Abstract
This paper describes the initial clinical experience of ex vivo lung perfusion (EVLP) at the Fondazione Ca’ Granda in Milan between January 2011 and May 2013. EVLP was considered if donor PaO2/FiO2 was below 300 mmHg or if lung function was doubtful. Donors with massive lung contusion, aspiration, purulent secretions, pneumonia, or sepsis were excluded. EVLP was run with a low-flow, open atrium and low hematocrit technique. Thirty-five lung transplants from brain death donors were performed, seven of which after EVLP. EVLP donors were older (54 ± 9 years vs. 40 ± 15 years, EVLP versus Standard, P < 0.05), had lower PaO2/FiO2 (264 ± 78 mmHg vs. 453 ± 119 mmHg, P < 0.05), and more chest X-ray abnormalities (P < 0.05). EVLP recipients were more often admitted to intensive care unit as urgent cases (57% vs. 18%, P = 0.05); lung allocation score at transplantation was higher (79 [40–84] vs. 39 [36–46], P < 0.05). After transplantation, primary graft dysfunction (PGD72 grade 3, 32% vs. 28%, EVLP versus Standard, P = 1), mortality at 30 days (0% vs. 0%, P = 1), and overall survival (71% vs. 86%, EVLP versus Standard P = 0.27) were not different between groups. EVLP enabled a 20% increase in available donor organs and resulted in successful transplants with lungs that would have otherwise been rejected (ClinicalTrials.gov number: NCT01967953).
Keywords: brain death, ex vivo lung perfusion organ regeneration, lung transplantation, marginal donor criteria, outcome
Introduction
The recent introduction of ex vivo lung perfusion (EVLP) as a technique to assess and recondition lungs from marginal donors represents an exciting new era in the field of lung transplantation. Although the concept of extracorporeal evaluation and treatment of lung function before transplantation dates back to 1970 1, a renewed interest has arisen with the clinical experience of Steen 2, further fostered by the pivotal study of Cypel et al. that showed the feasibility of transplanting high-risk donor lungs that have undergone EVLP 3. Not only has EVLP enabled an expansion in the availability of transplantable organs; it has also deeply challenged the concept of lung suitability itself. In fact, organs previously not considered for transplantation are now safely used with outcomes similar to those of standard donor lungs 3–9.
Given the huge possibilities of this technique, the Fondazione IRCCS Ca’ Granda in Milan, having previously completed a preclinical study 10,11, has commenced a clinical program of EVLP to increase the number of lungs available for transplantation by improving donor lung function. Here, we present the initial clinical results of our program and discuss the data we obtained with reference to the available literature.
Patients and methods
Study design
The clinical outcome of subjects receiving EVLP lungs was compared to that of recipients undergoing standard transplantation (Standard) within the same time frame. At the time of listing, potential recipients were asked to sign an informed consent regarding the possibility of receiving EVLP-treated lungs. At the end of the EVLP procedure, if lungs were deemed suitable for transplantation, subjects were informed that they were receiving reconditioned lungs and were asked for a second consent. The investigation was approved by the Ethics Committee of our Institution (3307/2011) and is registered at ClinicalTrials.gov (Identifier: NCT01967953).
Lung donor inclusion and exclusion criteria
Any donor lung allocated toward a potential recipient listed at the Fondazione IRCCS Ca’ Granda was considered for EVLP if donor PaO2/FiO2 was below 300 mmHg with 5 cmH2O of positive end-expiratory pressure (PEEP) after optimization of donor ventilation or if lung function was doubtful despite oxygenation above 300 mmHg. Donors with massive lung contusion, aspiration, pneumonia, or sepsis were excluded.
Recipient inclusion criteria
Following a discussion with the ethics committee of the institution, the decision was to select, as potential EVLP lung candidates, only those recipients whose clinical condition was rapidly deteriorating. Conversely, lungs procured from standard donors were offered to all recipients, irrespective of their clinical status.
Donor logistics
In the Italian system, whenever a donor becomes available, the local Organ Procurement Organization (OPO) allocates lungs to blood group and size-matched potential recipients. Lungs are offered in rotation to the various transplantation centers that have the prerogative to accept or refuse the organ. Lungs refused by one center are then offered to the next. Urgent cases take priority and will be preferentially offered any compatible lung. The rotational allocation system still applies if more than one center has an urgent case.
According to the above mentioned allocation logistics, when lungs offered to the Fondazione Ca’ Granda were suitable for transplantation based on standard criteria, and in the absence of contraindications, after cross-clamp lungs were flushed with Perfadex® solution in an antegrade manner, recovered, cold-stored on ice after retrograde perfusion, and transplanted in a standard fashion. If donor lung function was unacceptable according to standard criteria or dubious despite optimized donor treatment, lung recovery went on as described above, but lungs underwent extracorporeal lung perfusion. EVLP was run in an operating room next to the theater where the transplantation procedure was planned. If deemed suitable for transplantation after EVLP, lungs were cooled, flushed with Perfadex® solution and cold-stored on ice. At this time, recipients underwent anesthesia. Transplantation and follow-up were performed following the same procedure in both Standard and EVLP recipients.
EVLP technique
The circuit used to perfuse the isolated lungs consisted of a blood reservoir connected to a gas oxygenator with a built-in heat exchanger (Dideco-Sorin, Milano, Italy), a centrifugal pump (Biomedicus, Medtronic, Minneapolis, MN, USA), a leukocyte arterial filter (Dideco-Sorin), and 0.375-inch non-heparin-coated polyvinyl tubing. The system was primed with 2000 ml of Steen solution™ (Vitrolife, Gothenburg, Sweden), methylprednisolone (Solu-Medrol 1 g, Pfizer, New York, NY, USA), cefazolin (Cefamezin 1 g, Pfizer), and heparin (Pharepa 20 000 UI). Packed red blood cells obtained from the blood bank, compatible to the recipient, were also added to the perfusate (150 ml, yealding an hematocrit of 3–5%). Lung perfusion was performed after de-airing the circuit and connecting the pulmonary artery. Blood flow was gradually increased up to a target of 40% of the estimated cardiac output (calculated as CO = 3*body surface area) while monitoring pulmonary artery pressure (PAP; Siemens SC6820XL, Sweden). Temperature of the perfusate was gradually increased from 25 °C to a left atrium target temperature of 37 °C over approximately 30 min (Medi-Therm II, Gaymar Industries Inc, Orchard Park, NY, USA). Once the lung outflow temperature exceeded 32 °C, a gas mix of air and CO2 (Sapio, Milano, Italy, 5–8% CO2) was connected to the circuit oxygenator (Dideco-Sorin) and mechanical ventilation was started (Datex-Ohmeda Inc, General Electric, Madison, WI, USA). Lungs were ventilated with a target tidal volume of 7 ml/kg of donor ideal weight, with PEEP of 5 cmH2O, respiratory rate of 7 bpm, and inspiratory fraction of oxygen (FiO2) of 0.4. Recruitment maneuvres were performed by inflating the lungs at 25 cmH2O airway pressure and holding inspiratory pressure for 10 s. At the end of the procedure, a final evaluation was performed. This was done setting the ventilator with FiO2 at 1 and connecting to the circuit oxygenator a gas mix of N2 and CO2. Details of the EVLP procedure can be found in Figure S1 of the supporting information.
Parameters of lung perfusion (perfusate flow, temperature, and pulmonary artery pressure) and ventilation (tidal volume, airway pressure, respiratory rate, PEEP, and FiO2) were measured throughout the experiments at time intervals. Pulmonary vascular resistance and dynamic compliance were calculated according to standard formulas. Analysis of partial pressures of oxygen (PO2) and carbon dioxide (PCO2) was performed on samples drawn from the pulmonary artery and left atrium (Radiometer ABL 800 Flex, Radiometer Medical ApS, Brønshøj, Denmark).
Clinical endpoints
To assess the characteristics of donor lungs undergoing EVLP and to compare them to those transplanted with a standard procedure, the Oto donor score was used 12. This includes age, smoking history, chest X-ray, secretions, and PaO2/FiO2 ratio to describe donor lungs attributing numerical scores; Oto score ranges from 0 to 18 (worse score).
Endpoints of EVLP assessment of lungs suitability were oxygenation, respiratory mechanics, and pulmonary vascular resistance; chest X-ray and fibrobronchoscopy contributed to evaluate lung suitability for transplantation.
Primary graft dysfunction 72 h after transplantation (PGD72), defined as grade 3 according to the International Society of Heart and Lung Transplantation classification 13, duration of mechanical ventilation after transplantation, Intensive Care Unit (ICU) length of stay after transplantation, and mortality at 30 days were considered the recipient's main outcome measures. Quantitative analysis of graft lung Computed Tomography (CT) scan was performed in a subset of recipients.
Statistical analysis
Data are presented as mean ± standard deviation or as median [interquartile range] when not normally distributed (Shapiro–Wilk test). Comparisons of continuous data between Standard and EVLP groups were performed with Student's t-test; the rank-sum test was used when data were not normally distributed. Differences between categorical data were analyzed with the chi-square test. Linear regression analysis was also conducted. Donor oxygenation, stratified by Standard and EVLP groups, was analyzed by one-way analysis of variance (anova), followed by Bonferroni test, when appropriate. Kaplan–Meier log-rank analysis was conducted to assess survival. Data were analyzed by using sigma stat 11.0 software (SPSS Inc, Chicago, IL, USA) and accepting P < 0.05 as significant.
Results
From January 2011 to May 2013, 35 lung transplants from brain death donors were performed at the Fondazione IRCCS Ca’ Granda. Donor lungs were disposed as shown in the diagram of Fig.1: Of 39 offers, three were excluded because fibrobronchoscopy at the time of recovery revealed the presence of purulent secretions. Of a total of eight EVLP procedures, seven lung transplants were performed (six double transplant, and one single transplant). Six EVLP procedures were run to recondition the lungs of donors whose PaO2/FiO2 was below 300 mmHg. Two procedures were run to evaluate lungs with uncertain function: In one case (3 in Table2), oxygenation of a 63-year-old donor was doubtful because of a suspected low cardiac output; there also was evidence of lung contusion, and bronchoaspirate was positive for Staphilococcus aureus (105 CFU/ml). Another EVLP was run on lungs of a donor who was on full veno-arterial extra-corporeal life support assistance because of refractory cardiogenic shock when brain death was diagnosed (case 8 in Table2). PaO2/FiO2 was 158 mmHg despite pressure controlled ventilation of +18 cmH2O and PEEP 12 cmH2O.
Figure 1.
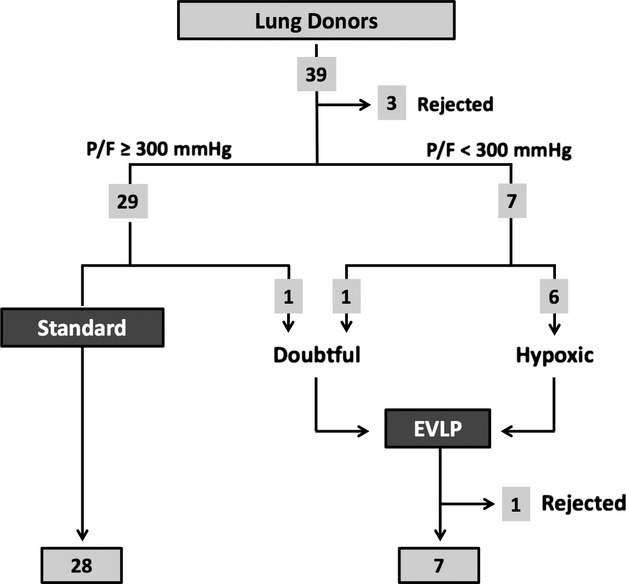
Disposition of donor lungs.
Table 2.
Characteristics of donors of lungs that underwent EVLP.
| Cause of death | Age | Smoking | CXR | Secretions | BAS | OTO | P/F | MV | CVP | WBC | Comorbidities | Vasopressors | CCA | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Cerebrovascular | 45 | 50 | + | – | – | 11 | 180 | 7 | 14 | 10.3 | – | NA, DP | NO |
| 2 | Cerebrovascular | 53 | 48 | + | – | – | 10 | 188 | 1 | 15 | 7.42 | DB, LD, AH | NA, DP | NO |
| 3 | Postanoxic | 63 | 10 | – | + | S.Aureus | 6 | 395 | 5 | 13 | 18.2 | HT | NA, DB | YES |
| 4 | Cerebrovascular | 59 | 0 | + | + | – | 10 | 280 | 1 | 13 | 16.5 | – | NA, DP | NO |
| 5 | Cerebrovascular | 48 | 10 | – | ++ | P.Aerug | 9 | 155 | 3 | 13 | 2.66 | LD, AH | NA | NO |
| 6 | Cerebrovascular | 60 | 56 | + | ++ | K.Pn;K.Ox | 14 | 258 | 3 | 8 | 14.3 | AH | NA | NO |
| 7 | Cerebrovascular | 38 | 54 | + | + | H.Infl | 11 | 283 | 2 | 13 | 12.7 | – | NA, DP | YES |
| 8 | Cerebrovascular | 59 | 0 | + | ++ | – | 12 | 158* | 8 | 17 | 17.1 | IHD, CS, AH | NA, DB | NO |
Age, years; Smoking, smoking history (packs/year); CXR, chest X-ray: (–) clear, (+), abnormal; Secretions: (–) none, (+) minor, (++) moderate; BAS, bronchoaspirate; OTO, Oto score 12; P/F, PaO2/FiO2 ratio; MV, duration of mechanical ventilation (days); CVP, central venous pressure; WBC, white blood cells; CAA, cardio-circulatory arrest; DB, diabetes; AH, arterial hypertension; HT, hypothyroidism; LD, liver disease; IHD, ischemic heart disease; CS, cardiogenic shock; NA, noradrenaline; DP, dopamine; DB, dobutamine; L, levosimendan. S.Aureus, Staphylococcus aureus; P.Aerug, Pseudomonas Aeruginosa; K.Pn, Klebsiella pneumoniae; K.Ox, Klebsiella oxytoca; H.Inf, Haemofilus influenzae.
While on full veno-arterial Extracorporeal Membrane Oxygenation (ECMO) support for cardiogenic shock treatment.
Table1 shows the characteristics of the investigated cohort of donors stratified according to study groups (Standard versus EVLP). Donors in the EVLP group were older (P < 0.05), with less favorable chest X-rays (P < 0.05), lower PaO2/FiO2 (P < 0.05), and higher Oto scores (P < 0.05). As detailed in Table2, together with poor oxygenation, four EVLP donors were older than 55 years, three had a smoking history of >20 packs per year, and three had major secretions. In fact, the Oto score was higher in the EVLP group even when oxygenation was not taken into account: 3 ± 2 vs. 5 ± 2, Standard versus EVLP, respectively (P < 0.05).
Table 1.
Characteristics of the investigated cohort of donors.
| All (n = 35) | Standard (n = 28) | EVLP (n = 7) | P | |
|---|---|---|---|---|
| Age, years | 43 ± 15 | 40 ± 15 | 54 ± 9 | <0.05 |
| BMI | 24 ± 5 | 23 ± 5 | 26 ± 2 | 0.11 |
| Cause of death | ||||
| Cerebrovascular accident, n (%) | 23 (66) | 17 (61) | 6 (86) | 0.21 |
| Postanoxic encephalopathy, n (%) | 3 (8) | 2 (7) | 1 (14) | |
| Trauma, n (%) | 9 (26) | 9 (32) | 0 (0) | |
| ICU, days | 2 [1–5] | 2 [1–4] | 3 [1–7] | 0.67 |
| Smoking history, n (%) | 12 (34) | 8 (29) | 4 (57) | 0.20 |
| Abnormal CXR, n (%) | 17 (49) | 11 (39) | 6 (85) | <0.05 |
| Secretions, n (%) | 21 (60) | 16 (57) | 5 (71) | 0.68 |
| PaO2/FiO2, mmHg | 419 ± 133 | 453 ± 119 | 264 ± 78 | <0.05 |
| Oto score | 6 [3–10] | 5 [3–7] | 11 [10–12] | <0.05 |
Data of the entire cohort of investigated donors (all) are presented, comparing data of Standard donors with EVLP lung donors (Student's t-test; P < 0.05). Data are presented as mean ± standard deviation, or median [25°–75°].
n, number of patients; BMI, body mass index; ICU, intensive care unit; CXR, chest X-ray; Oto score 12.
Donor oxygenation (PaO2/FiO2 measured at PEEP 5 cmH2O and FiO2 of 1) stratified by studied groups is shown in Fig.2. The best PaO2/FiO2 recorded during the entire process of brain death diagnosis was significantly higher in the Standard group as opposed to the EVLP one (P < 0.05). In both Standard and EVLP donors, oxygenation worsened over time (P < 0.05), being the cumulative time from brain death assessment to lung recovery similar between groups (727 ± 191 min vs. 735 ± 190 min, EVLP versus Standard group, respectively, P = 0.92). Central venous pressure (CVP) was significantly higher at the time of recovery in the EVLP group: 13 ± 3 cmH2O vs. 8 ± 4 cmH2O, EVLP versus Standard group, respectively (P < 0.05). CVP was also correlated with PaO2/FiO2 (R2 = 0.25, P < 0.05).
Figure 2.
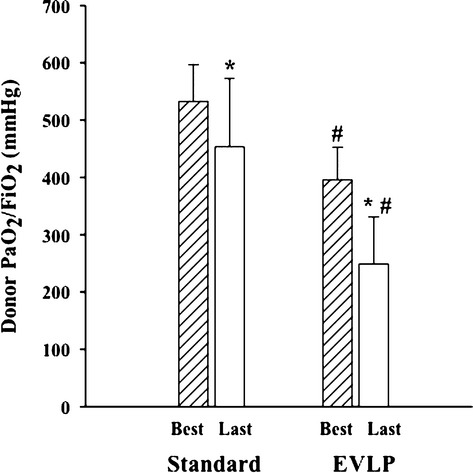
Donor oxygenation stratified by studied groups. Arterial partial pressure of oxygen (PaO2) was measured at 5 cmH2O of positive end-expiratory pressure, and with an inspiratory fraction of oxygen (FiO2) of 1. “Best” represents the best PaO2/FiO2 recorded during the entire process of brain death determination; “Last” represents the last PaO2/FiO2 value recorded before the recovery of lungs. *P < 0.05. Best versus Last, #P < 0.05 Standard versus EVLP, One-way anova.
A comparison of ischemic times in the two study groups is shown in Table3: The time elapsed from cross-clamp to reperfusion of the second lung was longer when lungs were transplanted after EVLP (P < 0.05).
Table 3.
Cross-clamp to reperfusion times.
| Standard (n = 28) | EVLP (n = 7) | P | |
|---|---|---|---|
| Cold ischemia pre-EVLP | – | 281 ± 58 | |
| Normothermic lung perfusion | – | 268 ± 104 | |
| Cold ischemia pre-LTx | 356 ± 140 | 345 ± 154 | 0.84 |
| Warm ischemia | 89 ± 15 | 94 ± 24 | 0.56 |
| Cross-clamp to reperfusion | 446 ± 140 | 968 ± 180 | <0.05 |
Lungs of the Standard group were procured at the time of donor cross-clamp and used for transplantation after cold storage (cold ischemia pre-LTx). Lungs of the EVLP group were cold-stored at the time of donor cross-clamp (cold ischemia pre-EVLP), underwent normothermic lung perfusion, and, if deemed suitable for transplantation, cooled down once more before transplantation (cold ischemia pre-LTx).
Data in the table refer to the time (min) elapsed within the above-described intervals and refer to the second lung in bilateral transplantations. To compare data, Student's t-test analysis was conducted. Data are presented as mean ± standard deviation.
LTx: lung transplantation.
All of the EVLP procedures were run on two-lung blocks. In case 3 of Table2, a progressive dysfunction of oxygenation and compliance of the right lung became evident by the end of the EVLP procedure, further confirmed by chest X-ray. In this case, only the left lung was transplanted. The lungs of case 5 were rejected because of a progressive rise of pulmonary vascular resistance, persistent secretions, and pathological chest X-ray at the end of the EVLP procedure. The average EVLP settings and functional parameters over time are shown in Table4. At the time of evaluation of lung suitability, left atrium PO2/FiO2 ratio of the transplanted lungs was 518 ± 55 mmHg (P < 0.05), with pulmonary arterial PO2/FiO2 of 55 ± 8 mmHg and arterial to venous difference in O2 of 463 ± 55 mmHg. No signs of deterioration over time were present; chest X-ray and bronchoscopy were negative.
Table 4.
Functional parameters during EVLP.
| Reperfusion | Reconditioning | Evaluation | P | |
|---|---|---|---|---|
| LA Temp, °C | 31.3 ± 7.0 | 36.6 ± 0.4 | 36.7 ± 0.8 | <0.05 |
| Vt, ml/kg | 5.7 ± 0.8 | 6.5 ± 1 | 6.3 ± 0.9 | <0.05 |
| Perfusate flow, l/min | 1.6 ± 0.4 | 2.2 ± 0.5 | 2.4 ± 0.4 | <0.05 |
| PAPm, cmH2O | 8 ± 3 | 11 ± 3 | 11 ± 3 | <0.05 |
| PVR, dine*s/cm5 | 435 ± 136 | 389 ± 137 | 363 ± 87 | 0.46 |
| Pawm, cmH2O | 7 ± 1 | 6 ± 0.2 | 6 ± 1 | 0.18 |
| Paw peak, cmH2O | 12 ± 3 | 10 ± 4 | 11 ± 1 | 0.11 |
| Cpldyn, ml/cmH2O | 98 ± 39 | 123 ± 40 | 129 ± 39 | <0.05 |
| PO2 IN, mmHg | 155 ± 7 | 157 ± 10 | 55 ± 8 | <0.05 |
| PO2 OUT, mmHg | 247 ± 59 | 278 ± 59 | 518 ± 55 | <0.05 |
Functional data during successful EVLP are presented, comparing data over time (one-way anova; P < 0.05). Average data measured during the Reperfusion phase were significantly different from data measured during Reconditioning and Evaluation (see Supporting Information). Data are presented as mean ± standard deviation. In the upper part of the table, set parameters are presented, whereas derived parameters are presented in the lower part of the table. LA Temp, left atrium temperature (°C); Vt, tidal volume (ml/kg donor ideal weight); PAPm, mean pulmonary arterial pressure (mmHg); PVR, pulmonary vascular resistance (dyne*s/cm5); Pawm, mean airways pressure (cmH2O); Pawpeak, peak airways pressure (cmH2O); Cpldyn, dynamic lung compliance (ml/cmH2O). PO2 IN, partial pressure of oxygen from a sample of perfusate taken from the pulmonary artery cannula (mmHg); PO2 OUT, partial oxygen pressure from a sample taken from the left atrium (mmHg).
The characteristics of the recipients are shown in Table5. Anthropometric characteristics of the two groups were similar; EVLP recipients were somewhat younger. Recipients of the EVLP group were more often admitted to the ICU at the time of transplantation (P = 0.05) and more often on urgent listing (P = 0.05). Three of them were on Extracorporeal Membrane Oxygenation (ECMO) bridge to lung transplantation, and overall, EVLP recipients were more frequently on ECMO during the surgical procedure (P = 0.07). Lung allocation score calculated at the time of transplantation was significantly higher in the EVLP group (P < 0.05). EVLP recipients were also characterized by a worse performance status, as assessed by Karnofsky index (P < 0.05).
Table 5.
Characteristics of the investigated cohort of recipients.
| All (n = 35) | Standard (n = 28) | EVLP (n = 7) | P | |
|---|---|---|---|---|
| Age, years | 47 ± 15 | 49 ± 14 | 38 ± 15 | 0.09 |
| BMI, kg/m2 | 22 [18–25] | 23 [18–25] | 20 [17–23] | 0.28 |
| Diagnosis | ||||
| Cystic fibrosis, n (%) | 14 (40) | 10 (36) | 4 (57) | 0.20 |
| Pulmonary fibrosis, n (%) | 11 (31) | 11 (40) | 0 (0) | |
| Other, n (%) | 10 (29) | 7 (25) | 3 (43) | |
| ICU admission pretransplant, n (%) | 9 (26) | 5 (18) | 4 (57) | 0.05 |
| Urgent listing, n (%) | 9 (26) | 5 (18) | 4 (57) | 0.05 |
| ECMO bridge to transplant, n (%) | 8 (23) | 5 (18) | 3 (43) | 0.31 |
| Noninvasive ventilation (%) | 5 (14) | 4 (14) | 1 (14) | 1.00 |
| Invasive ventilation (%) | 2 (6) | 1 (4) | 1 (14) | 0.37 |
| LAS transplantation | 39 [36–58] | 39 [36–46] | 79 [40–84] | <0.05 |
| Karnofsky index | 60 [13–70] | 65 [40–70] | 20 [10–50] | <0.05 |
| Lung transplant procedure | ||||
| Single, n (%) | 15 (43) | 14 (50) | 1 (14) | 0.20 |
| Double, n (%) | 20 (57) | 14 (50) | 6 (86) | |
| Total surgical time, min | 617 ± 157 | 609 ± 169 | 647 ± 93 | 0.58 |
| Intraoperative ECMO, n (%) | 13 (37) | 8 (26) | 5 (71) | 0.07 |
| Intraoperative ECMO-VA, n (%) | 6 (17) | 4 (14) | 2 (29) | 0.58 |
Data of the entire cohort of investigated recipients (All) are presented, comparing data of Standard recipients with EVLP recipients (Student's t-test; P < 0.05). Data are presented as mean ± standard deviation or median and 25°–75° percentile in square brackets.
n, number of patients; BMI, body mass index; ICU, intensive care unit; LAS, lung allocation score; ECMO, extracorporeal membrane oxygenation; VA, venous arterial.
Clinical outcome of transplantation was similar between Standard and EVLP recipients. In fact, as shown in Table6, graft function on day 3 after transplantation (P between 1 and 0.30), the duration of mechanical ventilation (P = 0.57), and ICU length of stay (P = 0.30) were similar. On day 30 after transplantation, graft function (P between 0.50 and 0.76) and mortality (P = 1.0) were similar between groups. Kaplan–Meier analysis showed that overall survival was similar in the two groups (86% vs. 71%, Standard versus EVLP groups, respectively, P = 0.27, Fig.3). Median follow-up was 260 [114–590] days, with no difference between Standard and EVLP groups (P = 0.39). In the EVLP group, one recipient died on day 39 after transplantation. At the time of transplantation, the subject was on ECMO bridge (7 days) and mechanically ventilated (2 days). After the surgical procedure, there were no signs of PGD, and the ECMO support was withdrawn on day 2 after surgery. However, the recipient went on to die because of invasive Scedosporium apiospermum, known to have colonized the tracheobronchial tree before surgery. Another subject died on day 357 from a pulmonary embolism. Table S1 of the supporting information shows that quantitative CT scan analysis of transplanted lungs was not different between EVLP recipients and a matched subset of Standard recipients.
Table 6.
Outcome after transplantation.
| All (n = 35) | Standard (n = 28) | EVLP (n = 7) | P | |
|---|---|---|---|---|
| 3rd day after LTx | ||||
| PGD grade 3, n (%) | 11 (35) | 9 (32) | 2 (28) | 1.00 |
| ECMO, n (%) | 3 (9) | 2 (7) | 1 (14) | 0.49 |
| SB/CPAP, n (%) | 21 (60) | 18 (64) | 3 (43) | 0.40 |
| PEEP, mmHg | 4 [0–10] | 1 [0–10] | 5 [1–7] | 0.49 |
| FiO2, % (as in FEV1) | 0.35 [0.21–0.40] | 0.33 [0.21–0.40] | 0.4 [0.35–0.44] | 0.30 |
| PaO2/FiO2, mmHg | 243 [195–281] | 247 [192–273] | 227 [211–311] | 0.57 |
| Duration of MV, days | 2 [1–18] | 1.5 [1–18] | 3 [1.5–16.5] | 0.57 |
| ICU LOS post-Ltx, days | 6 [4–21] | 5.5 [4–21.5] | 10 [5–18] | 0.30 |
| 30th day after LTx | ||||
| FEV1,% | 64 [52–74] | 64 [52–74] | 61 [51–68] | 0.60 |
| FVC,% | 57 [50–66] | 58 [50–67] | 56 [47–63] | 0.50 |
| FEV1/ FVC | 93 [88–97] | 92 [87–97] | 93 [85–96] | 0.76 |
| Mortality, n (%) | 0 (0) | 0 (0) | 0 (0) | 1.00 |
Outcomes of the investigated recipients (All) are presented, comparing outcomes of Standard recipients with outcomes of EVLP recipients (Student's t-test; P < 0,05). Data are presented as mean ± standard deviation or median and 25°–75° percentile in square brackets.
n, number of patients; LTx, lung transplantation; PGD, primary graft dysfuction (PGD grade 3); ECMO, extracorporeal membrane oxygenation; SB, spontaneous breathing; CPAP, continuous positive airways pressure; PEEP, positive end-expiratory pressure; MV, mechanical ventilation; ICU LOS post-LTx, intensive care unit length of stay after lung transplantation; FEV1, forced expiratory volume in one second; FVC, forced vital capacity.
Figure 3.
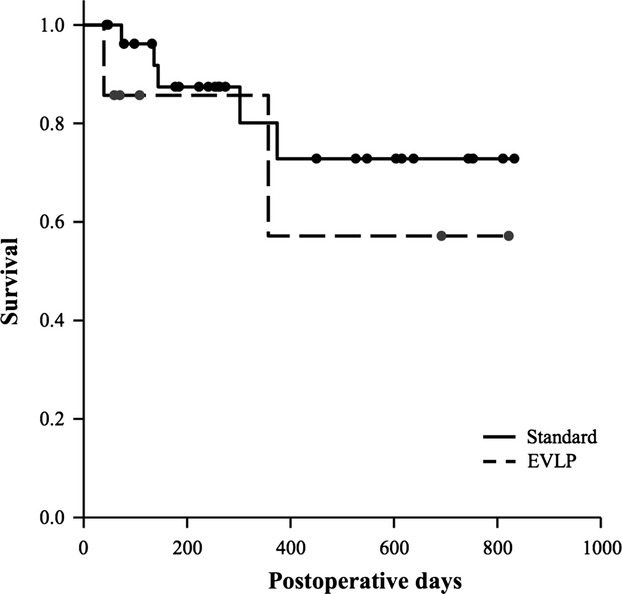
Kaplan–Meier curves show that overall survival after transplantation was similar between groups (86% vs. 71%, P = 0.27).
Discussion
Lungs recovered from multi-organ donors rarely meet ideal criteria 14,15. For this reason, over the last decade, several authors have extended standard criteria to expand the pool of donor organs 16–19. Unfortunately, recipient outcome is negatively influenced by the use of extended criteria or the use of out-of-protocol donors, especially when lungs from these donors are transplanted to out-of-protocol recipients 20–24. Therefore, lung donor selection and recipient matching remain uncertain 15,22. The recent introduction of EVLP has changed the scenario. The possibility offered by EVLP to evaluate and recondition lungs recovered from marginal donors before transplantation has not only allowed an expansion in the pool of lungs available for transplantation, but it has also deeply challenged the concept of lung suitability itself 25. Organs that previously were not considered for transplantation are now safely used with outcomes similar to those of standard donor lungs 3–9.
Our initial clinical experience with EVLP is in line with available data relating to transplanted lungs that have undergone EVLP. Given the cohort of EVLP recipients, the study also suggests that EVLP-treated lungs may be safely transplanted to recipients whose clinical condition is rapidly deteriorating. In fact, EVLP recipients in our study were more often admitted to ICU as urgent cases, their performance status was worse, three of them were on ECMO bridge to lung transplantation, and overall, EVLP recipients were more frequently on ECMO during the surgical procedure. Nevertheless, primary graft dysfunction on day 3 after transplantation, 30-day mortality, and overall survival were similar between groups, and comparable with those reported in other investigations that included unselected or low-risk recipients.
Donors included in the EVLP group were selected from donors with high Oto donor scores. Even if factors other than oxygenation contributed to make EVLP donor lungs far from ideal (see Oto score without oxygenation), lung edema mostly contributed to donor hypoxia, as a lower PaO2/FiO2 ratio at recovery was associated with higher CVP values. Hypoxemia and fluid overload are common findings during brain-death donor treatment 25–28 and often contribute to lung rejection 29. Pulmonary edema, in fact, was one of the inclusion criteria in the EVLP trial of Cypel et al. 3 We deem that the true potential of EVLP is to repair previously injured lungs and is not intended to substitute optimal donor treatment 30. However, in our case series, EVLP allowed to improve the function of lungs otherwise too poor to accept for transplantation. Of note, total cold ischemic time was longer, and this did not have a deleterious effect.
Taking into account our preclinical experience 10,11 and taking into consideration factors from the Toronto and the Lund protocols, we have adopted a low-flow, open atrium EVLP model. A debate exists on the question of adopting a closed or open atrium technique during EVLP. Physiological left atrium pressure (i.e., slightly positive) is known to be protective to the pulmonary vasculature in isolated, perfused, and ventilated lung preparations 31–33. This is clearly an advantage of the closed atrium and a theoretical disadvantage of the open atrium technique 33,34. However, EVLP procedures have been run with the open atrium technique leading to safe lung transplantations 2,6–8, possibly downplaying to some extent the debate. We have positioned pressure probes in the pulmonary veins during three EVLP procedures and confirmed the presence of positive values (2–4 mmHg), even with the relatively low flow that we adopt in our protocol. Clearly, the closed atrium is a better choice to guarantee continuous positive pressure in the left atrium. However, the technique is more prone to both deleterious inadvertent high left atrium pressures 32,34 or negative pressures that are harmful per se and draw air to into the system 35. Moreover, the open atrium allows an easier selective evaluation of right/left lung function 8.
We have also used a cellular perfusate. There are theoretical advantages of adding red blood cells to the perfusate: Oxygen delivery is significantly higher 36; red cells couple oxygen and carbon dioxide transport so that the uptake of carbon dioxide by red cells and chemical reactions with hemoglobin facilitate the release of oxygen from hemoglobin 36; hemoglobin contributes to the buffer power of the perfusate 37. Evaluation of oxygenation during EVLP is also easier to interpret 8,34,38,39. Moreover, there are rheological advantages on microvasculature dynamics 40–42: Capillary recruitment and vascular distension that is protective to the lung 33 are both favoured by the presence of red blood cells. To use a cellular perfusate inevitably adds to the complexity of the procedure. In fact, to obtain packed red blood cells from the transfusion laboratory may be difficult. Moreover, hemolysis may occur over time 43,44, which is why we have decided upon a low hematocrit strategy.
Technical aspects of EVLP deserve further consideration; however, we believe that the major contribution of EVLP to transplantation derives from its use rather than from procedural details 38. Nevertheless, the view that lung-repair protocols might substantially differ from those orientated toward short-term evaluation, seems reasonable.
We realize that a major limitation of our study relates to the low number of subjects included. For this reason, we are not able to draw any definitive conclusions. However, although the number of Standard recipients is far less than those reported in other series, the number of EVLP procedures is similar. Moreover, even if we did not run a controlled study, we report data on the transplantation of EVLP-treated lungs to a selected cohort of recipients.
If anything, the clinical experience with EVLP we have reported shows that a low-volume transplantation center can definitely benefit from building an EVLP program. Indeed, running a EVLP protocol resulted in a 20% increase in the number of lungs transplanted at the Fondazione IRCCS Ca’ Granda.
Conclusions
In conclusion, the treatment of suboptimal lungs recovered from brain death donors using an EVLP protocol allows the safe transplantation of organs that have been rejected by other centers and would not have otherwise been transplanted. The practice of organ reconditioning represents an exciting and important breakthrough in the field of lung transplantation.
Authorship
FV, LR, LS, LG: contributed to the design and implementation of the EVLP program. AP, DT, PM: contributed to lung procurement. FV, LR, SC, SF: contributed to running the EVLP procedures. GMR, JF, AV, MN: contributed to data collection. FV, VS, LR, MN: contributed to data analysis. FV, VS, LG: contributed to manuscript writing.
Funding
This study was funded by Fondazione IRCCS Ca’ Granda–Ospedale Maggiore Policlinico, Milano, Italy, and by Regione Lombardia. A contribution was also provided by Lega Lombarda Fibrosi Cistica.
Acknowledgments
Authors are in debt to Agelica Perazzoli for her logistical support to the implementation of the EVLP program.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Synoptic description of the EVLP procedure. PRBC, packed red blood cells; Vt, tidal volume (ml/kg); RR, respiratory rate; PEEP, positive end-expiratory pressure (cmH2O); CO, cardiac output (l/min); PAPm, mean pulmonary arterial pressure (mmHg); RM, recruitment maneuver; FBS, fibrobronchoscopy; TLC, total lung capacity; RX, chest X-ray.
Quantitative analysis of graft CT scan.
References
- 1.Jirsch DW, Fisk RL, Couves CM. Ex vivo evaluation of stored lungs. Ann Thorac Surg. 1970;10:163. doi: 10.1016/s0003-4975(10)65582-8. [DOI] [PubMed] [Google Scholar]
- 2.Steen S, Ingemansson R, Eriksson L, et al. First human transplantation of a nonacceptable donor lung after reconditioning ex vivo. Ann Thorac Surg. 2007;83:2191. doi: 10.1016/j.athoracsur.2007.01.033. [DOI] [PubMed] [Google Scholar]
- 3.Cypel M, Yeung JC, Liu M, et al. Normothermic ex vivo lung perfusion in clinical lung transplantation. N Engl J Med. 2011;364:1431. doi: 10.1056/NEJMoa1014597. [DOI] [PubMed] [Google Scholar]
- 4.Aigner C, Slama A, Hotzenecker K, et al. Clinical ex vivo lung perfusion–pushing the limits. Am J Transplant. 2012;12:1839. doi: 10.1111/j.1600-6143.2012.04027.x. [DOI] [PubMed] [Google Scholar]
- 5.Cypel M, Yeung JC, Machuca T, et al. Experience with the first 50 ex vivo lung perfusions in clinical transplantation. J Thorac Cardiovasc Surg. 2012;144:1200. doi: 10.1016/j.jtcvs.2012.08.009. [DOI] [PubMed] [Google Scholar]
- 6.Ingemansson R, Eyjolfsson A, Mared L, et al. Clinical transplantation of initially rejected donor lungs after reconditioning ex vivo. Ann Thorac Surg. 2009;87:255. doi: 10.1016/j.athoracsur.2008.09.049. [DOI] [PubMed] [Google Scholar]
- 7.Wallinder A, Ricksten SE, Hansson C, et al. Transplantation of initially rejected donor lungs after ex vivo lung perfusion. J Thorac Cardiovasc Surg. 2012;144:1222. doi: 10.1016/j.jtcvs.2012.08.011. [DOI] [PubMed] [Google Scholar]
- 8.Wallinder A, Ricksten SE, Silverborn M, et al. Early results in transplantation of initially rejected donor lungs after ex vivo lung perfusion: a case-control study. Eur J Cardiothorac Surg. 2014;45:40. doi: 10.1093/ejcts/ezt250. [DOI] [PubMed] [Google Scholar]
- 9.Zych B, Popov AF, Stavri G, et al. Early outcomes of bilateral sequential single lung transplantation after ex-vivo lung evaluation and reconditioning. J Heart Lung Transplant. 2012;31:274. doi: 10.1016/j.healun.2011.10.008. [DOI] [PubMed] [Google Scholar]
- 10.Valenza F, Rosso L, Pizzocri M, et al. The consumption of glucose during ex vivo lung perfusion correlates with lung edema. Transplant Proc. 2011;43:993. doi: 10.1016/j.transproceed.2011.01.122. [DOI] [PubMed] [Google Scholar]
- 11.Valenza F, Rosso L, Coppola S, et al. Beta-adrenergic agonist infusion during extracorporeal lung perfusion: effects on glucose concentration in the perfusion fluid and on lung function. J Heart Lung Transplant. 2012;31:524. doi: 10.1016/j.healun.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 12.Oto T, Levvey BJ, Whitford H, et al. Feasibility and utility of a lung donor score: correlation with early post-transplant outcomes. Ann Thorac Surg. 2007;83:257. doi: 10.1016/j.athoracsur.2006.07.040. [DOI] [PubMed] [Google Scholar]
- 13.Christie JD, Carby M, Bag R, et al. Report of the ISHLT Working Group on Primary Lung Graft Dysfunction part II: definition. A consensus statement of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 2005;24:1454. doi: 10.1016/j.healun.2004.11.049. [DOI] [PubMed] [Google Scholar]
- 14.Orens JB, Boehler A, de Perrot M, et al. A review of lung transplant donor acceptability criteria. J Heart Lung Transplant. 2003;22:1183. doi: 10.1016/s1053-2498(03)00096-2. [DOI] [PubMed] [Google Scholar]
- 15.Van Raemdonck D, Neyrinck A, Verleden GM, et al. Lung donor selection and management. Proc Am Thorac Soc. 2009;6:28. doi: 10.1513/pats.200808-098GO. [DOI] [PubMed] [Google Scholar]
- 16.Aigner C, Winkler G, Jaksch P, et al. Extended donor criteria for lung transplantation–a clinical reality. Eur J Cardiothorac Surg. 2005;27:757. doi: 10.1016/j.ejcts.2005.01.024. [DOI] [PubMed] [Google Scholar]
- 17.Bhorade SM, Vigneswaran W, McCabe MA, Garrity ER. Liberalization of donor criteria may expand the donor pool without adverse consequence in lung transplantation. J Heart Lung Transplant. 2000;19:1199. doi: 10.1016/s1053-2498(00)00215-1. [DOI] [PubMed] [Google Scholar]
- 18.Kron IL, Tribble CG, Kern JA, et al. Successful transplantation of marginally acceptable thoracic organs. Ann Surg. 1993;217:518. doi: 10.1097/00000658-199305010-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meers C, Van Raemdonck D, Verleden GM, et al. The number of lung transplants can be safely doubled using extended criteria donors; a single-center review. Transpl Int. 2010;23:628. doi: 10.1111/j.1432-2277.2009.01033.x. [DOI] [PubMed] [Google Scholar]
- 20.Botha P, Trivedi D, Weir CJ, et al. Extended donor criteria in lung transplantation: impact on organ allocation. J Thorac Cardiovasc Surg. 2006;131:1154. doi: 10.1016/j.jtcvs.2005.12.037. [DOI] [PubMed] [Google Scholar]
- 21.Kawut SM, Reyentovich A, Wilt JS, et al. Outcomes of extended donor lung recipients after lung transplantation. Transplantation. 2005;79:310. doi: 10.1097/01.tp.0000149504.53710.ae. [DOI] [PubMed] [Google Scholar]
- 22.Pierre AF, Sekine Y, Hutcheon MA, Waddell TK, Keshavjee SH. Marginal donor lungs: a reassessment. J Thorac Cardiovasc Surg. 2002;123:421. doi: 10.1067/mtc.2002.120345. [DOI] [PubMed] [Google Scholar]
- 23.Schiavon M, Falcoz PE, Santelmo N, Massard G. Does the use of extended criteria donors influence early and long-term results of lung transplantation? Interact Cardiovasc Thorac Surg. 2012;14:183. doi: 10.1093/icvts/ivr079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Whitson BA, Nath DS, Johnson AC, et al. Risk factors for primary graft dysfunction after lung transplantation. J Thorac Cardiovasc Surg. 2006;131:73. doi: 10.1016/j.jtcvs.2005.08.039. [DOI] [PubMed] [Google Scholar]
- 25.Pego-Fernandes PM, Samano MN, Fiorelli AI, et al. Recommendations for the use of extended criteria donors in lung transplantation. Transplant Proc. 2011;43:216. doi: 10.1016/j.transproceed.2010.12.050. [DOI] [PubMed] [Google Scholar]
- 26.Avlonitis VS, Fisher AJ, Kirby JA, Dark JH. Pulmonary transplantation: the role of brain death in donor lung injury. Transplantation. 2003;75:1928. doi: 10.1097/01.TP.0000066351.87480.9E. [DOI] [PubMed] [Google Scholar]
- 27.Pennefather SH, Bullock RE, Dark JH. The effect of fluid therapy on alveolar arterial oxygen gradient in brain-dead organ donors. Transplantation. 1993;56:1418. doi: 10.1097/00007890-199312000-00028. [DOI] [PubMed] [Google Scholar]
- 28.Venkateswaran RV, Dronavalli V, Patchell V, et al. Measurement of extravascular lung water following human brain death: implications for lung donor assessment and transplantation. Eur J Cardiothorac Surg. 2013;43:1227. doi: 10.1093/ejcts/ezs657. [DOI] [PubMed] [Google Scholar]
- 29.Porro GA, Valenza F, Coppola S, et al. Use of the Oto lung donor score to analyze the 2010 donor pool of the Nord Italia Transplant program. Transplant Proc. 2012;44:1830. doi: 10.1016/j.transproceed.2012.06.024. [DOI] [PubMed] [Google Scholar]
- 30.Mascia L, Pasero D, Slutsky AS, et al. Effect of a lung protective strategy for organ donors on eligibility and availability of lungs for transplantation: a randomized controlled trial. JAMA. 2010;304:2620. doi: 10.1001/jama.2010.1796. [DOI] [PubMed] [Google Scholar]
- 31.Broccard AF, Vannay C, Feihl F, Schaller MD. Impact of low pulmonary vascular pressure on ventilator-induced lung injury. Crit Care Med. 2002;30:2183. doi: 10.1097/00003246-200210000-00002. [DOI] [PubMed] [Google Scholar]
- 32.Petak F, Habre W, Hantos Z, Sly PD, Morel DR. Effects of pulmonary vascular pressures and flow on airway and parenchymal mechanics in isolated rat lungs. J Appl Physiol. 1985;2002:169. doi: 10.1152/jappl.2002.92.1.169. [DOI] [PubMed] [Google Scholar]
- 33.Schutte H, Hermle G, Seeger W, Grimminger F. Vascular distension and continued ventilation are protective in lung ischemia/reperfusion. Am J Respir Crit Care Med. 1998;157:171. doi: 10.1164/ajrccm.157.1.9706029. [DOI] [PubMed] [Google Scholar]
- 34.Cypel M, Yeung JC, Hirayama S, et al. Technique for prolonged normothermic ex vivo lung perfusion. J Heart Lung Transplant. 2008;27:1319. doi: 10.1016/j.healun.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 35.Sanchez PG, D'Ovidio F. Ex-vivo lung perfusion. Curr Opin Organ Transplant. 2012;17:490. doi: 10.1097/MOT.0b013e328357f865. [DOI] [PubMed] [Google Scholar]
- 36.Hsia CC. Respiratory function of hemoglobin. N Engl J Med. 1998;338:239. doi: 10.1056/NEJM199801223380407. [DOI] [PubMed] [Google Scholar]
- 37.West JB. Hemoglobin O2 affinity and tissue hypoxia. J Appl Physiol. 1985;1989:2163. doi: 10.1152/jappl.1989.67.5.2163. [DOI] [PubMed] [Google Scholar]
- 38.Aigner C. Growing experience with ex vivo lung perfusion: many ways leading to the same goal. Eur J Cardiothorac Surg. 2014;45:45. doi: 10.1093/ejcts/ezt255. [DOI] [PubMed] [Google Scholar]
- 39.Yeung JC, Cypel M, Machuca TN, et al. Physiologic assessment of the ex vivo donor lung for transplantation. J Heart Lung Transplant. 2012;31:1120. doi: 10.1016/j.healun.2012.08.016. [DOI] [PubMed] [Google Scholar]
- 40.Baskurt OK, Meiselman HJ. Blood rheology and hemodynamics. Semin Thromb Hemost. 2003;29:435. doi: 10.1055/s-2003-44551. [DOI] [PubMed] [Google Scholar]
- 41.Bucens D, Pain MC. Influence of hematocrit, blood gas tensions, and pH on pressure-flow relations in the isolated canine lung. Circ Res. 1975;37:588. doi: 10.1161/01.res.37.5.588. [DOI] [PubMed] [Google Scholar]
- 42.Pries AR, Secomb TW, Sperandio M, Gaehtgens P. Blood flow resistance during hemodilution: effect of plasma composition. Cardiovasc Res. 1998;37:225. doi: 10.1016/s0008-6363(97)00226-5. [DOI] [PubMed] [Google Scholar]
- 43.Lee SS, Antaki JF, Kameneva MV, et al. Strain hardening of red blood cells by accumulated cyclic supraphysiological stress. Artif Organs. 2007;31:80. doi: 10.1111/j.1525-1594.2007.00344.x. [DOI] [PubMed] [Google Scholar]
- 44.Watanabe N, Sakota D, Ohuchi K, Takatani S. Deformability of red blood cells and its relation to blood trauma in rotary blood pumps. Artif Organs. 2007;31:352. doi: 10.1111/j.1525-1594.2007.00392.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Synoptic description of the EVLP procedure. PRBC, packed red blood cells; Vt, tidal volume (ml/kg); RR, respiratory rate; PEEP, positive end-expiratory pressure (cmH2O); CO, cardiac output (l/min); PAPm, mean pulmonary arterial pressure (mmHg); RM, recruitment maneuver; FBS, fibrobronchoscopy; TLC, total lung capacity; RX, chest X-ray.
Quantitative analysis of graft CT scan.


