Abstract
Anthropogenic increases in atmospheric CO2 over this century are predicted to cause global average surface ocean pH to decline by 0.1–0.3 pH units and sea surface temperature to increase by 1–4°C. We conducted controlled laboratory experiments to investigate the impacts of CO2-induced ocean acidification (pCO2 = 324, 477, 604, 2553 µatm) and warming (25, 28, 32°C) on the calcification rate of the zooxanthellate scleractinian coral Siderastrea siderea, a widespread, abundant and keystone reef-builder in the Caribbean Sea. We show that both acidification and warming cause a parabolic response in the calcification rate within this coral species. Moderate increases in pCO2 and warming, relative to near-present-day values, enhanced coral calcification, with calcification rates declining under the highest pCO2 and thermal conditions. Equivalent responses to acidification and warming were exhibited by colonies across reef zones and the parabolic nature of the corals' response to these stressors was evident across all three of the experiment's 30-day observational intervals. Furthermore, the warming projected by the Intergovernmental Panel on Climate Change for the end of the twenty-first century caused a fivefold decrease in the rate of coral calcification, while the acidification projected for the same interval had no statistically significant impact on the calcification rate—suggesting that ocean warming poses a more immediate threat than acidification for this important coral species.
Keywords: tropical scleractinian coral; calcification; ocean warming, ocean acidification; Siderastrea siderea; Caribbean
1. Introduction
Atmospheric pCO2 has increased from pre-industrial levels of ca 280 µatm to current levels exceeding 400 µatm [1,2], primarily due to the burning of fossil fuels, cement production and deforestation. This anthropogenic elevation of atmospheric pCO2 has already decreased surface ocean pH by ca 0.1 pH unit [3]. Atmospheric pCO2 is predicted to exceed 600 µatm by the end of the twenty-first century [4], which would cause surface ocean pH to decline by an additional 0.3 pH units [5,6]. This process of ‘ocean acidification’ reduces the carbonate ion concentration of seawater, which in turn reduces its saturation with respect to the calcium carbonate mineral aragonite, from which scleractinian corals and other marine invertebrates and algae build their protective shells and skeletons.
Atmospheric pCO2 is also a greenhouse gas and its elevation has caused sea surface temperatures within the habitats of tropical scleractinian corals to increase by as much as 0.7°C over the past several decades [7,8]. The relationship between seawater temperature and calcification rates of tropical corals has been well explored [9–12]. In general, calcification rate increases with increasing seawater temperature up to an optimal temperature, which typically coincides with the mean summer seawater temperature of the coral's natural habitat [11]. At sufficiently elevated temperatures, corals lose their symbionts through a process known as bleaching, resulting in a further decline in calcification. Because maximum summertime temperatures on tropical reefs already approach the temperature at which corals bleach [13], even a small increase in average seawater temperature may negatively impact their fitness.
The number of studies investigating the impacts of ocean acidification on coral calcification has increased exponentially [14–20], with several reviews published on the subject [21–23]. With each additional study, it is increasingly apparent that the calcification response of scleractinian corals to ocean acidification varies widely among taxa [16,20,24,25], and can vary within the same coral species when other experimental parameters (e.g. feeding, light, temperature, method of acidification) are modified [20,24]. Many of these experimental studies have shown that calcification rates of scleractinian corals decline relatively linearly with reductions in seawater pH [15,16,19,26–35]. However, other experimental studies have shown that scleractinian corals can also exhibit no response, a nonlinear threshold response or even a positive response to CO2-induced reductions in seawater pH [14,18,36–39]. The complexities of the relationship between seawater pH and calcification rates of scleractinian corals are compounded by interactions between thermal and pH stress that are still not fully understood. For example, the negative effects of reduced seawater pH on coral calcification have been shown to increase under elevated temperatures, suggesting a synergistic effect [30,36,40], while other studies have shown that elevated temperature has either no effect or a mitigating effect on the response of scleractinian corals to ocean acidification [25,29,38,41,42]. This variability in corals’ calcification response to ocean acidification, compounded by the interactive effects of other stressors, complicates efforts to predict and potentially mitigate the impacts of CO2-induced ocean acidification on coral reefs.
Although the most adverse impacts on corals may arise from the combined effects of acidification and warming, the objective of this study was to isolate the impacts of these two stressors. Here, we present results of 95-day laboratory experiments designed to investigate the impacts of CO2-induced ocean acidification (pCO2 (s.d.); 324 (89), 477 (83), 604 (107) and 2553 (506) µatm) and warming (temperature (s.d.); 25 (0.14), 28 (0.24) and 32 (0.17)°C) on calcification rates of the tropical reef-building zooxanthellate coral Siderastrea siderea—an important and ubiquitous component of Caribbean reef systems [43].
2. Material and methods
(a). Coral collection, transportation and maintenance
In July 2011, eighteen 20–30-year-old colonies of S. siderea were collected by hammer and chisel at 3–5 m depth from near shore, backreef and forereef reef zones in southern Belize [8] (see the electronic supplementary material for a detailed description of coral collection sites). Whole corals were transported to the Aquarium Research Center at the University of North Carolina at Chapel Hill by aeroplane. At UNC-Chapel Hill, each coral colony was sectioned into 18 comparatively sized specimens (surface area: 3 × 2 cm; thickness: 1 cm) with a diamond-embedded petrographic saw and glued with cyanoacrylate to acrylic microscope slides. The coral specimens were allowed to recover for 30 days under laboratory conditions in two 500 l recirculating artificial seawater systems maintained at a salinity of 35, temperature of 28°C and an irradiance of ca 250 µmol photons m−2 s−1. The corals were visually inspected each day of the recovery period and no evidence of bleaching or disease was observed. The corals were then acclimated for 15 days following the recovery period, after which the coral specimens were incrementally exposed to the modified pCO2 and thermal conditions.
(b). Growth conditions
(i). Ocean acidification experiment
Siderastrea siderea coral specimens from each of the 18 colonies were reared for 95 days (5 August–8 November 2011) in each of twelve 38 l glass tanks (18 specimens per tank; 216 specimens in total) filled with artificial seawater formulated at a salinity (s.d.) of 35.13 (0.32) with Instant Ocean Sea Salt and deionized water. Although the trace elemental composition of Instant Ocean Sea Salt differs subtly from that of natural seawater, its major and minor elemental composition and its carbonate chemistry are the most similar to natural seawater when compared with eight other commercial sea salt mixes [44]. Four CO2 partial pressures (s.d.) (324 (89), 477 (83), 604 (107), 2553 (506) µatm)), corresponding to a near-pre-industrial, a near-present-day, an end-of-century and an extreme year 2500 pCO2 level were selected to define the shape of the pCO2-calcification response curve for S. siderea. CO2 partial pressures were established by mixing pure CO2 with CO2-free compressed air (CO2 was removed with a Parker Hannifan FTIR Purge Gas Generator) using high-precision digital solenoid-valve-based mass flow controllers (Aalborg Instruments and Controls; Orangeburg, NY, USA). The experimental seawater was bubbled with microporous ceramic airstones into triplicate glass tanks (12 total). The pCO2 of the mixed gases was measured with a Qubit S151 infrared pCO2 analyser (Qubit Systems; Kingston, Ontario, Canada) calibrated with certified air-CO2 gas standards (precision = ±2.0%; accuracy = ±1.8%). Coral specimens from the 18 colonies were reared in each of the 12 replicate tanks. The pCO2 treatments were maintained at an average temperature (s.d.) of 28.10 (0.28)°C.
(ii). Temperature experiment
Experimental growth conditions for the temperature experiment were similar to those for the acidification experiment described above. Siderastrea siderea coral specimens from each of the 18 colonies were reared for 95 days (5 August–8 November 2011) in each of nine 38 l glass tanks (18 specimens per tank; 162 specimens in total) maintained at seawater temperatures (s.d.) of 25.01 (0.14), 28.16 (0.24), and 32.01 (0.17)°C. Salinity (s.d.) was maintained at 35.01 (0.12) by dissolving Instant Ocean Sea Salt in deionized water. These temperatures correspond to the corals' approximate annual minimum, mean and maximum seawater temperature as determined from more than 10 years (2002–2014) of in situ seawater temperature records obtained near the coral collection sites [8,45,46]. Thus, this range of temperatures was selected to capture this species' calcification response to the temperature variability occurring at present within a given year, as well as to the range of average annual seawater temperatures predicted for the next century. Coral specimens were reared in triplicate glass tanks at each of the three temperatures (nine tanks total). Mixed gas with an average pCO2 (s.d.) of 488 (88) µatm was bubbled with microporous ceramic airstones into the tanks. The pCO2 of the temperature treatments were slightly higher than present-day atmospheric value of 400 µatm due to slightly elevated pCO2 in the aquarium culture laboratory. Nevertheless, the pH range in the temperature experiment (7.9–8.0) was within the range observed for present-day reefs [47].
(c). Tank conditions
Seawater within each tank was continuously filtered (757 l h−1) with a power filter. Circulation and turbulence of seawater was enhanced with a 400 l h−1 powerhead. Each tank was covered with a transparent 3-mm Plexiglas sheet and both the tank and filtration system were wrapped with cellophane to promote equilibration between the gas mixtures and the experimental seawaters and to minimize evaporative water loss. The tanks were illuminated for 12 h each day with compact fluorescent lights (ultra-actinic and white; 96 W, 10 000 K) and with standard white fluorescent lights (32 W, T8 6500 K), with a maximum photosynthetically active radiation (PAR) of ca 250 µmol photons m−2 s−1. The intensity and timing of the prescribed irradiance within the tanks was designed to replicate the light cycle of the corals' native habitat (see the electronic supplementary material for a detailed description of light conditions). PAR in the field and in the experimental tanks was measured using a LI-1400 datalogger affixed with a LI-192 underwater quantum sensor (LI-COR; Lincoln, Nebraska; see the electronic supplementary material, figures S1 and S2).
The nominal 28 and 32°C temperature treatments were maintained with 50-W heaters, while the 25°C treatment was maintained with a 1-hp aquarium chiller paired with a 50-W heater for stability. Seventy-five per cent seawater changes were performed weekly. Seawater pH and temperature returned to target values within 60 min of water changes. Each week, 250 ml seawater samples were obtained in ground-glass-stoppered borosilicate glass bottles for analysis of dissolved inorganic carbon and total alkalinity (TA). Seawater samples were obtained midway between weekly water changes in order to acquire average values for the water chemistry parameters in the treatment tanks. Small aliquots of deionized water were periodically added to the experimental tanks in order to replenish water lost through evaporation, thereby maintaining target salinity (35). Each coral specimen was hand-fed 20 mg of frozen Artemia sp. every other day using a 1-ml transfer pipette. Feeding trials conducted prior to the start of the experiment revealed that this amount of food was sufficient to adequately nourish the coral specimens.
(d). Measurement and calculation of carbonate system parameters
Weekly seawater samples were analysed for DIC via coulometry (UIC 5400) and for TA via closed-cell potentiometric Gran titration calibrated with certified TA/DIC standards (see the electronic supplementary material for detailed methods). Temperature, salinity, and pH were determined via standard methods [48] approximately every other day. Additional carbonate system parameters (seawater pCO2, pH, carbonate ion concentration, bicarbonate ion concentration, aqueous CO2, and aragonite saturation state) were calculated with the program CO2SYS [49], using Roy et al. [50] values for K1 and K2 carbonic acid constants, the Mucci [51] value for the stoichiometric aragonite solubility product and an atmospheric pressure of 1.015 atm (see tables S1 and S2 and figures S3 and S4 of the electronic supplementary material for seawater chemistry data).
(e). Quantification of calcification rates via buoyant weighing
Siderastrea siderea calcification rates were estimated using an empirically calibrated buoyant weight technique [14,52] (see the electronic supplementary material for empirical derivation of the buoyant weight–dry weight relationship for this species (figure S5)).
Calcification rates were estimated from the change in the coral specimen's dry weight normalized to its surface area and observational interval. Coral surface area was quantified from scaled top-view photographs of each coral specimen using the imaging software Image J.
(f). Statistical analyses
Hierarchical mixed-effects models were employed to account for the combined repeated-measures/split-plot design to assess the overall effect of treatment on S. siderea calcification rates for the 95-day experiments and the impact of treatment duration on coral calcification response to warming and acidification (see the electronic supplementary material for details of statistical methods employed and tables S3 and S4 for description of observational intervals). All mixed models were estimated with the lme4 package [53] of R 3.0.2 [54].
Data are archived in the US National Science Foundation's Biological and Chemical Oceanography Database at (http://data.bco-dmo.org/jg/dir/test/OA_MarineCalcifiers/).
3. Results
(a). Ocean acidification experiment
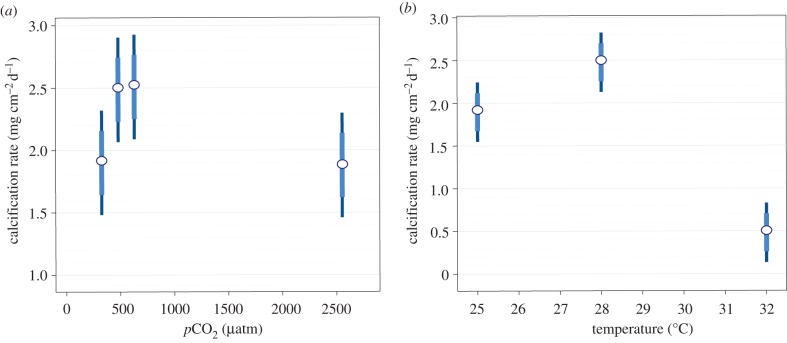
Calcification rates for the coral S. siderea exhibited a parabolic response to increasing atmospheric pCO2 (figure 1a). Over the entire 95-day experiment, calcification rates increased from the near-pre-industrial pCO2 value of 324 µatm to the near-present-day value of 477 µatm, remained relatively unchanged at the predicted end-of-century value of 604 µatm and returned to near-pre-industrial rates at six-times the modern pCO2 value of 2553 µatm (see the electronic supplementary material, table S5).
Figure 1.
Parabolic calcification responses of the coral S. siderea to elevated pCO2 and temperature across the 95-day experiments. (a) Calcification rates for corals at mean pCO2 (s.d.) of 324 (89), 477 (83), 604 (107) and 2553 (506) µatm and at mean temperature (s.d.) of 28.10 (0.28)°C. (b) Calcification rates at mean temperatures (s.d.) of 25.01 (0.14), 28.16 (0.24) and 32.01 (0.17)°C and at mean pCO2 (s.d.) of 488 (88) µatm. Ninety-five per cent (thin bars) and 83.5% (thick bars) confidence intervals of the means are shown.
(b). Temperature experiments
A parabolic calcification response pattern was also exhibited by the coral S. siderea in response to increasing seawater temperature (figure 1b). Over the entire 95-day experiment, calcification rates increased from the lower end of the corals' temperature range of 25°C to their average annual temperature of 28°C and then declined under a temperature of 32°C, near the upper end of their annual thermal range (see the electronic supplementary material, table S6).
(c). Effect of exposure duration on coral calcification response to CO2-induced acidification and warming
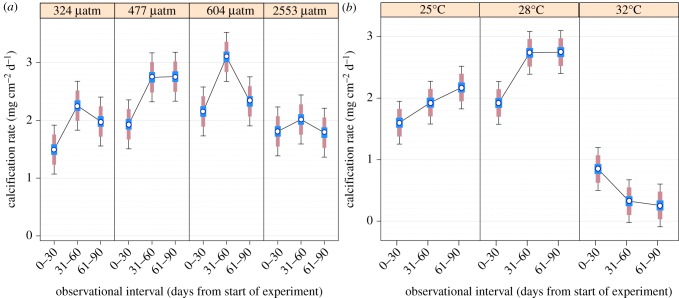
Differences in coral calcification rates were also assessed across three ca 30-day observational intervals (0–30, 31–60 and 61–90 days) using difference-adjusted confidence intervals [55,56] to assess the impact of duration of exposure to pCO2 (see figure 2a and electronic supplementary material, table S7) and temperature treatments (see figure 2b and electronic supplementary material, table S8) on S. siderea calcification rates.
Figure 2.
Effects of exposure duration on S. siderea coral calcification response to pCO2 and temperature. (a) Calcification rates at three monthly observational intervals for S. siderea corals reared at mean pCO2 (s.d.) of 324 (89), 477 (83), 604 (107) and 2553 (506) µatm and maintained at mean temperature (s.d.) of 28.10 (0.28)°C. (b) Calcification rates at three monthly observational intervals for S. siderea corals reared at temperatures (s.d.) of 25.01 (0.14), 28.16 (0.24) and 32.01 (0.17)°C and at mean pCO2 (s.d.) of 488 (88). Ninety-five per cent confidence intervals (black bars) show precision of estimated calcification rates. Eighty-three and one-half per cent confidence intervals (pink bars) are for across-panel (i.e. across treatment) comparison. Forty-two and one-half per cent confidence intervals (blue bars) are for within-panel (i.e. within treatment) comparison.
(i). Ocean acidification experiment
Comparisons within pCO2 treatments (i.e. within-panel comparisons; figure 2a; confidence interval = blue bars) reveal that calcification rates for S. siderea corals reared at 324, 477, 604 and 2553 µatm increased significantly between the first observational interval (0–30 days) and the second observational interval (31–60 days), but declined (except for corals reared at 477 µatm, which remained constant) between the second observational interval and the third observation interval (61–90 days). Notably, calcification rates for the third observational interval were significantly greater than at the first observational interval for the two lowest pCO2 treatments, but not for the two highest pCO2 treatments.
Comparisons between pCO2 treatments (i.e. across-panel comparisons; figure 2a; confidence interval = pink bars) reveal that calcification response patterns to acidification are parabolic for each of the three observational intervals.
(ii). Temperature experiment
Comparisons within temperature treatments (i.e. within-panel comparisons; figure 2b; confidence interval = blue bars) reveal that calcification rates increased across the three observational intervals for corals reared at 25°C, increased across the first two observational intervals for corals reared at 28°C and decreased across the first two observational intervals for corals reared at 32°C. However, coral calcification rates were constant between the second and third observational intervals for corals reared at 28 and 32°C.
Comparisons between temperature treatments (i.e. across-panel comparisons; figure 2b; confidence interval = pink bars) reveal that calcification response patterns to warming are parabolic for each of the three observational intervals.
(d). Effect of reef zone on coral calcification response to CO2-induced acidification and warming
Calcification rates of S. siderea corals were not significantly different across reef zones (i.e. forereef versus backreef versus near shore colonies) within any of the pCO2 or temperature treatments (see the electronic supplementary material, figures S7 and S8, and tables S9 and S10).
4. Discussion
(a). Parabolic calcification response to acidification
Calcification rates within the coral S. siderea increased with moderate elevations in pCO2, but declined with extreme elevation, yielding a parabolic response to CO2-induced ocean acidification. Previous experimental studies, most of which did not use a pre-industrial pCO2 level, showed that corals exhibit either no response [16,25,30,40], a threshold-negative response [14] or a linear negative response to CO2-induced ocean acidification [15,16,20,25,30], although in a recent study the cold-water coral Lophelia pertusa exhibited slightly enhanced calcification under acidified conditions [57].
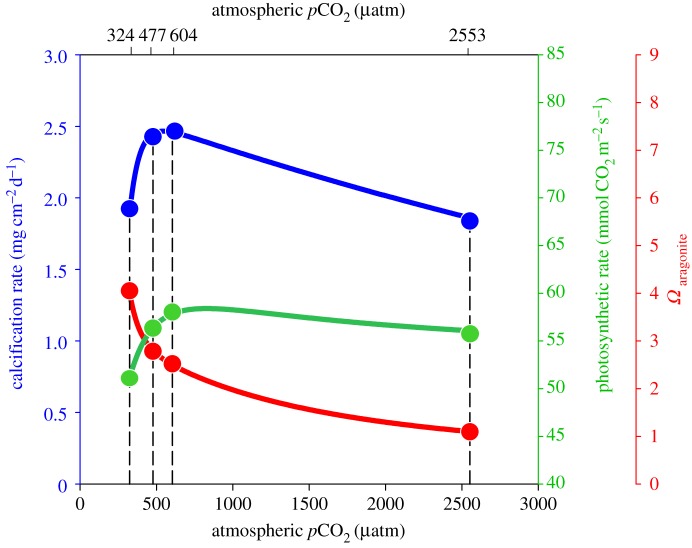
There are two important factors involved in the process of coral calcification that are impacted by CO2-induced ocean acidification in potentially opposite ways: seawater saturation state with respect to the calcium carbonate mineral aragonite (ΩA) and photosynthesis (figure 3). Increasing pCO2 causes seawater pH to decline, which results in a reduction in carbonate ion concentration ([CO3−2]) and thus ΩA, which should impair calcification (red curve, figure 3). Conversely, increasing pCO2 causes the amount of CO2 dissolved in seawater, i.e. aqueous CO2 (CO2-aq), to increase, which should fertilize photosynthesis by the coral's algal symbionts, yielding more photosynthate and thus more energy for coral calcification [39] (green curve, figure 3). Recent studies on Symbiodinium phylotypes previously isolated from reef-building corals suggest that the diffusive uptake of CO2-aq from the external medium within at least one of four Symbiodinium phylotypes is at least partially dependent upon the concentration of CO2-aq [59,60]. Thus, CO2-induced ocean acidification may increase the concentration of CO2-aq available to this symbiont type, potentially elevating photosynthetic capacity of the coral holobiont that could confer supplemental energy for calcification.
Figure 3.
Conceptual diagram (constrained by study results) illustrating how photosynthesis (green curve; estimated from measured Fv/Fm using empirical Fv/Fm–ETRmax relationship from Frade et al. [58] (see the electronic supplementary material, figure S6)) and aragonite saturation state (ΩA) (red curve; data from this study) interact to generate the corals' parabolic calcification response (blue curve; data from this study) to rising atmospheric pCO2.
A generalized model of the relationship between aragonite saturation state (red curve), rate of photosynthesis (green curve) and rate of coral calcification (blue curve)—each constrained by measurements from the present experiment (solid circles)—is rendered in figure 3. Aragonite saturation states and rates of calcification were measured directly, while rates of photosynthesis were estimated indirectly from pulse amplitude modulated fluorometry (see the electronic supplementary material (figure S6) for details of how photosynthetic rates were estimated). This analysis reveals that rates of symbiont photosynthesis (green curve in figure 3) increase with increasing pCO2 from 324 to 604 µatm, and then decline slightly between 604 and 2553 µatm. Thus, moderate elevations in pCO2 (324–604 µatm) appear to enhance photosynthesis of Symbiodinium within S. siderea, while extreme elevations cause symbiont photosynthesis to plateau or slightly decline [59], perhaps because CO2 is no longer limiting for photosynthesis at these elevated levels. The model (figure 3) suggests that calcification rates for S. siderea corals may increase (figure 1a) as pCO2 rises from 324 to 477 µatm because the challenge of calcifying under lower ΩA is outweighed by the benefits of enhanced symbiont photosynthesis (e.g. increased energy and/or more favourable carbonate chemistry at the site of calcification) under moderately elevated pCO2 (324–477 µatm; figure 3). As pCO2 rises from 477 to 604 µatm, ΩA continues to decrease while the benefits to calcification conferred by CO2-enhanced photosynthesis should continue to increase. It is therefore possible that the observed lack of change in coral calcification rate from 477 to 604 µatm (figure 1a) results from the benefit of enhanced photosynthesis being effectively neutralized, in terms of its impact on coral calcification rate, by the decline of ΩA towards undersaturated conditions. Likewise, the increase in pCO2 from 604 µatm to the ultra-high value of 2553 µatm translates to an extreme decrease in ΩA—nearly to the point of undersaturation (ΩA < 1)—which may outweigh the now relatively minor benefit of CO2-enhanced photosynthesis as the corals' symbionts transition away from strict CO2-limitation [30,61] (figure 3), resulting in the substantial decline in coral calcification rate observed across the 604–2553 µatm range (figure 1a).
The surprising ability of S. siderea corals to continue building new skeletal material under all experimental treatments, even at the nearly undersaturated (ΩA < 1) level of 2553 µatm, may arise from the corals' capacity to manipulate the carbonate chemistry at their site of calcification [14,31,62–64]. Some calcifying organisms, by elevating pH of their calcifying fluid, facilitate the deprotonation of bicarbonate ions―whose concentrations are increased under conditions of elevated pCO2―resulting in elevated carbonate ion concentrations and ΩA at the site of calcification. Indeed, in situ microelectrode measurements of pH within the calcifying medium of the tropical scleractinian coral Galaxea fascicularis reveal greater than one pH unit increase above that of ambient seawater [65]. Similar increases in pH have been measured within the calcifying fluid of the temperate scleractinian coral Astrangia poculata [15] and inferred for the tropical scleractinian corals Stylophora pistillata [66], Porites sp.[31], Cladocora caespitosa [67], Desmophyllum dianthus [68], Favia fragum [69] and various species of cold-water scleractinia [70]. A recent study also reveals spatial variations in the calcifying fluid pH of the coral S. pistillata, with polyp tissue exhibiting apparently greater control over calcifying fluid pH than coenosarc tissue [71].
Yet, despite the ability of S. siderea corals to continue building new skeletal material at pCO2 of 2553 µatm, the decline in calcification rate from 604 to 2553 µatm reveals there is a limit to the extent that they can manipulate carbonate chemistry at their site of calcification under conditions of elevated pCO2—beyond which coral calcification rates will decline.
(b). Parabolic calcification response to warming
A parabolic response pattern was also exhibited by the S. siderea corals in response to increasing seawater temperature, with calcification increasing from 25 to 28°C, reaching a maximum at 28°C, and then decreasing from 28 to 32°C (figure 1b). This is consistent with a typical thermal performance curve, in which biological performance increases with rising temperature, reaches a maximum at an optimal temperature, and then declines as temperature continue to rise [72–74].
The parabolic shape of the thermal performance curve is usually attributed to a combination of thermodynamic effects of temperature on reaction rates and the destabilizing effects of temperature on a range of intermolecular interactions [75]. Specifically, the increase in coral calcification from 25 to 28°C may result from thermal acceleration of coral metabolism, including acceleration of zooxanthellate photosynthesis or increased rates of respiration by the coral animal, which would increase thermal energy (as described by the Arrhenius equation) and thus increase rates of chemical reactions involved in calcification [76]. The thermally driven increase in aragonite saturation state may also contribute to the increase in calcification rate observed between 25°C and 28°C. The waning phase of the thermal performance curve results from the destabilizing effects of temperature on a range of intermolecular interactions, ultimately leading to the destruction of the coral–dinoflagellate symbiosis—a process known as coral bleaching [13,77].
The parabolic shape of S. siderea's calcification response to both warming and acidification suggests that parabolic responses to environmental stressors may be the norm and that linear responses arise when the range of the independent stress variable (e.g. temperature, pCO2) is too narrow to capture the full parabolic geometry of the response pattern. However, our observation that the calcification responses of S. siderea to both warming and acidification are parabolic does not necessarily mean that the corals' response to future combined warming and acidification will be parabolic.
Although target temperature and pCO2 levels were generally maintained throughout the 95-day experimental interval, there was moderate variability in TA and associated carbonate system parameters within both sets of experiments. These variations in TA were driven by progressive sequestration of carbonate ions through the coral calcification process. Although weekly water changes were performed, only 75% of the experimental seawater was exchanged in order to avoid shocking the corals. Thus, 25% of the TA drawdown was passed on to the next week's treatment, causing the weekly drawdown in TA to be semi-cumulative throughout the duration of the experiment. This resulted in two trends in TA among treatments: variability in weekly TA within treatments and variability in average TA among treatments (see the electronic supplementary material, tables S1 and S2, and figures S3 and S4).
These trends were most pronounced in the temperature experiment due to the relatively large difference in average calcification rates between the 32°C (TA = 2725 µM) and 28°C (TA = 1951 µM) treatments, which translated to proportional differences in TA (and associated carbonate system parameters) between the treatments. However, after controlling for the effect of temperature on pH, the elevated TA in the 32°C only imparts an approximately 0.1 unit effect on pH relative to pH of the 28°C treatment. Differences in calcification rates between the high-calcification-rate pCO2 treatments (i.e. 477, 604 µatm) and the low-calcification-rate pCO2 treatments (i.e. 324, 2553 µatm) yielded similar but more muted trends in TA for the pCO2 experiment.
Since elevated calcification was causing the decline in TA in both the temperature and pCO2 experiments (rather than depressed TA causing the decline in calcification), corals exhibiting the slowest calcification rates occupied treatments with the highest, most geochemically favourable TA. Therefore, it is reasonable to conclude that the observed differences in TA among treatments only dampened the fundamental calcification trends that were observed, rather than modifying their directions. Had the intermediate pCO2 and temperature treatments that supported the faster calcifying corals been fixed at the higher TAs that were maintained for the low and high pCO2 and temperature treatments, then the faster calcifying corals in the intermediate treatments would have experienced higher aragonite saturation states and thus presumably exhibited even higher calcification rates—thereby enhancing the parabolic shape of the calcification trends observed in both experiments.
Many studies on coral calcification [78,79] use such coral-induced drawdown of TA in a closed system to estimate coral calcifications rates (2 moles of TA = 1 mole of CaCO3 produced), an approach known as the ‘alkalinity anomaly technique’. Indeed, this approach is recommended as one of the ‘best practices' for quantifying calcification rates in ocean acidification research [80]. Nevertheless, the observed differences in coral-induced drawdown of TA and associated carbonate system parameters among treatments should be duly considered in the interpretation of these results.
(c). Duration of exposure to CO2-induced acidification impacts coral calcification rate
The increase in calcification rates of S. siderea between the 0–30-day and the 31–60-day observational intervals suggests that the corals continued acclimating to their treatment conditions throughout these intervals (figure 2a), despite the prescribed acclimation period and gradual adjustment of temperature and pCO2 to the treatment levels. The difference in coral calcification rate between these two observational intervals suggests that a coral's response to an ocean acidification experiment is impacted by its duration of exposure, and may partly explain the wide range of calcification response patterns exhibited by identical or similar organisms in experiments that differ in their duration [14,16,22,23]. Despite these within-treatment differences in calcification rate across the three observational intervals, the corals exhibited comparably parabolic response patterns to acidification within each of the three observational intervals.
Although corals reared at 477 µatm maintained constant calcification after the second observational interval, calcification rates for corals reared at 324, 604 and 2553 µatm declined between the second and third observational intervals. Perhaps during shorter term exposure of these corals to elevated (604, 2553 µatm) or reduced (324 µatm) pCO2, the corals are able to maintain their calcifying medium at a suitable ΩA via pH regulation of the calcifying medium [66], which requires energy. More prolonged exposure to pCO2 perturbation, however, may deplete the corals' lipid energy reserves, which would limit their ability to regulate ΩA at the site of calcification, resulting in the reduced calcification rates evident in the third observational interval (61–90 days). Siderastrea siderea corals reared at the near-present-day pCO2 level of 477 µatm would have experienced the least change in energetic demands associated with regulating carbonate chemistry at their site of calcification, which is consistent with their calcification rates remaining constant between the second and third observational intervals. Although it is assumed that calcification consumes more energy under acidified conditions [23,37], a recent study [25] shows that lipid reserves of four coral species did not decline after approximately 30 days as pCO2 was elevated from 382 to 741 µatm. Thus, the findings of that study are not consistent with our assertion that S. siderea lipid reserves are progressively depleted when the corals are exposed to prolonged periods of acidification. These disparities may arise from interspecific differences in energetic demands of calcification or from differences in the duration of the corals' exposure to elevated pCO2.
(d). Duration of exposure to warming impacts coral calcification rate
The increase in calcification rates across the three observational intervals for corals reared at 25°C suggests that they continued to acclimate to the low temperature conditions throughout the duration of the experiment. Conversely, the relative stabilization in calcification rates between the second and third observational intervals for corals reared at 28 and 32°C suggests that they had fully acclimated by the end of the second interval (figure 2b). Yet, despite these within-treatment differences in calcification rate across observational intervals, the corals' general calcification response patterns to warming were parabolic within each of the three observational intervals.
It is unlikely that the effects of exposure duration on the calcification response of S. siderea corals in this study simply arose from the corals' experimental conditions differing from their natural habitat as such effects should have been constant among treatments and thus impacted corals in all treatments in approximately the same manner. This was not borne out in the experiments, as exposure duration generally had less of an impact on corals in the control treatments than on corals in the high/low pCO2 and temperature treatments—suggesting that the variable effects of exposure duration were indeed linked to the experiments' independent variables (temperature and pCO2).
(e). Near shore, backreef and forereef colonies exhibit equivalent responses to ocean acidification and warming
No statistically significant differences in calcification rates were observed among forereef, backreef and near shore colonies reared under replicate treatments in this study (see the electronic supplementary material, figures S7 and S8, and tables S9 and S10). However, it is possible that a longer experiment, across narrower ranges and finer increments of temperature, would reveal the differential responses among S. siderea corals from different reef zones that were evident in recently obtained cores of this species [8].
(f). Ocean warming poses a more immediate threat than ocean acidification for the coral Siderastrea siderea
This experimental study shows that calcification rates of S. siderea corals exposed to IPCC projected end-of-century tropical seawater temperatures (32°C) declined nearly 80% relative to the control treatment (28°C), while calcification rates for corals reared at IPCC projected end-of-century pCO2 levels (604 µatm) were unchanged relative to the control treatment (477 µatm). Thus, given IPCC's projections for end-of-century climate and oceanic change [81], the results of this study suggest that ocean warming poses a more immediate threat than ocean acidification for the coral S. siderea. That said, interpretation of these isolated impacts of warming and acidification on coral calcification should be tempered by the understanding that these two stressors are occurring and will continue to occur in tandem.
Supplementary Material
Acknowledgements
J. Weiss is acknowledged for providing guidance on the statistical analyses. We thank B. Elder, E. Chow, K. Patel, R. Yost and D. Shroff for helping to maintain the experimental tanks.
Ethics statement
We thank the Belize Fisheries Department for providing permits for collecting and exporting coral samples.
Data accessibility
Data are archived in the US National Science Foundation's Biological and Chemical Oceanography Database at (http://data.bco-dmo.org/jg/dir/test/OA_MarineCalcifiers/).
Funding statement
This research was supported by the Carolina Postdoctoral Fellowship for Faculty Diversity (to K.D.C.), Seeding Postdoctoral Innovators in Research and Education (SPIRE; to K.D.C.), NOAA award nos. NA11OAR431016 and NA13OAR4310186 (to J.B.R. and K.D.C.) and NSF award nos. 1031995 and 1357665 (to J.B.R.). This is publication number 381 from the Northeastern University Marine Science Center.
References
- 1.Keeling R, Piper S, Bollenbacher A, Walker J. 2009. Atmospheric CO2 records from sites in the SIO air sampling network. In Trends: a compendium of data on Global Change, Carbon Dioxide Information Analysis Center. Oak Ridge, TN: Oak Ridge National Laboratory, US Department of Energy. [Google Scholar]
- 2.Rahmstorf S, Cazenave A, Church JA, Hansen JE, Keeling RF, Parker DE, Somerville RCJ. 2007. Recent climate observations compared to projections. Science 316, 709 ( 10.1126/science.1136843) [DOI] [PubMed] [Google Scholar]
- 3.Raven J, Caldeira K, Elderfield H, Hoegh-Guldberg O, Liss P, Riebesell U, Shephard J, Turley C, Watson A. 2005. Ocean acidification due to increasing atmospheric carbon dioxide. Policy Document 12/05 London, UK: The Royal Society. [Google Scholar]
- 4.Trenberth KE, et al. 2007. In Climate Change 2007: The Physical Science Basis Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change (eds S Solomon, D Qin, M Manning, Z Chen, M Marquis, KB Averyt, M Tignor, HL Miller). Cambridge, UK and New York, NY, USA: Cambridge University Press. [Google Scholar]
- 5.Caldeira K, Wickett ME. 2003. Oceanography: anthropogenic carbon and ocean pH. Nature 425, 365 ( 10.1038/425365a) [DOI] [PubMed] [Google Scholar]
- 6.Orr JC, et al. 2005. Anthropogenic ocean acidification over the twenty-first century and its impact on calcifying organisms. Nature 437, 681–686. ( 10.1038/nature04095) [DOI] [PubMed] [Google Scholar]
- 7.Kleypas JA, Danabasoglu G, Lough JM. 2008. Potential role of the ocean thermostat in determining regional differences in coral reef bleaching events. Geophys. Res. Lett. 35, L03613 ( 10.1029/2007GL032257) [DOI] [Google Scholar]
- 8.Castillo KD, Ries JB, Weiss JM, Lima FP. 2012. Decline of forereef corals in response to recent warming linked to history of thermal exposure. Nat. Clim. Change 2, 756–760. ( 10.1038/nclimate1577) [DOI] [Google Scholar]
- 9.Lough JM, Barnes DJ. 2000. Environmental controls on growth of the massive coral Porites. J. Exp. Mar. Biol. Ecol. 245, 225–243. ( 10.1016/S0022-0981(99)00168-9) [DOI] [PubMed] [Google Scholar]
- 10.De'ath G, Lough JM, Fabricius KE. 2009. Declining coral calcification on the Great Barrier Reef. Science 323, 116–119. ( 10.1126/science.1165283) [DOI] [PubMed] [Google Scholar]
- 11.Jokiel PL, Coles SL. 1977. Effects of temperature on the mortality and growth of Hawaiian reef corals. Mar. Biol. 43, 201–208. ( 10.1007/BF00402312) [DOI] [Google Scholar]
- 12.Cooper TF, De'ath G, Fabricius KE, Lough JM. 2008. Declining coral calcification in massive Porites in two nearshore regions of the northern Great Barrier Reef. Glob. Change Biol. 14, 529–538. ( 10.1111/j.1365-2486.2007.01520.x) [DOI] [Google Scholar]
- 13.Brown BE. 1997. Coral bleaching: causes and consequences. Coral Reefs 16, S129–S138. ( 10.1007/s003380050249) [DOI] [Google Scholar]
- 14.Ries J, Cohen A, McCorkle D. 2010. A nonlinear calcification response to CO2-induced ocean acidification by the coral Oculina arbuscula. Coral Reefs 29, 661–674. ( 10.1007/s00338-010-0632-3) [DOI] [Google Scholar]
- 15.Holcomb M, Cohen A, McCorkle D. 2012. An investigation of the calcifiation response of the scleractinian coral Astrangia poculata to elevated pCO2 and the effects of nutrients, zooxanthellae and gender. Biogeosciences 9, 29–39. ( 10.5194/bg-9-29-2012) [DOI] [Google Scholar]
- 16.Comeau S, Edmunds P, Spindel N, Carpenter R. 2013. The response of eight coral reef calcifiers to increasing partial pressure of CO2 do not exhibit a tipping point. Limnol. Oceanogr. 58, 388–398. ( 10.4319/lo.2013.58.1.0388) [DOI] [Google Scholar]
- 17.Bramanti L, et al. 2013. Detrimental effects of ocean acidification on the economically important Mediterranean red coral (Corallium rubrum). Glob. Change Biol. 19, 1897–1908. ( 10.1111/gcb.12171) [DOI] [PubMed] [Google Scholar]
- 18.Jury CP, Whitehead RF, Szmant AM. 2010. Effects of variations in carbonate chemistry on the calcification rates of Madracis auretenra (=Madracis mirabilis sensu Wells, 1973): bicarbonate concentrations best predict calcification rates. Glob. Change Biol. 16, 1632–1644. ( 10.1111/j.1365-2486.2009.02057.x) [DOI] [Google Scholar]
- 19.Edmunds PJ, Brown D, Moriarty V. 2012. Interactive effects of ocean acidification and temperature on two scleractinian corals from Moorea, French Polynesia. Glob. Change Biol. 18, 2173–2183. ( 10.1111/j.1365-2486.2012.02695.x) [DOI] [Google Scholar]
- 20.Anthony KRN, Kleypas AJ, Gattuso J-P. 2011. Coral reefs modify their seawater carbon chemistry— implications for impacts of ocean acidification. Glob. Change Biol. 17, 3655–3666. ( 10.1111/j.1365-2486.2011.02510.x) [DOI] [Google Scholar]
- 21.Hoegh-Guldberg O, et al. 2007. Coral reefs under rapid climate change and ocean acidification. Science 318, 1737–1742. ( 10.1126/science.1152509) [DOI] [PubMed] [Google Scholar]
- 22.Doney SC, Fabry VJ, Feely RA, Kleypas JA. 2009. Ocean acidification: the other CO2 problem. Annu. Rev. Mar. Sci. 1, 169–192. ( 10.1146/annurev.marine.010908.163834) [DOI] [PubMed] [Google Scholar]
- 23.Erez J, Reynaud S, Silverman J, Schneider K, Allemand D. 2011. Coral calcification under ocean acidification and global change. In Coral reefs: an ecosystem in transition (eds Z Dubinsky, N Stambler), pp. 151–176. Berlin, Germany: Springer. [Google Scholar]
- 24.Chan NCS, Connolly SR. 2013. Sensitivity of coral calcification to ocean acidification: a meta-analysis. Glob. Change Biol. 19, 282–290. ( 10.1111/gcb.12011) [DOI] [PubMed] [Google Scholar]
- 25.Schoepf V, et al. 2013. Coral energy reserves and calcification in a high-CO2 world at two temperatures. PLoS ONE 8, e75049 ( 10.1371/journal.pone.0075049) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marubini F, Atkinson MJ. 1999. Effects of lowered pH and elevated nitrate on coral calcification. Mar. Ecol. Prog. Ser. 188, 117–121. ( 10.3354/meps188117) [DOI] [Google Scholar]
- 27.Langdon C, Takahashi T, Sweeney C, Chipman D, Goddard J, Marubini F, Aceves H, Barnett H, Atkinson MJ. 2000. Effect of calcium carbonate saturation state on the calcification rate of an experimental coral reef. Glob. Biogeochem. Cycles 14, 639–654. ( 10.1029/1999gb001195) [DOI] [Google Scholar]
- 28.Leclercq NIC, Gattuso JEANP, Jaubert JEAN. 2000. CO2 partial pressure controls the calcification rate of a coral community. Glob. Change Biol. 6, 329–334. ( 10.1046/j.1365-2486.2000.00315.x). [DOI] [Google Scholar]
- 29.Langdon C, Atkinson MJ. 2005. Effect of elevated pCO2 on photosynthesis and calcification of corals and interactions with seasonal change in temperature/irradiance and nutrient enrichment. J. Geophys. Res. 110, C09S07 ( 10.1029/2004jc002576) [DOI] [Google Scholar]
- 30.Anthony KRN, Kline DI, Diaz-Pulido G, Dove S, Hoegh-Guldberg O. 2008. Ocean acidification causes bleaching and productivity loss in coral reef builders. Proc. Natl Acad. Sci. USA 105, 17 442–17 446. ( 10.1073/pnas.0804478105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Krief S, Hendy EJ, Fine M, Yam R, Meibom A, Foster GL, Shemesh A. 2010. Physiological and isotopic responses of scleractinian corals to ocean acidification. Geochim. Cosmochim. Acta 74, 4988–5001. ( 10.1016/j.gca.2010.05.023) [DOI] [Google Scholar]
- 32.Marubini F, Ferrier-Pagès C, Furla P, Allemand D. 2008. Coral calcification responds to seawater acidification: a working hypothesis towards a physiological mechanism. Coral Reefs 27, 491–499. ( 10.1007/s00338-008-0375-6) [DOI] [Google Scholar]
- 33.Schneider K, Erez J. 2006. The effect of carbonate chemistry on calcification and photosynthesis in the hermatypic coral Acropora eurystoma. Limnol. Oceanogr. 51, 1284–1293. ( 10.4319/lo.2006.51.3.1284) [DOI] [Google Scholar]
- 34.Renegar DA, Riegl BM. 2005. Effect of nutrient enrichment and elevated CO2 partial pressure on growth rate of Atlantic scleractinian coral Acropora cervicornis. Mar. Ecol. Prog. Ser. 293, 69–76. ( 10.3354/meps293069) [DOI] [Google Scholar]
- 35.Jokiel PL, Rodgers KS, Kuffner IB, Andersson AJ, Cox EF, Mackenzie FT. 2008. Ocean acidification and calcifying reef organisms: a mesocosm investigation. Coral Reefs 27, 473–483. ( 10.1007/s00338-008-0380-9) [DOI] [Google Scholar]
- 36.Reynaud S, Leclercq N, Romaine-Lioud S, Ferrier-Pagés C, Jaubert J, Gattuso J-P. 2003. Interacting effects of CO2 partial pressure and temperature on photosynthesis and calcification in a scleractinian coral. Glob. Change Biol. 9, 1660–1668. ( 10.1046/j.1365-2486.2003.00678.x) [DOI] [Google Scholar]
- 37.Cohen A, Holcomb M. 2009. Why corals care about ocean acidification: uncovering the mechanism. Oceanography 22, 118–127. ( 10.5670/oceanog.2009.102) [DOI] [Google Scholar]
- 38.Rodolfo-Metalpa R, Martin S, Ferrier–Pages C, Gattuso JEANP. 2010. Response of the temperate coral Cladocora caespitosa to mid-and long-term exposure to pCO2 and temperature levels projected for the year 2100 AD. Biogeoscience 7, 289–300. ( 10.5194/bg-7-289-2010) [DOI] [Google Scholar]
- 39.Holcomb M, McCorkle DC, Cohen AL. 2010. Long-term effects of nutrient and CO2 enrichment on the temperate coral Astrangia poculata (Ellis and Solander, 1786). J. Exp. Mar. Biol. Ecol. 386, 27–33. ( 10.1016/j.jembe.2010.02.007) [DOI] [Google Scholar]
- 40.Rodolfo-Metalpa R, et al. 2011. Coral and mollusc resistance to ocean acidification adversely affected by warming. Nat. Clim. Change 1, 308–312. ( 10.1038/nclimate1200) [DOI] [Google Scholar]
- 41.Edmunds PJ. 2011. Zooplanktivory ameliorates the effects of ocean acidification on the reef coral Porites spp. Limnol. Oceanogr. 56, 2402–2410. ( 10.4319/lo.2011.56.6.2402) [DOI] [Google Scholar]
- 42.Muehllehner N, Edmunds PJ. 2008. Effects of ocean acidification and increased temperature on skeletal growth of two scleractinian corals, Pocillopora meandrina and Porites rus In Proc. of the 11th Int. Coral Reef Symp., pp. 57–61. Fort Lauderdale, FL: International Coral Reef Symposium (ICRS). [Google Scholar]
- 43.Veron J, Stafford-Smith M. 2000. Corals of the world, p. 463 Townville, SC: Australian Institute of Marine Sciences. [Google Scholar]
- 44.Atkinson MJ, Bingman C. 1998. Elemental composition of commercial seasalts. J. Aquariculture Aquat. Sci. 8, 39–43. [Google Scholar]
- 45.Castillo KD, Helmuth BST. 2005. Influence of thermal history on the response of Montastraea annularis to short-term temperature exposure. Mar. Biol. 148, 261–270. ( 10.1007/s00227-005-0046-x) [DOI] [Google Scholar]
- 46.Castillo K, Lima F. 2010. Comparison of in situ and satelite-derived (MODIS-Aqua/Terra) methods for assessing temperatures on coral reefs. Limnol. Oceanogr. Methods 8, 107–117. ( 10.4319/lom.2010.8.0107) [DOI] [Google Scholar]
- 47.Hofmann GE, et al. 2011. High-frequency dynamics of ocean pH: a multi-ecosystem comparison. PLoS ONE 6, e28983 ( 10.1371/journal.pone.0028983) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dickson A, Sabine C, Christian J. 2007. Guide to best practices for ocean CO2 measurements. Sidney, Canada: PICES Special Publication 3. [Google Scholar]
- 49.Lewis E, Wallace D. 1998. CO2SYS: program developed for CO2 system calculations, ORNL/CDIAC-105. Oak Ridge, TN: Oak Ridge National Laboratory, US Department of Energy. [Google Scholar]
- 50.Roy R, Roy L, Vogel K, Porter-Moore C, Pearson T, Good C, Millero FJ, Campbell D. 1993. The dissociation constants of carbonic acid in seawater at salinities 5 to 45 and temperatures 0 to 45°C. Mar. Chem. 44, 249–267. ( 10.1016/0304-4203(93)90207-5) [DOI] [Google Scholar]
- 51.Mucci A. 1983. The solubility of calcite and aragonite in seawater at various salinities, temperatures, and one atmosphere total pressure. Am. J. Sci. 283, 780–799. ( 10.2475/ajs.283.7.780) [DOI] [Google Scholar]
- 52.Spencer Davies P. 1989. Short-term growth measurements of corals using an accurate buoyant weighing technique. Mar. Biol. 101, 389–395. ( 10.1007/BF00428135) [DOI] [Google Scholar]
- 53.Bates D, Martin M, Ben B, Walker S. 2014. lme4: Linear mixed effects models using Eigen and S4. (R package v. 1.0–6). See http://CRAN.R-project.org/package=lme4.
- 54.R Development Core Team. 2014. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 55.Baguley T. 2012. Calculating and graphing within-subject confidence intervals for ANOVA. Behav. Res. Methods 44, 158–175. ( 10.3758/s13428-011-0123-7) [DOI] [PubMed] [Google Scholar]
- 56.Baguley T. 2012. Serious stats: a guide to advanced statistics for the behavioral sciences. Basingstoke, UK: Palgrave. [Google Scholar]
- 57.Form AU, Riebesell U. 2012. Acclimation to ocean acidification during long-term CO2 exposure in the cold-water coral Lophelia pertusa. Glob. Change Biol. 18, 843–853. ( 10.1111/j.1365-2486.2011.02583.x) [DOI] [Google Scholar]
- 58.Frade PR, Bongaerts P, Winkelhagen AJS, Tonk L, Bak RPM. 2008. In situ photobiology of corals over large depth ranges: a multivariate analysis on the roles of enviornment, host, and algal symbiont. Limnol. Oceanogr. 53, 2711–2723. ( 10.4319/lo.2008.53.6.2711) [DOI] [Google Scholar]
- 59.Brading P, Warner ME, Davey P, Smith DJ, Achterberg EP, Suggett DJ. 2011. Differential effects of ocean acidification on growth and photosynthesis among phylotypes of Symbiodinium (Dinophyceae). Limnol. Oceanogr. 56, 927–938. ( 10.4319/lo.2011.56.3.0927) [DOI] [Google Scholar]
- 60.Brading P, Warner ME, Smith DJ, Suggett DJ. 2013. Contrasting modes of inorganic carbon acquisition amongst Symbiodinium (Dinophyceae) phylotypes. New Phytol. 200, 432–442. ( 10.1111/nph.12379) [DOI] [PubMed] [Google Scholar]
- 61.Crawley A, Kline DI, Dunn S, Anthony KEN, Dove S. 2010. The effect of ocean acidification on symbiont photorespiration and productivity in Acropora formosa. Glob. Change Biol. 16, 851–863. ( 10.1111/j.1365-2486.2009.01943.x) [DOI] [Google Scholar]
- 62.McCulloch M, Falter J, Trotter J, Montagna P. 2012. Coral resilience to ocean acidification and global warming through pH up-regulation. Nat. Clim. Change 2, 623–627. ( 10.1038/nclimate1473) [DOI] [Google Scholar]
- 63.Ries JB. 2011. A physicochemical framework for interpreting the biological calcification response to CO2-induced ocean acidification. Geochim. Cosmochim. Acta 75, 4053–4064. ( 10.1016/j.gca.2011.04.025) [DOI] [Google Scholar]
- 64.Venn AA, Tambutté E, Holcomb M, Laurent J, Allemand D, Tambutté S. 2013. Impact of seawater acidification on pH at the tissue–skeleton interface and calcification in reef corals. Proc. Natl Acad. Sci. USA 110, 1634–1639. ( 10.1073/pnas.1216153110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Al-Horani FA, Al-Moghrabi SM, de Beer D. 2003. The mechanism of calcification and its relation to photosynthesis and respiration in the scleractinian coral Galaxea fascicularis. Mar. Biol. 142, 419–426. ( 10.1007/s00227-002-0981-8) [DOI] [Google Scholar]
- 66.Venn A, Tambutté E, Holcomb M, Allemand D, Tambutté S. 2011. Live tissue imaging shows reef corals elevate pH under their calcifying tissue relative to seawater. PLoS ONE 6, e20013 ( 10.1371/journal.pone.0020013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Trotter J, et al. 2011. Quantifying the pH ‘vital effect’ in the temperate zooxanthellate coral Cladocora caespitosa: validation of the boron seawater pH proxy. Earth Planet. Sci. Lett. 303, 163–173. ( 10.1016/j.epsl.2011.01.030) [DOI] [Google Scholar]
- 68.Anagnostou E, Huang KF, You CF, Sikes EL, Sherrell RM. 2012. Evaluation of boron isotope ratio as a pH proxy in the deep sea coral Desmophyllum dianthus: evidence of physiological pH adjustment. Earth Planet. Sci. Lett. 349–350, 251–260. ( 10.1016/j.epsl.2012.07.006) [DOI] [Google Scholar]
- 69.Cohen A, McCorkle D, de Putron S, Rose KA. 2009. Morphological and compositional changes in the skeletons of new coral recruits reared in acidified seawater: insights into the biomineralization response to ocean acidification. Geochem. Geophys. Geosyst. 10, Q07005 ( 10.1029/2009GC002411) [DOI] [Google Scholar]
- 70.McCulloch M, et al. 2012. Resilience of cold-water scleractinian corals to ocean acidification: boron isotopic systematics of pH and saturation state up-regulation. Geochim. Cosmochim. Acta 87, 21–34. ( 10.1016/j.gca.2012.03.027) [DOI] [Google Scholar]
- 71.Holcomb M, Venn AA, Tambutte E, Tambutte S, Allemand D, Trotter J, McCulloch M. 2014. Coral calcifying fluid pH dictates response to ocean acidification. Sci. Rep. 4, 5207 ( 10.1038/srep05207) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Huey RB, Kingsolver JG. 1989. Evolution of thermal sensitivity of ectotherm performance. Trends Ecol. Evol. 4, 131–135. ( 10.1016/0169-5347(89)90211-5) [DOI] [PubMed] [Google Scholar]
- 73.Pörtner HO, et al. 2006. Trade-offs in thermal adaptation: the need for a molecular to ecological integration. Physiol. Biochem. Zool. 79, 295–313. ( 10.1086/499986) [DOI] [PubMed] [Google Scholar]
- 74.Angilletta MJ. 2009. Thermal adaptation, a theoretical and empirical synthesis. Oxford, UK: Oxford University Press. [Google Scholar]
- 75.Hochachka P, Somero G. 2002. Biochemical adaptations: mechanism and process in physiological evolution. New York, NY: Oxford University Press. [Google Scholar]
- 76.Saxby T, Dennison WC, Hoegh-Guldberg O. 2003. Photosynthetic responses of the coral Montipora digitata to cold temperature stress. Mar. Ecol. Prog. Ser. 248, 85–97. ( 10.3354/meps248085) [DOI] [Google Scholar]
- 77.Moya A, Ferrier-Pagès C, Furla P, Richier S, Tambutté E, Allemand D, Tambutté S. 2008. Calcification and associated physiological parameters during a stress event in the scleractinian coral Stylophora pistillata. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 151, 29–36. ( 10.1016/j.cbpa.2008.05.009) [DOI] [PubMed] [Google Scholar]
- 78.Chisholm JRM, Gattuso J-P. 1991. Validation of the alkalinity anomaly technique for investigating calcification and photosynthesis in coral reef communities. Limnol. Oceanogr. 36, 1232–1239. ( 10.4319/lo.1991.36.6.1232) [DOI] [Google Scholar]
- 79.Murillo LJA, Jokiel PL, Atkinson MJ. 2014. Alkalinity to calcium flux ratios for corals and coral reef communities: variances between isolated and community conditions. Peer J. 2, e249 ( 10.7717/peerj.249) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Riebesell U, Fabry VJ, Hansson L, Gattuso J-P. (eds) 2010. Guide to best practices for ocean acidification research and data reporting, p. 260 Luxembourg: Publication Office of the European Union. [Google Scholar]
- 81.Cubasch U, Wuebbles D, Chen D, Facchini M, Frame D, Mahowald D, Winther J-G. 2013. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. In Climate Change 2013: The Physical Science Basis (eds Stocker TF, Qin D, Plattner G-K, Tignor M, Allen SK, Boschung J, Nauels A, Xia Y, Bex V, Midgley PM.), pp. 119–158 UK and New York, NY, USA: Cambridge University Press. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are archived in the US National Science Foundation's Biological and Chemical Oceanography Database at (http://data.bco-dmo.org/jg/dir/test/OA_MarineCalcifiers/).