenced predominantly by direct selection, female mate preferences can, at least to some degree, be driven also by sexual selection for indirect fitness costs and benefits [49]. Therefore, selection for species recognition might be the main force on male mate preferences, whereas female preferences might instead be shaped by a selective conflict between the need for species recognition in sympatry and selection for high-quality mates in allopatry (figure 3a,c).
One potential outcome of different selection pressures on the mate preferences in the two sexes could be that male mate preferences might evolve more slowly and show a higher degree of phylogenetic inertia in the degree of species recognition. This was actually recently found in a large-scale comparative phylogenetic study of species recognition across several taxa from different classes and orders [50]. Another consequence of sex-specific selection pressures on mate preferences could be weakened selection on female mate preferences since learning should shield these plastic preferences from selection (cf. [13]). We also hypothesize that male mate preferences have become more canalized, as selection for species recognition to avoid heterospecific matings might have an overriding effect in males compared with females, relative to the strength of selection to obtain high-quality mates among conspecifics. Strong male discrimination against heterospecifics among both naive (figure 4a,c) and sexually experienced males (figure 3b,c) challenges the assumption that matings and courtship are cheap in males and suggests fitness costs of matings with C. virgo females.
That males and females should differ in the degree of plasticity and canalization has previously been suggested for morphological traits [18], but to our knowledge this has seldom been discussed in the context of sex differences in mate preferences. Here, we have presented experimental data showing that males and females differ in the degree of canalization and plasticity in their mate preferences (figure 4). These sex differences in plasticity and canalization are presumably responsible for the sex differences in population divergence in males and females (figure 3c). Sex differences in plasticity and learning can therefore maintain substantial but sex-specific population divergence in mate preference phenotypes despite gene flow (figure 2). An interesting evolutionary implication of this sex difference is that the plastic sex (females) might become more shielded from genetic evolution of mate preferences than the less plastic sex (males) [13], a prediction that should be investigated in future quantitative genetic and selection studies in this area.
A recent study on sex-specific mate preferences in birds found evidence for an evolutionary constraint in the form of an intersexual genetic correlation and shared inheritance of mating behaviours of males and females [51]. By contrast, another recent study on the fruit fly Drosophila serrata found no evidence of a genetic correlation between male and female mate preferences, strongly suggesting that in this insect, different sets of loci govern male and female mate preferences [52]. The results presented in this study have revealed a remarkable flexibility of mate preferences and striking sex and population differences in how males and females respond towards heterospecifics. This flexibility allows males and females in populations with different species composition to develop distinctly different, but locally adaptive mate preferences. (figure 3). Theory suggests that developmental plasticity and canalization might have key roles in local adaptation and in spatially and temporally variable environments [16,17]. Our results strongly suggest that sex differences in developmental plasticity and canalization jointly influence population divergence in mate preferences and variation in local responses to heterospecifics. These sex differences in learning, plasticity and canalization are likely to play a crucial role in population divergence and speciation processes, particularly in damselflies and other odonates, where ecological speciation through niche differentiation plays a minor role [25,26] compared with the stronger diversifying effects of social selection, sexual selection and mating conflicts between males and females [27–29].
More generally, there is a growing interest in non-ecological speciation processes, such as mutation-order speciation, as alternative scenarios to the more widely recognized process of ecological speciation [30]. Social and sexual mating interactions and sex differences in developmental plasticity and learning can play a key role in such non-ecological speciation processes [29]. We therefore strongly encourage researchers using other organisms to pay more attention to these problems in future experimental investigations. In addition, plasticity and learned mate preferences might also more rapidly allow local populations to develop locally adaptive mate preferences, e.g. to avoid costly heterospecific matings in sympatry, depending on local community context. In such situations, learning and plastic mate preferences can potentially act as an evolutionary rescue [53] to avoid local species extinction and facilitate further coexistence. By contrast, genetic evolution of mate preferences might not be fast enough to prevent extinction if the fitness costs of heterospecific matings are high, as in the case for the species we have studied here. Finally, a recent theoretical study took a metapopulation ecological perspective and linked sexual selection to the evolution of plasticity and canalization [54]. This study is an empirical contribution to a much-needed integrative research programme of the consequences of sexual selection in heterogeneous environments [55].
Supplementary Material
Acknowledgements
We are grateful to E. Björk, L. Corlatti, F. Eroukhmanoff, M. Friberg, K. Karlsson-Green, J. Nelson and S. Scobell for valuable help with the field work and experiments in the early stages of this study, and K. Karlsson-Green and F. Eroukhmanoff for comments on an early draft of this manuscript. We also wish to thank H. Rosenquist and A. Berg for assistance with the molecular laboratory work.
Ethics statement
All field and laboratory experiments on animals described in this study have been legally approved by local and regional Swedish authorities, and all the necessary permissions to carry out these experiments have been obtained.
Data accessibility
Data in all the analyses in this article are available in the online Digital Data Repository Dryad: http://doi:10.5061/dryad.sr687.
Funding statement
Funding for this study was provided by the Swedish Research Council (VR), Carl Tryggers Stiftelse and the Crafoord Foundation to E.I.S., a Wenner-Gren postdoctoral scholarship to M. N.V. and a Marie Curie Intra-European fellowship to M.W.
References
- 1.Dobzhansky T. 1937. Genetics and the origin of species. New York, NY: Columbia University Press. [Google Scholar]
- 2.Saetre GP, Moum T, Bures S, Král M, Adamjan M, Moreno J. 1997. A sexually selected character displacement in flycatchers reinforces premating isolation. Nature 387, 589–592. ( 10.1038/42451) [DOI] [Google Scholar]
- 3.Higgie M, Chenoweth S, Blows MW. 2000. Natural selection and the reinforcement of mate recognition. Science 290, 519–521. ( 10.1126/science.290.5491.519) [DOI] [PubMed] [Google Scholar]
- 4.Servedio MR, Noor MAF. 2003. The role of reinforcement in speciation: theory and data. Annu. Rev. Ecol. Evol. Syst. 34, 339–364. ( 10.1146/annurev.ecolsys.34.011802.132412) [DOI] [Google Scholar]
- 5.Coyne JA, Orr HA. 2004. Speciation. Sinauer, MA: Sinauer Associates Inc. [Google Scholar]
- 6.Noor MA. 1995. Speciation driven by natural selection in Drosophila. Nature 375, 674–675. ( 10.1038/375674a0) [DOI] [PubMed] [Google Scholar]
- 7.Noor MAF. 1999. Reinforcement and other consequences of sympatry. Heredity 83, 503–508. ( 10.1038/sj.hdy.6886320) [DOI] [PubMed] [Google Scholar]
- 8.Dukas R. 2006. Learning in the context of sexual behaviour in insects. Anim. Biol. 56, 125–141. ( 10.1163/157075606777304258) [DOI] [Google Scholar]
- 9.Verzijden MN, ten Cate C. 2007. Early learning influences species assortative mating preferences in Lake Victoria cichlid fish. Biol. Lett. 3, 134–136. ( 10.1098/rsbl.2006.0601) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Svensson EI, Eroukhmanoff F, Karlsson K, Runemark A, Brodin A. 2010. A role for learning in population divergence of mate preferences. Evolution 64, 3101–3113. ( 10.1111/j.1558-5646.2010.01085.x) [DOI] [PubMed] [Google Scholar]
- 11.Verzijden MN, Lachlan RF, Servedio MR. 2005. Female mate-choice behavior and sympatric speciation. Evolution 59, 2097–2108. ( 10.1111/j.0014-3820.2005.tb00920.x) [DOI] [PubMed] [Google Scholar]
- 12.Verzijden MN, ten Cate C, Servedio MR, Kozak GM, Boughman JW, Svensson EI. 2012. The impact of learning on sexual selection and speciation. Trends Ecol. Evol. 27, 511–519. ( 10.1016/j.tree.2012.05.007) [DOI] [PubMed] [Google Scholar]
- 13.Servedio MR, Dukas R. 2013. Effects on population divergence of within-generational learning about prospective mates. Evolution 67, 1–13. ( 10.1111/evo.12127) [DOI] [PubMed] [Google Scholar]
- 14.Verzijden MN, Korthof REM, ten Cate C. 2008. Females learn from mothers and males learn from others. The effect of mother and siblings on the development of female mate preferences and male aggression biases in Lake Victoria cichlids, genus Mbipia. Behav. Ecol. Sociobiol. 62, 1359–1368. ( 10.1007/s00265-008-0564-x) [DOI] [Google Scholar]
- 15.Svensson EI, Karlsson K, Friberg M, Eroukhmanoff F. 2007. Gender differences in species recognition and the evolution of asymmetric sexual isolation. Curr. Biol. 22, 1943–1947. ( 10.1016/j.cub.2007.09.038) [DOI] [PubMed] [Google Scholar]
- 16.Sultan SE, Spencer HG. 2002. Metapopulation structure favors plasticity over local adaptation. Am. Nat. 160, 271–283. ( 10.1086/341015) [DOI] [PubMed] [Google Scholar]
- 17.Lively CM. 1986. Canalization versus developmental conversion in a spatially variable environment. Am. Nat. 128, 561–572. ( 10.1086/284588) [DOI] [Google Scholar]
- 18.Stillwell RC, Blanckenhorn WU, Teder T, Davidowitz G, Fox CW. 2010. Sex differences in phenotypic plasticity affect variation in sexual size dimorphism in insects: from physiology to evolution. Annu. Rev. Entomol. 55, 227–245. ( 10.1146/annurev-ento-112408-085500) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Svensson EI, Waller JT. 2013. Ecology and sexual selection: evolution of wing pigmentation in calopterygid damselflies in relation to latitude, sexual dimorphism and speciation. Am. Nat. 182, E174–E195. ( 10.1086/673206) [DOI] [PubMed] [Google Scholar]
- 20.Svensson EI, Kristoffersen L, Oskarsson K, Bensch S. 2004. Molecular population divergence and sexual selection on morphology in the banded demoiselle (Calopteryx splendens). Heredity 93, 423–433. ( 10.1038/sj.hdy.6800519) [DOI] [PubMed] [Google Scholar]
- 21.Svensson EI, Eroukhmanoff F, Friberg M. 2006. Effects of natural and sexual selection on adaptive population divergence and premating isolation in a damselfly. Evolution 60, 1242–1253. ( 10.1111/j.0014-3820.2006.tb01202.x) [DOI] [PubMed] [Google Scholar]
- 22.Svensson EI, Friberg M. 2007. Selective predation on wing morphology in sympatric damselflies. Am. Nat. 170, 101–112. ( 10.1086/518181) [DOI] [PubMed] [Google Scholar]
- 23.Kuchta SR, Svensson EI. 2014. Predator-mediated natural selection on the wings of the damselfly Calopteryx splendens: differences in selection among trait types. Am. Nat. 184, 91–109. ( 10.1086/676043) [DOI] [PubMed] [Google Scholar]
- 24.Servedio MR, Van Doorn GS, Kopp M, Frame AM, Nosil P. 2011. Magic traits in speciation: ‘magic’ but not rare? Trends Ecol. Evol. 26, 389–397. ( 10.1016/j.tree.2011.04.005) [DOI] [PubMed] [Google Scholar]
- 25.Siepielski AM, Hung J, Bein EB, McPeek MA. 2010. Experimental evidence for neutral community dynamics governing an insect assemblage. Ecology 91, 847–857. ( 10.1890/09-0609.1) [DOI] [PubMed] [Google Scholar]
- 26.Wellenreuther M, Larson KW, Svensson EI. 2012. Climatic niche divergence or conservatism? Environmental niches and range limits in ecologically similar damselflies. Ecology 93, 1353–1366. ( 10.1890/11-1181.1) [DOI] [PubMed] [Google Scholar]
- 27.McPeek MA, Gavrilets S. 2006. The evolution of female mating preferences: differentiation from species with promiscuous males can promote speciation. Evolution 60, 1967–1980. ( 10.1111/j.0014-3820.2006.tb01835.x) [DOI] [PubMed] [Google Scholar]
- 28.McPeek MA, Shen L, Torrey JZ, Farid H. 2008. The tempo and mode of three-dimensional morphological evolution in male reproductive structures. Am. Nat. 171, E158–E178. ( 10.1086/587076) [DOI] [PubMed] [Google Scholar]
- 29.Svensson EI. 2012. Nonecological speciation, niche conservatism and thermal adaptation: how are they connected? Org. Div. Evol. 12, 229–240. ( 10.1007/s13127-012-0082-6) [DOI] [Google Scholar]
- 30.Mendelson TC, Martin MD, Flaxman SM. 2014. Mutation-order divergence by sexual selection: diversification of sexual signals in similar environments as a first step in speciation. Ecol. Lett. 17, 1053–1066. ( 10.1111/ele.12313) [DOI] [PubMed] [Google Scholar]
- 31.Tynkkynen K, Rantala MJ, Suhonen J. 2004. Interspecific aggression and character displacement in the damselfly Calopteryx splendens. J. Evol. Biol. 17, 759–767. ( 10.1111/j.1420-9101.2004.00733.x) [DOI] [PubMed] [Google Scholar]
- 32.Karjalainen S, Hämäläinen M. 2013. Demoiselle damselflies - winged jewels of silvery streams. Helsinki, Finland: Caloptera Publishing. [Google Scholar]
- 33.Rüppell G, Rüpell DH, Rehfeldt G, Schütte C. 2005. Die Prachtlibellen Europas. Hohenwarsleben: Westarp Wissenschaften-Verlagsgesellschaft. Die Neue Brehm-Bücherei; [In German.] [Google Scholar]
- 34.Paulson DR. 1974. Reproductive isolation in damselflies. Syst. Zool. 23, 40–49. ( 10.2307/2412238) [DOI] [Google Scholar]
- 35.Waage JK. 1975. Reproductive isolation and potential for character displacement in damselflies, Calopteryx maculata and Calopteryx aequabilis (Odonata: Calopterygidae). Syst. Zool. 24, 24–36. ( 10.2307/2412695) [DOI] [Google Scholar]
- 36.Corbet PS. 1999. Dragonflies: behaviour and ecology of Odonata. Colchester, UK: Harley Books. [Google Scholar]
- 37.Wellenreuther M, Tynkkynen K, Svensson EI. 2010. Simulating range expansion: male species recognition and loss of premating isolation in damselflies. Evolution 64, 242–252. ( 10.1111/j.1558-5646.2009.00815.x) [DOI] [PubMed] [Google Scholar]
- 38.Wellenreuther M, Vercken E, Svensson EI. 2010. A role for ecology in male mate discrimination of immigrant females in Calopteryx damselflies? Biol. J. Linn. Soc. 100, 506–518. ( 10.1111/j.1095-8312.2010.01464.x) [DOI] [Google Scholar]
- 39.R Development Core Team. 2008. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing: See http://www.R-project.org. [Google Scholar]
- 40.Sambrook J, Fritsch EF, Maniatis T. 1989. Molecular cloning: a laboratory manual, 2nd edn. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press. [Google Scholar]
- 41.Austin JD, et al. 2011. Permanent genetic resources added to Molecular Ecology Resources Database 1 February 2011–31. Mol. Ecol. Resour. 11, 757–758. ( 10.1111/j.1755-0998.2011.03028.x) [DOI] [PubMed] [Google Scholar]
- 42.Geneious version 6.0. 2013. Created by biomatters See http://www.geneious.com<.
- 43.Van Oosterhout C, Hutchinson WF, Willis DPM, Shipley P. 2004. MICRO-CHECKER: software for identifying and correcting genotyping errors in microsatellite data. Mol. Ecol. Resour. 4, 535–538. [Google Scholar]
- 44.Goudet J. 2001. FSTAT: a program to estimate and test gene diversities and fixation indices, v. 2.9.3 See http://www unilch/popgen/softwares/fstathtm.
- 45.Pritchard JK, Stephens M, Donnelly P. 2000. Inference of population structure using multilocus genotype data. Genetics 155, 945–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Earl DA, Vonholdt BM. 2012. STRUCTURE HARVESTER: a website and program for visualizing STRUCTURE output and implementing the Evanno method. Conserv. Gen. Resour. 4, 359–361. ( 10.1007/s12686-011-9548-7) [DOI] [Google Scholar]
- 47.Evanno G, Regnaut S, Goudet J. 2005. Detecting the number of clusters of individuals using the software STRUCTURE: a simulation study. Mol. Ecol. 14, 2611–2620. ( 10.1111/j.1365-294X.2005.02553.x) [DOI] [PubMed] [Google Scholar]
- 48.Wilson GA, Rannala B. 2003. Bayesian inference of recent migration rates using multilocus genotypes. Genetics 163, 1177–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Servedio MR. 2007. Male versus female mate choice: sexual selection and the evolution of species recognition via reinforcement. Evolution 61, 2772–2789. ( 10.1111/j.1558-5646.2007.00247.x) [DOI] [PubMed] [Google Scholar]
- 50.Ord TJ, King L, Young AR. 2011. Contrasting theory with the empirical data of species recognition. Evolution 65, 2572–2591. ( 10.1111/j.1558-5646.2011.01319.x) [DOI] [PubMed] [Google Scholar]
- 51.Forstmeier W, Martin K, Bolund E, Schielzeth H, Kempenaers B. 2011. Female extrapair mating behavior can evolve via indirect selection on males. Proc. Natl Acad. Sci. USA 108, 10 608–10 613. ( 10.1073/pnas.1103195108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gosden TP, Rundle HD, Chenoweth SF. 2014. Testing the correlated response hypothesis for the evolution and maintenance of male mating preferences in Drosophila serrata . J. Evol. Biol. 27, 2106–2112. ( 10.1111/jeb.12461) [DOI] [PubMed] [Google Scholar]
- 53.Chevin LM, Gallet R, Gomulkiewicz R, Holt RD, Fellous S. 2013. Phenotypic plasticity in evolutionary rescue experiments. Phil. Trans. R. Soc. B 368, 20120089 (doi:10/1098/rstb.2012.0089) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Harts AMF, Schwanz LE, Kokko H. 2014. Demography can favour female-advantageous alleles. Proc. R. Soc. B 281, 20140005 ( 10.1098/rspb.2014.0005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Miller CW, Svensson EI. 2014. Sexual selection in complex environments. Annu. Rev. Entomol. 59, 427–445. ( 10.1146/annurev-ento-011613-162044) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data in all the analyses in this article are available in the online Digital Data Repository Dryad: http://doi:10.5061/dryad.sr687.