Abstract
This prospective cohort study sought to identify predictors of functional decline in patients aged 65 years or older who underwent major, nonemergent abdominal or thoracic surgery in our tertiary hospital from 2006 to 2008. We used the Stanford Health Assessment Questionnaire–Disability Index (HAQ-DI) to evaluate functional decline; a 0.1 or greater increase was used to indicate a clinically significant decline. The preoperative Duke Activity Status Index (DASI) and a physical function score (PFS), assessing gait speed, grip strength, balance, and standing speed, were evaluated as predictors of decline. We enrolled 215 patients (71.2 ± 5.2 years; 56.7% female); 204 completed follow-up HAQ assessments (71.1 ± 5.3 years; 57.8% female). A significant number of patients had functional decline out to 1 year. Postoperative HAQ-DI increases of 0.1 or greater occurred in 45.3 per cent at 1 month, 30.1 per cent at 3 months, and 28.3 per cent at 1 year. Pre-operative DASI and PFS scores were not predictors of functional decline. Male sex at 1 month (odds ratio [OR], 3.05; 95% confidence interval [CI], 1.41 to 6.85); American Society of Anesthesiologists class (OR, 3.41; 95% CI, 1.31 to 8.86), smoking (OR, 3.15; 95% CI, 1.27 to 7.85), and length of stay (OR, 1.09; 95% CI, 1.01 to 1.16) at 3 months; and cancer diagnosis at 1 year (OR, 2.6; 95% CI, 1.14 to 5.96) were associated with functional decline.
The fastest growing segment of the American population is the group of people who are 65 years of age or older. According to the National Center for Health Statistics, “baby boomers” started to become 65 years old in 2011; 71 million people will be 65 years of age or older by 2030, representing approximately 20 per cent of the U.S. population.1 Approximately 16 million inpatient surgical procedures were conducted in patients aged 65 years or older in 2006, and 2.3 million of those procedures were abdominal surgeries.1 Older adults undergo surgery twice as often as their younger counterparts, and as the growing American population ages even more, many older patients will undergo both elective and emergency surgeries.2
Previous studies3–6 have shown that older age is associated with increased mortality and morbidity after surgery. Older patients have decreased functional reserve in most organ systems, making compensation from the stresses of surgery more challenging with increased frequency of postoperative complications.2, 7 Although preoperative risk factors for postoperative morbidity and mortality are well established,8, 9 little is known about postoperative functional decline and disability independent of morbidity. Like with postoperative morbidity and mortality, the stress of a surgical intervention—anesthesia, operation, and complicated or uncomplicated recovery—appears to have a major influence on functional decline in many older patients. Our group previously evaluated the impact of the most common abdominopelvic procedures among patients aged 65 years or older in Washington State. We found that 11 per cent of these patients were discharged to an institutional care facility (ICF) and that the risk of death within a year after surgery was 3.5 times higher for patients discharged to an ICF compared with patients discharged to home.10 Therefore, it is imperative to understand and intervene on preoperative factors that are associated with functional decline.
Accordingly, a focus of research in older patients undergoing surgery has been to identify preoperative factors that predict postoperative functional decline and, through intervention, to potentially reduce postoperative morbidity and mortality. Several studies11–14 have been conducted to define preoperative risk factors for complications in older patients by using geriatric-specific markers such as frailty. However, the disadvantages in using frailty as a marker are that frailty has a multidimensional component, and it is inconsistently associated with decline. In addition, a simple, standardized function-based metric, if predictive, might be more helpful.
To understand functional decline and the potentially modifiable factors associated with decline, we performed a prospective study of patients 65 years of age or older undergoing general and thoracic surgical procedures. The objective of the study was to quantify the degree of functional decline in older patients and to determine the demographic, clinical, and—most specifically—the functional factors, based on standardized measures of preoperative function, that predict functional decline after surgery.
Methods
Study Subjects
This was a prospective, observational study. All study participants were enrolled consecutively from the University of Washington Medical Center (UWMC) clinics from June 2006 through August 2008 and followed for at least 12 months. Inclusion criteria were age 65 years or older, English-speaking, undergoing nonemergent abdominal or thoracic surgery, and willingness to be followed for 1 year after surgery. Exclusion criteria were inguinal/abdominal wall hernia repairs, lung volume reduction surgeries, organ transplantation, cardiac procedures, and dementia as measured by the Mini-Cog test.15
Screening Procedure
Eligible patients were identified through the UWMC scheduling system and approached by trained research staff during their initial consult or preanesthesia visit in the surgical clinic. Patients who were interested in the study were given a Mini-Cog test. Written informed consent was obtained from all participants, and the study protocol and procedures were approved by the University of Washington Human Subjects Review Committee.
Study Protocol
Data Classification
Eligible procedures were identified by the International Classification of Diseases, Ninth Edition (ICD-9) procedural code that represented gastrointestinal, genitourinary, and thoracic operations as the primary procedure.
Sociodemographic and Clinical Characteristics
Before surgery, demographic information and social history were obtained for each subject. The participants’ self-reported medical histories and total number of medications taken within the past 30 days before surgery were also ascertained. The participants were specifically asked whether or not they had been diagnosed with cancer. Each subject’s weight, height, and body mass index were measured before surgery.
Preoperative Exercise Tolerance
The following questionnaires were answered by the participants before surgery: Five-Item Geriatric Depression Scale,16 Duke Activity Status Index (DASI),17 and Stanford Health Assessment Questionnaire (HAQ).18, 19 Baseline functional performance was assessed using the DASI questionnaire. Participants were asked if they could perform certain activities (and if so, whether with no difficulty or some difficulty). A score for an activity was given only if the participant could perform the activity with no difficulty. The scores were summed and ranged semicontinuously from 0 to 52.95; a score of 52.95 indicated the highest level of functioning and exercise tolerance.17
Objective Physical Performance Measures
Preoperative physical performance was measured in four areas: gait speed, grip strength, balance, and standing speed. For gait speed, each participant was asked to complete two 10-foot linear walks down a corridor at the person’s normal pace. The average of the two walks was scored as follows: greater than 4.5 seconds (men) or greater than 5.0 seconds (women), a score of 1; 4.0 to 4.5 seconds (men) or 4.0 to 5.0 seconds (women), a score of 2; 3.0 to 3.9 seconds (men and women), a score of 3; and 3.0 seconds or less (men and women), a score of 4. For grip strength, each participant was asked to use a Jamar® Hydraulic Hand Dynamometer. The greatest of the three attempts in the participant’s dominant hand, measured to the nearest 0.1 kg, was recorded. Quartiles for this measure were scored as follows: less than 25 kg (men) or less than 15 kg (women), a score of 1; 25 to 29.9 kg (men) or 15 to 19.9 kg (women), a score of 2; 30 to 39.9 kg (men) or 20 to 24.9 kg (women), a score of 3; and 40 kg or greater (men) or 25 kg or greater (women), a score of 4. For balance, each participant was asked to stand fully tandem for 10 seconds. Quartile scores were the same for both sexes and were scored as follows: If the participant was able to stand in full tandem (heel of one foot contiguous to toes of opposite foot) for 10 seconds, a score of 4; if the participant was only able to hold full tandem between 1 and 9 seconds, a score of 3; if the participant could hold only a semitandem position (heel of one foot adjacent to toes of opposite foot) for 10 seconds, a score of 2; and if the participant could hold only a side-to-side position for 10 seconds, a score of 1. For standing speed, each participant was asked to sit in an upright position and cross arms over the chest. Chair size was adjusted to the participant’s height so that the thighs were parallel to the ground with feet flat to the floor. The participant was asked to stand straight up as quickly as possible and sit back down five sequential times. Timing began at the start of the initial movement up and stopped at the moment the posterior was in contact with the chair on the third trial. Quartile scores were given as follows: 20 seconds or greater (men) or 21 seconds or greater (women), a score of 1; 17 to 19.9 seconds (men) or 18 to 20.9 seconds (women), a score of 2; 11 to 16.9 seconds (men) or 12 to 17.9 seconds (women), a score of 3; and 11 seconds or less (men) or 12 seconds or less (women), a score of 4.
Performance quintiles were based on previously validated measures controlled for sex and used as cutoff points.20–22 If a participant could not perform a physical test, a score of 0 was given for that measure. A physical function score (PFS) was obtained for each participant by summing the scores from each domain, creating a total scale ranging from 0 to 16. A score of 0 indicated inability to perform any of the tasks, and a score of 16 indicated no physical limitations. The participants were divided into tertiles: the high, middle, and low PFS groups.
Disability and Functional Status Measure
Functional status and disability after surgery were evaluated using the Stanford HAQ and were assessed in eight realms (dressing, arising, eating, walking, hygiene, reach, grip, and performing errands). Each question was scored as follows: no difficulty in performing a task, a score of 0; some difficulty, a score of 1; much difficulty, a score of 2; and unable to do, a score of 3. The highest score indicated the disability level for that category; the cumulative score for each category was averaged to create the disability index (DI). After surgery, each subject was followed for 1 year. The HAQ was administered over the telephone at 1 month, 3 months, and 1 year after surgery to assess each participant’s functional status and quality of life after the surgical procedure.
Clinical Outcomes
Relevant clinical parameters and outcomes were abstracted by trained abstractors from the electronic medical record. These included: length of anesthesia, length of surgery, estimated blood loss, American Society of Anesthesiologists (ASA) classification, complications, length of in-patient stay (in days), and discharge location. Laboratory values obtained pre-operatively included the most recent creatinine (mg/dL) and albumin (g/dL) within 90 days before surgery and the lowest postoperative, inpatient hematocrit (%) before discharge. Procedural type was classified as open or laparoscopic; any laparoscopic procedure converted to an open procedure was classified as open.
Complications were classified into five domains: cardiac complications (ischemic events, heart failure, arrhythmia, hypotension), pulmonary complications (acute respiratory distress syndrome, hypoxemia, pneumonia, bronchospasm), infectious complications (superficial wound, deep wound, abscess wound, bacteremia, urinary infection), general complications (delirium, stroke/transient ischemic attacks, deep vein thrombosis, pulmonary embolism, gastrointestinal bleed, fall, renal failure, death), and surgical complications (wound dehiscence, anastomotic leak, reoperation).
Statistical Analysis
Prior studies23, 24 have determined that an increase in the HAQ-DI score of at least 0.1 is clinically important (equivalent to patient-reported overall status of improvement or worsening). We used this parameter to classify whether patients experienced functional decline after surgery (i.e., whether no significant worsening or functional improvement) at 1, 3, and 12 months follow-up.
Patient characteristics were summarized using frequency distributions, Pearson χ2 statistics for categorical variables, means and standard deviations using the Wilcoxon rank sum test, and Student’s t test for continuous variables stratified by HAQ-DI score increase of 0.1 or greater at 1, 3, and 12 months postoperatively. Characteristics associated with a risk of HAQ-DI score increase of 0.1 or greater at 1 month, at 3 months and at 1 year were initially assessed by univariate comparison. Logistic regression models were created to evaluate the association between significant (P < 0.05) preoperative predictors and outcomes and variables expected to be related based on previous studies. STATA software was used for all analyses (Version 11; STATA Corp., College Station, TX).
Results
Four hundred patients were identified as eligible for the study, 370 were approached for participation, and 285 gave written informed consent. There were 72 potential participants who were excluded for the following reasons: did not proceed to surgery (n = 36); failed preoperative cognitive screening (n = 14); underwent a nonqualifying procedure (n = 2); withdrew consent before the 3-month assessment (n = 10); had incomplete baseline data (n = 4); failed to undergo preoperative physical assessments (n = 2); needed emergent surgery (n = 1); or died before surgery (n = 3). There remained 213 patients who were included in the analysis; of these, 204 completed HAQ and PFS questionnaires at the 3-month follow-up assessment. Some participants’ data were missing at 3 months for the following reasons: death before the 3-month postoperative assessment (n = 4); lost to follow-up (n = 5); and failure to complete all HAQ-DI domains.
The average age of the subjects was 71.1 ± 5.3 years and 57.8 per cent were female. Patients who had an increase in their HAQ-DI scores of 0.1 or greater (clinically significant worsened functional status) after 3 months were older (72.3 ± 6.1 years vs 70.6 ± 4.8 years, P = 0.03), more likely to be current or former smokers (72.9 vs 55.3%, P = 0.02), and more likely to be in ASA Class 3 or above (86.7 vs 62.2%, P = 0.001) (Table 1). Patients whose HAQ-DI score increased by 0.1 or greater had longer length of stay (LOS) (7.4 ± 8.2 days vs 5.3 ± 5.8 days, P = 0.04) and were more likely to be discharged to an ICF (14.8 vs 6.3%, P = 0.05) (Table 1).
Table 1.
Baseline Clinical Characteristics of Study Participants with HAQ-DI Score Increase of 0.1 or Greater at 3 Months Postoperatively
| Characteristics | Overall | No Decline | Decline | P |
|---|---|---|---|---|
| Sociodemographic Variables | ||||
| No. | 204 | 143 | 61 | |
| Age (mean ± SD) | 71.1 ± 5.3 | 70.6 ± 4.8 | 72.3 ± 6.1 | 0.03 |
| Percent female | 118 (57.8%) | 86 (60.1%) | 32 (52.5%) | 0.3 |
| Percent married | 138 (69.0%) | 100 (71.9%) | 38 (62.3%) | 0.2 |
| Education level (%) | 0.4 | |||
| Elementary | 7 (3.7%) | 4 (3.0%) | 3 (5.2%) | |
| High school | 52 (27.4%) | 40 (30.3%) | 12 (20.7%) | |
| College | 121 (63.7%) | 80 (60.6%) | 41 (70.7%) | |
| Other | 10 (5.3%) | 8 (6.1%) | 2 (3.5%) | |
| Smoking status | 0.02 | |||
| Current/former | 121 (60.5%) | 78 (55.3%) | 43 (72.9%) | |
| Never | 79 (39.5%) | 64 (44.7%) | 16 (27.1%) | |
| Clinical Variables | ||||
| Cancer diagnosis | 110 (55.6%) | 75 (53.6%) | 35 (60.3%) | 0.4 |
| Laparoscopic procedures | 87 (43.3%) | 63 (44.7%) | 24 (40.0%) | 0.5 |
| ASA class | 0.001 | |||
| I–II | 59 (30.3%) | 51 (37.8%) | 8 (13.3%) | |
| III–IV | 136 (69.7%) | 84 (62.2%) | 52 (86.7%) | |
| Medical history | ||||
| Congestive heart failure | 14 (7.0%) | 7 (5.0%) | 7 (11.7%) | 0.09 |
| Hypertension | 109 (53.7%) | 75 (52.8%) | 34 (55.7%) | 0.7 |
| Diabetes | 48 (23.8%) | 36 (25.4%) | 12 (20.0%) | 0.4 |
| Ischemic heart disease | 21 (10.3%) | 13 (9.1%) | 8 (13.1%) | 0.4 |
| COPD/emphysema | 22 (10.9%) | 13 (9.2%) | 9 (15.0%) | 0.2 |
| Depression | 45 (22.2%) | 28 (19.7%) | 17 (27.9%) | 0.2 |
| DVT/PE | 27 (14.0%) | 20 (14.7%) | 7 (12.3%) | 0.7 |
| Charlson’s comorbidity index | 0.7 | |||
| 0 | 42 (21.4%) | 30 (21.4%) | 12 (21.4%) | |
| 10 | 86 (43.9%) | 63 (45.0%) | 23 (41.1%) | |
| 2 | 36 (18.4%) | 27 (19.3%) | 9 (16.1%) | |
| 3+ | 32 (16.3%) | 20 (14.3%) | 12 (21.4%) | |
| Mean ± SD | 1.4 ± 1.4 | 1.4 ± 1.3 | 1.6 ± 1.5 | |
| Preoperative hematocrit (%) | 37.1 ± 5.2 | 37.0 ± 5.0 | 37.3 ± 5.6 | 0.7 |
| Creatinine greater than 2 mg/dL | 12 (7.2%) | 3 (7.8%) | 3 (5.8%) | 0.6 |
| Albumin less than 3 g/dL | 14 (13.0%) | 12 (16.0%) | 2 (6.1%) | 0.2 |
| Postoperative Variables | ||||
| Postoperative complications | 48 (23.7%) | 33 (23.2%) | 15 (24.6%) | 0.8 |
| Length of stay | 6.0 ± 6.7 | 5.3 ± 5.8 | 7.4 ± 8.2 | 0.04 |
| Discharge to assisted facility | 18 (8.8%) | 9 (6.3%) | 9 (14.8%) | 0.05 |
HAQ-DI, Health Assessment Questionnaire–Disability Index; SD, standard deviation; ASA, American Society of Anesthesiologists; COPD, chronic obstructive pulmonary disease; DVT, deep vein thrombosis; PE, pulmonary embolism.
Proportion with Functional Decline
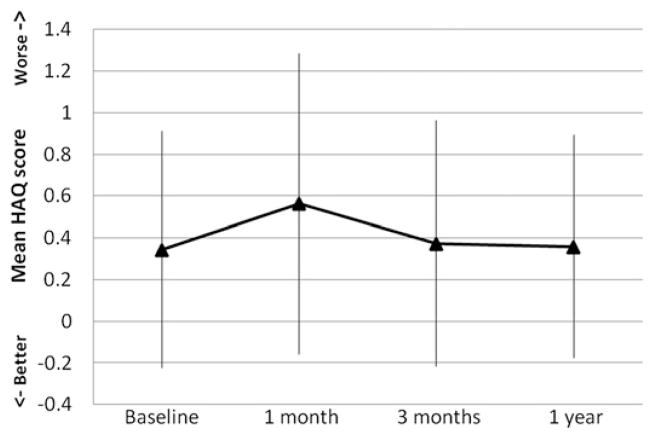
Overall, the mean HAQ-DI scores increased from baseline by 0.22 at 1 month and by 0.03 at 3 months. Eventually, the mean HAQ-DI scores returned down to baseline with a 0.36 mean HAQ-DI score at 1 year (Fig. 1). There was significant variability in HAQ scores at all time points with a range of −0.16 to 1.29 at 1 month, −0.22 to 0.96 at 3 months, and −0.18 to 0.89 at 1 year. A significant proportion of patients had an HAQ-DI score increase of 0.1 or greater: 45.3 per cent at 1 month, 30.1 per cent at 3 months, and 28.3 per cent at 1 year. Patients undergoing pancreatic and thoracic resections had the greatest functional decline at 1 month (80.0 and 50.0%, respectively), but by 3 months, the proportion declined (33.3 and 22.7% at 1 year, respectively) (Table 2). Patients undergoing hepatobiliary operations did not recover as well from functional disability with 40 per cent of the patients still having an HAQ-DI score increase of 0.1 or greater at 1 year compared to 38.9 per cent at 1 month.
Fig. 1.
Mean Health Assessment Questionnaire (HAQ) scores (with standard deviation) at baseline and at 1 month, 3 months, and 1 year postoperatively.
Table 2.
HAQ-DI Score Increase of 0.1 or Greater Overall and by Type of operation Over Different Time Periods Postoperatively
| Type of Operation | HAQ-DI Increase at 1 Month | HAQ-DI Increase at 3 Months | HAQ-DI Increase at 1 Year |
|---|---|---|---|
| Overall (n = 204) | 91/201 (45.3%) | 61/203 (30.1%) | 51/180 (28.3%) |
| Endocrine and renal (n = 5) | 1 (20%) | 1 (20%) | 2 (40%) |
| Intestinal (n = 45) | 21 (48.8%) | 17 (39.5%) | 13 (34.2%) |
| Esophageal (n = 45) | 20 (46.5%) | 11 (24.4%) | 10 (23.8%) |
| Gastric (n = 26) | 8 (30.8%) | 4 (15.4%) | 3 (12.0%) |
| Pancreatic (n = 16) | 12 (80.0%) | 6 (40.0%) | 4 (33.3%) |
| Hepatobiliary (n = 38) | 14 (38.9%) | 14 (38.9%) | 12 (40.0%) |
| Abdominal wall procedures (n = 7) | 2 (28.6%) | 1 (14.3%) | 2 (33.3%) |
| Thoracic (n = 26) | 13 (50.0%) | 7 (26.9%) | 5 (22.7%) |
HAQ-DI, Health Assessment Questionnaire–Disability Index.
Relationship of Preoperative Parameters and Decline
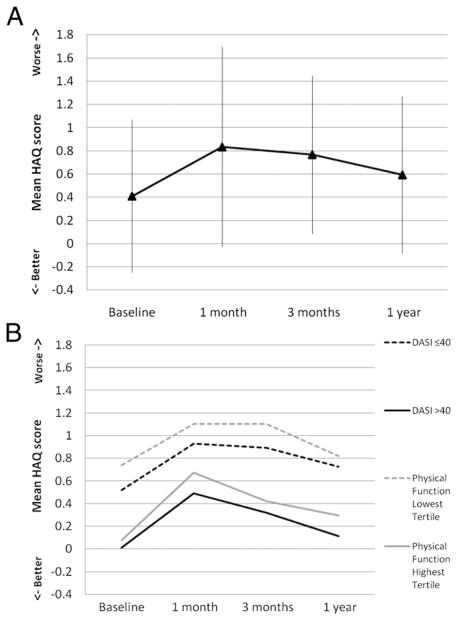
Subjects with higher preoperative functional status (DASI greater than 40 and highest PFS tertile) had a similar pattern of functional decline at all time points after surgery compared with subjects who had lower preoperative functional status (DASI 40 or lower and lowest PFS tertile). We compared preoperative characteristics for subjects who experienced clinically significant functional decline at 3 months. Subjects with higher functional status at baseline recovered quicker (i.e., improvement of HAQ scores starting at 3 months) compared with those with lower functional status, but neither group returned to their baseline functional status at 1 year (Fig. 2).
Fig. 2.
Mean Health Assessment Questionnaire (HAQ) scores for study participants with HAQ score worsening (increase) by 0.1 or greater at 3 months: (A) overall mean HAQ scores (with standard deviation) at baseline, at 1 month, at 3 months, and at 1 year postoperatively. (B) Mean HAQ scores at each time period stratified by study participants with Duke Activity Status Index (DASI) scores greater than 40 versus DASI scores 40 or less at baseline, and study participants with physical function score in the highest tertile versus lowest tertile at baseline.
Univariate analysis showed that age, sex, depression, cancer, LOS, discharge to an ICF, postoperative complications, and laparoscopic procedures were associated with functional decline. Higher preoperative DASI and total PFS scores (highest tertile vs lowest tertile) were associated with a small but statistically significant reduced risk of functional decline at 1 month (odds ratio [OR], 0.98; 95% confidence interval [CI], 0.97 to 0.99 for DASI and OR, 0.46; 95% CI, 0.23 to 0.92) for PFS) and 3 months (OR, 0.98; 95% CI, 0.96 to 0.99 for DASI and OR, 0.32; 95% CI, 0.14 to 0.73 for PFS) but not at 1 year (OR, 0.99; 95% CI, 0.97 to 1.01 for DASI and OR, 0.61; 95% CI, 0.27 to 1.39 for PFS). For each of the functional measures (gait speed, grip strength, balance, and standing speed), only the higher preoperative balance score was associated with lower odds of functional decline at 3 months (OR, 0.5; 95% CI, 0.26 to 0.94). Adjusting for important patient, clinical, and perioperative characteristics, ASA class (OR, 3.41; 95% CI, 1.31 to 8.86), smoking status (OR, 3.15; 95% CI, 1.27 to 7.85), and LOS (OR, 1.09; 95% CI, 1.01 to 1.16) were associated with functional decline at 3 months (Table 3). Male sex was associated with functional decline at 1 month but not afterward, and cancer diagnosis was associated with decline only at 1 year (Table 3).
Table 3.
Multivariate Logistic Regression Analysis of Study Participants with Functional Decline (as measured by HAQ-DI score increase of 0.1 or greater) at 1 Month, 3 Months, and 1 Year Postoperatively
| Baseline Characteristics
| |||
|---|---|---|---|
| Characteristics | Functional decline at 1 month (OR, 95% CI) | Functional decline at 3 months (OR, 95% CI) | Functional decline at 1 year (OR, 95% CI) |
| Age | 1.07 (0.99–1.15) | 1.02 (0.95–1.09) | 1.03 (0.96–1.11) |
| Sex | 3.05 (1.41–6.58) | 2.23 (0.98–5.08) | 1.17 (0.49–2.78) |
| ASA | 1.29 (0.58–2.87) | 3.41 (1.31–8.86) | 1.41 (0.59–3.34) |
| Depression | 2.04 (0.95–4.36) | 1.5 (0.63–3.6) | 1.5 (0.66–3.39) |
| Cancer | 1.52 (0.68–3.39) | 1.29 (0.58–2.89) | 2.6 (1.14–5.96) |
| Smoker | 1.67 (0.79–3.53) | 3.15 (1.27–7.85) | 1.04 (0.46–2.36) |
| Length of stay | 1.13 (1.04–1.23) | 1.09 (1.01–1.16) | 1.01 (0.94–1.09) |
| Discharge to assisted facility | 0.37 (0.06–2.08) | 1.02 (0.2–5.09) | 0.11 (0.01–1.35) |
| Postoperative complications | 1.26 (0.52–3.03) | 0.59 (0.21–1.62) | 0.45 (0.18–1.15) |
| Laparoscopic procedures | 1.89 (0.89–4.02) | 0.47 (0.22–1.01) | 1.46 (0.68–3.15) |
| DASI | 1.01 (0.98–1.03) | 1.01 (0.98–1.04) | 0.99 (0.96–1.02) |
| Physical functional score* | |||
| Middle tertile | 0.68 (0.28–1.66) | 0.51 (0.19–1.36) | 0.74 (0.29–1.92) |
| Highest tertile | 0.67 (0.23–1.95) | 0.45 (0.14–1.41) | 0.66 (0.23–1.89) |
HAQ-DI, Health Assessment Questionnaire–Disability Index; OR, odds ratio; CI, confidence interval; ASA, American Society of Anesthesiologists; DASI, Duke Activity Status Index.
For physical functional score, the middle and highest tertile groups are compared against the lowest tertile group.
Discussion
A significant proportion of older patients undergoing major surgery experienced functional decline at 1 month (45.3%), 3 months (30.1%), and 1 year (28.3%). Although other investigators11, 13, 25, 26 have suggested that preoperative functional and physical function might be a modifiable predictor of decline, we found no significant association between baseline functional and physical status and functional decline. Among participants who had an initial functional decline, higher preoperative functional status was related to a quicker recovery, but no subgroups returned to baseline functional status. ASA class, smoking, LOS, cancer, and male sex were associated with significant functional decline at some point after surgery.
Functional decline is commonly noted in older adults after surgery. A case series study27 of nonagenarians reported a 27 per cent loss of the ability to ambulate independently after all major surgery. A more recently published retrospective cohort study28 found that 16 per cent of all adults were unable to return directly home after noncardiac surgery, increasing to 39 per cent in adults aged 80 years and older. Modifiable preoperative factors that are associated with functional decline after surgery still need to be identified. Small studies25, 29 in older patients undergoing orthopedic surgery have shown that preoperative functional status is an important predictor of postoperative function. Seymour and Pringle30 found that in patients undergoing nonorthopedic surgery, higher preoperative activity level, defined as “those who left their homes as a result of their own efforts at least twice a week,” was associated with fewer complications. Recently, Lawrence et al.31 conducted a prospective cohort study investigating the natural history of functional recovery in older adults after major elective abdominal surgery. These authors showed that some patients experience significant functional decline in activities of daily living (ADL) after 1 week postoperatively with 50 per cent of patients reporting dependency for dressing and bathing and 30 per cent requiring assistance for eating and grooming.31 Some patients failed to recover their preoperative ADL functioning even after 6 months. Lawrence et al.31 noted that poor preoperative physical performance (measured by gait speed, functional reach, and handgrip strength), depression, and serious postoperative complications were associated with poor ADL recovery after abdominal surgery. Our study builds on previous studies by quantifying the degree of postoperative functional decline using the HAQ-DI score, describing functional decline and recovery over time, and determining the preoperative factors associated with physical performance and functional decline/disability after surgery. We did not see a similar relationship between preoperative functional status and outcome. This may be because the DASI and PFS scores are inconsistent predictors of functional decline in certain populations or are not effective descriptors of preoperative function.
Maintenance of baseline levels of function and independence and reduction in premature disability after a surgical procedure are critical from a physical, psychological, social, and economic standpoint. As the population ages, functional decline and the subsequent need for extended medical care after surgery affects an important proportion of the two million Americans in nursing homes.32 Surgery is a major stressor to functional status and must be considered in the context of a general decline in functional status associated with “healthy aging.” A healthy 65 year old is expected to have 9.5 years of functional independence, whereas an 85 year old is expected to have an average of 3.3 years of independent function.33 For all aging patients, modifiable risk factor identification may provide important information about risk reduction.
Studies examining predictors of functional decline in community-dwelling and hospitalized older adults have evaluated medications,34 age,35 low social activity,36 increasing comorbidity,36–39 cognitive impairment,35–37, 39 depression,40, 41 prior disability,35–37 lack of physical activity,42, 43 and poor physical performance.20, 44 We analyzed most of these variables and found that only ASA scores, LOS, smoking, cancer, and male sex were associated with significant functional decline at some point after surgery. Limited self-reported exercise tolerance and frailty at baseline have been associated with an increased risk for complications.13, 26 Carli et al.45 demonstrated an association between preoperative improvement in functional status and postoperative recovery regardless of the type of exercise program (complex vs simple). Identifying a good exercise tolerance measure is needed because interventional trials suggest the benefits of preoperative rehabilitation programs.45 To identify good candidates for such an intervention, our findings do not support the use of the DASI (self-reported) and PFS (objective) questionnaires.
There are several limitations to our study. Because the study subjects were recruited at an academic medical center and 97 per cent of those enrolled identified themselves as non-Hispanic whites, our results may not be generalized beyond patients with similar characteristics. Additionally, patients who were too ill to undergo surgery or showed signs of cognitive decline at screening were not included. Although some research has concluded that frailty occurs before the onset of cognitive impairment, some investigators contend that frailty and cognitive impairment have similar etiologies and occur in tandem.16, 31 The HAQ-DI has been validated in numerous studies and has demonstrated substantial content and face validity compared with other measures of disability.18, 19 However, the HAQ-DI has been used mostly in patients with rheumatoid arthritis and has never been evaluated in patients undergoing major surgery. There is a possibility that some of the decline seen in our study was a consequence of postoperative events not reported by the participants. Lastly, any preoperative frailty status of the patients may have been known to the patients’ providers, and we could not determine if interventions were adjusted to facilitate postoperative function.
A major focus in caring for aging patients is preventing functional decline and increasing the number of disability-free years. In this study, 45 per cent of older adults experienced functional decline after surgery. The search for modifiable factors to prevent decline is important; but although we discovered certain clinical factors to be associated with decline, we found that a determination of baseline functional physical decline (as measured by the DASI and PFS scores) was not helpful in predicting functional decline. Research on nonpredictive factors of post-surgical functional decline will be vital for guiding future investigations to find modifiable factors to prevent functional decline.
Acknowledgments
Supported by the National Institutes of Health (NIH) and the Hartford/American Foundation for Aging Research Grant K12HD049100. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
References
- 1.National Center for Health Statistics, Centers for Disease Control and Prevention. [Accessed November 11, 2010]; Available at: www.cdc.gov/nchs.
- 2.Thomas DR, Ritchie CS. Preoperative assessment of older adults. J Am Geriatr Soc. 1995;43:811–21. doi: 10.1111/j.1532-5415.1995.tb07058.x. [DOI] [PubMed] [Google Scholar]
- 3.Stamou SC, Dangas G, Dullum MK, et al. Beating heart surgery in octogenarians: perioperative outcome and comparison with younger age groups. Ann Thorac Surg. 2000;69:1140–5. doi: 10.1016/s0003-4975(99)01430-7. [DOI] [PubMed] [Google Scholar]
- 4.Kirchhoff P, Dincler S, Buchmann P. A multivariate analysis of potential risk factors for intra- and postoperative complications in 1316 elective laparoscopic colorectal procedures. Ann Surg. 2008;248:259–65. doi: 10.1097/SLA.0b013e31817bbe3a. [DOI] [PubMed] [Google Scholar]
- 5.Finlayson EV, Birkmeyer JD. Operative mortality with elective surgery in older adults. Eff Clin Pract. 2001;4:172–7. [PubMed] [Google Scholar]
- 6.Alexander KP, Anstrom KJ, Muhlbaier LH, et al. Outcomes of cardiac surgery in patients > or = 80 years: results from the National Cardiovascular Network. J Am Coll Cardiol. 2000;35:731–8. doi: 10.1016/s0735-1097(99)00606-3. [DOI] [PubMed] [Google Scholar]
- 7.Brennan TA, Leape LL, Laird NM, et al. Incidence of adverse events and negligence in hospitalized patients. Results of the Harvard Medical Practice Study I. N Engl J Med. 1991;324:370–6. doi: 10.1056/NEJM199102073240604. [DOI] [PubMed] [Google Scholar]
- 8.Khuri SF, Daley J, Henderson W, et al. Risk adjustment of the postoperative mortality rate for the comparative assessment of the quality of surgical care: results of the National Veterans Affairs Surgical Risk Study. J Am Coll Surg. 1997;185:315–27. [PubMed] [Google Scholar]
- 9.Daley J, Khuri SF, Henderson W, et al. Risk adjustment of the postoperative morbidity rate for the comparative assessment of the quality of surgical care: results of the National Veterans Affairs Surgical Risk Study. J Am Coll Surg. 1997;185:328–40. [PubMed] [Google Scholar]
- 10.Legner VJ, Massarweh NN, Symons RG, et al. The significance of discharge to skilled care after abdominopelvic surgery in older adults. Ann Surg. 2009;249:250–5. doi: 10.1097/SLA.0b013e318195e12f. [DOI] [PubMed] [Google Scholar]
- 11.Robinson TN, Eiseman B, Wallace JI, et al. Redefining geriatric preoperative assessment using frailty, disability and comorbidity. Ann Surg. 2009;250:449–55. doi: 10.1097/SLA.0b013e3181b45598. [DOI] [PubMed] [Google Scholar]
- 12.Fukuse T, Satoda N, Hijiya K, Fujinaga T. Importance of a comprehensive geriatric assessment in prediction of complications following thoracic surgery in elderly patients. Chest. 2005;127:886–91. doi: 10.1378/chest.127.3.886. [DOI] [PubMed] [Google Scholar]
- 13.Makary MA, Segev DL, Pronovost PJ, et al. Frailty as a predictor of surgical outcomes in older patients. J Am Coll Surg. 2011;210:901–8. doi: 10.1016/j.jamcollsurg.2010.01.028. [DOI] [PubMed] [Google Scholar]
- 14.Turrentine FE, Wang H, Simpson VB, Jones RS. Surgical risk factors, morbidity, and mortality in elderly patients. J Am Coll Surg. 2006;203:865–77. doi: 10.1016/j.jamcollsurg.2006.08.026. [DOI] [PubMed] [Google Scholar]
- 15.Borson S, Scanlan J, Brush M, et al. The Mini-Cog: a cognitive ‘vital signs’ measure for dementia screening in multi-lingual elderly. Int J Geriatr Psychiatry. 2000;15:1021–7. doi: 10.1002/1099-1166(200011)15:11<1021::aid-gps234>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 16.Rinaldi P, Mecocci P, Benedetti C, et al. Validation of the five-item geriatric depression scale in elderly subjects in three different settings. J Am Geriatr Soc. 2003;51:694–8. doi: 10.1034/j.1600-0579.2003.00216.x. [DOI] [PubMed] [Google Scholar]
- 17.Hlatky MA, Boineau RE, Higginbotham MB, et al. A brief self-administered questionnaire to determine functional capacity (the Duke Activity Status Index) Am J Cardiol. 1989;64:651–4. doi: 10.1016/0002-9149(89)90496-7. [DOI] [PubMed] [Google Scholar]
- 18.Bruce B, Fries JF. The Stanford Health Assessment Questionnaire: dimensions and practical applications. Health Qual Life Outcomes. 2003;1:20. doi: 10.1186/1477-7525-1-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bruce B, Fries JF. The Stanford Health Assessment Questionnaire: a review of its history, issues, progress, and documentation. J Rheumatol. 2003;30:167–78. [PubMed] [Google Scholar]
- 20.Guralnik JM, Ferrucci L, Simonsick EM, et al. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332:556–61. doi: 10.1056/NEJM199503023320902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ostir GV, Volpato S, Fried LP, et al. Reliability and sensitivity to change assessed for a summary measure of lower body function: results from the Women’s Health and Aging Study. J Clin Epidemiol. 2002;55:916–21. doi: 10.1016/s0895-4356(02)00436-5. [DOI] [PubMed] [Google Scholar]
- 22.Wang L, van Belle G, Kukull WB, Larson EB. Predictors of functional change: a longitudinal study of nondemented people aged 65 and older. J Am Geriatr Soc. 2002;50:1525–34. doi: 10.1046/j.1532-5415.2002.50408.x. [DOI] [PubMed] [Google Scholar]
- 23.Colangelo KJ, Pope JE, Peschken C. The minimally important difference for patient reported outcomes in systemic lupus erythematosus including the HAQ-DI, pain, fatigue, and SF-36. J Rheumatol. 2009;36:2231–7. doi: 10.3899/jrheum.090193. [DOI] [PubMed] [Google Scholar]
- 24.Pope JE, Khanna D, Norrie D, Ouimet JM. The minimally important difference for the health assessment questionnaire in rheumatoid arthritis clinical practice is smaller than in randomized controlled trials. J Rheumatol. 2009;36:254–9. doi: 10.3899/jrheum.080479. [DOI] [PubMed] [Google Scholar]
- 25.MacWilliam CH, Yood MU, Verner JJ, et al. Patient-related risk factors that predict poor outcome after total hip replacement. Health Serv Res. 1996;31:623–38. [PMC free article] [PubMed] [Google Scholar]
- 26.Reilly DF, McNeely MJ, Doerner D, et al. Self-reported exercise tolerance and the risk of serious perioperative complications. Arch Intern Med. 1999;159:2185–92. doi: 10.1001/archinte.159.18.2185. [DOI] [PubMed] [Google Scholar]
- 27.Cohen JR, Johnson H, Eaton S, et al. Surgical procedures in patients during the tenth decade of life. Surgery. 1988;104:646–51. [PubMed] [Google Scholar]
- 28.Polanczyk CA, Marcantonio E, Goldman L, et al. Impact of age on perioperative complications and length of stay in patients undergoing noncardiac surgery. Ann Intern Med. 2001;134:637–43. doi: 10.7326/0003-4819-134-8-200104170-00008. [DOI] [PubMed] [Google Scholar]
- 29.Holtzman J, Saleh K, Kane R. Effect of baseline functional status and pain on outcomes of total hip arthroplasty. J Bone Joint Surg Am. 2002;84:1942–8. doi: 10.2106/00004623-200211000-00006. [DOI] [PubMed] [Google Scholar]
- 30.Seymour DG, Pringle R. Post-operative complications in the elderly surgical patient. Gerontology. 1983;29:262–70. doi: 10.1159/000213125. [DOI] [PubMed] [Google Scholar]
- 31.Lawrence VA, Hazuda HP, Cornell JE, et al. Functional independence after major abdominal surgery in the elderly. J Am Coll Surg. 2004;199:762–72. doi: 10.1016/j.jamcollsurg.2004.05.280. [DOI] [PubMed] [Google Scholar]
- 32.Evans JM, Chutka DS, Fleming KC, et al. Medical care of nursing home residents. Mayo Clin Proc. 1995;70:694–702. doi: 10.4065/70.7.694. [DOI] [PubMed] [Google Scholar]
- 33.Katz S, Branch LG, Branson MH, et al. Active life expectancy. N Engl J Med. 1983;309:1218–24. doi: 10.1056/NEJM198311173092005. [DOI] [PubMed] [Google Scholar]
- 34.Magaziner J, Cadigan DA. Community resources and mental health of older women living alone. J Aging Health. 1989;1:35–49. doi: 10.1177/089826438900100103. [DOI] [PubMed] [Google Scholar]
- 35.Sager MA, Rudberg MA, Jalaluddin M, et al. Hospital Admission Risk Profile (HARP): identifying older patients at risk for functional decline following acute medical illness and hospitalization. J Am Geriatr Soc. 1996;44:251–7. doi: 10.1111/j.1532-5415.1996.tb00910.x. [DOI] [PubMed] [Google Scholar]
- 36.Inouye SK, Wagner DR, Acampora D, et al. A predictive index for functional decline in hospitalized elderly medical patients. J Gen Intern Med. 1993;8:645–52. doi: 10.1007/BF02598279. [DOI] [PubMed] [Google Scholar]
- 37.Zureik M, Lombrail P, Davido A, et al. Predicting the outcome in elderly patients of hospital admission for acute care in Paris, France: construction and initial validation of a simplex index. J Epidemiol Community Health. 1997;51:192–8. doi: 10.1136/jech.51.2.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guralnik JM, LaCroix AZ, Abbott RD, et al. Maintaining mobility in late life. I. Demographic characteristics and chronic conditions. Am J Epidemiol. 1993;137:845–57. doi: 10.1093/oxfordjournals.aje.a116746. [DOI] [PubMed] [Google Scholar]
- 39.Narain P, Rubenstein LZ, Wieland GD, et al. Predictors of immediate and 6-month outcomes in hospitalized elderly patients. The importance of functional status. J Am Geriatr Soc. 1988;36:775–83. doi: 10.1111/j.1532-5415.1988.tb04259.x. [DOI] [PubMed] [Google Scholar]
- 40.Moritz DJ, Kasl SV, Berkman LF. Cognitive functioning and the incidence of limitations in activities of daily living in an elderly community sample. Am J Epidemiol. 1995;141:41–9. doi: 10.1093/oxfordjournals.aje.a117344. [DOI] [PubMed] [Google Scholar]
- 41.Gill TM, Williams CS, Richardson ED, et al. A predictive model for ADL dependence in community-living older adults based on a reduced set of cognitive status items. J Am Geriatr Soc. 1997;45:441–5. doi: 10.1111/j.1532-5415.1997.tb05168.x. [DOI] [PubMed] [Google Scholar]
- 42.Clark DO. The effect of walking on lower body disability among older blacks and whites. Am J Public Health. 1996;86:57–61. doi: 10.2105/ajph.86.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Seeman TE, Berkman LF, Charpentier PA, et al. Behavioral and psychosocial predictors of physical performance: MacArthur studies of successful aging. J Gerontol A Biol Sci Med Sci. 1995;50:M177–83. doi: 10.1093/gerona/50a.4.m177. [DOI] [PubMed] [Google Scholar]
- 44.Tinetti ME, Inouye SK, Gill TM, Doucette JT. Shared risk factors for falls, incontinence, and functional dependence. Unifying the approach to geriatric syndromes. JAMA. 1995;273:1348–53. [PubMed] [Google Scholar]
- 45.Vlessides M. [Accessed June 23, 2011];Presurgery Exercise May Boost Postoperative Recovery. Available at: www.generalsurgerynews.com.