Abstract
Objectives
Congenital hepatic fibrosis (CHF) is an important cause of morbidity and mortality in patients with Autosomal Recessive Polycystic Kidney Disease (ARPKD). The pathogenesis of CHF remains undefined. Several recent studies suggest that the reninangiotensin system (RAS) is an important mediator of progressive hepatic fibrosis, through activation of pro-fibrotic mediators, such as transforming growth factor-β (TGF-β). RAS activation has not previously been studied in CHF patients or animal models. The aim of the current study was to characterize RAS expression during the course of CHF in the PCK rat.
Methods
Studies were conducted in the PCK rat, an orthologous ARPKD/CHF model, and age matched normal control Sprague Dawley rats. Expression of the RAS components, renin, angiotensinogen (AGT), angiotensin converting enzyme (ACE) and angiotensin II type 1 receptor (AT1R) as well as the pro-fibrotic mediator TGF-β was examined in cystic PCK and control rat livers at 2, 4 and 6 months of age by quantitative real time PCR (qRT-PCR). Angiotensin II (ANG II) was examined by immunohistochemistry (IHC). Fibrosis was assessed by IHC using reticulin staining and Masson’s trichrome. Collagen content was determined by hydroxyproline analysis.
Results
Progressive fibrosis and increased hepatic collagen content occurred in PCK rats with age. In 4 and 6 month old PCK rats livers, ACE gene expression was markedly increased, 8 and 17-fold, respectively, compared to agematched control livers. Expression of the other RAS components, renin, AGT and AT1R were not significantly different. IHC demonstrated prominent ANG II protein expression in periportal regions in PCK rats. In contrast, no expression was noted in control livers. TGF-β expression was also increased in PCK rat livers with progressive disease.
Conclusions
The current study demonstrates, for the first time, RAS upregulation in an orthologous rat ARPKD/CHF model. Increases in ACE and ANG II, as well as the downstream target, the pro-fibrotic mediator TGF-β, suggest that RAS activation may be an important mediator of CHF disease progression. The findings also suggest that treatment with RAS inhibitors, specifically ACE inhibitors or AT1R blockers, could be therapeutic in slowing disease progression in CHF.
Keywords: Congenital hepatic fibrosis, renin-angiotensin system, angiotensin converting enzyme
INTRODUCTION
Congenital hepatic fibrosis (CHF) is an important cause of morbidity and mortality in patients with Autosomal Recessive Polycystic Kidney Disease (ARPKD) as well as those with other inherited and/or congenital syndromes such as nephronophthisis and Meckel syndrome.1-3 CHF is a biliary ductal plate malformation characterized by bile duct proliferation, dilatation and periportal fibrosis. 2, 4 Although it is invariably present histologically at birth, the liver disease may not become clinically evident for many years. More severely affected patients develop portal hypertension and are at risk for potentially life-threatening complications, including gastrointestinal bleeding due to varices and recurrent cholangitis.4 Recent reports suggest that CHF is an important cause of mortality and morbidity in ARPKD patients who have undergone kidney transplantation.5, 6 Although the gene for ARPKD, PKHD1, has been identified, the mechanisms by which the gene defect results in CHF are poorly defined.3, 7
The renin-angiotensin system (RAS) is a key mediator of fibrosis in many organs, including the kidney, heart and vessels. Several recent studies suggest that the RAS is also an important mediator of progressive hepatic fibrosis through activation of pro-fibrotic mediators such as transforming growth factor-β (TGF-β)8-11. However, RAS expression in ARPKD-associated CHF has not been studied in any ARPKD animal models or human disease.
The goal of this study was to determine expression of the RAS and its downstream target, TGF-β, in progressive fibrocystic liver disease in the PCK rat, an orthologous model of human ARPKD that develops polycystic kidney disease, as well as significant periportal fibrosis and bile duct dilatation similar to that of human ARPKD-associated CHF.
MATERIAL AND METHODS
Animal Models
The PCK rat model arose out of a spontaneous mutation in a colony of Sprague-Dawley rats. This model has a spontaneous mutation in the rat orthologue of the human ARPKD gene, PKHD1,7 and develops not only polycystic kidney disease but also significant biliary abnormalities including bile duct dilatation and fibrosis.12 Liver disease in this model is observed histologically by 19 days gestation and intrahepatic bile duct dilatation and overgrowth of portal connective tissue progresses with aging. This is very similar to the hepatic phenotype of affected ARPKD patients.13 For this study, livers from 2, 4, 6 and 7 month old male PCK rats and age-matched Sprague Dawley (SD) rats were used.
All experiments were conducted according to policies of the Institutional Animal Care and Use Committee (IACUC) of Case Western Reserve University School of Medicine.
Clinical and Pathologic Assessments of Disease Severity
PCK and control Sprague Dawley (SD) rats were sacrificed at the timepoints listed above. Liver weight (LW), body weight (BW) and LW/BW were measured at sacrifice. For determination of the fibrotic area, the left lateral lobe of the liver was fixed in 4% paraformaldehyde and embedded in paraffin. Five micron sections were obtained and stained with Masson’s Trichrome (Richard Allen Scientific). Sections were visualized by light microscopy and five random fields per section were imaged at 10x and digitally captured. The percentage of positive staining, corresponding to collagen, was assessed by pixel counting and expressed as the percent of total parenchyma imaged in each section. Means and standard deviations for each section were obtained and then averaged across the 5 sections studied. Reticulin staining, which identifies glycosaminoglycan-bound type III collagen fibrils, was also performed on sections obtained as above from 2 and 7 month PCK and control SD rats.
For measurement of hydroxyproline content, livers were flash frozen in liquid nitrogen at sacrifice. One hundred mg (wet weight) of each liver was homogenized in 6N HCL then incubated for 24 hours at 110°C to allow for complete hydrolysis. Aliquots (50 μl) were resuspended in 1 ml of Chloramine T solution (Fisher Scientific) and incubated at room temperature for 20 minutes. 1 ml of Ehrlich’s solution was added and the samples incubated at 65°C for an additional 15 minutes. Samples were allowed to return to room temperature, and absorbance was measured at 550 nm by spectrophotometry. The hydroxyproline content was determined using a standard curve with known concentrations of hydroxyproline (Sigma, St Louis MO) and was normalized to mg of wet tissue weight.
Serum levels of gamma-glutamyl transferase (GGT) were quantified using a commercially available enzymatic kit (Stanbio Laboratory, Boerne, TX), according to the manufacturer’s instructions.
Measurement of RAS Components and TGF-β Expression by Quantitative-Real time PCR (qRT-PCR)
Renin, angiotensinogen (AGT), angiotensin converting enzyme (ACE), angiotensin type IA receptor (AT1R) and TGF-β gene expression levels were measured by qRT-PCR of whole liver samples. Samples were flash frozen at sacrifice and total liver RNA was extracted using TRIZOL reagent (Invitrogen) according to manufacturer’s instructions. cDNA was generated by reverse transcription using MMLV reverse transcriptase (Promega) in accordance with manufacturer’s protocols. Real-time PCR was performed using the ABI Prism 7000 Sequence Detection System (SDS). Relative quantification was assessed using the ΔΔCT method with samples standardized to expression of the housekeeping gene, 18s. Samples were run in triplicate and at least three livers were studied for each experimental age/group.
Primer sequences used were: renin, F-TTC TCT CCC AGA GGG TGC TA; R-CCC TCC TCA CAC AAC AAG GT; AGT, F-CAC GGA CAG CAC CCT ATT TT; R-GCT GTT GTC CAC CCA GAA CT; ACE, F-GAG CCA TCC TTC CCT TTT TC; R-GGC TGC AGC TCC TGG TAT AG; AT1R, F: CGT CAT CCA TGA CTG TAA AAT TTC;R-GGG CAT TAC ATT GCC AGT GTG; TGF-β, F- CCG CAA CAA CGC AAT CTA TG; R-CTC TGC ACG GGA CAG CAA T; 18s, F- CGC GGT TCT ATT TTG TTG; R-AGT CGG CAT CGT TTA TGG TC.
Immunohistochemistry (IHC) for Angiotensin II (ANG II)
IHC was performed on 5 μm serial sections obtained as above from the livers of 2 and 7 month old PCK rats and age-matched control SD rats, as well as rats 2 weeks post-BDL or sham surgery. Sections were deparaffinized with xylene and rehydrated with graded ethanol. IHC was performed using the ABC Vectastain kit (Zymed) for peroxidase conjugated secondary antibodies according to manufacturer’s instructions. Sections were immersed in 3% H2O2 for inhibition of endogenous peroxidase and blocked with PBS/1% serum from the secondary antibody host, then incubated with primary antibodies overnight at 4°C. Antigen retrieval was performed by heating in 10mM citrate buffer (pH6). All sections were counterstained with hematoxylin and mounted with Permount (Sigma). Anti-ANG II (Bachem 1:2000) was used as the primary antibody. Negative controls included omission of primary antibody and staining with an irrelevant species-matched antibody.
Statistical Analysis
Data were expressed as mean +/− SD. Differences in gene expression between normal and PCK rats were assessed by 2-tailed Student’s T-test and a p value of <0.05 was considered statistically significant.
RESULTS
Collagen deposition in progressive congenital hepatic fibrosis (CHF) in 2 and 7 month-old PCK and normal rat livers
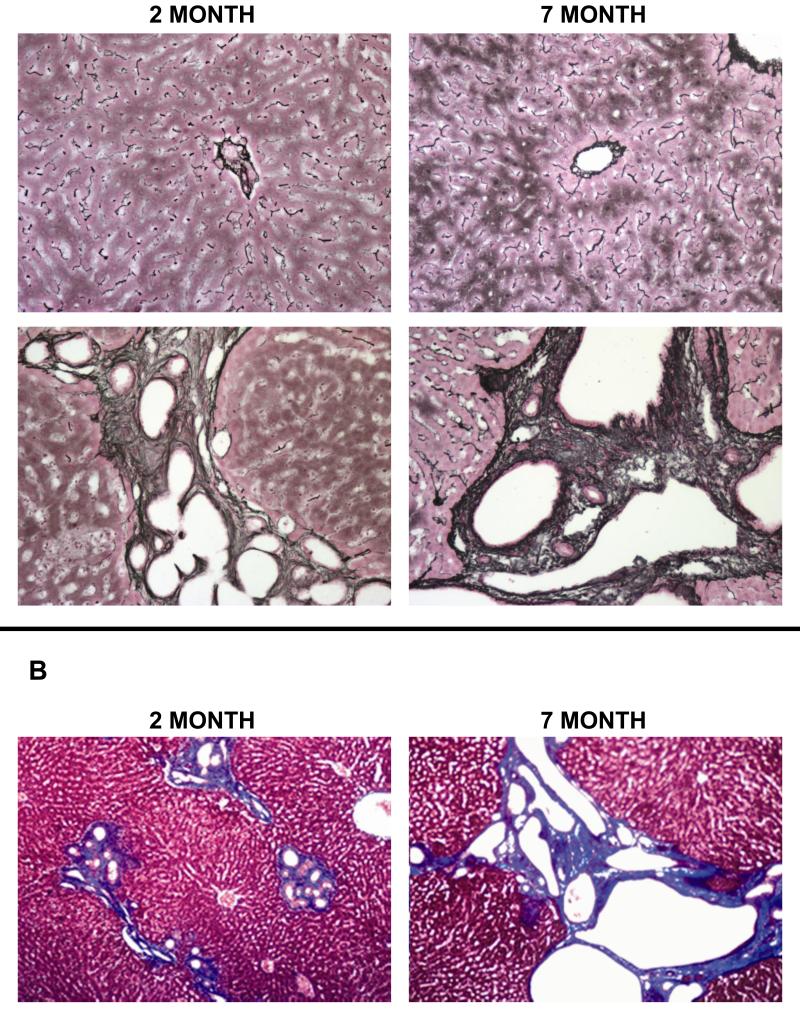
As illustrated by reticulin and Masson’s trichrome staining (Figure 1), PCK rat livers have dilated bile ducts with surrounding periportal fibrosis (collagen shown as black staining for reticulin, blue staining for Masson’s). Intrahepatic bile duct dilatation and collagen deposition increased with age, illustrating the progressive fibrosis present in this model.
Figure 1. Collagen deposition in progressive congenital hepatic fibrosis (CHF) in 2 and 7 month-old PCK and normal rat livers.
(A) Reticulin (black=collagen) staining of PCK rat livers (lower panels) at 2 months (left panel) and 7 months (right panel) illustrates the increasing intrahepatic bile duct dilatation and collagen deposition with progressive liver disease in this model. Normal rat livers at both ages (upper panels) are shown as comparison. (B) Masson’s trichrome (blue = collagen) staining of PCK rat livers highlights the progressive worsening of periportal fibrosis and bile duct dilatation with advancing disease. 10× original magnification.
Clinical, histologic and biochemical features of progressive CHF in PCK rats
To further characterize progressive liver disease in the PCK rat, both PCK and normal control Sprague Dawley (SD) rats were sacrificed at 2, 4 and 6 months of age both clinical features (e.g., liver weight) and histologic scoring for biliary duct dilatation and fibrosis (by trichrome staining) were assessed. As shown in Table 1, LW is greater in PCK versus control rats at 4 and 6 months; however, the LW and LW/BW% level off at 4 months and remain constant at that point, despite progressive disease. Gamma-glutamyl transferase (GGT) values are similar in PCK and age-matched SD rats. Published data in this model have also reported that alkaline phosphatase and bilirubin levels are similar in PCK rats when compared to normal SD rats.12
TABLE 1. CLINICAL FEATURES OF PCK AND CONTROL RAT LIVERS.
| 2 month | 4 month | 6 month | ||
|---|---|---|---|---|
|
Liver weight
(LW), g |
PCK | 11.6 +/− 1.8 | 18.9 +/− 2.5 | 20.2 +/− 3.2 |
| SD | 11.9 +/− 0.8 | 13.0 +/− 1.2 | 15.5 +/− 1.0 | |
|
Body weight
(BW), g |
PCK | 188 +/− 37 | 423+/−27 | 445 +/− 20 |
| SD | 251 +/− 6 | 446 +/− 18 | 448 +/− 16 | |
| LW/BW (%) | PCK | 6.2 +/− 0.3 | 4.5 +/− 0.6 | 4.6 +/− 0.6 |
| SD | 4.8 +/− 0.3 | 2.9 +/− 0.2 | 3.5 +/− 0.2 | |
| GGT (U/L) | PCK | 4 +/− 1 | 4 +/− 2 | 5 +/−2 |
| SD | 3 +/− 2 | 4 +/− 2 | 4 +/−1 |
In contrast to the absence of significant changes in these clinical parameters with progressive disease, both the fibrosis score and hydroxyproline content increase significantly over the course of disease (Table 2). Results are similar to reported fibrosis scores using picrosirius red staining.14 These findings mirror the progressive nature of the liver disease in ARPKD patients.
TABLE 2. PATHOLOGIC MEASURES OF PCK AND CONTROL RAT LIVERS.
| 2 month | 4 month | 6 month | ||
|---|---|---|---|---|
|
Fibrosis score
(% of total liver positive for collagen) |
PCK | 5.0 +/− 0.6 * | 7.1 +/− 0.9 * | 11.1+/− 2.1 * |
| SD | 0.8 +/− 0.3 | 0.7 +/− 0.3 | 0.8 +/− 0.4 | |
|
Hydroxyproline content
(ug/mg wet tissue) |
PCK | 0.16 +/− 0.03 | 0.25 +/− 0.05 * | 0.36 +/− 0.08 * |
| SD | 0.11 +/− 0.03 | 0.13+/− 0.02 | 0.11 +/− 0.003 |
p<0.05 PCK vs. age-matched SD
The RAS gene expression in PCK and normal rat livers
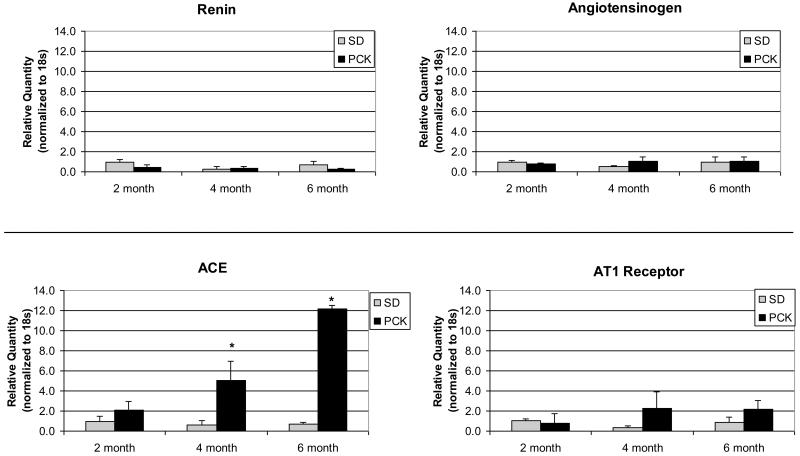
Renin, AGT, ACE and AT1R gene expression were studied in PCK and SD rat livers at the ages indicated (Figure 2). ACE expression in 4 month PCK rat livers was significantly increased (8 fold higher) when compared to age-matched SD rat livers. The ACE upregulation in 6 month PCK livers was even more dramatic, with a greater than 17-fold higher level of ACE expression than for 6-month SD livers. In contrast, renin, AGT and AT1R expression levels were not significantly different in PCK compared to SD livers at each age studied.
Figure 2. RAS gene expression in PCK and normal rat livers.
Livers were obtained at sacrifice at the ages indicated and flash frozen in liquid nitrogen. RNA was extracted using TRIZOL and cDNA generated. qRT-PCR for renin, AGT, ACE and AT1R gene expression was performed using the ABI Prism system 7700 and normalized to the housekeeping gene, 18s. Expression of renin, AGT and AT1R did not differ significantly in the groups studied. However, ACE gene expression was markedly increased in PCK rat livers compared to control livers at 4 months and rose further by 6 months. (* p<0.05)
ANG II expression and localization in PCK and normal rat livers
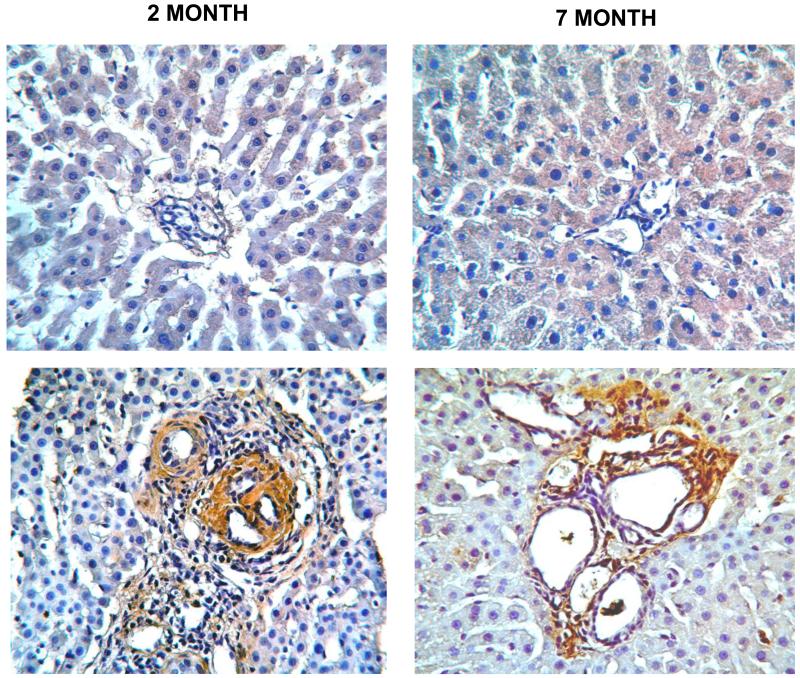
IHC for ANG II protein demonstrates increased expression in the PCK rat livers, which localizes to the regions around dilated bile ducts and within fibrotic portal tracts (Figure 3). With progressive disease, increased numbers of portal triads show ANG II expression. In contrast, normal rat livers showed no apparent ANG II expression by IHC.
Figure 3. Ang II expression in PCK and normal SD rat livers.
IHC was performed on paraformaldehyde-fixed, paraffin-embedded liver sections obtained from PCK and Normal SD rats at 2 and 7 months of age as described in the text. In the normal SD liver (upper panels), minimal to no ANG II protein (indicated by brown staining) is seen at either 2 or 7 months. In contrast, in PCK rat livers at both ages (lower panels), there is a marked increase in ANG II expression in areas surrounding dilated bile ducts within fibrotic portal areas. Original magnification 20×.
TGF-β gene expression in PCK rat livers
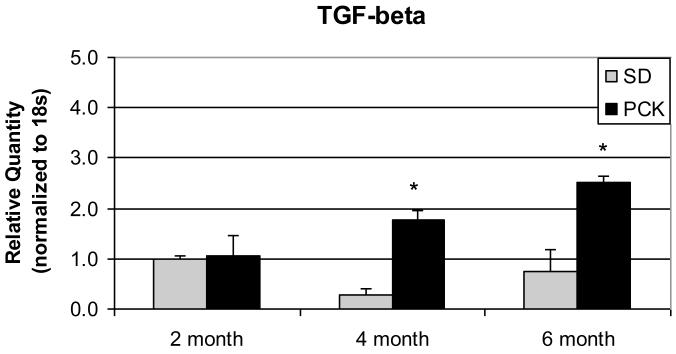
A major consequence of RAS activation is production of pro-fibrotic mediators, notably, TGF-β. In parallel to the ACE gene and ANG II protein upregulation seen in 4 and 6 month PCK livers, TGF-β gene expression was also significantly increased in PCK livers at the same ages (7-fold and 3-fold increase in 4 and 6-month old PCK livers, respectively) (Figure 4).
Figure 4. TGF-β gene expression in PCK and normal rat livers.
Livers were obtained at sacrifice at the ages indicated and cDNA generated as described in Figure 2. qRT-PCR for TGF-β gene expression was performed using the ABI Prism system 7700 and normalized to the housekeeping gene, 18s. TGF-β gene expression is increased in PCK rat livers compared to control livers at 4 and 6 months (* p<0.05).
DISCUSSION
CHF is an important cause of morbidity and mortality in patients with ARPKD and can also occur in patients with other inherited and/or congenital syndromes such as Meckel syndrome.1,2 The mediators of disease progression in CHF, as opposed to other forms of biliary fibrosis, however, are poorly delineated. The presence of a “local” (versus systemic) RAS, in which all of the components necessary for RAS activation are present within the tissue of interest, has emerged as an important mechanism for tissue and organ-specific regulation of RAS.15 In the liver, a growing body of literature in supports the concept of local hepatic RAS activation. Paizis, et al reported that ACE and AT1R gene expression is markedly upregulated in the bile-duct ligation (BDL) model of hepatic fibrosis, and increased AT1R binding was seen in fibrotic areas, despite no differences in liver AGT or renin levels.10 In that same BDL model, systemic ANG II infusion exacerbated hepatic fibrosis.8 Review of the liver fibrosis literature suggests that the key fibrosis mediator TGF-β is downstream of ANG II in fibrosis.8, 11, 16, 17 and that infusion of ANG II induces TGF-β upregulation even in normal rat livers.18 This suggests that upregulation of RAS is sufficient to cause damage even in the absence of an underlying liver disease process. Our results are consistent with a RAS-mediated mechanism of TGF-β upregulation, although additional pathways downstream of ACE may also play an important role, regulating TGF-β more indirectly.
RAS activation has not previously been studied in CHF. The current study demonstrates, for the first time, RAS upregulation in an orthologous rat ARPKD/CHF model. Increases in ANG II, as well as its downstream target, the pro-fibrotic mediator TGF-β, suggest that RAS activation may be an important mediator of CHF disease progression. As systemic levels of ANG I and II were not significantly elevated (data not shown), these findings suggest that it is local, not systemic, RAS activation that is driving the upregulation. CHF is an outlier in the category of biliary fibrosis given the specific mutation, the dilated ducts in the absence of a ductular reaction, and the relatively slow rate of progression. In this context it is particularly interesting that RAS activation is common to ARPKD/CHF and other forms of biliary fibrosis.
IHC demonstrates increased ANG II staining around dilated bile ducts. This pattern suggests that ANG II could be produced by either the biliary epithelial cells (BECs) themselves or by surrounding cells such as portal fibroblasts. The fact that BECs produce the abnormal protein fibrocystin/polyduction, as well as the localization of ANG II around bile ducts, suggest that at least the earliest RAS components are made in BECs. Abnormal fibrocystin is associated with increased expression of cAMP, which can activate the ACE promoter.19-21 Thus, we speculate that in the presence of abnormal fibrocystin, BECs produce increased ACE. This, in turn, results in increased production of ANG II, which can readily diffuse out of the cell and exert its effects locally on portal fibroblasts and other potentially fibrogenic cells in the portal and periportal regions.22 Our current study’s primary limitation is that all of the experiments were performed on whole liver specimens and thus, conclusions about the role of specific cell types in this newly identified pathogenic RAS activation are speculative. Additional in vitro studies are currently underway to more fully define these pathways/processes.
Finally, it is important to note that there are currently no disease-specific therapies for CHF other than standard medical management of complications such as portal hypertension and varices. The data in the current study suggest that RAS inhibition, using ACE inhibitors or angiotensin receptor blockers (ARBs), may be therapeutic in the treatment of progressive CHF in ARPKD patients.
Acknowledgements
The authors would like to thank Dr. Samir El-Dahr, Tulane University for his helpful suggestions regarding RAS assessments.
This research was supported by the NIH/NIDDK (DK-058123 to RGW), the National Kidney Foundation of Ohio, MetroHealth Medical Center, and a pilot award from the UAB Recessive PKD Center Core (to KMD). RGW is funded in part by a pilot award from the ARPKD/CHF Alliance. JW is supported by NIH training grant T32-DK007006.
REFERENCES
- 1.Guay-Woodford LM, Desmond RA. Autosomal recessive polycystic kidney disease: the clinical experience in North America. Pediatrics. 2003;111:1072–80. doi: 10.1542/peds.111.5.1072. [DOI] [PubMed] [Google Scholar]
- 2.Kamath BM, Piccoli DA. Heritable disorders of the bile ducts. Gastroenterol Clin North Am. 2003;32:857–75. vi. doi: 10.1016/s0889-8553(03)00054-2. [DOI] [PubMed] [Google Scholar]
- 3.Dell KM, Avner ED. GeneClinics: Clinical Genetic Information Resource [database online] Copyright, University of Washington; Seattle: 2001. Autosomal recessive polycystic kidney disease. Available at http://www.geneclinics.org. Initial posting July 2001, updated 2008. [Google Scholar]
- 4.Shneider BL, Magid MS. Liver disease in autosomal recessive polycystic kidney disease. Pediatr Transplant. 2005;9:634–9. doi: 10.1111/j.1399-3046.2005.00342.x. [DOI] [PubMed] [Google Scholar]
- 5.Davis ID, Ho M, Hupertz V, Avner ED. Survival of childhood polycystic kidney disease following renal transplantation: the impact of advanced hepatobiliary disease. Pediatr Transplant. 2003;7:364–9. doi: 10.1034/j.1399-3046.2003.00094.x. [DOI] [PubMed] [Google Scholar]
- 6.Khan K, Schwarzenberg SJ, Sharp HL, Matas AJ, Chavers BM. Morbidity from congenital hepatic fibrosis after renal transplantation for autosomal recessive polycystic kidney disease. Am J Tranplant. 2002;2:360–365. doi: 10.1034/j.1600-6143.2002.20412.x. [DOI] [PubMed] [Google Scholar]
- 7.Ward CJ, Hogan MC, Rossetti S, et al. The gene mutated in autosomal recessive polycystic kidney disease encodes a large, receptor-like protein. Nat Genet. 2002;30:259–69. doi: 10.1038/ng833. [DOI] [PubMed] [Google Scholar]
- 8.Bataller R, Gabele E, Parsons CJ, et al. Systemic infusion of angiotensin II exacerbates liver fibrosis in bile duct-ligated rats. Hepatology. 2005;41:1046–55. doi: 10.1002/hep.20665. [DOI] [PubMed] [Google Scholar]
- 9.Bataller R, Brenner DA. Liver fibrosis. J Clin Invest. 2005;115:209–18. doi: 10.1172/JCI24282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paizis G, Cooper ME, Schembri JM, Tikellis C, Burrell LM, Angus PW. Up-regulation of components of the renin-angiotensin system in the bile duct-ligated rat liver. Gastroenterology. 2002;123:1667–76. doi: 10.1053/gast.2002.36561. [DOI] [PubMed] [Google Scholar]
- 11.Wells RG, Fibrogenesis V. TGF-beta signaling pathways. Am J Physiol Gastrointest Liver Physiol. 2000;279:G845–50. doi: 10.1152/ajpgi.2000.279.5.G845. [DOI] [PubMed] [Google Scholar]
- 12.Lager DJ, Qian Q, Bengal RJ, Ishibashi M, Torres VE. The pck rat: a new model that resembles human autosomal dominant polycystic kidney and liver disease. Kidney Int. 2001;59:126–136. doi: 10.1046/j.1523-1755.2001.00473.x. [DOI] [PubMed] [Google Scholar]
- 13.Sanzen T, Harada K, Yasoshima M, Kawamura Y, Ishibashi M, Nakanuma Y. Polycystic kidney rat is a novel animal model of Caroli’s disease associated with congenital hepatic fibrosis. Am J Pathol. 2001;158:1605–12. doi: 10.1016/S0002-9440(10)64116-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Masyuk TV, Masyuk AI, Torres VE, Harris PC, Larusso NF. Octreotide inhibits hepatic cystogenesis in a rodent model of polycystic liver disease by reducing cholangiocyte adenosine 3′,5′-cyclic monophosphate. Gastroenterology. 2007;132:1104–16. doi: 10.1053/j.gastro.2006.12.039. [DOI] [PubMed] [Google Scholar]
- 15.Paul M, Poyan Mehr A, Kreutz R. Physiology of local renin-angiotensin systems. Physiol Rev. 2006;86:747–803. doi: 10.1152/physrev.00036.2005. [DOI] [PubMed] [Google Scholar]
- 16.Bataller R, Schwabe RF, Choi YH, et al. NADPH oxidase signal transduces angiotensin II in hepatic stellate cells and is critical in hepatic fibrosis. J Clin Invest. 2003;112:1383–94. doi: 10.1172/JCI18212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yoshiji H, Kuriyama S, Fukui H. Blockade of renin-angiotensin system in antifibrotic therapy. J Gastroenterol Hepatol. 2007;22(Suppl 1):S93–5. doi: 10.1111/j.1440-1746.2006.04663.x. [DOI] [PubMed] [Google Scholar]
- 18.Ishizaka N, Saito K, Noiri E, et al. Administration of ANG II induces iron deposition and upregulation of TGF-beta1 mRNA in the rat liver. Am J Physiol Regul Integr Comp Physiol. 2005;288:R1063–70. doi: 10.1152/ajpregu.00281.2004. [DOI] [PubMed] [Google Scholar]
- 19.Eyries M, Agrapart M, Alonso A, Soubrier F. Phorbol ester induction of angiotensin-converting enzyme transcription is mediated by Egr-1 and AP-1 in human endothelial cells via ERK1/2 pathway. Circ Res. 2002;91:899–906. doi: 10.1161/01.res.0000042703.39845.b4. [DOI] [PubMed] [Google Scholar]
- 20.Xavier-Neto J, Pereira AC, Junqueira ML, Carmona R, Krieger JE. Rat angiotensin-converting enzyme promoter regulation by beta-adrenergics and cAMP in endothelium. Hypertension. 1999;34:31–8. doi: 10.1161/01.hyp.34.1.31. [DOI] [PubMed] [Google Scholar]
- 21.Xavier-Neto J, Pereira AC, Oliveira EM, Miyakawa AA, Junqueira ML, Krieger JE. Control of the rat angiotensin I converting enzyme gene by CRE-like sequences. Braz J Med Biol Res. 2004;37:1441–53. doi: 10.1590/s0100-879x2004001000002. [DOI] [PubMed] [Google Scholar]
- 22.Guyot C, Lepreux S, Combe C, et al. Hepatic fibrosis and cirrhosis: the (myo)fibroblastic cell subpopulations involved. Int J Biochem Cell Biol. 2006;38:135–51. doi: 10.1016/j.biocel.2005.08.021. [DOI] [PubMed] [Google Scholar]