Abstract
Essential oils obtained from different populations of Viola etrusca from Italy have been analysed to verify the phenotypic discontinuity observed in a previous study. All of the essential oils contained methyl salicylate as a main constituent. However, multivariate analysis showed differences among some populations, in particular between northern and southern ones. Results suggest that this species could be undergoing a slow schizogenetic differentiation process due to its genetic isolation.
Key words: Viola etrusca, Violaceae, essential oil variability, GC‐MS, multivariate analysis
INTRODUCTION
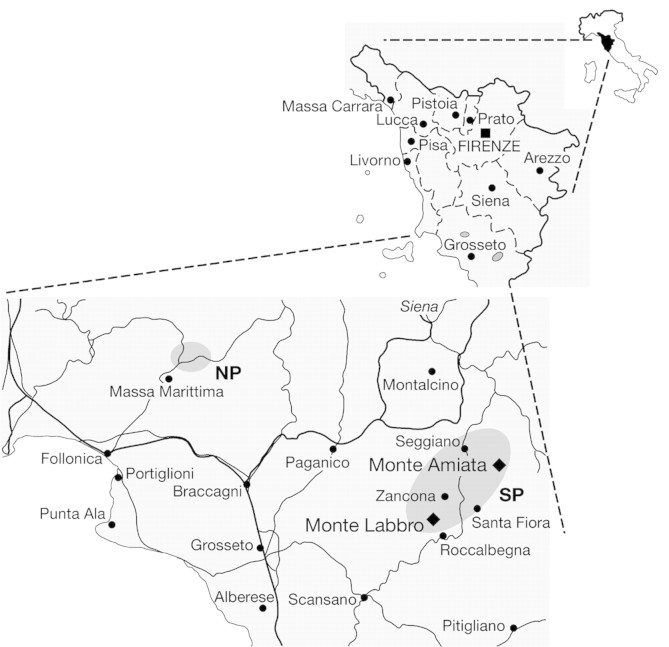
Viola etrusca Erben is endemic to south Tuscany (Italy), having two separate groups of populations: a northern group on Colline Metallifere, and a southern one on Monte Amiata and Monte Labbro (Fig. 1). The two groups are separated by approx. 60 km of fluvial plains and lowlands. Together with other Viola species, V. etrusca is used in local folk medicine as a diuretic, diaphoretic, laxative and emollient (Mazza, 2000).
Fig. 1. Populations of Viola etrusca. NP, Northern populations; SP, southern populations.
The great variability of the Viola species of the series Calcaratae has resulted in many controversial interpretations of the various populations which are known today as V. etrusca; in fact, these species have undergone many revisions and misapplied names: V. gigantea, V. bertolonii, V. hetrophylla, V. gracilis, V. grandiflora and V. calcarata var. grandiflora (Santi, 1795; Caruel, 1860; Arcangeli, 1889; Bertoloni, 1889; Terracciano, 1889; Parlatore, 1890; Fiori, 1924; Valentine et al., 1968; Zangheri, 1976). Finally, Erben (1986) described the entity as V. etrusca. Recently, Foggi et al. (1993) confirmed the study of Erben by carrying out a chorological and karyological investigation of the plants, demonstrating also that both groups of populations have the same chromosome number.
In this genus, volatile compounds have been studied only in V. odorata (Werkhoff et al., 1990; Cu, 1992), which contains mainly non‐terpenic hydrocarbons, alcohols and aldehydes. Only one essential oil composition, and an in vivo SPME (solid phase micro‐extraction) analysis of a sample from Monte Labbro, have been reported in the literature (Flamini et al., 2002). This paper describes the variability of the essential oil obtained from different populations collected both from the northern and the southern part of the range. Moreover, because of the phenotypic discontinuity between the two populations observed by Selvi et al. (1995), a multivariate analysis using chemical variables was carried out to verify this discontinuity.
MATERIALS AND METHODS
Field sampling
The northern part of the range is represented by two populations, at Montieri and Cornate, whereas eight populations were sampled from the larger Amiata‐Labbro southern area, namely Altore, Capo Vetra, Aia dei Venti and Sasso dei Tre Confini from the trachitic volcanic dome of Monte Amiata, and Aquilaia, Poggio Fabbrazzoni, Poggio le Forche and Poggio dell’Allodola from the calcareous soils of Monte Labbro. Groups of individuals 2 km apart were considered to be distinct populations, as defined by Selvi et al. (1995). There were no problems in the spatial delimitation of populations since individuals were grouped in dense clumps; Selvi et al. (1995) attributed this to being the result of entomophilous pollination and rhizome propagation.
Essential oil analyses
Fresh flowering aerial parts (100 g) of V. etrusca were coarsely ground and hydrodistilled in a Clevenger‐like apparatus for 2 h. GC‐analyses were accomplished using an HP‐5890 Series II instrument equipped with HP‐WAX and HP‐5 capillary columns (30 m × 0·25 mm, 0·25 µm film thickness), working with the following temperature programme: 60 °C for 10 min, ramp of 5 °C min–1 up to 220 °C; injector and detector temperatures 250 °C; carrier gas nitrogen (2 ml min–1); detector dual FID; split ratio 1 : 30; injection of 0·5 µl). For both columns, components were identified by comparing their retention times with those of pure authentic samples and by means of their linear retention indices (l.r.i.) relative to the series of n‐hydrocarbons. The relative proportions of the essential oil constituents were percentages obtained by FID peak‐area normalization, all relative response factors being taken as one.
GC/EIMS (Electronic Ionization Mass Spectrometry) analyses were performed using a Varian CP‐3800 gas chromatograph equipped with a DB‐5 capillary column (30 m × 0·25 mm; coating thickness 0·25 µm) and a Varian Saturn 2000 ion trap mass detector. Analytical conditions were as follows: injector and transfer line temperatures 220 and 240 °C, respectively; oven temperature programmed from 60 to 240 °C at 3 °C min–1; carrier gas helium at 1 ml min–1; injection of 0·2 µl (10 % hexane solution); split ratio 1 : 30. Identification of constituents was based on comparison of the retention times with those of authentic samples, comparing their linear retention indices relative to the series of n‐hydrocarbons, and on computer matching against commercial (NIST 98 and ADAMS 95) and home‐made library mass spectra built up from pure substances and components of known oils and MS literature data (Stenhagen et al., 1974; Massada, 1976; Jennings and Shibamoto, 1980; Swigar and Silverstein, 1981; Davies, 1990; Adams, 1995). Moreover, molecular weights of all the substances identified were confirmed by GC/CIMS (Chemical Ionization Mass Spectrometry), using MeOH as the CI ionizing gas. All analyses were performed in triplicate.
RESULTS AND DISCUSSION
Analysis of the essential oils identified 107 constituents, accounting for 83·31–99·94 % of the total oils (only compounds >0·1 % are reported in Table 1). All of the populations had essential oils with a very high methyl salicylate content (Table 1): in samples collected from Aquilaia and Aia dei Venti, methyl salicylate constituted about 75 % of the total oil; in those from Altore and Poggio le Forche it represented between 80 and 90 % of the total oil; and in samples from Montieri, Cornate, Poggio dell’Allodola, Capo Vetra, Sasso dei Tre Confini and Poggio Fabbrazzoni it exceeded 90 %. Besides methyl salicylate, other compounds were present in smaller amounts. Almost all of the populations produced many non‐terpenic aldehydes, such as heptanal, (E)‐2‐heptenal, benzaldehyde, phenylacetaldeyde, nonanal, (E,Z)‐2,6‐nonadienal, (E)‐2‐nonenal and (E)‐2‐decenal. Many common monoterpenes were present, but normally in very small amounts (less than 0·1 %). Among these compounds, α‐pinene, camphene, β‐pinene, limonene, 1,8‐cineole, camphor and 4‐terpineol were most frequently represented. Sesquiterpenes were numerically more abundant than monoterpenes, with the most common being β‐caryophyllene, α‐humulene, farnesene, germacrene D, bicyclogermacrene and caryophyllene oxide.
Table 1.
Essential oil composition* of different populations of Viola etrusca
| Constituents | l.r.i.† | Montieri | Cornate | Allodola | Aquilaia | Altore | Vetra | Fabbraz. | Confini | Forche | Venti |
| (E)‐3‐Hexen‐1‐ol | 852 | 0·31 | 0·27 | 0·32 | 0·28 | – | 0·36 | 0·34 | 0·10 | 0·55 | 0·36 |
| (E)‐2‐Hexenal | 854 | – | – | – | – | 0·86 | – | – | – | – | – |
| Hexanol | 869 | – | – | – | – | – | 0·35 | – | – | – | – |
| Nonane | 900 | – | – | – | – | – | 0·16 | – | – | – | – |
| (Z)‐4‐Heptenal | 902 | – | – | – | – | 0·11 | – | – | – | – | – |
| Heptanal | 903 | – | – | – | – | 0·76 | 0·26 | – | – | 0·15 | 0·10 |
| α‐Pinene | 941 | 0·29 | 0·31 | – | 2·15 | 0·70 | – | 0·62 | – | 2·26 | – |
| Camphene | 955 | – | – | – | 0·82 | 0·10 | – | – | – | 0·11 | – |
| Benzaldehyde | 963 | – | – | – | – | 0·17 | – | 0·12 | – | 0·11 | – |
| (E)‐2‐Heptenal | 978 | – | – | – | – | 0·21 | 0·11 | 0·16 | – | – | – |
| 1‐Octen‐3‐ol | 980 | – | – | 0·10 | – | – | – | – | – | – | – |
| β‐Pinene | 982 | 0·26 | 0·29 | – | 1·46 | 0·65 | – | 0·27 | – | 0·18 | – |
| 6‐Methyl‐5‐hepen‐2‐one | 987 | – | – | – | – | 0·15 | – | – | – | – | – |
| Myrcene | 991 | – | – | – | – | – | – | 0·28 | – | – | – |
| 2‐Pentyl furane | 993 | – | – | 0·11 | 0·64 | 1·50 | 0·46 | – | – | 0·36 | 0·18 |
| 6‐Methyl‐5‐hepen‐2‐ol | 994 | – | – | 0·13 | 0·90 | 2·08 | 1·07 | 0·27 | – | 0·82 | 0·49 |
| Octanal | 1003 | 0·17 | 0·15 | – | 0·15 | 0·24 | 0·10 | – | – | – | – |
| p‐Cymene | 1028 | – | – | – | 0·26 | – | – | – | – | – | – |
| Limonene | 1033 | 0·10 | 0·11 | – | 1·37 | 0·19 | – | – | – | 0·13 | – |
| 1,8‐Cineole | 1035 | 1·53 | 1·63 | – | 7·29 | 1·13 | – | – | – | – | – |
| Phenylacetaldehyde | 1045 | – | – | – | – | 0·29 | 0·15 | 0·19 | – | 0·14 | – |
| γ‐Terpinene | 1064 | – | – | – | 0·15 | – | – | – | – | – | – |
| Undecane | 1100 | – | – | 0·10 | – | – | – | – | – | – | – |
| Nonanal | 1104 | – | 0·26 | 0·25 | 1·43 | 2·44 | 1·07 | 1·37 | 0·53 | 0·94 | 0·46 |
| trans‐Pinocarveol | 1141 | – | – | – | – | – | – | – | – | 0·39 | – |
| cis‐Verbenol | 1142 | – | – | – | – | – | – | – | – | 0·30 | – |
| Camphor | 1145 | – | 0·38 | – | 1·63 | 0·18 | – | – | – | – | – |
| (E,Z)‐2,6‐Nonadienal | 1158 | – | – | – | 0·60 | 1·55 | – | – | – | 0·22 | 0·26 |
| (E)‐2‐Nonenal | 1165 | – | – | – | 0·18 | 0·83 | – | – | – | 0·11 | – |
| Pinocarvone | 1166 | – | – | – | – | – | – | – | – | – | 0·10 |
| Borneol | 1167 | 1·27 | 0·27 | – | – | 0·20 | 0·11 | – | – | – | 0·18 |
| 4‐Terpineol | 1179 | 0·21 | – | – | – | – | – | – | – | – | – |
| Methyl salicylate | 1192 | 93·24 | 90·44 | 95·99 | 75·04 | 80·88 | 92·21 | 77·42 | 90·52 | 87·10 | 92·91 |
| Decanal | 1206 | – | – | – | – | – | 0·14 | – | – | – | – |
| (E)‐2‐Decenal | 1263 | – | – | – | – | – | 0·10 | – | – | – | – |
| Ethyl salicylate | 1269 | – | – | – | – | – | – | – | – | 0·16 | – |
| Isobornyl acetate | 1286 | 0·33 | – | – | – | – | – | – | – | – | – |
| α‐Terpinyl acetate | 1351 | – | – | – | – | 0·12 | 0·10 | – | – | – | – |
| Methyl eugenol | 1402 | – | – | – | – | – | – | – | 0·14 | – | – |
| Longifolene | 1404 | 0·12 | – | – | – | 0·16 | 0·35 | – | – | – | – |
| β‐Caryophyllene | 1420 | 0·49 | – | – | – | 0·15 | 0·12 | 0·27 | 0·79 | 0·16 | 0·7 |
| (E)‐Geranylacetone | 1454 | – | – | – | – | – | – | – | 0·17 | – | – |
| α‐Humulene | 1456 | – | – | – | – | – | – | – | 0·47 | – | 0·10 |
| (E)‐β‐Farnesene | 1459 | – | – | – | – | – | – | – | 0·40 | – | – |
| Farnesane | 1462 | – | 0·45 | – | 0·50 | 0·36 | 1·66 | 0·64 | 0·93 | 1·18 | 0·4 |
| γ‐Muurolene | 1478 | – | – | – | – | – | – | – | – | – | 0·16 |
| γ‐Himachalene | 1479 | – | – | – | – | – | – | – | 0·71 | – | – |
| Germacrene D | 1482 | 0·36 | – | – | 0·80 | – | 0·25 | 0·57 | 1·69 | 0·29 | 1·66 |
| Bicyclogermacrene | 1496 | – | – | – | 0·21 | – | 0·14 | 0·16 | 0·32 | – | – |
| (E,E)‐α‐Farnesene | 1509 | – | – | – | 0·11 | – | – | – | – | – | – |
| trans‐γ‐Cadinene | 1514 | – | – | – | – | – | – | – | – | – | 0·10 |
| δ‐Cadinene | 1525 | – | – | – | – | – | – | – | 0·39 | – | 0·26 |
| (Z)‐3‐Hexenyl benzoate | 1572 | – | – | – | – | – | – | – | – | 0·15 | – |
| Spathulenol | 1578 | – | – | – | – | – | – | – | 0·20 | – | – |
| Caryophyllene oxide | 1582 | 0·16 | – | – | – | – | – | – | – | – | 0·16 |
| Hexahydrofarnesylacetone | 1843 | – | – | – | 0·17 | – | – | – | – | 0·22 | – |
| Nonadecane | 1900 | – | – | – | – | – | – | – | – | 0·26 | 0·12 |
| Manoyl oxide | 1990 | – | – | – | – | – | – | – | 1·78 | 0·85 | – |
| epi‐13‐Manoyl oxide | 2011 | – | – | – | – | – | – | – | 0·16 | – | – |
| Eneicosane | 2100 | – | – | – | – | – | – | – | – | 0·16 | 0·10 |
Only compounds >0·1 % are reported.
* Percentages obtained by FID peak‐area normalization, all relative response factors being taken as one (HP‐5 column). Mean of three analyses.
† Linear retention indices (HP‐5 column).
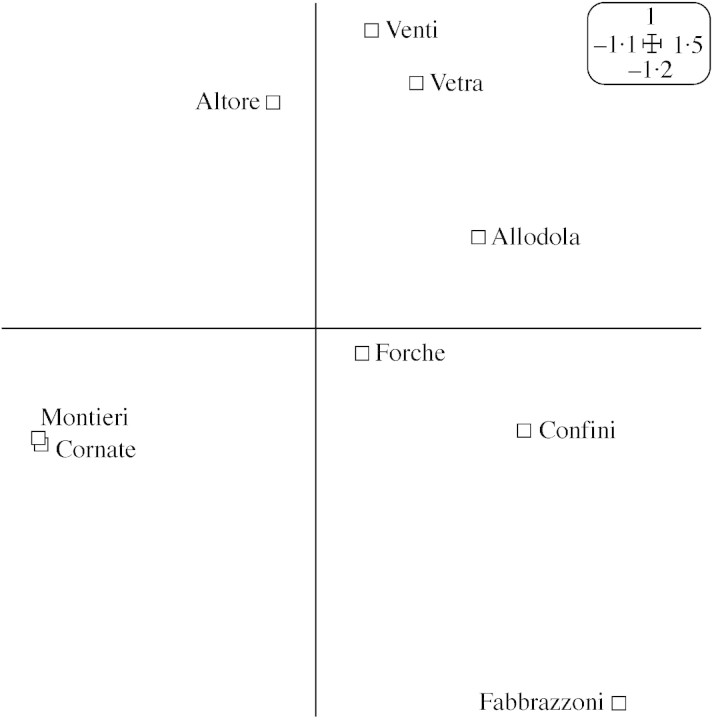
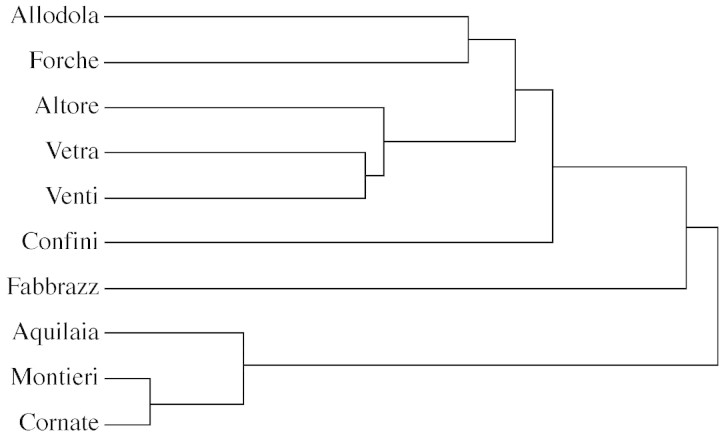
To evaluate whether the essential oil constituents identified reflected ecological relationships among the different populations, components of all the essential oils were subjected to principal component analysis (PCA) and cluster analysis. In PCA (Fig. 2), the horizontal axis explained about 46 % of the total variance, and the vertical axis a further 37 %. In the absence of information about the shape of the groupings and/or statistical distribution of values, Euclidean distances combined with unweighted pair group average linking were used for cluster analysis (Dunlop et al., 1997). The resulting dendrogram is presented in Fig. 3.
Fig. 2. PCA analysis of the different populations of Viola etrusca.
Fig. 3. Cluster analysis of the different populations of Viola etrusca.
As is evident from these statistical analyses, the northern populations of V. etrusca stand out clearly, forming a separate group in PCA and a deep dicotomy in cluster analysis. Only the sample from Aquilaia, one of the southern populations, showed any affinity with samples from the northern populations, in agreement with a phenotypic investigation (Selvi et al., 1995) in which an unspecified southern population showed the same clustering. It is difficult to conclude whether the southern Aquilaia population is atypical or whether other members of the southern populations could show similar behaviour. The former hypothesis seems to be the most probable, as the northern populations grow in a very small area, spanning a few square kilometres. Other samples collected in this area, which were sampled at less than the stipulated 2 km apart, showed almost identical essential oil compositions, with distinct affinities to the Montieri or to the Cornate populations.
The dendrogram and PCA (Figs 2 and 3) confirmed the observations of Selvi et al. (1995) on the effect of calcareous and acidic substrates. Among the southern populations, there were no clear distinctions between the western populations growing on calcareous soil and the eastern ones growing on trachitic soil; there was a clustering pattern, but the members of the two subgroups were not consistently divided.
The Viola etrusca populations growing in Montieri (northern population) and on the volcanic dome in Amiata (eastern southern population) grow in very similar environments, namely on the edges of chestnut forests on siliceous soil. Conversely, populations in Cornate (northern population) and Monte Labbro (western southern population) grow on arid and calcareous soil. If environmental factors were the only agents that induced chemical convergence in essential oil composition, then cross‐similarities should be observed instead of the geographical differentiation between northern and southern populations (i.e. Amiata and Montieri populations should have very similar essential oil compositions). Thus this work confirms the hypothesis of Selvi et al. (1995) that this species could be undergoing slow schizogenetic differentiation due to its genetic isolation. Schizogenesis followed by hybridization is considered the main mechanism of speciation in Viola sect. Melanium (Küpfer, 1971).
ACKNOWLEDGEMENT
This paper is dedicated to the memory of Professor Serena Catalano, 1945–2002.
Supplementary Material
Received: 18 September 2002; Returned for revision: 14 October 2002; Accepted: 22 November 2002 Published electronically: 16 January 2003
References
- AdamsRP.1995. Identification of essential oil components by gas chromatography/mass spectroscopy. Carol Stream, NC, USA: Allured. [Google Scholar]
- ArcangeliG.1889. Sopra alcune piante raccolte al Monte Amiata. Nuovo Giornale Botanico Italiano 21: 119. [Google Scholar]
- BertoloniA.1889. Flora Italica. Bologna, Italy: Masi. [Google Scholar]
- CaruelT.1860. Prodromo della flora toscana. Firenze, Italy: Le Monnier. [Google Scholar]
- CuJQ.1992. Volatile components of violet leaves. Phytochemistry 31: 571–573. [Google Scholar]
- DaviesNW.1990. Gas chromatographic retention indexes of monoterpenes and sesquiterpenes on methyl silicone and Carbowax 20M phases. Journal of Chromatography 503: 1–24. [Google Scholar]
- DunlopPJ, Bignell CM, Hibbert DB.1997. Use of gas chromatograms of the essential leaf oils of the genus Eucalyptus for taxonomic purposes. Australian Journal of Botany 45: 1–13. [Google Scholar]
- ErbenM.1986. Viola acrocerauniensis und Viola etrusca Zwei neue Viola arten aus der section Melanium Mitteilungen der Botanische Staatssammlung München 22: 493–506. [Google Scholar]
- FioriA.1924. Nuova Flora Analitica Italiana Bologna: Edagricole, Ristampa anastatica 1974. [Google Scholar]
- FlaminiG, Cioni PL, Morelli I.2002. Analysis of the essential oil of the aerial parts of Viola etrusca from Monte Labbro (South Tuscany, Italy) and in vivo analysis of flower volatiles using SPME. Flavour and Fragrance Journal 17: 147–149. [Google Scholar]
- FoggiB, Di Fazio L, Selvi F, Clauser M.1993. Chorological and caryological investigation on Viola etrusca Erben (Violaceae). Giornale Botanico Italiano 127: 755–763. [Google Scholar]
- JenningsW, Shibamoto T.1980. Qualitative analysis of flavor and fragrance volatiles by glass capillary chromatography. New York: Academic Press. [Google Scholar]
- KüpferPM.1971. Contribution à l’étude cytologique et phylogénétique de la section Melanium du genre Viola L. Comptes Rendus de l’Académie des Sciences 272: 1085–1088. [Google Scholar]
- MassadaY.1976. Analysis of essential oils by gas chromatography and mass spectrometry. New York: J. Wiley & Sons. [Google Scholar]
- MazzaF.2000. Itinerari alla Scoperta delle Erbe Officinali del Monte Amiata Abbadia S. Salvatore, Siena, Italy: Stampa 2000. [Google Scholar]
- ParlatoreF.1890. Flora Italiana. Firenze, Italy: Le Monnier. [Google Scholar]
- SantiG.1795. Viaggio al Montamiata. Pisa, Italy: Prosperi. [Google Scholar]
- SelviF, Foggi B, Di Fazio L.1995. Patterns of phenotypic variation in Viola etrusca Erben (Violaceae). Candollea 50: 309–319. [Google Scholar]
- StenhagenE, Abrahamsson S, McLafferty FW.1974. Registry of mass spectral data. New York: J. Wiley & Sons. [Google Scholar]
- SwigarAA, Silverstein RM.1981. Monoterpenes. Milwaukee, WI, USA: Aldrich Chemical Company. [Google Scholar]
- TerraccianoA.1889. Le viole italiane spettanti alla sezione Melanium D.C. Appunti di studi filogenetico‐sistematici. Nuovo Giornale Botanico Italiano 21: 320–331. [Google Scholar]
- ValentineDH, Merzmüller H, Schmidt A.1968. Genus Viola In: Tutin TG, eds. Flora Europaea. Cambridge: Cambridge University Press. [Google Scholar]
- WerkhoffP, Bretschneider W, Guentert M, Hopp R, Surburg H.1990. Chiral analysis in flavor and essential oil chemistry. Part B. Direct enantiomer resolution of (E)‐α‐ionone and (E)‐α‐damascone by inclusion gas chromatography. 6th Flavour Science and Technology Symposium, Chichester, UK. [Google Scholar]
- ZangheriP.1976. Flora Italica. Padova, Italy: Cedam. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.