Abstract
Background
Worldwide, streptokinase remains the most used thrombolytic agent for the treatment of myocardial infarction. Recombinant streptokinase, from E. coli, is increasingly used in developing countries as a biosimilar of native streptokinase; however, potency assignments relative to the WHO International Standard (IS) are highly variable with potentially dangerous consequences. A proportion of recombinant streptokinase appears to be incompletely processed, retaining the amino-terminal methionine engineered for intracellular expression.
Objectives
To investigate and quantify the impact of an amino-terminal methionine on streptokinase activity.
Methods
Mature native streptokinase (rSK) was cloned and a novel variant constructed to include an amino-terminal methionine (rSK-Met) that is not susceptible to processing during expression. Potencies of rSK and rSK-Met were determined relative to the WHO IS using a chromogenic solution (European Pharmacopoeia) assay, and fibrin-based assays.
Results
In the chromogenic solution assay there was no measurable difference between rSK and rSK-Met activities. In the fibrin-based methods, however, potency estimates for rSK-Met were greatly reduced compared with rSK, and fibrinolytic activity for rSK-Met was shown to increase over time with methionine aminopeptidase treatment. This apparent difference in activity and fibrin selectivity was consistent with potency estimates for several different batches of commercial recombinant streptokinase products also tested; consequently, different potencies would be assigned to therapeutic recombinant streptokinase products depending on the degree of amino-terminal methionine processing, and on the pharmacopoeial assay method used, affecting the dosage patients receive. This has serious health implications and provides an example of the danger in the unregulated clinical use of biosimilars.
Keywords: biosimilar harmaceuticals, DNA, recombinant, fibrinolytic agents, reference standards, thrombolytic therapy
Introduction
The bacterial plasminogen activator streptokinase was the first licensed thrombolytic drug. Despite advances with new fibrinolytic agents 1, streptokinase is still widely used, particularly in developing countries, largely because of patent expiry and the availability of biosimilars (or follow-on biologicals). Discussions on the problems associated with manufacturing biosimilars, and their susceptibility to changes in the manufacturing process (often described as ‘the process is the product’), are widely available 2 yet there is currently no globally agreed regulatory approval pathway. While streptokinase produced and sold in Europe is maintained and well regulated 3 there are concerns over the regulatory frameworks in developing countries 4. Independent surveys on the quality of streptokinase from around the world have found wide variations in activity, purity and composition 3,5.
The originator streptokinase product is purified from culture filtrates of the H46A isolate of group C Streptococcus equisimilis 6. Many biosimilar products are also produced this way, although increasingly recombinant streptokinase is being manufactured as a cheaper alternative. A specific issue associated with recombinant streptokinase has been identified, whereby differences in activity were found to depend on the assay system used and on the presence or absence of fibrin 7–9. This is unexpected because the gene sequence should be the same as the originator product, and the presence of fibrin in the assay system was previously found to make no difference to streptokinase potency estimates 10.
The difference in activity between native and some recombinant streptokinase products has not been fully explained, although amino-terminal sequencing of different recombinant products suggests that gene cloning and protein expression strategies may be issues 5. Commercial production of recombinant streptokinase is typically through intracellular E. coli expression, which requires a methionine translation start signal in place of the secretory signal peptide sequence. This amino-terminal methionine may be cleaved during post-translational processing by host amino-peptidase enzymes; however, the efficiency of this process is variable and influenced by the culture growth and expression conditions 11. Incomplete methionine processing has been identified in one recombinant streptokinase product by amino-terminal sequencing 5. The significance of this for functional activity is not known, but could be expected to have an impact based on the known ‘molecular sexuality’ mechanism of plasminogen activation used by streptokinase 12. In this paper we investigate the impact of incomplete amino-terminal methionine processing on recombinant streptokinase activity, using representative recombinant streptokinase variants generated through targeted mutation. The activity of these controlled variants was determined in different assay systems, and the results compared with commercial recombinant streptokinase products.
Materials and methods
Cloning, expression and purification of recombinant streptokinase
The coding region for mature recombinant streptokinase from the H46A strain of Streptococcus equisimilis (rSK) protein (414 amino acids, amino-terminal isoleucine) was amplified from genomic DNA (a gift from Biocon, Bangalore, India) by PCR and cloned in frame with the amino-terminal SUMOstar fusion tag in the vector pI-SUMOstar (Lifesensors, Malvern, PA, USA). An alternative sense primer including an ATG codon was used to incorporate an amino-terminal methionine for the expression of rSK-Met. SUMOstar-streptokinase fusion sequences were sub-cloned into the EcoRV site of the expression vector pET-Blue-1 (Novagen (Merck), Darmstadt, Germany) by PCR.
Recombinant streptokinase variants were expressed in T7 Express lysY E. coli (NEB, Herts, UK) and cells were lysed using B-PER Direct with enzymes (Thermo Fisher Scientific, Loughborough, UK). Recombinant SUMO star-streptokinase fusion proteins were purified by Ni-affinity and cleaved with SUMOstar Protease I (Lifesensors) over 4 h (assessed by SDS PAGE) at 30°C in the recommended buffer. Cleaved SUMOstar tag and SUMOstar protease I were removed by Ni-affinity.
Protein purity was determined visually by SDS PAGE, and protein concentrations were determined using Coomassie plus reagent (Thermo Fisher Scientific) relative to a standard curve of rSK of known protein concentration determined by amino acid analysis (Alta Bioscience, Birmingham, UK). Amino-terminal protein sequencing was performed by Alta Bioscience.
Chromogenic solution assay
Chromogenic streptokinase activity was measured against glu-plasminogen according to the European Pharmacopoeia (EP) monograph for Streptokinase Concentrated Solution 13. Briefly, microplate assays were performed in 100 μL reactions in 10 mM Tris-HCl (pH 7.4 at 37°C), 100 mM NaCl and 0.01% Tween 20. Streptokinase samples, pre-diluted in buffer containing 1 mg mL−1 human serum albumin (HSA), were included over a concentration range of 0.5–5.0 IU mL−1 with 100 nM glu-plasminogen (Chromogenix, Milan, Italy) and 0.6 mM H-D-Valyl-L-leucyl-L-lysine p-Nitroaniline dihydrochloride (S2251, Chromogenix), the chromogenic substrate for plasmin. Absorbance at 405 nm was monitored kinetically for 90 min at 37°C. Initial rates of plasminogen activation were calculated from plots of absorbance vs. seconds squared for absorbance values <0.2, to give results in pM s−1 plasmin generation.
Fibrin clot overlay assay
Fibrin clot overlay assays were performed as described previously 14 to measure rates of fibrin-bound plasminogen activation by following S-2251 hydrolysis. Briefly fibrin clots (60 μL) were formed in microplates at 37°C for 30 min, made by mixing 9 μM fibrinogen (Merck Millipore; cat. 341578) in 40 mM Tris HCl, 75 mM NaCl and 0.01% Tween 20, with 0.1 U bovine thrombin (Diagnostic Reagents Ltd, Oxon, UK) incorporating 165 nM glu-plasminogen. The assay was initiated by the addition of 40 μL of a mixture of streptokinase and 0.6 mM S2251. Streptokinase samples were pre-diluted in buffer containing 1 mg mL−1 HSA, over a concentration range of 0.4–4.0 IU mL−1. Absorbance at 405 nm was monitored kinetically for 90 min at 37°C. Initial rates of plasminogen activation were calculated from plots of absorbance vs. seconds squared for absorbance values <0.2, and results converted to pM s−1 plasmin generation.
Fibrin clot lysis assays
Fibrin clot lysis assays were performed in microplates using changes in fibrin opacity to monitor fibrin clot formation and lysis. A mixture of 2.5 nM human thrombin reagent 01/578 (NIBSC, Herts, UK), 100 nM glu-plasminogen, 8 μM fibrinogen and streptokinase (concentration range 0.2–2 IU mL−1) was added to microplate wells to a final volume of 100 μL. Absorbance at 405 nm was monitored kinetically at 37°C. Fibrinolysis rates were taken as the time to the minimum first derivative (maximum lysis rate), calculated from absorbance vs. time data as previously described 15.
Statistical analysis
Potencies and 95% confidence intervals were calculated in IU relative to the 3rd International Standard (IS) for streptokinase (00/464; NIBSC) based on a parallel line bioassay analysis using the statistical program Combistats (EDQM, Strasbourg, France) following EP guidelines on the statistical analysis of results of biological tests and assays 16. For each assay a minimum of three streptokinase doses were included, with replicates, and overall potencies were calculated from the combined analysis of multiple assays (n ≥ 3).
Methionine aminopeptidase treatment of rSK-Met
rSK-Met (290 ng) was incubated at 37°C in 10 mM Tris-HCl, pH 7.5, 0.01% Tween 20 and 0.1 mM CoCl2, for 1 h ± 0.45 mU recombinant methionine aminopeptidase from Pyrococcus furiosus (Sigma-Aldrich, St Louis, MO, USA). Aliquots were snap frozen in liquid nitrogen immediately upon the addition of the enzyme, and at regular intervals throughout the incubation, and stored at −40°C.
Results and discussion
To investigate the impact of an additional amino-terminal methionine on streptokinase activity, the mature native streptokinase sequence (rSK) was cloned and a novel variant constructed to include an amino-terminal methionine (rSK-Met) that is not susceptible to processing during expression. Control over the amino-terminal residue was achieved using an E. coli expression system to produce proteins with amino-terminal SUMOstar tag. The tag was removed by SUMOstar protease, which cleaves precisely to preserve the streptokinase amino-terminal residue, and the presence of the amino-terminal methionine in rSK-Met was confirmed by protein sequencing.
Potency estimates for rSK and rSK-Met were made relative to the 3rd IS for streptokinase (00/464) in three different assay systems: the European Pharmacopoeia (EP) method, in which initial rates of glu-plasminogen activation were measured in solution using the chromogenic substrate for plasmin S-2251; a fibrin clot overlay assay, which also measured initial plasminogen activation rates but at the surface of a preformed fibrin clot; and a fibrin clot lysis assay, which provided a measure of fibrinolytic activity by monitoring clot formation and lysis through fibrin absorbance changes. For each method, rSK and rSK-Met were diluted to an appropriate concentration range for the assay to give an equivalent response to the IS, determined empirically for each recombinant streptokinase preparation in each assay system. For direct comparison between different rSK preparations results were expressed as specific activities in terms of International Units (IU) per μg protein.
Potency estimates obtained for rSK (Fig.1(A) left panel) were consistent with native streptokinase, whereby the presence of fibrin had no significant effect on activity 10. When rSK-Met was compared in the same three assay systems (Fig.1(A) right panel) a markedly different activity profile was obtained. In the solution assay there was no measurable difference between potencies calculated for rSK and rSK-Met. In the fibrin clot overlay assay, however, potency estimates for rSK-Met were more than 80% lower than those for rSK, and those calculated in the solution assay. When fibrin lysis rates were compared the difference was even greater, with a further 5-fold decrease in rSK-Met potency estimates, 96% lower than in those calculated for the solution assay. Potency estimates were also obtained for 11 different batches of commercial therapeutic recombinant streptokinase products, from two different manufacturers, using the chromogenic and fibrin clot overlay assays (Fig.1B). In the presence of fibrin, potency estimates of all of these products were approximately 50% lower than the chromogenic result.
Figure 1.
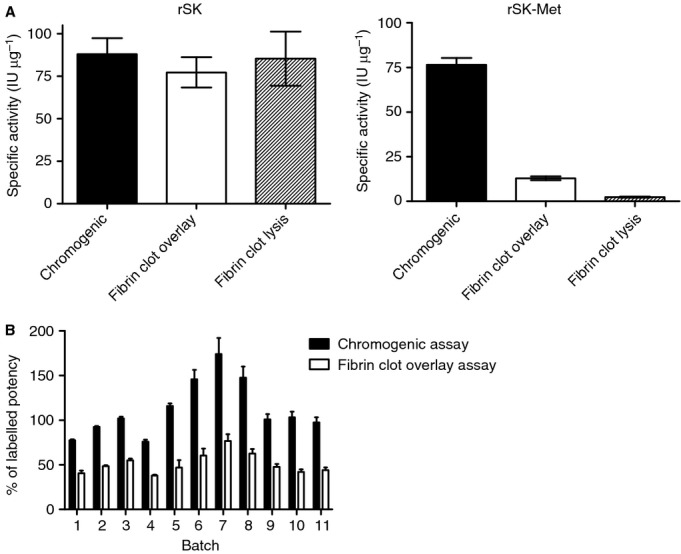
(A) Specific activities (IU μg−1) of native sequence recombinant streptokinase (rSK) and an engineered variant with an amino-terminal methionine (rSK-Met), measured in three different assay systems: a chromogenic assay, a fibrin clot overlay assay and a fibrin clot lysis assay. All potency estimates were calculated relative to the WHO 3rd International Standard for Streptokinase (00/464) using a parallel line bioassay model. (B) Potency estimates for 11 batches of commercial streptokinase from two different manufacturers, based on chromogenic and fibrin clot overlay assay methods and presented as a percentage of the labeled potency.
Following incubation with methionine aminopeptidase, an enzyme that specifically cleaves amino-terminal methionine residues, the fibrinolytic activity of rSK-Met was shown to improve over time through increased fibrin clot lysis rates (Fig.2). These observations clearly implicate the amino-terminal methionine as being responsible for the different activity profiles of rSK and rSK-Met.
Figure 2.
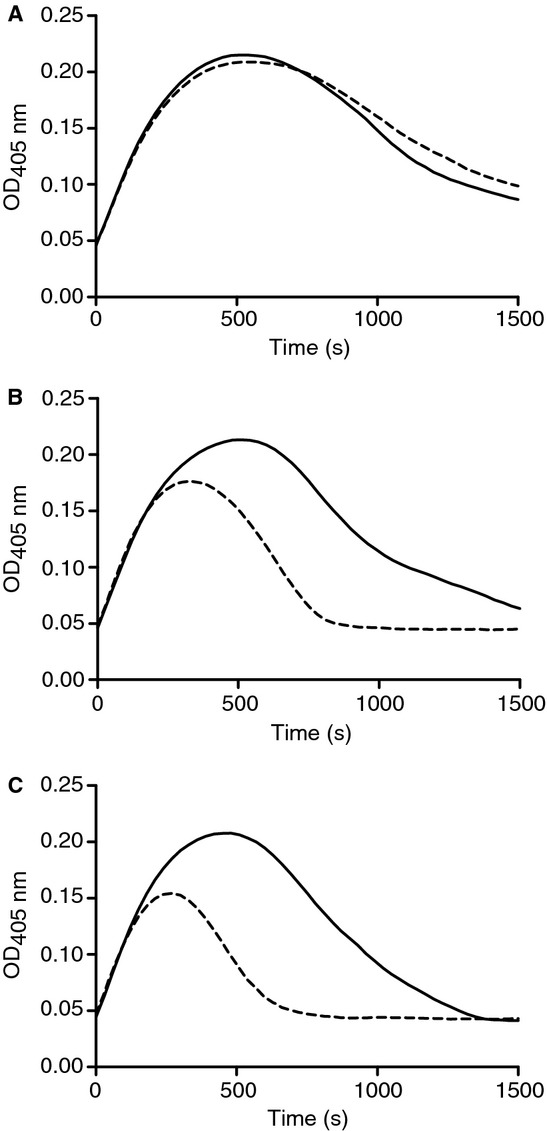
Recovery of activity of rSK-Met upon the removal of amino-terminal methionine. Fibrin clot lysis curves represent rSK-Met pretreated with (dashed line) or without (solid line) methionine aminopeptidase, for 0 min (A), 30 min (B) and 60 min (C).
The results obtained for recombinant streptokinase, generated under controlled conditions, demonstrate that the presence of amino-terminal methionine results in greatly reduced potency estimates where fibrin is included in the assay. For the commercial recombinant streptokinase batches tested (Fig.1B), activity with fibrin was approximately 50% of the chromogenic result. Each of these recombinant products was manufactured in E. coli, with intracellular expression of the mature protein facilitated by the addition of an artificial amino-terminal methionine. Quantitative amino-terminal sequencing of a sample from batch 11 (Fig.1B) confirmed the presence of both native N-terminal Ile and Met, with the amino-terminal methionine variant representing approximately 48% of the total as expected based on activity. The reduced activity in fibrin of the commercial recombinant batches shown in Fig.1(B) is therefore consistent with products being a heterogeneous mixture of both forms as a result of partial methionine processing during manufacture.
Batches of therapeutic recombinant streptokinase produced in E. coli, with significant proportions of amino-terminal methionine, will therefore be assigned different potencies relative to the WHO IS for streptokinase depending on the degree of methionine processing, and on the assay system used. This highlights the truth in the adage ‘the process is the product’, and is of particular concern for streptokinase as different pharmacopoeial assay methods for potency testing are variable in their requirement for fibrin (e.g. the EP method (no fibrin) and Indian Pharmacopoeia method (with fibrin)).
This discrepancy has serious implications for the dosage patients receive in treatment for acute myocardial infarction. As dosages (in IU) can only be determined relative to the WHO IS for streptokinase, it follows that potencies of recombinant streptokinase should not be labelled in IU when there is likely to be a significant proportion of molecules with an amino-terminal methionine present. There is a narrow ‘therapeutic window’ for fibrinolytic-antithrombotic regimens and the potential for adverse outcomes is high if incorrect doses are administered. The administration of a too low dose is associated with decreased rates of reperfusion in the infarct-related artery, and higher doses associated with increased intracranial hemorrhage 17. These findings highlight the dangers in the unregulated use of biosimilars and have serious health implications.
Addendum
C. Thelwell designed the study, performed experiments, analyzed data and wrote the manuscript. C. Longstaff performed experiments, analyzed data and critically reviewed the manuscript.
Disclosure of Conflict of Interests
The authors state that they have no conflict of interests.
References
- 1.Flemmig M, Melzig MF. Serine-proteases as plasminogen activators in terms of fibrinolysis. J Pharm Pharmacol. 2012;64:1025–39. doi: 10.1111/j.2042-7158.2012.01457.x. [DOI] [PubMed] [Google Scholar]
- 2.Tsiftsoglou A, Ruiz S, Schneider C. Development and regulation of Biosimilars: current status and future challenges. BioDrugs. 2013;27:203–11. doi: 10.1007/s40259-013-0020-y. [DOI] [PubMed] [Google Scholar]
- 3.Longstaff C, Thelwell C, Whitton C. The poor quality of streptokinase products in use in developing countries. J Thromb Haemostat. 2005;3:1092–3. doi: 10.1111/j.1538-7836.2005.01271.x. [DOI] [PubMed] [Google Scholar]
- 4.Longstaff C, Thelwell C, Whitton C. Regulatory frameworks in developing countries. Nat Biotechnol. 2005;23:413. doi: 10.1038/nbt0405-413a. [DOI] [PubMed] [Google Scholar]
- 5.Hermentin P, Cuesta-Linker T, Weisse J, Schmidt KH, Knorst M, Scheld M, Thimme M. Comparative analysis of the activity and content of different streptokinase preparations. Eur Heart J. 2005;26:933–40. doi: 10.1093/eurheartj/ehi093. [DOI] [PubMed] [Google Scholar]
- 6.Sikri N, Bardia A. A history of streptokinase use in acute myocardial infarction. Tex Heart Inst J. 2007;34:318–27. [PMC free article] [PubMed] [Google Scholar]
- 7.Couto LT, Donato JL, De Nucci G. Analysis of five streptokinase formulations using the euglobulin lysis test and the plasminogen activation assay. Braz J Med Biol Res. 2004;37:1889–94. doi: 10.1590/s0100-879x2004001200015. [DOI] [PubMed] [Google Scholar]
- 8.Mahboubi A, Sadjady SK, Abadi MMS, Azadi S, Solaimanian R. Biological activity analysis of native and recombinant streptokinase using clot lysis and chromogenic substrate assay. Iran J Pharm Res. 2012;11:1087–93. [PMC free article] [PubMed] [Google Scholar]
- 9.Longstaff C, Whitton CM, Stebbings R, Gray E. How do we assure the quality of biological medicines? Drug Discov Today. 2009;14:50–5. doi: 10.1016/j.drudis.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 10.Sands D, Whitton CM, Longstaff C. International collaborative study to establish the 3rd International Standard for Streptokinase. J Thromb Haemostat. 2004;2:1411–5. doi: 10.1111/j.1538-7836.2004.00814.x. [DOI] [PubMed] [Google Scholar]
- 11.Covalt J, Cao TB, Magdaroag JRC, Gross LA, Jennings PA. Temperature, media, and point of induction affect the N-terminal processing of interleukin-1. Protein Expr Purif. 2005;41:45–52. doi: 10.1016/j.pep.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 12.Boxrud PD, Verhamme IMA, Fay WP, Bock PE. Streptokinase triggers conformational activation of plasminogen through specific interactions of the amino-terminal sequence and stabil-izes the active zymogen conformation. J Biol Chem. 2001;276:26084–9. doi: 10.1074/jbc.M101966200. [DOI] [PubMed] [Google Scholar]
- 13.Monograph. Streptokinase bulk solution. 2008. . Eur.Pharm 07/2008:0356.
- 14.Longstaff C, Whitton CM. A proposed reference method for plasminogen activators that enables calculation of enzyme activities in SI units. J Thromb Haemostat. 2004;2:1416–21. doi: 10.1111/j.1538-7836.2004.00816.x. [DOI] [PubMed] [Google Scholar]
- 15.Silva MMCG, Thelwell C, Williams SC, Longstaff C. Regulation of fibrinolysis by C-terminal lysines operates through plasminogen and plasmin but not tissue-type plasminogen activator. J Thromb Haemostat. 2012;10:2354–60. doi: 10.1111/j.1538-7836.2012.04925.x. [DOI] [PubMed] [Google Scholar]
- 16.Monograph. Statistical analysis of results of biological tests and assays. 2008. . Eur.Pharm 01/2008:50300.
- 17.Cannon CP. Exploring the issues of appropriate dosing in the treatment of acute myocardial infarction: potential benefits of bolus fibrinolytic agents. Am Heart J. 2000;140:S154–60. doi: 10.1067/mhj.2000.111605. [DOI] [PubMed] [Google Scholar]


