Abstract
Human epidermal growth factor receptor 2 (HER2) testing in gastric and gastroesophageal junction cancer is an evolving area in clinical practice that has particular relevance to Asia-Pacific countries, which face a high incidence of these diseases. A growing body of evidence demonstrates that HER2-targeted therapy improves survival for patients with HER2-positive advanced disease, and drives the need for high-quality testing procedures to identify patients who will respond to treatment. However, various factors challenge day-to-day testing of gastric specimens in these countries, to a degree greater than that observed for breast specimens. Recommendations for HER2 testing of gastric cancer specimens were published as a result of the Trastuzumab for Gastric Cancer (ToGA) trial. The guidelines proposed in this manuscript build on these recommendations and emphasize local testing environments, particularly in Asia-Pacific countries. A multidisciplinary task force comprising experts from Asia-Pacific who actively work and provide education in the area was convened to assess the applicability of existing recommendations in the Asia-Pacific region. The resulting recommendations reported here highlight and clarify aspects of testing that are of particular relevance to the region, and notably emphasize multidisciplinary collaborations to optimize HER2 testing quality.
Keywords: gastric cancer, human epidermal growth factor receptor 2, immunohistochemistry, in situ hybridization, quality control
INTRODUCTION
Following the publication of the Trastuzumab for Gastric Cancer (ToGA) trial results1 and the approval of trastuzumab for the treatment of human epidermal growth factor receptor 2 (HER2)-positive advanced gastric cancer, the quality of HER2 testing in gastric cancer emerged as an area of interest in the Asia-Pacific region, which reports a high incidence of gastric cancer. A multidisciplinary panel of experts from the Asia-Pacific region convened to discuss the applicability of current HER2 testing recommendations in gastric cancer. Experts were selected based on scientific merit, on recognition as regional key opinion leaders in gastric cancer management, and on advocacy of HER2 testing quality through programs such as the Scientific Partnership for HER2 testing Excellence (SPHERE). SPHERE is an ongoing educational initiative that engages with pathology laboratories in the Asia-Pacific region to achieve the highest standards in HER2 testing. The program also runs advocacy activities to engage members of HER2 testing teams to coordinate diagnosis, treatment plans and overall patient care. These expert discussions assessed latest research and real-world practice in the region to supplement Rüschoff et al.'s recommendations on HER2 testing in gastric cancer, which have been developed primarily based on experience from Europe.2 Where necessary, Rüschoff et al.'s recommendations have been modified or expanded upon to reflect the experience of Asia-Pacific laboratories in routine testing. It should be noted that the following recommendations are not binding and should be adapted to reflect national level reimbursement plans.
EPIDEMIOLOGY
Worldwide, gastric cancer is the fourth most commonly diagnosed cancer3,4 and the second leading cause of cancer death in men and women.4 The Asia-Pacific region records among the highest disease rates globally, with 50% of all cases reported originating in East Asian countries.4 The estimated mortality rates in East Asia, the highest in the world, are 28.1 per 100 000 in men and 13.0 per 100 000 in women.4
Gastric cancer is associated with a poor prognosis.1 Patients with stage III and IV advanced or metastatic gastric cancer have a 5-year survival rate between 5.0 and 26.9%; the median overall survival is typically less than 1 year.1,5 These poor survival rates are more often reported in countries that do not have government-supported nationwide screening programs, such as the United States (<25.0%).3 In countries with screening programs, such as Korea and Japan, gastric cancer is more likely to be detected at the early stages of the disease, thus resulting in more favorable 5-year survival rates of 55.6–66.0%6 and 50.0%, respectively.7
THE CLINICAL SIGNIFICANCE OF HER2 IN GASTRIC CANCER
Across Asian populations, HER2 overexpression in gastric cancer is reported in approximately 9.8–23.0% of cases,8–15 with higher rates generally observed in gastroesophageal junction (GEJ) cancer.14,15 HER2 is a key driver of tumorigenesis through its association with tumor cell proliferation, apoptosis, adhesion, migration, and differentiation, and is suggested to be associated with aggressive disease.1 In gastric cancer, HER2 overexpression is predictive of response to HER2-targeted therapy;1 however, its prognostic significance remains controversial. While some studies failed to establish the prognostic value of HER2 positivity,12 other studies suggested that there were correlations between HER2 positivity and poorer outcomes, as evidenced by higher HER2 positivity rates in advanced gastric carcinomas and prognostic significance found in early gastric carcinomas.2,9,16 A large cohort study by Cho et al. suggested that frequent HER2 positivity observed in advanced gastric carcinomas is indicative that HER2 may be involved in tumor progression and poor prognosis.17
Further analyses found that poorer prognosis in HER2-positive cases was significantly correlated with the patient's age and histopathologic type,9,18 whereby intestinal-type carcinomas are more likely to demonstrate HER2 overexpression compared with diffuse- or mixed-type carcinomas.9,16,19
TOGA'S SIGNIFICANCE ON THE TREATMENT OF HER2-POSITIVE GASTRIC CARCINOMAS
ToGA was an open-label, multicenter, randomized controlled trial that investigated trastuzumab in combination with chemotherapy for the first-line treatment of patients with HER2-positive disease with advanced gastric or GEJ cancer.1
Results showed that the addition of trastuzumab to chemotherapy provided the greatest survival benefit to patients with high HER2 protein expression (defined as immunohistochemistry [IHC] modified HercepTest [Dako Corp, Carpinteria, CA, USA] score of 2+ and fluorescence in situ hybridization [FISH]-determined HER2 gene amplification, or IHC modified HercepTest score of 3+). The median overall survival was 16.0 months for patients treated with combination chemotherapy and trastuzumab versus 11.8 months for those treated with chemotherapy alone.1 To date, ToGA is the only trial to evidence a treatment strategy that extends overall survival beyond the 12-month median mark in advanced gastric/GEJ cancer patients who are HER2 positive.1
Trastuzumab has approved indications for the treatment of HER2-positive gastric cancer in five East Asian countries and areas, as shown in Table 1.
Table 1.
Approved trastuzumab indications for HER2-positive gastric cancer in East Asian countries
| Country | Indication |
|---|---|
| Japan | Advanced or recurrent gastric cancer overexpressing HER2, not amenable to curative resection |
| Hong Kong | Combination with cisplatin and capecitabine or 5-fluorouracil for HER2-positive metastatic gastric adenocarcinoma in treatment-naive patients |
| South Korea and China | Combination with capecitabine or 5-fluorouracil and cisplatin for the treatment of patients with HER2-positive metastatic or unresectable adenocarcinoma of the stomach or GEJ, who have not received prior anticancer treatment for their metastatic or unresectable disease |
| Taiwan | Combination with capecitabine or 5-fluorouracil and cisplatin is indicated for the treatment of patients with HER2-positive (IHC2+/ISH+ or IHC3+) metastatic adenocarcinoma of the stomach (or GEJ) who have not received prior chemotherapy for their metastatic disease |
GEJ, gastroesophageal junction; HER2, human epidermal growth factor receptor 2; IHC, immunohistochemistry; ISH, in situ hybridization.
THE HER2 TESTING ALGORITHM IN GASTRIC CANCER
HER2 status in gastric cancer is determined through IHC testing for HER2 protein overexpression and in situ hybridization (ISH) testing for HER2 gene amplification.
The ToGA trial demonstrated that trastuzumab provides the greatest benefit for patients with high HER2 protein overexpression, thereby establishing IHC as the primary testing method for gastric cancer.1 ISH testing is reserved for HER2 status confirmation of cases with equivocal HER2 protein expression (IHC 2+) (Fig. 1).2,20 Bright-field ISH methodologies are now preferred to effectively identify regions of HER2 amplification; however, selection of ISH methodology should adhere to national level guidelines and reimbursement policies.
Figure 1.
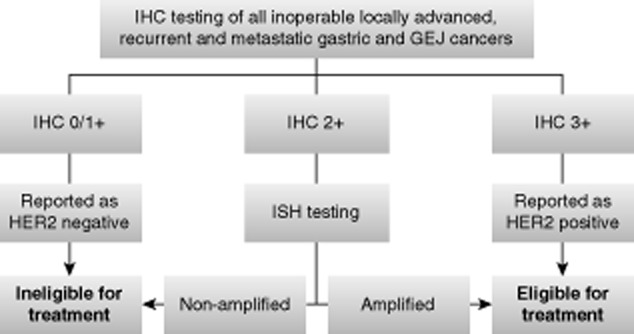
Recommended HER2 testing algorithm in gastric cancer. IHC, immunohistochemistry; GEJ, gastroesophageal junction; HER2, human epidermal growth factor receptor 2; ISH, in situ hybridization.
While IHC is the primary test for all gastric cancers, there is a growing interest in the role of ISH techniques. The Australian Gastric HER2 Testing Study (GaTHER), which examined expanding the application of ISH to determine HER2 positivity in gastric cancer, concluded that an IHC 3+ score strongly predicts a positive ISH result, and that IHC triage before ISH testing was the most cost-effective strategy.21 Given the unique features of gastric cancers and the difficulty of ensuring consistency of HER2 staining in the community setting, when the laboratory initiates a testing program for HER2 in advanced gastric/GEJ cancer, it should test IHC and ISH in parallel until the results are consistent.21 Gómez-Martin et al. considered hybridization techniques in general, and dual-color silver-enhanced ISH (dc-SISH) in particular, may optimize the selection of patients with HER2-positive gastric cancer who will respond to HER2-targeted therapy.22 However, due to limited resources, ISH's role in the HER2 testing algorithm within the Asia-Pacific context will most likely be applied for the confirmation of HER2 status in all HER2 IHC 2+ cases.
PRINCIPLES FOR A MULTIDISCIPLINARY HER2 TESTING TEAM
HER2 testing in gastric cancer requires multidisciplinary coordination to ensure accurate and timely diagnosis of patients' HER2 status. The multidisciplinary team, which involves core members of the surgical, oncology and pathology teams, should collectively promote several key principles toward achieving the goal of quality testing.
First, all inoperable, locally advanced, recurrent and metastatic gastric and GEJ cancers should be routinely tested for HER2 overexpression at diagnosis. While the Task Force accepts that access to HER2-targeted therapy is an important factor in clinical decision making, it should not influence decisions to test patients.
Second, turnaround time from histological diagnosis of gastric cancer to determination of HER2 status should be as short as possible. Appropriate early treatment is important given the rapid progression of disease and high relapse rates in gastric or GEJ cancers.
Third, treatment plans must be formulated with full knowledge of a patient's HER2 status. HER2 test reports should therefore provide a conclusive diagnosis; an equivocal result should not be submitted or accepted.
Finally, HER2 testing should be automated if possible to minimize the risks of testing variations and to produce more reliable and accurate results.
RECOMMENDATIONS FOR GASTRIC HER2 TESTING
Appropriate specimen type
Sampling is a crucial early determinant in the accuracy of HER2 testing. Surgical resection and biopsies are both accepted. However, biopsies are more commonly provided because advanced and metastatic gastric cancers are generally inoperable.
Current recommendations state that six to eight biopsies should be taken to account for intratumoral heterogeneity and to provide sufficient tissue for testing.2 This range may not necessarily be achievable in clinical settings; the Task Force considers four to six biopsies as acceptable. Endoscopic teams should be aware that insufficient tumor tissue cannot account for heterogeneity and increases the risk of false-negative results.23,24 To reduce sampling errors and necrotic tissue, which limit the available volume of tissue for testing, endoscopists should be careful to acquire sufficient viable tumor samples and routinely record the number of biopsy fragments submitted.
Tissue microarrays are not recommended for routine specimen assessment because they cannot fully represent heterogeneity.
Handling and fixation
Gastric biopsy specimens are susceptible to rapid dehydration. Stringent procedures should be in place to transfer biopsy samples as quickly as possible after removal into the recommended fixative 10% neutral buffered formalin (NBF), preferably within 20 min of excision, as proposed by Rüschoff et al.2
Surgical excisions should be handled under similar stringent procedures with the transfer of the specimen into 10% NBF within 1 h of resection. Pre-analytic cold ischemia time, the time between tissue removal and initiation of formalin fixation, can affect accurate measurement of protein expression patterns in tissues, leading to inaccurate HER2 test interpretation and false-negative results.25,26 In some clinical situations, a longer cold ischemia time may be acceptable. For example, in one laboratory's practical experiments with gastric specimens showing HER2 protein overexpression, delayed time to fixation not exceeding 4 h provided surprisingly acceptable IHC and ISH results (Professor Kyoung-Mee Kim, pers. comm., 2013). This is however not recommended as a guideline without revalidation in the original laboratory as well as further validation in other laboratories.
The Task Force recommends that laboratories validate the cold ischemia time for biopsies and surgical excisions separately to provide evidence for appropriate tissue handling protocols. Validations must reflect whether specimens are to be transferred fresh to the laboratory or whether fixation is to be initiated in the operating suite.
If initiated in the operating suite, the time that specimens are removed and placed into 10% NBF should be noted in the testing request form. Specimens should be placed in sturdy, appropriate-sized containers and submerged in 10% NBF; surgical specimens should be opened and oriented prior to submersion (Fig. 2). While laboratory staff should ideally lead in process validation, surgical and other operating suite staff should confirm adherence to validated tissue handling procedures.
Figure 2.
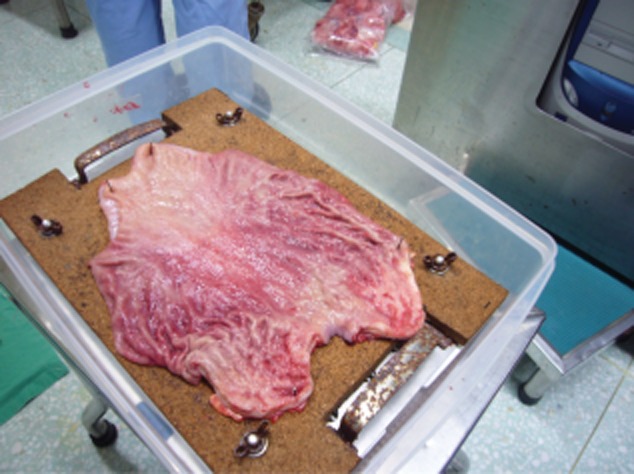
An example of a custom-designed container provided to an operating theater in Taiwan. The container allows surgical staff to open, orientate and pin resected specimens to a corkboard prior to submersion in 10% neutral buffered formalin. (Image courtesy of J. Wang).
RECOMMENDATIONS FOR LABORATORIES: PARAMETERS COMMONLY ASSOCIATED WITH TESTING VARIATIONS
Fixation
Task Force members have noted that variable and non-validated fixation procedures are commonly used in the Asia-Pacific region. This has caused testing variations, as noted in confidential feedback provided to individual laboratories by the United Kingdom National External Quality Assessment Service (UK NEQAS), the quality assurance program to which many laboratories subscribe. This section highlights the accepted standards that should benchmark the validation of fixation procedures.
Fixation is influenced by a number of factors: temperature, penetration rate, specimen dimensions (preferably no more than 4 mm thick), fixative-to-tissue volume ratio, pH and duration of fixation. Optimizing these parameters will ensure that cross-linkages are appropriately formed between tissue elements and fixative, and that specimens are stable for processing.
Fresh supplies of 10% NBF, the only fixative associated with consistent testing results, should be used where possible in a fixative-to-tissue volume ratio of 10:1. Even when fixation is initiated in operating theaters, the Task Force suggests that laboratory staff monitor the process to ensure compliance with validated procedures, and to ensure appropriate fixative type and volume upon receipt in the laboratory.
The duration of fixation for biopsies and surgical excisions should be validated separately within the recommended range of 8–72 h (Fig. 3). Access to HER2 testing centers is an important issue in the Asia-Pacific region. The Task Force recognizes that tissue sampling may be performed in remote settings and some distance away from HER2 testing centers, which are more commonly located in urban areas. HER2 testing centers should work closely with local laboratories in these remote settings to appropriately prepare tissues for transportation. If specimens will reach the HER2 testing laboratory within the validated fixation duration, local laboratories should initiate fixation by submerging specimens in sufficient volume of 10% NBF and sealing them within containers that are appropriate for long-distance transportation. If specimens will reach the laboratory after the validated fixation duration, specimens should be processed and embedded in paraffin blocks prior to transportation, in accordance with validated protocols.
Figure 3.
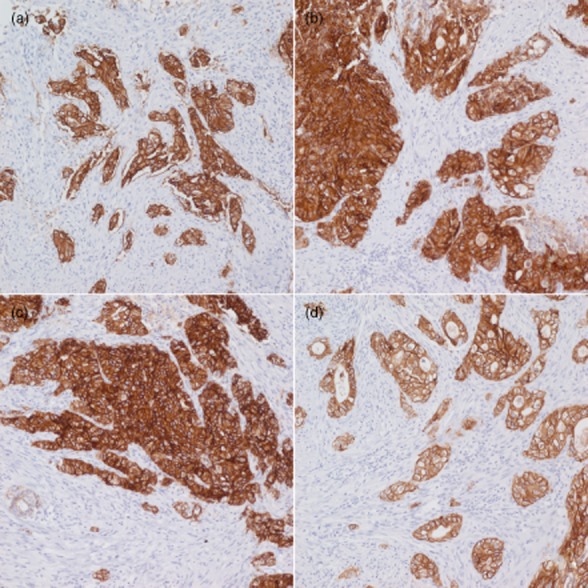
Staining of human epidermal growth factor receptor 2 (HER2) immunohistochemistry (IHC) 3 + specimen fixed for 8 h (a), 48 h (b), 72 h (c), and 96 h (d). The HER2 IHC 3+ specimen that was fixed for 96 h shows relatively weak staining compared with the other specimens. (Image courtesy of K-M. Kim).
Antigen retrieval
Automated, temperature-controlled antigen retrieval, optimized and validated based on the instrument manufacturer's recommendations, is recommended. Poor retrieval techniques are a common cause of false results. Over-retrieval, for instance, may activate endogenous biotin, leading to high levels of background staining with avidin-biotin reaction-based detection and may produce false-positive results.27
Assays
Standardized, commercially available IHC and ISH assays are preferred over in-house assays to ensure reliability and reproducibility of HER2 testing results. Assays should ideally be validated and approved by regional regulatory agencies for testing specifically in gastric cancer specimens. In-house validation of assays is critical, as based on the Task Force's collective experiences, different IHC antibodies may produce different staining intensities, and positivity rates may vary.13 The validation process should produce reference images for no, low and high levels of HER2 protein expression to standardize day-to-day practice in scoring in laboratories.
Controls
Wherever possible, controls with defined HER2 expression levels should be present on each specimen slide to detect false results. For IHC testing, controls should include sections with no (IHC 0) and strong IHC staining (IHC 3+), and if possible, equivocal staining (IHC 2+). Controls, preferably those recommended and/or provided in test kits, should be prepared using similar fixation, processing and paraffin embedding techniques as specimens.
RECOMMENDATIONS FOR HER2 TEST INTERPRETATION AND SCORING IN GASTRIC CANCER
The unique features of gastric carcinomas
Reflecting the unique features of gastric carcinomas, gastric interpretation and scoring guidelines differ from those for breast cancer.2 Unlike breast carcinomas, gastric carcinomas are gland-forming, mucin-producing carcinomas that demonstrate incomplete, basolateral/lateral staining patterns (Fig. 4). When strongly stained, this U-shaped pattern is classified as HER2 positive.20,28 If breast guidelines, which require complete membrane staining, are applied to gastric carcinomas, there would be a significant number of gastric specimens that would be incorrectly scored as HER2 negative.
Figure 4.
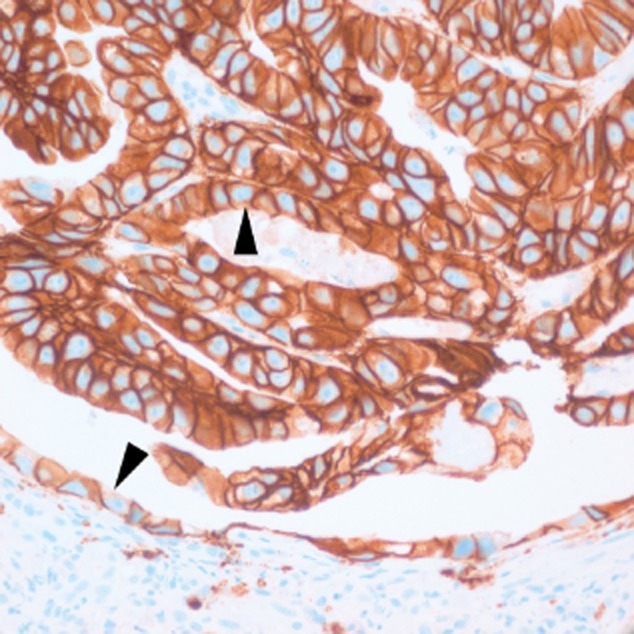
Incomplete lateral/basolateral membrane staining produces the characteristic U-shaped patterns (arrowheads) associated with gastric cancer cells. (Image courtesy of J. Wang).
Heterogeneous staining is a true biological feature that is far more common in gastric and GEJ carcinomas than in breast carcinomas20,28 and is estimated to be present in up to 30% of HER2-positive gastric cases.2 Heterogeneity is more often detected in IHC 2+ cases or in mixed histologic type,17 and overexpression of HER2 protein is associated with histologic types (differentiated or intestinal) and aggressive biological behaviors (lymphovascular invasion and lymph node metastasis).12,13,17,28
Heterogeneity impacts each phase of testing, from sampling to scoring, and may cause HER2-positive foci to be missed in gastric specimens (Fig. 5). The heterogeneity issue has raised questions on whether tumors with <10% positively stained cells would respond to HER2-targeted therapy, despite classification as HER2 negative with current scoring criteria. In tumors in which strong complete/basolateral or lateral membrane staining is seen in <10% of the cells, IHC staining should be repeated on a different paraffin block section, and if still inconclusive, an ISH test should be performed to determine HER2 gene amplification and final HER2 status.
Figure 5.
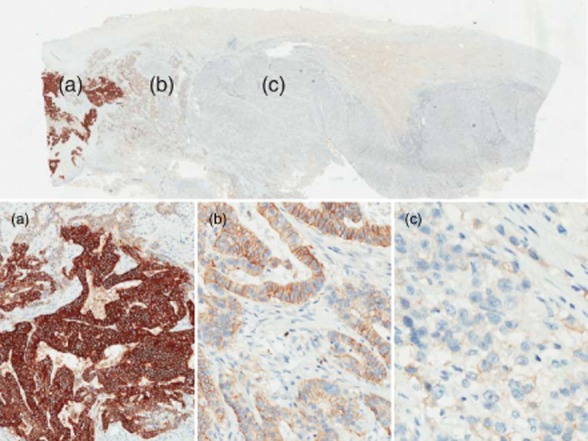
Heterogeneous human epidermal growth factor receptor 2 (HER2) protein expression in a gastric specimen. (a) Strong staining characterizing a HER2-positive foci under low (4×) magnification; (b) weak-to-moderate staining intensity under 10× magnification, classified as immunohistochemistry (IHC) 2+; (c) barely perceptible staining under higher (20×) magnification, which, if seen in isolation, would give the specimen a score of IHC 1+. (Images courtesy of J. Ying).
Besides intratumoral heterogeneity, discordant HER2 status between primary and metastatic gastric tumors is not uncommon in gastric carcinoma13,24 and could also impact patients' eligibility for HER2-targeted therapy. Therefore, in stage IV disease, HER2 testing should ideally be performed on samples from both primary and distant metastatic sites, subject to the availability of specimens. Evidence of discordance between the primary and metastatic sites should prompt a discussion between the pathologist and oncologist, prior to making a final treatment decision.
ASSESSMENT OF IHC HER2 STAINING
When assessing a specimen following IHC staining, care should be taken to interpret an invasive area of the carcinoma. Nonspecific staining and artifacts should not be assessed. Tests should be repeated on different samples when faced with poorly preserved tissue.
Table 2 summarizes the widely accepted IHC scoring criteria for gastric carcinomas that were developed from validation studies, including the preclinical validation phase of the ToGA trial.1,2,20,30 Rüschoff et al. further suggest that the microscopic magnification rule can complement the interpretation process.2 The rule correlates staining intensity and microscopic magnification with IHC scores as outlined below.
IHC 3+: any membranous staining visible at low (×2.5–5) magnification.
IHC 2+: membranous staining visible at medium (×10–20) magnification.
IHC 1+: staining visible only at high (×40) magnification.
Table 2.
HER2 IHC and ISH scoring criteria in gastric cancer
| IHC scoring criteria† | ISH scoring criteria‡ | |||
|---|---|---|---|---|
| Score | Surgical specimen staining pattern | Biopsy specimen staining pattern | Diagnosis | Scoring criteria |
| IHC 0 (negative) | No reactivity or membranous reactivity in <10% of tumor cells | No reactivity or no membranous reactivity in any tumor cell | ISH positive | HER2:CEP17 ratio ≥2.0; if present, record polysomic amplified |
| IHC 1+ (negative) | Faint/barely perceptible membranous reactivity in ≥10% of tumor cells; cells are reactive only in part of their membrane | Tumor cell cluster§ with a faint/barely perceptible membranous reactivity, irrespective of percentage of tumor cells stained | ISH negative | HER2:CEP17 ratio <2.0; if present, record polysomic not amplified |
| IHC 2+ (equivocal) | Weak-to-moderate, complete, basolateral or lateral membranous reactivity in ≥10% of tumor cells | Tumor cell cluster§ with a weak-to-moderate, complete, basolateral or lateral membranous reactivity, irrespective of percentage of tumor cells stained | ISH borderline amplification (ratio close to 2.0) |
HER2:CEP17 ratio 1.8–2.2
|
| IHC 3+ (positive) | Strong, complete, basolateral or lateral membranous reactivity in ≥10% of tumor cells | Tumor cell cluster§ with a strong, complete, basolateral or lateral membranous reactivity, irrespective of percentage of tumor cells stained | ||
Adapted from Bang et al.1
Adapted from Rüschoff et al.2
For biopsies, there is no percentage cutoff; however, a cluster of at least five positive cells is required. CEP17, chromosome enumeration probe 17; HER2, human epidermal growth factor receptor 2; IHC, immunohistochemistry; ISH, in situ hybridization.
Using this magnification rule in conjunction with IHC scoring criteria may improve concordance between IHC and ISH test results.2 In the ToGA trial, as many as 18.6% of IHC 1+ gastric carcinomas were HER2 gene amplified.1 However, in the opinion of the Task Force, this rule should not be used in isolation without the scoring criteria listed in Table 2, as fluctuations in temperature, microscope settings and environmental brightness could affect staining visibility.
If a surgical excision specimen shows <10% strongly stained tumor cells, a different section of the specimen that contains intestinal-type carcinoma by the Lauren classification system should be re-stained. The Task Force further recommends that if a specimen's HER2 status is in doubt, an ISH test should be performed for confirmation.12,17,31 If the ISH test confirms the discordance, a HER2-positive diagnosis should be reported in the presence of HER2-positive foci.
The final HER2 status is that of the invasive carcinoma only. HER2 positivity confined to the dysplasia of Barrett's esophagus or in situ gastric carcinoma can be recorded in the report notes.
Assessment of HER2 gene amplification
Due to the heterogeneous nature of gastric carcinomas, hematoxylin and eosin and IHC stains should guide screening and selection of possible HER2-amplified areas for assessment. The entire specimen is screened to confirm successful hybridization, detection and visualization, and a selection of normal cells is formally counted before interpreting tumor cells.29 Approximately 20 adjacent or continuous cells from IHC 2+ areas should be counted based on the principles outlined in Table 2. It should be noted that polysomy of chromosome 17 (detected using chromosome enumeration probe 17 [CEP17]), defined as ≥3 CEP17 signals per nuclei, is often associated with IHC 2+ cases.17 While its occurrence should be noted in the report, it should not affect accuracy of the diagnosis with assessment of the HER2/CEP17 ratio.
Caution should be exercised when counting overlapping nuclei, because the signals cannot be accurately counted or assigned to a particular cell in the region of the overlap. Nuclei that are not intact and contain central unstained areas should not be counted, since the missing nuclear area may have contained signals that cannot be assessed. Cells with weak signal intensity should also be excluded from assessment.
With dark-field methodologies, cells within regions of nonspecific or high-background staining should not be counted. When using dual probes, only cells with at least one ISH signal for each probe should be counted. FISH-stained slides will fade with time. It is recommended that representative images are taken and that slides are stored at −20°C for a minimum of 12 months.16
A test should be rejected in the presence of the following factors, which could impact the accuracy of assessment.
Pre-analytic parameters, particularly fixation, are not in accordance with validated procedures.
Analytic parameters are not as expected due to microtome artifacts, intensity of hybridization signals and/or presence of both signals in the dual-probe test.
Unsatisfactory results in the controls.
Lack of tumor component in the histological section.
Negative results in sections prepared during a period in excess of 4–6 weeks (which have not been paraffinized and/or stored at 4°C).
The GaTHER study concluded that good agreement across laboratories for HER2 copy number, as determined by single-probe chromogenic ISH or silver ISH (SISH), suggests that this is the optimal HER2 ISH testing method in gastric/GEJ cancer.21 This recommendation differs from United Kingdom guidelines, which no longer recommend modalities that do not include CEP17 probes.29 While the Task Force considers all ISH tests to provide accurate test results following validation, there is a preference for bright-field, dual-probe tests, which produce signals that are easier to score and interpret.
QUALITY CONTROL AND QUALITY ASSURANCE
Quality control and quality assurance measures implemented at each phase of testing will ensure reliable and consistent results. The Task Force recognizes that experience contributes significantly to accuracy of testing, and recommends that HER2 testing laboratories in the region should routinely perform at least 10 IHC tests per day for any antigen, including HER2. Where laboratories are unable to comply with the recommendations, they should consider referring cases to central testing laboratories for HER2 testing.
For laboratories with both IHC and ISH testing capabilities that are performing HER2 testing in gastric cancer for the first time, 25–50 gastric cancer cases should be analyzed in parallel, using IHC and ISH, with the aim of achieving concordance of greater than 90%. At least five HER2-positive cases (10–20% validation cases) should be included.
HER2 testing in gastric cancer must be validated independently of breast cancer protocols, and the optimized procedures documented in in-house HER2 testing protocols that are specific for gastric cancer specimens. If procedures vary from these protocols, records must be made detailing these variations. Revalidation must be performed if there is a change to testing conditions, reagents, materials and methods.
Testing in the laboratory should be performed by a designated team specifically trained in gastric HER2 testing; members should be reevaluated once a year. Besides competency in laboratory procedures, staff involved in testing and scoring should learn to recognize histological features of gastric cells, and identify and assess the invasive component of the tumor. Prior HER2 testing experience in breast cancer should not be considered an indicator of competency in gastric cancer testing. The team's pathologists should score a minimum of 50 gastric cancer cases per year to build expertise and accuracy of scoring and interpretation, verified by participation in ring studies.
Ongoing quality assurance programs can also help laboratories to ensure standards are consistently achieved in day-to-day testing. Internally, laboratories can maintain an audit of HER2 test results to detect changes in testing trends, such as positivity rates and scoring distributions, and to provide evidence when investigating sources of variations. Externally, laboratories may consider participation in proficiency testing and external quality assurance programs, like UK NEQAS. These external programs allow laboratories to continuously monitor HER2 testing quality against peers/published positivity rates.
CONCLUSION
HER2 testing laboratories in Asia-Pacific countries see a high incidence of gastric cancer and as such perform thousands of tests on unselected patients outside the context of clinical trials. The experience of a multidisciplinary task force from Asia-Pacific countries has been utilized to provide HER2 testing recommendations that are of particular relevance to the Asia-Pacific region. Multidisciplinary collaborations are the cornerstone of best practice to ensure patients receive the best possible treatment for their disease. Laboratories should make every effort to comply with best practice guidelines in order to improve the accuracy and reliability of HER2 testing in gastric cancer.
Acknowledgments
Support for third-party writing assistance for this manuscript was provided by F. Hoffmann-La Roche Ltd.
Conflicts of interest: KMK, MB, BSK, YSP and MHR have received speaker's and/or consultancy fees from Roche. KMC has received speaker's and/or consultancy fees from Roche and has had clinical trial involvement with Roche, Bristol Myers Squibb, ImClone Systems, Merck Serono and Novartis. WHK and JW have received speaker's and/or consultancy fees and travel grants from Roche. WS, YC, JMY and SZ have no conflicting interests to disclose. KMK, MB, BSK, YSP, MHR, KMC, WHK and JW received speaker's and/or consultancy fees or travel grants as part of SPHERE. SPHERE is an educational initiative that promotes and facilitates excellence in HER2 testing in breast and gastric cancer across the Asia-Pacific region. It aims to achieve this by conducting regional pathology workshops and by highlighting the importance of multidisciplinary collaboration between surgeons, oncologists, pathologists and laboratory scientists in ensuring accurate test results. This program is funded by F. Hoffmann-La Roche Ltd.
REFERENCES
- 1.Bang YJ, Van CE, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687–697. doi: 10.1016/S0140-6736(10)61121-X. [DOI] [PubMed] [Google Scholar]
- 2.Rüschoff J, Hanna W, Bilous M, et al. HER2 testing in gastric cancer: a practical approach. Mod Pathol. 2012;25:637–650. doi: 10.1038/modpathol.2011.198. [DOI] [PubMed] [Google Scholar]
- 3.American Cancer Society. Global Facts and Figures. 2nd edn. Atlanta: American Cancer Society; 2011. [Google Scholar]
- 4.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 5.SEER Cancer Statistics Review. 1975–2009 (Vintage 2009 Populations) Bethesda, MD: National Cancer Institute; 2013. [Google Scholar]
- 6.Park JM, Kim YH. Current approaches to gastric cancer in Korea. Gastrointest Cancer Res. 2008;2:137–144. [PMC free article] [PubMed] [Google Scholar]
- 7.Inoue M, Tsugane S. Epidemiology of gastric cancer in Japan. Postgrad Med J. 2005;81:419–424. doi: 10.1136/pgmj.2004.029330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choritz H, Busche G, Kreipe H. Quality assessment of HER2 testing by monitoring of positivity rates. Virchows Arch. 2011;459:283–289. doi: 10.1007/s00428-011-1132-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim KC, Koh YW, Chang HM, et al. Evaluation of HER2 protein expression in gastric carcinomas: comparative analysis of 1,414 cases of whole-tissue sections and 595 cases of tissue microarrays. Ann Surg Oncol. 2011;18:2833–2840. doi: 10.1245/s10434-011-1695-2. [DOI] [PubMed] [Google Scholar]
- 10.Park DI, Yun JW, Park JH, et al. HER-2/neu amplification is an independent prognostic factor in gastric cancer. Dig Dis Sci. 2006;51:1371–1379. doi: 10.1007/s10620-005-9057-1. [DOI] [PubMed] [Google Scholar]
- 11.Yano T, Doi T, Ohtsu A, et al. Comparison of HER2 gene amplification assessed by fluorescence in situ hybridization and HER2 protein expression assessed by immunohistochemistry in gastric cancer. Oncol Rep. 2006;15:65–71. [PubMed] [Google Scholar]
- 12.Yoon HH, Shi Q, Sukov WR, et al. Association of HER2/ErbB2 expression and gene amplification with pathologic features and prognosis in esophageal adenocarcinomas. Clin Cancer Res. 2012;18:546–554. doi: 10.1158/1078-0432.CCR-11-2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cho EY, Park K, Do I, et al. Heterogeneity of ERBB2 in gastric carcinomas: a study of tissue microarray and matched primary and metastatic carcinomas. Mod Pathol. 2013;26:677–684. doi: 10.1038/modpathol.2012.205. [DOI] [PubMed] [Google Scholar]
- 14.Shan L, Ying J, Lu N. HER2 expression and relevant clinicopathological features in gastric and gastroesophageal junction adenocarcinoma in a Chinese population. Diagn Pathol. 2013;8:76. doi: 10.1186/1746-1596-8-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sheng WQ, Huang D, Ying JM, et al. HER2 status in gastric cancers: a retrospective analysis from four Chinese representative clinical centers and assessment of its prognostic significance. Ann Oncol. 2013;24:2360–2364. doi: 10.1093/annonc/mdt232. [DOI] [PubMed] [Google Scholar]
- 16.Gómez-Martin C, Concha A, Corominas JM, et al. Consensus of the Spanish Society of Medical Oncology (SEOM) and Spanish Society of Pathology (SEAP) for HER2 testing in gastric carcinoma. Clin Transl Oncol. 2011;13:636–651. doi: 10.1007/s12094-011-0709-7. [DOI] [PubMed] [Google Scholar]
- 17.Cho J, Jeong J, Sung J, et al. A large cohort of consecutive patients confirmed frequent HER2 positivity in gastric carcinomas with advanced stages. Ann Surg Oncol. 2013;20:477–484. doi: 10.1245/s10434-012-2818-0. [DOI] [PubMed] [Google Scholar]
- 18.Tanner M, Hollmen M, Junttila TT, et al. Amplification of HER-2 in gastric carcinoma: association with Topoisomerase IIalpha gene amplification, intestinal type, poor prognosis and sensitivity to trastuzumab. Ann Oncol. 2005;16:273–278. doi: 10.1093/annonc/mdi064. [DOI] [PubMed] [Google Scholar]
- 19.Gravalos C, Jimeno A. HER2 in gastric cancer: a new prognostic factor and a novel therapeutic target. Ann Oncol. 2008;19:1523–1529. doi: 10.1093/annonc/mdn169. [DOI] [PubMed] [Google Scholar]
- 20.Hofmann M, Stoss O, Shi D, et al. Assessment of a HER2 scoring system for gastric cancer: results from a validation study. Histopathology. 2008;52:797–805. doi: 10.1111/j.1365-2559.2008.03028.x. [DOI] [PubMed] [Google Scholar]
- 21.Fox SB, Kumarasinghe MP, Armes JE, et al. Gastric HER2 Testing Study (GaTHER): an evaluation of gastric/gastroesophageal junction cancer testing accuracy in Australia. Am J Surg Pathol. 2012;36:577–582. doi: 10.1097/PAS.0b013e318244adbb. [DOI] [PubMed] [Google Scholar]
- 22.Gómez-Martin C, Garralda E, Echarri MJ, et al. HER2/neu testing for anti-HER2-based therapies in patients with unresectable and/or metastatic gastric cancer. J Clin Pathol. 2012;65:751–757. doi: 10.1136/jclinpath-2012-200774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Warneke VS, Behrens HM, Boger C, et al. Her2/neu testing in gastric cancer: evaluating the risk of sampling errors. Ann Oncol. 2013;24:725–733. doi: 10.1093/annonc/mds528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee HE, Park KU, Yoo SB, et al. Clinical significance of intratumoral HER2 heterogeneity in gastric cancer. Eur J Cancer. 2013;49:1448–1457. doi: 10.1016/j.ejca.2012.10.018. [DOI] [PubMed] [Google Scholar]
- 25.Yildiz-Aktas IZ, Dabbs DJ, Bhargava R. The effect of cold ischemic time on the immunohistochemical evaluation of estrogen receptor, progesterone receptor, and HER2 expression in invasive breast carcinoma. Mod Pathol. 2012;25:1098–1105. doi: 10.1038/modpathol.2012.59. [DOI] [PubMed] [Google Scholar]
- 26.Neumeister VM, Anagnostou V, Siddiqui S, et al. Quantitative assessment of effect of preanalytic cold ischemic time on protein expression in breast cancer tissues. J Natl Cancer Inst. 2012;104:1815–1824. doi: 10.1093/jnci/djs438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bussolati G, Gugliotta P, Volante M, Pace M, Papotti M. Retrieved endogenous biotin: a novel marker and a potential pitfall in diagnostic immunohistochemistry. Histopathology. 1997;31:400–407. doi: 10.1046/j.1365-2559.1997.3020895.x. [DOI] [PubMed] [Google Scholar]
- 28.Jouret-Mourin A, Hoorens A, De HG, Vanderveken J, Demetter P, Van CE. Analysis of HER2 expression and gene amplification in adenocarcinoma of the stomach and the gastro-oesophageal junction: rationale for the Belgian way of working. Acta Gastroenterol Belg. 2012;75:9–13. [PubMed] [Google Scholar]
- 29.Bartlett JM, Starczynski J, Atkey N, et al. HER2 testing in the UK: recommendations for breast and gastric in-situ hybridisation methods. J Clin Pathol. 2011;64:649–653. doi: 10.1136/jcp.2011.089847. [DOI] [PubMed] [Google Scholar]
- 30.Ruschoff J, Dietel M, Baretton G, et al. HER2 diagnostics in gastric cancer-guideline validation and development of standardized immunohistochemical testing. Virchows Arch. 2010;457:299–307. doi: 10.1007/s00428-010-0952-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim MA, Lee HJ, Yang HK, Bang YJ, Kim WH. Heterogeneous amplification of ERBB2 in primary lesions is responsible for the discordant ERBB2 status of primary and metastatic lesions in gastric carcinoma. Histopathology. 2011;59:822–831. doi: 10.1111/j.1365-2559.2011.04012.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


