Abstract
Recently, we described a patient with severe lactic acidosis due to congenital complex I (NADH-ubiquinone oxidoreductase) deficiency. We now report further enzymatic and immunological characterizations. Both NADH and ferricyanide titrations of complex I activity (measured as NADH-ferricyanide reductase) were distinctly altered in the mitochondria from the patient's tissues. In addition, antisera against complex I immunoprecipitated NADH-ferricyanide reductase from the control but not the patient's mitochondria. However, immunoprecipitation and sodium dodecyl sulfate-polyacrylamide gel electrophoresis of complex I polypeptides demonstrated that the majority of the 25 polypeptides comprising complex I were present in the affected mitochondria. A more detailed analysis using subunit selective antisera against the main polypeptides of the iron-protein fragments of complex I revealed a selective absence of the 75- and 13-kD polypeptides. These findings suggest that the underlying basis for this patient's disease was a congenital deficiency of at least two polypeptides comprising the iron-protein fragment of complex I, which resulted in the inability to correctly assemble a functional enzyme complex.
Full text
PDF


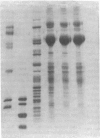
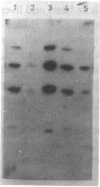
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boustany R. N., Aprille J. R., Halperin J., Levy H., DeLong G. R. Mitochondrial cytochrome deficiency presenting as a myopathy with hypotonia, external ophthalmoplegia, and lactic acidosis in an infant and as fatal hepatopathy in a second cousin. Ann Neurol. 1983 Oct;14(4):462–470. doi: 10.1002/ana.410140411. [DOI] [PubMed] [Google Scholar]
- Capeillere-Blandin C., Ohnishi T. Investigation of the iron-sulfur clusters in some mitochondrial mutants of Saccharomyces cerevisiae. A possible correlation between Rieske's iron-sulfur cluster and cytochrome b. Eur J Biochem. 1982 Feb;122(2):403–413. doi: 10.1111/j.1432-1033.1982.tb05895.x. [DOI] [PubMed] [Google Scholar]
- Darley-Usmar V. M., Kennaway N. G., Buist N. R., Capaldi R. A. Deficiency in ubiquinone cytochrome c reductase in a patient with mitochondrial myopathy and lactic acidosis. Proc Natl Acad Sci U S A. 1983 Aug;80(16):5103–5106. doi: 10.1073/pnas.80.16.5103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiMauro S., Mendell J. R., Sahenk Z., Bachman D., Scarpa A., Scofield R. M., Reiner C. Fatal infantile mitochondrial myopathy and renal dysfunction due to cytochrome-c-oxidase deficiency. Neurology. 1980 Aug;30(8):795–804. doi: 10.1212/wnl.30.8.795. [DOI] [PubMed] [Google Scholar]
- DiMauro S., Nicholson J. F., Hays A. P., Eastwood A. B., Papadimitriou A., Koenigsberger R., DeVivo D. C. Benign infantile mitochondrial myopathy due to reversible cytochrome c oxidase deficiency. Ann Neurol. 1983 Aug;14(2):226–234. doi: 10.1002/ana.410140209. [DOI] [PubMed] [Google Scholar]
- Heiman-Patterson T. D., Bonilla E., DiMauro S., Foreman J., Schotland D. L. Cytochrome-c-oxidase deficiency in a floppy infant. Neurology. 1982 Aug;32(8):898–901. doi: 10.1212/wnl.32.8.898. [DOI] [PubMed] [Google Scholar]
- LUFT R., IKKOS D., PALMIERI G., ERNSTER L., AFZELIUS B. A case of severe hypermetabolism of nonthyroid origin with a defect in the maintenance of mitochondrial respiratory control: a correlated clinical, biochemical, and morphological study. J Clin Invest. 1962 Sep;41:1776–1804. doi: 10.1172/JCI104637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Land J. M., Morgan-Hughes J. A., Clark J. B. Mitochondrial myopathy. Biochemical studies revealing a deficiency of NADH--cytochrome b reductase activity. J Neurol Sci. 1981 Apr;50(1):1–13. doi: 10.1016/0022-510x(81)90038-1. [DOI] [PubMed] [Google Scholar]
- Moreadith R. W., Batshaw M. L., Ohnishi T., Kerr D., Knox B., Jackson D., Hruban R., Olson J., Reynafarje B., Lehninger A. L. Deficiency of the iron-sulfur clusters of mitochondrial reduced nicotinamide-adenine dinucleotide-ubiquinone oxidoreductase (complex I) in an infant with congenital lactic acidosis. J Clin Invest. 1984 Sep;74(3):685–697. doi: 10.1172/JCI111484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan-Hughes J. A., Darveniza P., Kahn S. N., Landon D. N., Sherratt R. M., Land J. M., Clark J. B. A mitochondrial myopathy characterized by a deficiency in reducible cytochrome b. Brain. 1977 Dec;100(4):617–640. doi: 10.1093/brain/100.4.617. [DOI] [PubMed] [Google Scholar]
- Morgan-Hughes J. A., Darveniza P., Landon D. N., Land J. M., Clark J. B. A mitochondrial myopathy with a deficiency of respiratory chain NADH-CoQ reductase activity. J Neurol Sci. 1979 Sep;43(1):27–46. doi: 10.1016/0022-510x(79)90071-6. [DOI] [PubMed] [Google Scholar]
- Morgan-Hughes J. A., Hayes D. J., Clark J. B., Landon D. N., Swash M., Stark R. J., Rudge P. Mitochondrial encephalomyopathies: biochemical studies in two cases revealing defects in the respiratory chain. Brain. 1982 Sep;105(Pt 3):553–582. doi: 10.1093/brain/105.3.553. [DOI] [PubMed] [Google Scholar]
- RACKER E. Studies of factors involved in oxidative phosphorylation. Proc Natl Acad Sci U S A. 1962 Sep 15;48:1659–1663. doi: 10.1073/pnas.48.9.1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radda G. K., Bore P. J., Gadian D. G., Ross B. D., Styles P., Taylor D. J., Morgan-Hughes J. 31P NMR examination of two patients with NADH-CoQ reductase deficiency. Nature. 1982 Feb 18;295(5850):608–609. doi: 10.1038/295608a0. [DOI] [PubMed] [Google Scholar]
- Riggs J. E., Schochet S. S., Jr, Fakadej A. V., Papadimitriou A., DiMauro S., Crosby T. W., Gutmann L., Moxley R. T. Mitochondrial encephalomyopathy with decreased succinate-cytochrome c reductase activity. Neurology. 1984 Jan;34(1):48–53. doi: 10.1212/wnl.34.1.48. [DOI] [PubMed] [Google Scholar]
- Schotland D. L., DiMauro S., Bonilla E., Scarpa A., Lee C. P. Neuromuscular disorder associated with a defect in mitochondrial energy supply. Arch Neurol. 1976 Jul;33(7):475–479. doi: 10.1001/archneur.1976.00500070017003. [DOI] [PubMed] [Google Scholar]
- Smith S., Cottingham I. R., Ragan C. I. Immunological assays of the NADH dehydrogenase content of bovine heart mitochondria and submitochondrial particles. FEBS Lett. 1980 Feb 11;110(2):279–282. doi: 10.1016/0014-5793(80)80092-5. [DOI] [PubMed] [Google Scholar]
- Smith S., Ragan C. I. The organization of NADH dehydrogenase polypeptides in the inner mitochondrial membrane. Biochem J. 1980 Feb 1;185(2):315–326. doi: 10.1042/bj1850315. [DOI] [PMC free article] [PubMed] [Google Scholar]