Abstract
The labella of Maxillaria acuminata Lindl., M. cerifera Barb. Rodr. and M. notylioglossa Rchb.f., all members of the M. acuminata alliance, produce a viscid wax‐like secretion. Histochemical analysis revealed that the chemical composition of the secretion is similar in all three species, consisting largely of lipid and protein. Light microscopy and low‐vacuum scanning electron microscopy were used to investigate the secretory process. In a fourth taxon, M. cf. notylioglossa, transmission electron microscopy showed that lipid bodies are associated with smooth endoplasmic reticulum or occur as plastoglobuli within plastids. Lipid bodies vary in appearance and this may reflect differences in chemical composition. They become associated with the plasmalemma and eventually accumulate between the latter and the cell wall. The wall contains no pits or ectodesmata, and it is speculated that lipid passes through the wall as small lipid moieties before eventually reassembling to form lipid globules on the external surface of the cuticle. These globules are able to coalesce forming extensive viscid areas on the labellum. The possible significance of this process to pollination is discussed.
Key words: Histochemistry; labellum, lipids; low‐vacuum scanning electron microscopy; SEM; Maxillaria; papillae; pollination; transmission electron microscopy; TEM; ultrastructure; wax
INTRODUCTION
Plants have evolved a number of strategies for attracting and rewarding insect pollinators. Potential pollinators may visit flowers in search of floral nectar, pollen, oils or floral fragrances (Proctor and Yeo, 1975). Some flowers simulate carrion, thereby attracting dipteran flies or stingless bees (Roubik, 2000), whereas others have evolved more elaborate strategies involving pseudocopulation and pseudoantagonism (van der Pijl and Dodson, 1969). However complex the strategy, insects are initially attracted by means of visual and olfactory stimuli (Proctor et al., 1996) but, once the insect has alighted, other factors, such as tactile stimuli and rewards assume an equally important role.
Nectar is the most common reward amongst epidendroid orchids (van der Pijl and Dodson, 1969; Arditti, 1992; Dressler, 1993) since their pollen occurs in discrete masses within pollinia and is generally inaccessible to foraging insects. Despite its importance, it is estimated that as many as one‐third of orchid species produce little or no nectar (Porsch, 1908) and that a similar number provide no reward whatsoever (van der Pijl and Dodson, 1969; Ackerman, 1984). In the absence of nectar, representatives from a number of genera such as Maxillaria Ruiz & Pav. (Janse, 1886; Porsch, 1905; van der Pijl and Dodson, 1969; Davies and Winters, 1998; Davies et al., 2000), Polystachya Hook. (Porsch, 1905; Davies et al., 2002a), Eria Lindl. (Beck, 1914) and Dendrobium Sw. (Kjellsson and Rasmussen, 1987) produce ‘food‐hairs’ or pseudopollen (van der Pijl and Dodson, 1969).
The precise morphology of the flower and the relative dimensions and configuration of its parts, coupled with specific visual, olfactory and tactile stimuli as well as an appropriate reward all contribute towards the selection of a particular pollinator. Furthermore, some flowers exhibit even greater selectivity in that they attract only pollinators of a particular sex. For example, male euglossine bees are attracted to the flowers of certain genera such as Catasetum Rich., Cycnoches Lindl., Gongora Ruiz & Pav. and Stanhopea Frost ex Hook. (van der Pijl and Dodson, 1969).
At least three types of reward are produced by representatives of the genus Maxillaria: nectar, pseudopollen and a wax‐like substance. Nectar is copiously produced in a number of species including M. jenischiana (Rchb.f.) C. Schweinf., M. imbricata Barb. Rodr. (K. L. Davies unpubl. res.), M. coccinea (Jacq.) L.O. Williams ex Hodge (cited in Roubik, 2000), M. parviflora (Poepp. & Endl.) Garay and M. pendens Pabst. (R. Singer, pers. comm.). Recently, we have also observed nectar in M. sophronitis (Rchb.f.) Garay and this tested positive for reducing sugars when boiled with Benedict’s reagent (unpubl. res.).
Pseudopollen is also common, especially amongst members of the M. grandiflora alliance (Davies et al., 2000), although similar structures also occur in species of the M. splendens and M. discolor alliances as well as M. longissima Lindl. (Davies and Winters, 1998; Davies et al., 2000, 2002a, b). It is also significant that those species which produce pseudopollen tend to lack nectar (van der Pijl and Dodson, 1969). Pseudopollen is formed by the fragmentation of uniseriate, moniliform, multicellular hairs into individual spherical, lemon‐shaped or fusiform component cells. Much of the cytoplasm of these cells is usually occupied by a single protein body, rich in aromatic amino acids together with starch‐laden amyloplasts, but lipids are usually absent (Davies et al., 2000). Meliponini (stingless bees) are the main pollinators of Maxillaria (Roubik, 2000), but published accounts of bees actually collecting pseudopollen are rare. Nevertheless, bees have been observed collecting pseudopollen from the labella of M. grandiflora (H.B.K.) Lindl. and M. sanderiana Rchb.f. (Dodson and Frymire, 1961; Dodson, 1962), whereas Trigona spp. have been seen gathering pseudopollen from the labella of M. brasiliensis Brieger & Bicalho and M. ochroleuca Lodd. ex Lindl. (R. Singer, pers. comm.).
Wax‐like secretions occur on the labella of M. cerifera Barb. Rodr. and the closely related (or conspecific according to some authorities) M. divaricata (Barb. Rodr.) Cogn. and M. flavoviridis Barb. Rodr. (Porsch, 1905; van der Pijl and Dodson, 1969; Senghas, 1993). These secretions are said to be collected by bees for nest building (van der Pijl and Dodson, 1969). Flowers of M. divaricata lack nectar and it appears that production of the wax‐like secretion has replaced nectar formation in this species (van der Pijl and Dodson, 1969). Maxillaria notylioglossa Rchb.f. and M. acuminata Lindl. are closely related to M. cerifera and also produce a colourless or white (Roberto Vásquez and Dodson, 1982), viscid material upon their labella. Like those of M. cerifera, their flowers lack nectar and pseudopollen. To date, no detailed accounts of insect visitors to these flowers have been published although it is probable that their pollinators are small bees.
The present paper investigates the formation and chemical composition of labellar secretions in the M. acuminata alliance and discusses how these may function in attracting and rewarding pollinators.
MATERIALS AND METHODS
Labella of Maxillaria acuminata Lindl., M. cerifera Barb. Rodr. and M. notylioglossa Rchb.f. were examined using light microscopy and low‐vacuum scanning electron microscopy (SEM). Specimens of M. cf. notylioglossa were also studied using transmission electron microscopy (TEM). This taxon resembled M. notylioglossa in its possession of lime‐green flowers and a triangular labellum. However, the labellum lacked the white, waxy material characteristic of that species. Instead, a transparent, viscid material was present. Authorities for plant names follow Brummitt and Powell (1992). Plants with accession numbers prefixed with an ‘S’ were grown at Singleton Botanic Garden, Swansea, UK, whereas those prefixed ‘ED’ and ‘MM’ were obtained from Dr E. D. L. Schmidt, Wageningen, The Netherlands, and Dr Michael McIllmurray, the National Collection of Maxillarias, Shirley Croydon, UK, respectively (Table 1). Herbarium specimens were prepared and deposited at the National Museum and Gallery of Wales, UK.
Table 1.
Results of histochemical analyses based on light microscopy observations
| Foods present in labellar secretion | ||||
| Species | Accession number | Protein | Starch | Lipid |
| Maxillaria acuminata Lindl. | MM B14 | + | – | + |
| M. cerifera Barb. Rodr. | ED 95‐70 | + | ‐ | + |
| M. notylioglossa Rchb.f. | ED 97‐16 | + | – | + |
| M. cf. notylioglossa Rchb.f. | S19990147 | + | – | + |
Low‐vacuum SEM
Labella were removed, attached to aluminium stubs using double‐sided, sticky carbon tabs and examined immediately by means of back‐scattered electron‐imaging using a JSM 5200 LV‐SEM at an accelerating voltage of 20–25 kV.
TEM
Pieces of labella of M. cf. notylioglossa (accession no. S19990147) were removed and fixed in 2 % glutaraldehyde/1 % (w/v) paraformaldehyde in cacodylate buffer, pH 6·9, for 1h, washed in cacodylate buffer overnight, post‐fixed for 1 h in Millonig’s buffered 1 % (w/v) osmium tetroxide, and dehydrated through a graded ethanol series before infiltrating and embedding in Spurr resin. Sections were cut at 60–90 nm, stained with uranyl acetate/lead citrate and examined using a JEOL 1201 TEM at an accelerating voltage of 80 kV.
Semi‐thin sections were also cut at 2 µm, stained in 0·25 % (w/v) toluidine blue in 0·25 % (w/v) aqueous sodium tetraborate solution, air‐dried and mounted using DPX mountant.
Histochemistry
Labella of each of the species were tested for starch using a dilute iodine/potassium iodide solution, for lipids using a saturated ethanolic solution of Sudan III and for proteins using a modified xanthoproteic test (Purvis et al., 1964). This involved treating the labellum for a few minutes with concentrated nitric acid followed by 10 % (w/v) aqueous sodium hydroxide or potassium hydroxide solution. Blue–black and red reaction products and orange nitroso derivatives demonstrated the presence of starch, lipids and aromatic amino acids, respectively.
RESULTS
Light microscopy and low‐vacuum SEM
A waxy substance is produced distally on the labella of M. cerifera and M. notylioglossa, whereas a viscid substance of more liquid consistency occurs distally on the labellum of M. cf. notylioglossa. This viscid substance, however, occurs both proximally and distally on the labellum of M. acuminata. The labella of all species are papillose (Fig. 1A–C), but simple, two‐ to three‐celled hairs with pointed tips were occasionally observed on the labella of M. cerifera. Secretory globules are produced at the tips and along the length of papillae (Fig. 1B and C) and hairs. The papillae are shortly conical with pointed or rounded tips (Fig. 1A–C) but modified obpyriform papillae occur in M. cf. notylioglossa at the centre of the labellum tip directly beneath the region of greatest secretory activity (Figs 1D and 3A). Similar papillae also occur in M. acuminata. Secretions are copious and accumulate to such an extent that the tips of the papillae often barely show through the viscid layer. Eventually, all topographical labellar detail is obscured (Figs 1C, E and 2A, B). The secretion also penetrates between the labellar papillae and accumulates in intercellular spaces (Figs 2A and B and 3B).
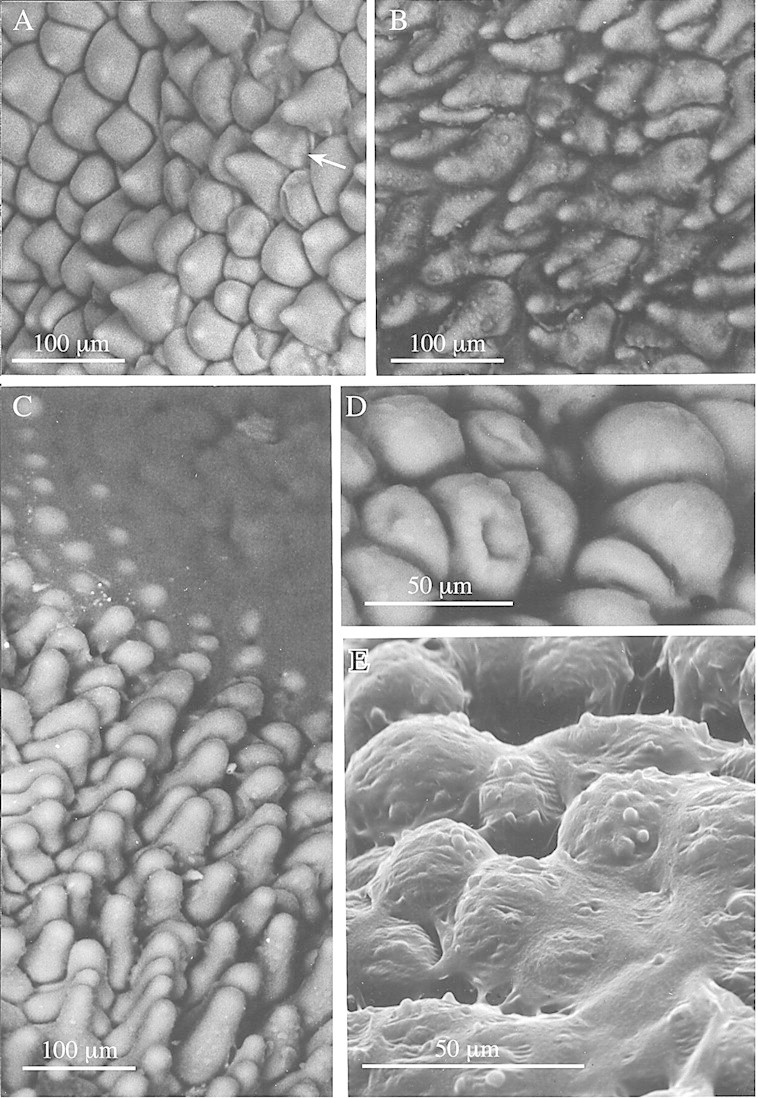
Fig. 1. Low‐vacuum SEMs showing labellar detail of members of the M. acuminata alliance. A, Conical papillae of M. cerifera with some indication of fine, longitudinal sculpturing of the wall (arrow). Bar = 100 µm. B, Conical papillae of M. acuminata showing globules of secretion at tips and along length of papillae. Bar = 100 µm. C, A similar SEM of M. cf. notylioglossa, showing the labellar papillae becoming engulfed by amorphous viscid secretion (top right). Bar = 100 µm. D, Obpyriform papillae from centre of labellum tip of M. cf. notylioglossa. Bar = 50 µm. E, Obpyriform papillae of M. cf. notylioglossa obscured by viscid secretion. Note also the small globules at the tips of the papillae. Bar = 50 µm.
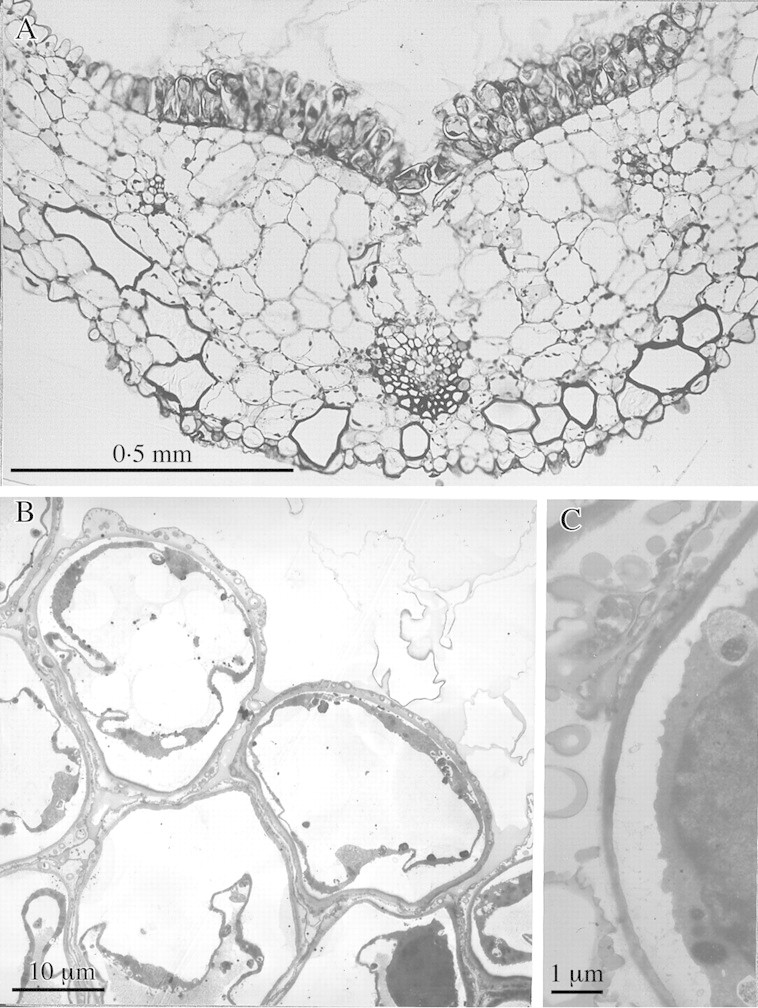
Fig. 3. Semi‐thin and TEM sections of labellum of M. cf. notylioglossa. A, Semi‐thin transverse section of labellum showing median obpyriform papillae and heavy secretion of viscid material. Note also the short conical papillae at the adaxial margin (top left) and similar papillae on the abaxial surface. Bar = 0·5 mm. B, TEM section of obpyriform papillae. Note the highly vacuolated cells with peripheral cytoplasm and nucleus (bottom right), also the heterogeneous nature of the viscid secretion which accumulates both on the outer tangential wall and intercellularly. Bar = 10 µm. C, Detail of cell showing dense cytoplasm with nucleus (right) and intercellular accumulation of viscid material. Note also that the cytoplasm appears to have withdrawn from the cell wall. Bar = 1 µm.
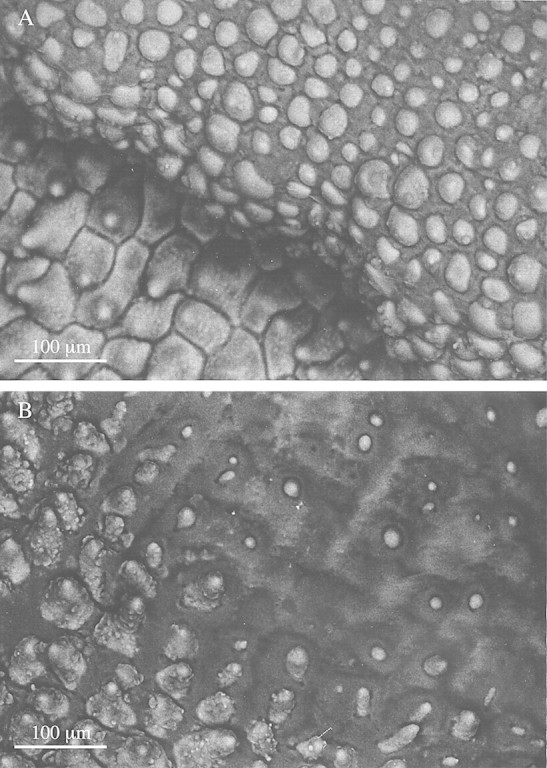
Fig. 2. Low‐vacuum SEMs of labellar surface of M. acuminata. A, Labellar papillae, some showing heavy intercellular deposition of viscid material. Bar = 100 µm. B, A similar SEM, showing papillae tips barely projecting through the thick viscid secretion which, in some cases, has completely engulfed the papillae, resulting in the loss of all topographical detail. Bar = 100 µm.
TEM
At maturity, the secretory cells of M. cf. notylioglossa are highly vacuolate and the cytoplasm is peripheral (Fig. 3B). Care was taken in preparing the material for TEM. Nevertheless, the cytoplasm invariably appeared to have withdrawn from the cell wall (Fig. 3B and C). Although it is possible that this may be an artefact of tissue processing, this is unlikely. Orchid flowers are renowned for their longevity and remain open for several weeks during which time their cells constantly lose water to the atmosphere and may become plasmolysed. As a result, the labella begin to shrivel. Our flowers, however, were fixed within 3–4 d of opening. Similar cytoplasmic withdrawal has been observed by other workers (e.g. Fahn, 1979) in freshly collected material.
Labellar papillae possess many features typical of secretory cells: a large nucleus (Fig. 3B and C); numerous mitochondria with well‐developed cristae (Fig. 4B); and an extensive internal membrane system of smooth endoplasmic reticulum (SER) (Fig. 4A and D). Small, osmiophilic, spherical bodies approx. 150 nm (49–375 nm) in diameter are associated with the latter (Fig. 4A and D). These are almost certainly lipid bodies as they are not membrane‐bound. Aggregations of lipid bodies approx. 150 nm (33–399 nm) in diameter also occur within plastids as plastoglobuli (Fig. 4B). Most are electron‐dense but others of similar size are less distinct (Fig. 4C). The centres of larger lipid bodies measuring about 200 nm (120–325 nm) in diameter are electron‐transparent but their peripheries are electron‐dense (Fig. 4C). This may indicate that the chemical composition of their contents changes as they develop.
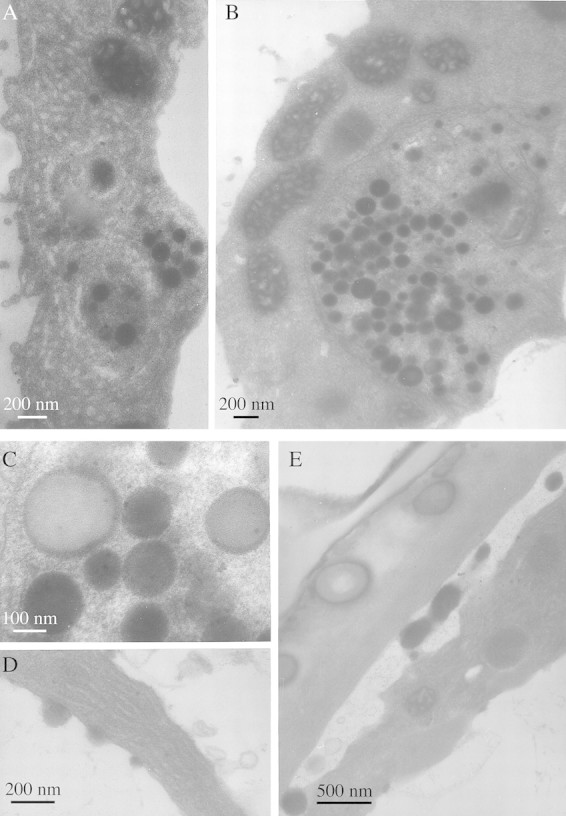
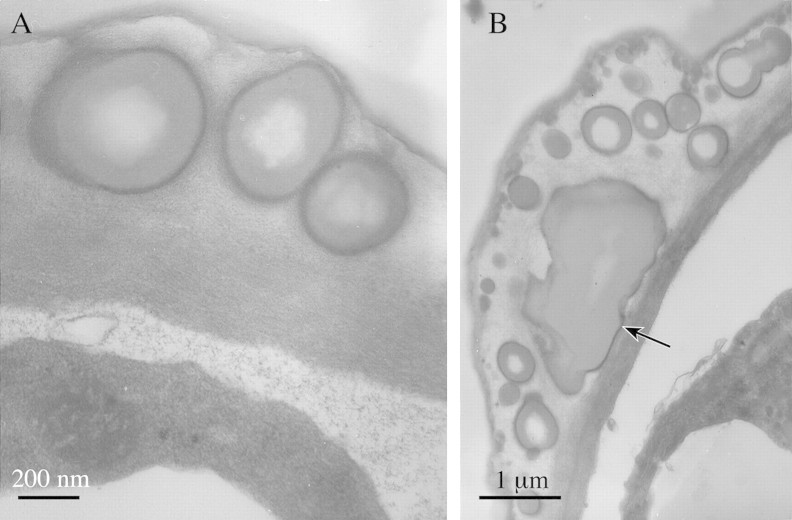
Fig. 4. TEM studies of labellar papillae of M. cf. notylioglossa. A, Smooth endoplasmic reticulum with associated lipid bodies. Bar = 200 nm. B, Plastid with disrupted membrane and plastoglobuli similar to cytoplasmic lipid bodies. Note also the abundant mitochondria with well‐developed cristae. Bar = 200 nm. C, Detail of plastid showing osmiophilic plastoglobuli, faint ‘ghost’ plastoglobuli and larger plastoglobuli with relatively electron‐transparent core and electron‐opaque margin. Bar = 100 nm. D, Smooth endoplasmic reticulum with dilated cisternae and associated lipid bodies. Bar = 200 nm. E, Accumulation of osmiophilic lipid bodies between the cytoplasm and cell wall. Note also the viscid material with globular lipid bodies (left) that is deposited on the labellar surface. Bar = 500 nm.
The viscid secretion on the surface of the cell wall (Figs 3C and 5B) and between the papillae (Fig. 3B) has a heterogeneous composition and consists of a relatively electron‐transparent matrix containing numerous spherical structures resembling the intracellular lipid bodies but often possessing a strongly osmiophilic outer region and an electron‐transparent core (Fig. 5A). These are much larger than the intracellular lipid bodies and have a mean diameter of 560 nm (130 nm–1·44 µm). The smaller globules are closely associated with the wall but they become progressively larger as they are able to coalesce (Fig. 5B). The surface edge of the secretion was also strongly osmiophilic (Fig 5A and B).
Fig. 5. TEM studies of labellar papillae of M. cf. notylioglossa. A, Detail of cell wall and associated viscid secretion. Note the accumulation of flocculent material and vesicles between the cytoplasm and cell wall and also the osmiophilic margin of the viscid secretion. Bar = 200 nm. B, Detail of labellar secretion showing stages in coalescence of lipid globules. Note also that the cuticle seems to be thinner directly beneath the region where secretion is greatest (arrow). Bar = 1 µm.
There is no evidence that papillae possess ectodesmata (Figs 4E and 5A) and no clear indication of how lipid bodies traverse the outer tangential wall and cuticle. However, lipid bodies become associated with the plasmalemma and are often found between the latter and the cell wall (Fig. 4E). Moreover, at intervals, the epidermal cuticle was thinner and this coincided with regions where secretion was visibly greatest (Fig. 5B).
Histochemistry
Labellar secretions of all species were insipid. Histochemical analysis revealed that they had a lipoidal composition in that they selectively stained with Sudan III. In the absence of detailed analytical data, we are reluctant to call these secretions ‘waxes’ in the strict biochemical sense of the term. The viscid material that occurs between the papillae also stained with Sudan III so that cellular outlines of the papillae became increasingly prominent. Globules both on the surface and within the papillae and hairs also stained with this reagent. In addition, the secretions tested positively for aromatic amino acids in all species, but not for starch (Table 1). However, starch was present in minute quantities in the papillae of M. acuminata whereas aromatic amino acids were found in the papillae of all species. Following staining with Sudan III, large, spherical or elliptical idioblasts with raphides were clearly visible in the labellar parenchyma of M. cerifera.
DISCUSSION
Histochemical tests revealed that labellar secretions in all species of the M. acuminata alliance studied, whether they be wax‐like, as in M. cerifera and M. notylioglossa, or the viscid material found in M. cf. notylioglossa and M. acuminata, had a similar chemical composition. In each case, they stained selectively with Sudan III. TEM showed that the labellar secretion of M. cf. notylioglossa has a heterogeneous composition of osmiophilic, spherical bodies embedded in an electron‐transparent matrix. Since osmium tetroxide also selectively stains lipids, it is likely that it is these spherical bodies that stain with Sudan III. Moreover, the extracellular lipid bodies may coalesce to form extensive globules.
It has been suggested that bees collect lipoidal material for nest building (van der Pijl and Dodson, 1969). While this may be true, very little has been published on the matter. However, the xanthoproteic test showed that in all cases the viscid material was also rich in aromatic amino acids and it may be that insects gather this substance for its nutritive value as well. Indeed, von Kirchner (1925) described a similar viscid substance on the labellum of Eria vulpina Rchb.f. It too was insipid, contained no reducing sugars and stained intense yellow with concentrated sulfuric acid and potassium hydroxide solution (compare with xanthoproteic test). It also stained red with Sudan–glycerin, and the edge of the secretion turned black with osmium tetroxide. This last result is comparable with the osmiophilic edge visible in TEM sections through the labellar secretion of M. cf. notylioglossa.
The labellar secretion of representatives of the M. acuminata alliance as well as that of E. vulpina (von Kirchner, 1925) is glossy and it is possible that its reflective nature may be involved in insect attraction much like the speculum of Ophrys spp. (Summerhayes, 1976). However, in Ophrys, the speculum is thought to simulate the folded wings of an insect but we know of no published account of pseudocopulation in Maxillaria. Nevertheless, the glistening labellar secretion could, at least in theory, still attract insects much like the mucilage on the leaves of insectivorous plants such as Drosera and Pinguicula. In the absence of relevant field studies, it remains to be seen whether labellar secretions in the M. acuminata alliance are involved both in attracting and rewarding potential visitors.
Contrary to an earlier SEM study (Senghas, 1993), the outer wall of the labellar papillae of M. cerifera did not show obvious fine sculpturing although in some papillae there was a slight hint that ridges extend from the tip to the base.
In some ways, the secretory labellar cells of M. cf. notylioglossa resemble pseudopollen cells of M. sanderiana (Davies et al., 2000) and nectar‐secreting cells (Stpiczynska, 1997; Stpiczynska and Matusiewicz, 2001) and osmophores (Stpiczynska, 1993, 2001) of orchids in that they possess an extensive ER system with dilated cisternae and numerous mitochondria with well‐developed cristae. However, the ER of the papillae cells studied here is of the smooth type and this is consistent with lipid metabolism. Dictyosomes were absent; this is not surprising since dictyosome activity, at least in nectary cells, is known to subside with the onset of secretion (Durkee, 1983).
Lipid bodies are often intimately associated with and probably formed by the SER. Initially, they are approx. 150 nm (49–375 nm) in diameter. Aggregates of similar osmiophilic bodies, each approx. 150 nm (33–399 nm) in diameter, are often encountered as plastoglobuli within plastids although, by this stage of anthesis, the plastid envelope invariably shows disruption. These plastids, which are considered to be elaioplasts, contain few internal membranes and lack starch grains. However, similar plastids (but with a more obvious profile) have been observed in the nectary cells of Platanthera bifolia L. (Stpiczynska, 1997). Also, starch typically disappears from the plastids of nectar‐secreting cells as secretion progresses (Durkee, 1983).
Osmiophilic bodies were also obvious in the labellar secretion. Similar bodies occur in the cytoplasm where they become associated with the plasmalemma or appear to accumulate between the plasmalemma and cell wall. This type of accumulation has also been observed in cells of the septal nectary of Musa (Fahn, 1979). Many of the extracellular globules have a peripheral electron‐dense region and an electron‐transparent core, much like some of the plastoglobuli, and this may indicate that the chemical composition of the lipid changes prior to secretion. Globules associated with the wall may only be approx. 130 nm in diameter but as they move outwards from the wall they coalesce to form globules measuring up to 1·44 µm in diameter. These may further coalesce to form extensive, irregular lipoidal bodies.
It is not clear how the secretion crosses the cell wall and cuticle. Pits with associated ectodesmata are absent from the outer tangential wall, and this observation confirms the results of an earlier study that showed that wax formed in the epidermal cells is secreted through intact cuticula (Porsch, 1905). It may be that the lipid passes through the wall as small lipid moieties which then reassemble to form larger globules on the surface of the labellum. There is also some indication that the cuticle may be thinner at points coinciding with regions of greatest secretory activity.
Although it has been known for some time that species of the M. acuminata alliance produce wax‐like substances upon their labella (Porsch, 1905) and that these flowers are visited by bees (van der Pijl and Dodson, 1969), no attempt has been made to correlate the orchid and insect species involved. Only when the insect pollinators are identified and their behaviour and life cycles studied will it be possible to fully understand the significance of these labellar secretions.
ACKNOWLEDGEMENTS
The authors are grateful to Drs E. D. L. Schmidt and M. McIllmurray for some of the flowers used in this study.
Supplementary Material
Received: 28 August 2002; Returned for revision: 4 November 2002; Accepted: 19 November 2002 Published electronically: 16 January 2003
References
- AckermanJD.1984. Pollination of tropical and temperate orchids. In: Tan KW, ed. Proceedings of the eleventh world orchid conference. Miami: American Orchid Society, 98–101. [Google Scholar]
- ArdittiJ.1992. Fundamentals of orchid biology. New York: John Wiley & Sons. [Google Scholar]
- BeckG.1914. Die Pollennachahmung in den Blüten der Orchideen‐Gattung Eria. Sitzungs Berichte Akadamie der Wissenschaften in Wien 123: 1033–1046. [Google Scholar]
- BrummittRK, Powell CE.1992. Authors of plant names. Kew: Royal Botanic Gardens, Kew. [Google Scholar]
- DaviesKL, Winters C.1998. Ultrastructure of the labellar epidermis in selected Maxillaria species (Orchidaceae). Botanical Journal of the Linnean Society 126: 349–361. [Google Scholar]
- DaviesKL, Winters C, Turner MP.2000. Pseudopollen: its structure and development in Maxillaria (Orchidaceae). Annals of Botany 85: 887–895. [Google Scholar]
- DaviesKL, Roberts DL, Turner MP.2002a Pseudopollen and food‐hair diversity in Polystachya Hook. (Orchidaceae) Annals of Botany 90: 477–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DaviesKL, Turner MP, Gregg A.2002b Atypical pseudopollen‐forming hairs in Maxillaria Ruiz & Pav. Lindleyana (in press). [Google Scholar]
- DodsonCH.1962. The importance of pollination in the evolution of the orchids of tropical America. American Orchid Society Bulletin 31: 525–534, 641,–649, 731–735. [Google Scholar]
- DodsonCH, Frymire GP.1961. Natural pollination of orchids. Missouri Botanical Garden Bulletin 49: 133–139. [Google Scholar]
- DresslerRL.1993. Phylogeny and classification of the orchid family. Cambridge Massachusetts: Dioscorides Press. [Google Scholar]
- DurkeeLT.1983. Ultrastructure of floral and extrafloral nectaries. In: Bentley B, Elias T, eds. The biology of nectaries. New York: Columbia University Press. [Google Scholar]
- FahnA.1979. Ultrastructure of nectaries in relation to nectar secretion. American Journal of Botany 66: 977–985. [Google Scholar]
- JanseJM.1886. Imitirte pollenkörner bei Maxillaria sp. Deutsche Botanische Gesellschaft Berichte 4: 277–283. [Google Scholar]
- KjellssonG, Rasmussen FN.1987. Does the pollination of Dendrobium unicum Seidenf. involve pseudopollen? Die Orchidee 38: 183–187. [Google Scholar]
- PorschO.1905. Beiträge zur ‘histologischen’ Blütenbiologie I. Österreichische Botanische Zeitschrift 55: 253–260. [Google Scholar]
- PorschO.1908. Neuere Untersuchungen über die Insektenanlockungs mittel der Orchideenblüte. Mittelungen Naturwissenschaftlichen Vereines für Steiermark 45: 346–370. [Google Scholar]
- ProctorM, Yeo P.1975. The pollination of flowers. London: Collins. [Google Scholar]
- ProctorM, Yeo P, Lark A.1996. The natural history of pollination. London: Harper Collins. [Google Scholar]
- PurvisMJ, Collier DC, Walls D.1964. Laboratory techniques in botany. London: Butterworths. [Google Scholar]
- Roberto VásquezC, Dodson CH.1982. Orchids of Bolivia. In: Dodson, CH, ed. Icones Plantarum Tropicarum. Sarasota: The Marie Selby Botanical Gardens, Series 1, Fascicle 6, Plate 5. [Google Scholar]
- RoubikDW.2000. Deceptive orchids with Meliponini as pollinators. Plant Systematics and Evolution 222: 271–279. [Google Scholar]
- SenghasK.1993. Subtribus Maxillariinae. In: Brieger FG, Maatsch R, Senghas K, eds. Rudolf Schlechter: Die Orchideen. Berlin: Blackwell Wissenschafts‐Verlag, 1766. [Google Scholar]
- StpiczynskaM.1993. Anatomy and ultrastructure of osmophores of Cymbidium tracyanum Rolfe (Orchidaceae). Acta Societatis Botanicorum Poloniae 62: 5–9. [Google Scholar]
- StpiczynskaM.1997. The structure of the nectary of Platanthera bifolia L. (Orchidaceae). Acta Societatis Botanicorum Poloniae 66: 5–11. [Google Scholar]
- StpiczynskaM.2001. Osmophores of the fragrant orchid Gymnadenia conopsea L. (Orchidaceae). Acta Societatis Botanicorum Poloniae 70: 91–96. [Google Scholar]
- StpiczynskaM, Matusiewicz J.2001. Anatomy and ultrastructure of the spur nectary of Gymnadenia conopsea L. (Orchidaceae). Acta Societatis Botanicorum Poloniae 70: 267–272. [Google Scholar]
- SummerhayesVS.1976. Wild orchids of Britain. London: Collins. [Google Scholar]
- van der PijlL, Dodson CH.1969. Orchid flowers: their pollination and evolution. Coral Gables, Florida: University of Miami Press. [Google Scholar]
- von KirchnerO.1925. Über die sogenannten Pollenblumen und die Ausbeutestoffe der Blüten. Flora 118/119: 312–330. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.