Abstract
Human activities are causing widespread changes in the species composition of natural and managed ecosystems, but the consequences of these changes are poorly understood. This paper presents a conceptual framework for predicting the ecosystem and regional consequences of changes in plant species composition. Changes in species composition have greatest ecological effects when they modify the ecological factors that directly control (and respond to) ecosystem processes. These interactive controls include: functional types of organisms present in the ecosystem; soil resources used by organisms to grow and reproduce; modulators such as microclimate that influence the activity of organisms; disturbance regime; and human activities. Plant traits related to size and growth rate are particularly important because they determine the productive capacity of vegetation and the rates of decomposition and nitrogen mineralization. Because the same plant traits affect most key processes in the cycling of carbon and nutrients, changes in plant traits tend to affect most biogeochemical cycling processes in parallel. Plant traits also have landscape and regional effects through their effects on water and energy exchange and disturbance regime.
Key words: Review, biomass, climate change, decomposition, ecosystem processes, energy exchange, functional types, global change, nitrogen mineralization, plant traits, productivity
INTRODUCTION
One of the greatest challenges facing humanity is to understand the mechanisms by which human activities are altering the environment and biota of our planet. Only through this understanding can we develop plausible projections of future changes and their impacts on society.
Many of the impacts of human activities are well documented and understood (Vitousek et al., 1997). Fossil fuel combustion and deforestation have increased concentrations of carbon dioxide by 30 % in the past three centuries (more than half of this in the last 40 years). We have more than doubled the concentration of methane and increased concentrations of other gases that contribute to climate warming, with the prospect that climate will change more rapidly than at any time since the end of the last glaciation 18 000 years ago. Industrial fixation of nitrogen for fertilizer and other human activities has more than doubled the rates of terrestrial fixation of gaseous nitrogen into biologically available forms. Humans have transformed 40–50 % of the ice‐free land surface and use about one‐third of terrestrial net primary productivity. We use 54 % of the available fresh water, with use projected to increase to 70 % by 2050. Finally, the mobility of people has transported organisms across geographic barriers that long kept the biotic regions of the earth separated. Consequently, over broad areas, many of the ecologically important plant and animal species have been introduced in historic time.
The most irreversible human impact has been the extinction of species. We are in the midst of the sixth major extinction event in the history of life on Earth. This extinction event is unique because it is biologically driven by human activities, in contrast to earlier extinction events that were caused by asteroid impacts or other physical events. Already we have caused the extinction of 5–20 % of the species in many groups of organisms, and current rates of extinction are estimated to be 100 to 1000 times greater than pre‐human rates (Pimm et al., 1995). Humanity is conducting this unprecedented global experiment without a clear understanding of its consequences.
In the absence of major changes in policy and human behaviour, our effects on the environment will continue to alter biodiversity. An analysis of principal causes of biodiversity change in the major biomes on Earth suggests that land‐use change will continue to have the largest global impact on biodiversity in the current century, followed by climate change, nitrogen deposition, species introductions and changing concentrations of atmospheric CO2 (Sala et al., 2000). Land‐use change is expected to be of particular importance in the tropics, climatic change at high latitudes and a multitude of interacting causes in other biomes.
An important reason for concern about the changes in abundance and diversity of organisms is that species differ substantially in their effects on ecosystem processes. These species effects are often as strong as, or stronger than, the direct effects of environment on ecosystems (Flanagan and Van Cleve, 1983). Organisms seldom affect a single process in isolation. Instead, effects often cascade through a broad range of ecosystem processes, due to the tight linkages among carbon, nutrient and water cycles of ecosystems (Tateno and Chapin, 1997). Species also influence landscape processes through their effects on the spread of materials, organisms, or disturbances among patches on the landscape. An improved understanding of the effects of organisms on ecosystem, landscape and regional processes is therefore essential to the development of policies that mitigate undesirable future changes in our planet.
In this paper, I present a general framework for understanding the mechanisms by which the traits characteristic of particular plant species or functional types affect ecosystem and regional processes. I emphasize those effects that influence processes at landscape and regional scales. These large‐scale effects of organisms should be incorporated into process‐based projections of the state of our planet in the coming decades to centuries.
Species traits are the attributes that most directly affect ecosystem processes. However, when examining evidence for the importance of these traits, it is virtually impossible to separate the effects of an individual trait from the effects of the species that has these traits. Because it is the abundance of individual organisms, rather than an individual trait, that changes with altered biodiversity, most of the evidence for impact of species traits on ecosystem processes comes from studies in which species composition differs among communities.
STATE FACTORS AND INTERACTIVE CONTROLS
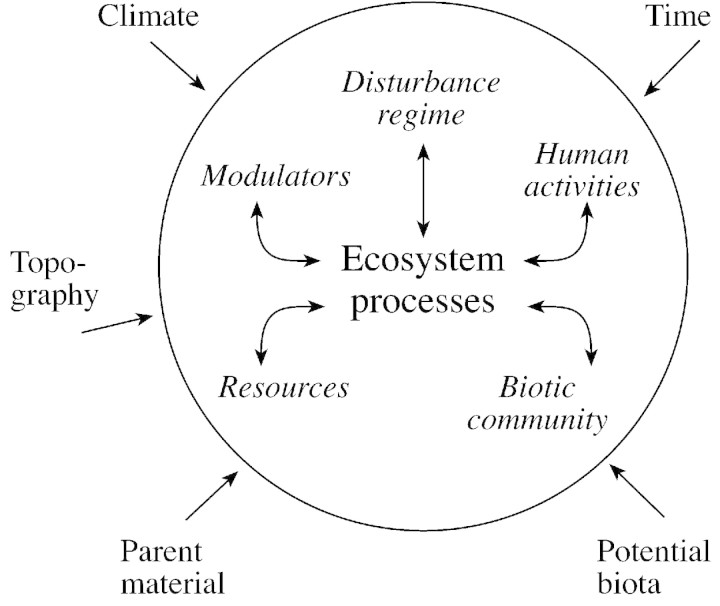
Dokuchaev (1879) and Jenny (1941) proposed that five independent state factors (climate, potential biota, parent material, topography and time) govern the properties of soils and ecosystems. The effects of these independent variables on ecosystems depend on the interactions among several interactive controls that both affect, and respond to, ecosystem processes (Field et al., 1992; Chapin et al., 1996) (Fig. 1). These interactive controls include the functional types of organisms that occupy the ecosystem, the resources (water, nutrients, oxygen, etc.) that are used by organisms to grow and reproduce, modulators (e.g. temperature and pH) that influence the activity of organisms but are not consumed by them, disturbance regime and human activities. These interactive controls respond dynamically to any external change in state factors and to any change in other interactive controls. The composition of a plant community, for example, is influenced both by the global changes in climate and regional biota (state factors) and by nitrogen deposition, livestock density, fire suppression and timber harvest (interactive controls). Many of the resulting changes in the characteristics of the plant community cause further changes in other interactive controls, including the ecosystem goods and services that benefit society. The dynamic interplay among changes in interactive controls governs the transient dynamics of ecosystems in a globally changing environment (Fig. 1).
Fig. 1. The relationship between state factors (outside the circle), interactive controls (inside the circle) and ecosystem processes. The circle represents the boundary of the ecosystem. Reprinted from Chapin et al. (2002), with the permission of Springer‐Verlag.
A FRAMEWORK FOR PREDICTING STRONG EFFECTS OF PLANT SPECIES
Because interactive controls are the factors that directly regulate ecosystem processes, species that alter these controls generally have strong effects on ecosystems. This provides a framework for predicting which species traits (or species) have the strongest effects on ecosystem processes.
Resource supply
The supply of resources required for plant growth is one of the interactive controls to which ecosystem processes are most sensitive. For this reason, ecosystem processes are often affected by the introduction or loss of species that alter resource supply. The introduction of the nitrogen‐fixing tree Myrica faya to nitrogen‐limited ecosystems in Hawaii, for example, led to a five‐fold increase in nitrogen inputs to the ecosystem, which in turn changed net primary production (NPP), species composition, and most other functional and structural properties of native forests (Vitousek et al., 1987). Nitrogen‐poor ecosystems are generally most vulnerable to invasion by nitrogen‐fixing exotic species because of the competitive advantage of these species under high‐light, low‐nitrogen conditions (Vitousek and Howarth, 1991). Because nitrogen fixation is recognized as a trait that enhances the supply of the most frequently limiting nutrient, introduction of nitrogen‐fixing species is a management tool that is often used to enhance the productive potential of degraded lands (Bradshaw, 1983).
Species also affect the resource base of an ecosystem by determining the volume of soil exploited. Eucalyptus forests and annual grasslands are two ecosystems that occur adjacent to one another across broad areas of Mediterranean California. Both vegetation types became established in about 1850 and now occupy slopes with similar climate, topography, parent material and potential colonizing biota. The two ecosystems are alternative stable states in which the eucalyptus forest is more deeply rooted, accesses more growth‐limiting resources, such as water and nutrients, and consequently supports greater leaf area and NPP (Robles and Chapin, 1995). Other deep‐rooted trees such as salt cedar (Tamarix) in the North American deserts have caused similar increases in productivity and alter many other ecosystem processes.
Differences between species in the timing of resource capture can also alter the pool of resources captured annually to support primary production. In mixed grasslands, for example, C4 species are generally active in the warmer, drier part of the growing season than are C3 species. Consequently, C3 species account for most early‐season production, C4 species account for most late‐season production, and the overall production is probably greater than if either C3 or C4 species were absent from the ecosystem.
Litter quality, which influences the turnover rate of nutrients in litter and soil organic matter, is perhaps the best documented mechanism by which species alter resource supply rate (Melillo et al., 1982; Hobbie, 1992). Litter from low‐nutrient‐adapted species decomposes slowly because of the negative effects on soil microbes of low concentrations of nitrogen and phosphorus and high concentrations of lignin, tannins, waxes and other recalcitrant or toxic compounds. This slow decomposition of litter from species characteristic of nutrient‐poor sites reinforces the low nutrient availability of these sites (Hobbie, 1992; Wilson and Agnew, 1992). In contrast, species from high‐resource sites produce rapidly decomposing litter owing to their higher nitrogen and phosphorus content and fewer recalcitrant compounds. This enhances rates of nutrient turnover in nutrient‐rich sites. These effects of litter quality on resource supply are most obvious in experimental gardens, where species with high litter quality enhance rates of nitrogen mineralization and therefore the nitrogen available to support production (Wedin and Tilman, 1990). Species differ in other traits that influence nutrient turnover (e.g. exudation rate and allocation), but the effects of these traits on nutrient supply are less well documented and appear less tightly linked to generalized suites of traits (Eviner and Chapin, 2003a).
In waterlogged soils, plants strongly influence soil oxygen availability by transporting oxygen through aerenchyma to soils (Armstrong et al., 1991). In these environments oxygen is a limiting resource for microbes and governs rates of decomposition, methane production and other processes (Verville et al., 1998).
Modulators
Traits of the dominant species in ecosystems strongly affect the microclimate of ecosystems because plants are located at the land–atmosphere interface, where water and energy exchange occur. Solar radiation is a directional resource that governs the energy input to ecosystems. The albedo (shortwave reflectance) of the vegetation–soil surface determines the proportion of incoming radiation that is absorbed, which in turn influences the quantity of heat that enters the soil and the energy available to drive water loss from the ecosystem through transpiration and soil evaporation. Forests dominated by conifers have a lower albedo (i.e. absorb more energy) than do deciduous‐dominated forests because of the low albedo of conifer leaves. In addition, the complex structure of conifer canopies and clumped distribution of leaves causes much of the reflected radiation in these forests to be absorbed by other leaves rather than being reflected directly back to space (Baldocchi et al., 2000; Chapin et al., 2000b).
Plant traits of the dominant species also influence the nature of energy transfer from the land surface to the atmosphere as a result of partitioning between sensible and latent heat fluxes. Sensible heat directly warms the air at the ecosystem–atmosphere boundary, where it affects all temperature‐sensitive processes in the ecosystem. Latent heat flux transfers heat and water vapour to the atmosphere. When water vapour condenses, it generates precipitation and heats the air in ways that influence downwind ecosystems, as described later. The size and density of tall plants govern surface roughness, which determines boundary layer (or aerodynamic) conductance, i.e. the efficiency with which energy is transferred from the vegetation surface to the atmosphere (Kelliher et al., 1995; Chapin et al., 2002). Rough canopies generate mechanical turbulence, allowing eddies of air from the bulk atmosphere to penetrate deep within the plant canopy. This efficiently carries water vapour and heat from the ecosystem to the atmosphere, minimizing surface build‐up of heat and water vapour. In contrast, short smooth canopies, such as those of crops or grasslands, exhibit more laminar flow of air across the surface, resulting in less efficient surface exchange and a build‐up of heat and water vapour at the surface. In this way the structural attributes of the dominant species in an ecosystem strongly influence surface temperature, humidity and water loss.
Species effects on microclimate have greatest impact in extreme environments, where ecosystem processes are particularly sensitive to climate (Wilson and Agnew, 1992; Hobbie, 1995). Arctic and boreal mosses, for example, are effective insulators that minimize heat transfer from the soil surface to the underlying soil. The resulting low soil temperature impedes decomposition and contributes to the low rates of nutrient cycling that characterize these ecosystems. In deserts, many plants germinate and establish in the shade of nurse plants that reduce surface temperature extremes. Plants indirectly influence microclimate through the quantity and structure of the litter layer. Grasses, for example, produce an insulative thatch that reduces the average temperature and the magnitude of temperature fluctuations in surface mineral soils (Cater and Chapin, 2000; Eviner and Chapin, 2001).
The chemical properties of plant litter and exudates strongly influence many chemical properties of soils that are critical to ecosystem functioning. Soil pH, for example, is generally lower beneath conifer plantations than beneath deciduous trees and influences availability of phosphorus and many other soil processes (Eviner and Chapin, 2003a). Chelates produced by plant roots also affect soil chemistry in ways that enhance the availability of phosphorus and other insoluble nutrients.
Disturbance regime
Disturbances such as fire, flooding, hurricanes and gophers radically alter all ecosystem processes by changing the balance between equilibrium processes, such as competition, and non‐equilibrium processes, such as colonization and recruitment. After disturbance there are increased opportunities for colonization by new individuals, and nutrient cycles are often more open and prone to leaching loss than in middle or late succession (Vitousek and Reiners, 1975).
The traits of plant species strongly influence the probability or severity of many disturbances. The introduction of grasses into a forest or shrubland, for example, can increase fire frequency and cause the replacement of forest by savanna (D’Antonio and Vitousek, 1992). Similarly, boreal conifers are more flammable than deciduous trees because of their large leaf and twig surface area, canopies that extend to the ground surface (acting as ladders for fire to move into the canopy), low moisture content and high resin content (Johnson, 1992). For this reason, the invasion of the boreal forests by black spruce in the middle Holocene caused an increase in fire frequency even though this occurred during a period when the climate was becoming cooler and wetter (Lynch et al., 2003). The resins in boreal conifers that promote fire also retard decomposition (Flanagan and Van Cleve, 1983) and contribute to fuel accumulation.
Traits that enable plants to colonize and spread on dry unvegetated soils are critical in soil stabilization. Introduced dune grasses, for example, have altered soil accumulation patterns and dune morphology in the western United States (D’Antonio and Vitousek, 1992). After plant cover increases, soil organic matter accumulates, and nutrient cycles become more closed.
At a finer scale, species differences in rooting density influence the suitability of soils for burrowing by gophers. Goatgrass (Aegilops triuncialis) produces an extensive fine root system that increases the structural integrity of grassland soils and attracts gophers, thus increasing the probability of gopher disturbance (Eviner and Chapin, 2003b). Species also differ in their vulnerability to strong winds, which influences the impact of hurricanes.
Species interactions and the functional types of organisms
Plant traits that influence species interactions are among the most profoundly important ways in which plant traits influence ecosystem processes. Interactions such as competition, mutualism and predation govern the abundance of species in an ecosystem and, therefore, the extent to which the traits of a species are represented in the ecosystem. The effects of species interactions are ubiquitous, but are often highly situation‐specific and idiosyncratic, so it is difficult to predict a priori the full range of ecosystem changes caused by the introduction or loss of a species (Carpenter and Kitchell, 1993). Development of a framework for predicting the effects of species interactions is an emerging challenge that will improve our capacity to predict and mitigate the effects of global changes in species composition and biodiversity (Chapin et al., 2000c).
Mutualistic species interactions contribute directly to many essential ecosystem processes, such as nutrient inputs through nitrogen fixation and mycorrhizal associations that govern phosphorus and organic nitrogen uptake by plants (Read, 1991). Other mutualisms, such as pollination and seed dispersal, have indirect effects, influencing the presence or abundance of species with traits that may have strong ecosystem effects.
Plant traits that influence herbivory affect virtually all ecosystem processes. In general, plants that characterize low‐fertility soils produce chemical defences that reduce the frequency of herbivory in these habitats; these compounds also retard decomposition and nutrient cycling. In contrast, plants characteristic of high‐fertility soils tend to invest preferentially in growth rather than chemical defence (Bryant et al., 1983), and herbivores are an important avenue of carbon and nutrient transfer from plants to soils. In this way, herbivores magnify inherent differences in soil fertility among ecosystems (Chapin, 1993). Herbivory has a major impact on ecosystem processes for several reasons (Chapin, 1993): first, herbivores transfer plant tissue to soils before nutrient resorption can occur, so approximately twice as much nitrogen and phosphorus is transferred per unit of plant biomass than would occur through litterfall. Secondly, herbivores preferentially select nutrient‐rich tissues, further enhancing nutrient transfer to soils. Finally, animal digestion, especially in homeotherms, uses much of the energy from ingested plant matter to support metabolism, resulting in the excretion of nutrients in readily available forms. In these ways, herbivory short‐circuits the decomposition process and speeds rates of nutrient cycling (Kielland and Bryant, 1998).
Competitive interactions among plant species influence the relative abundance of species in an ecosystem and therefore the traits that are expressed at an ecosystem scale. This is particularly evident in primary succession, where an early abundance of nitrogen fixers is critical to increasing nitrogen inputs. This directly determines the availability of nitrogen to support plant production and indirectly influences the stand structure and species composition in later stages of succession (Van Cleve et al., 1991; Chapin et al., 1994; Fastie, 1995).
Species effects on human activities
Human activities are playing an increasingly important role in the functioning of all ecosystems worldwide, through both direct and indirect effects. Traits of common plant species directly influence human use of natural and managed ecosystems by determining the abundance of food, fibre and other ecosystem goods and services to society. Hunting and gathering societies focus their activities on locations with desirable sources of food and fibre, and frequently enhance the abundance of, or cultivate, many of these species (Diamond, 1999). Timber harvest is concentrated in stands dominated by species with commercially valuable wood qualities. In diverse tropical rainforests or temperate woodlands, individual trees of valuable species are selectively eliminated from the forests, altering the forest composition and therefore the traits of vegetation that influence ecosystem processes.
INTEGRATED EFFECTS OF PLANT TRAITS ON ECOSYSTEMS
Given the large number of plant traits that influence interactive controls and the multiple interactive controls that influence ecosystem processes, how can one generalize about vegetation effects on ecosystem processes? The answer to this question depends on the spatial and temporal scales of comparison. In studies that compare current ecosystem processes across regions or continents, the traits of dominant plant species, particularly traits related to size and growth rate, have predictable effects on several ecosystem processes. These broad generalizations apply less readily to the transient dynamics of a single ecosystem, which depend on the properties of both the current dominants and the species that might become more common with community change. Even rare species may play an important role as changes in conditions alter species composition (Walker et al., 1999). In addition, at the scale of an individual ecosystem, there is substantial variation in the trait combinations found among species within a given functional type, making it essential to consider the traits and abundances of individual species in evaluating the impacts of changes in species composition in a community (Eviner and Chapin, 2003a). In this section I focus on trait combinations that contribute to broad‐scale patterns of ecosystem properties. This comparison suggests that certain suites of traits related to size and growth rate account for most of the global variation in plant effects on ecosystem processes.
Controls over NPP
Total NPP varies 14‐fold among mature stands of the major terrestrial biomes (Table 1). This variation correlates strongly with climate: ecosystems that are dry (e.g. deserts) or cold (e.g. tundra) are less productive than those that are warm and moist (e.g. tropical rain forests). Much of this variation in NPP simply reflects the length of the growing season. NPP that is averaged over the time that plants actively produce new biomass varies only four‐fold among biomes. When NPP is normalized by both growing‐season length and the quantity of leaf area available to fix carbon, there is no consistent relationship between NPP and climate. Biome differences in NPP per unit leaf area and time may reflect uncertainty in the data as much as any underlying climatic influence. To the extent that general patterns emerge, the climatic controls over NPP of mature stands can therefore be viewed as a combination of the climatic constraints on the length of growing season and the capacity of vegetation to produce and maintain leaf area. On average, plants in most mature stands produce 1–3 g biomass per square meter of leaf area per day during the growing season. This is consistent with the generalization that light use efficiency, i.e. the efficiency of converting absorbed radiation into plant biomass, is relatively constant (within a factor of two) among C3 plants (Chapin et al., 2002). Plants differ in leaf area in response to spatial variation in climate more strongly than they differ in photosynthesis per unit leaf area during the time that leaves are photosynthetically active. To the extent that photosynthetic rate per unit leaf area varies among ecosystems, it tends to be higher in high‐resource environments (Reich et al., 1997), particularly in sites recovering from recent disturbance, where leaf area is much less than would be expected to occur at equilibrium. Variation among ecosystems in the potential of leaf area to produce new biomass during the growing season primarily reflects regional variation in soil resources rather than variation in climate. Fine root length is probably just as important as leaf area in governing the productive potential of vegetation (Craine et al., 2001), but fewer comparative data are available for roots.
Table 1.
Productivity per day and per unit leaf area
| Biome | Total NPP (g m–2 year–1)* | Season length† (d) | Daily NPP per ground area (g m–2 d–1) | Total LAI‡ (m2 m–2) | Daily NPP per leaf area (g m–2 d–1) |
| Tropical forests | 2500 | 365 | 6·8 | 6·0 | 1·14 |
| Temperate forests | 1550 | 250 | 6·2 | 6·0 | 1·03 |
| Boreal forests | 380 | 150 | 2·5 | 3·5 | 0·72 |
| Mediterranean shrublands | 1000 | 200 | 5·0 | 2·0 | 2·50 |
| Tropical savannas and grasslands | 1080 | 200 | 5·4 | 5·0 | 1·08 |
| Temperate grasslands | 750 | 150 | 5·0 | 3·5 | 1·43 |
| Deserts | 250 | 100 | 2·5 | 1·0 | 2·50 |
| Arctic tundra | 180 | 100 | 1·8 | 1·0 | 1·80 |
| Crops | 610 | 200 | 3·1 | 4·0 | 0·76 |
| Range of values | 14‐fold | 3·7‐fold | 3·8‐fold | 6‐fold | 3·3‐fold |
* NPP is expressed in units of dry mass (Saugier et al., 2001).
† Estimated.
‡ Data from Gower (2002).
Many plant traits directly influence the capacity of plants to produce and maintain leaf and root area, and therefore NPP. In mature stands, plant size and leaf longevity are particularly important. Large plants such as trees support more leaf area than do smaller plants. Evergreen trees, including many conifers, support more leaf area than deciduous trees in the same environment. A suite of traits related to growth rate also strongly influences leaf area and NPP, particularly after disturbance. Small, slowly growing plants commonly dominate environments characterized by low availability of water and nutrients (Chapin, 1980; Lambers and Poorter, 1992). These species minimize their resource requirement by retaining leaves for a long time (Berendse et al., 1987). Persistence of leaves in turn requires an anatomy that resists desiccation and/or freezing and a chemistry that deters herbivores and pathogens. These leaves typically have a low specific leaf area (thick or dense leaves) and a low tissue nitrogen concentration that in turn constrains photosynthetic rate (Reich et al., 1997). Many of these growth‐related traits are functionally inter‐related; others may be linked through common developmental pathways (Chapin et al., 1993).
Other plant traits that influence NPP show a less consistent correlation with growth rate. These include stem and root architecture, vulnerability to pathogens and allocation to leaf, root, stem and reproductive parts.
Decomposition and nitrogen mineralization
Traits that govern plant growth rate and NPP also determine the microbial processing of carbon and nitrogen in soils. When plant leaves senesce, they resorb approximately half of their nitrogen and phosphorus pool and very little of the initial carbon pool, regardless of the environment in which they grow (Chapin and Kedrowski, 1983; Aerts and Chapin, 2000). The quality of leaf litter, as measured by litter C : N ratio and carbon quality, therefore correlates strongly with corresponding parameters in live leaves. The scant data available suggest that roots and stems resorb an even smaller proportion of nutrients during senescence than do leaves and also produce a litter whose chemistry reflects that of the corresponding live tissues (Aerts and Chapin, 2000). Chemical properties that promote high physiological activity and growth in plants (e.g. high tissue nitrogen concentration) and low lignin content (reflecting less sclerified leaves with a high ratio of cytoplasm to cell wall) also promote rapid decomposition (Melillo et al., 1982; Hobbie, 1992). Litter from species typical of productive environments (e.g. herbs and deciduous species) typically decomposes more rapidly than that from species from less productive environments (e.g. evergreens) (Cornelissen, 1996; Perez‐Harguindeguy et al., 2000).
The quantity of litter input provides a second critical link between NPP and decomposition because, at steady‐state, NPP governs the quantity of organic matter inputs to decomposers. When biomes are compared, heterotrophic respiration (i.e. the carbon released by processing of dead plant material by decomposer organisms and animals) is approximately equal to NPP. In other words, net ecosystem production (NEP), the rate of net carbon sequestration, is approximately zero, regardless of climate or ecosystem type. This indicates that the quantity and quality of organic matter inputs to soils, as determined by plant traits, are the major determinants of decomposition when ecosystems are compared at steady‐state. Environment exerts important additional controls on decomposition through effects on both NPP (quantity and quality of litter inputs) and the activity of decomposer organisms. Other factors that influence decomposition rate include pH and the composition of the microbial community. Any plant effects on these factors will also influence decomposition. Both successional dynamics and directional changes in land cover and environment resulting from human activities decouple this linkage between NPP and decomposition.
Litter properties that promote NPP and decomposition also facilitate net nitrogen mineralization. The activity of decomposer organisms, which depends strongly on the carbon quality of substrates and the nitrogen status of microbes (a function of litter nitrogen concentration), are the major effects of plant litter quality on net nitrogen mineralization (Paul and Clark, 1996). Litter with high concentrations of lignin or other recalcitrant compounds mineralizes nitrogen more slowly than does litter with more labile carbon compounds. High‐nitrogen litter shows greater net mineralization of nitrogen than does low‐nitrogen litter because microbes are seldom nitrogen‐limited below a C : N ratio of 25 : 1; the nitrogen in excess of microbial demands for growth is released into the soil where it becomes available to plants. As with decomposition, traits governing NPP strongly influence annual net nitrogen mineralization because productive ecosystems produce large quantities of high‐quality litter.
Evapotranspiration
Climate plays a more direct role in explaining spatial patterns of evapotranspiration than in the case of photosynthesis, decomposition and nitrogen mineralization because of ecosystem differences in water use efficiency. Climate directly governs the rate of evapotranspiration by determining the soil moisture supply and the radiation and temperature environment, which determine evaporative demand of the atmosphere. The size and structural complexity of plant canopies are the major vegetation traits that influence evapotranspiration, primarily through their effects on boundary layer (aerodynamic) conductance, as described earlier. Surface conductance, which describes the potential of leaf and soil surfaces to lose water, is surprisingly insensitive to vegetation properties under moist conditions (Kelliher et al., 1995; Chapin et al., 2002). As soil moisture declines, stomatal conductance and associated changes in surface conductance become increasingly important controls over evapotranspiration. Because stomatal conductance correlates with photosynthetic capacity (Schulze et al., 1994), and because NPP correlates with soil moisture availability (Lieth, 1975), evapotranspiration tends to be greatest in productive ecosystems, although the link to NPP is less direct than in the case of photosynthesis, decomposition and nitrogen mineralization.
Disturbance regime
Vegetation traits that determine disturbance regime are highly specific to the nature of prevailing disturbances and are therefore less consistently linked with the plant traits that regulate NPP, decomposition and net nitrogen mineralization. In grasslands, for example, productive stands produce more leaf litter and carry fire readily, whereas in boreal forests unproductive conifer stands produce dry, resin‐rich fuels that are most fire‐prone.
EFFECT OF PLANT TRAITS ON LANDSCAPE AND REGIONAL PROCESSES
Plant traits that affect the movement of energy, materials, organisms or disturbance among patches within a landscape have consequences that extend beyond the ecosystem in which the plant occurs. There is increasing evidence that plant traits affecting water and energy exchange and fire probability have large landscape and regional effects.
Water and energy exchanges by an ecosystem not only affect the local microclimate of an ecosystem, but also the input of heat and moisture to the atmosphere, which can affect temperature and precipitation in downwind ecosystems. Simulations using a general circulation model suggest that if South America were dominated by shallow‐rooted pasture grasses, it would have a warmer, drier climate than a continent dominated by tropical forests (Shukla et al., 1990). Grasses would have a lower evapotranspiration rate than deep‐rooted trees and would therefore transfer more energy to the atmosphere as sensible heat.
Observations and measurements in arctic Alaska also suggest that strong effects of plant traits on regional climate generate a positive feedback that augments high‐latitude warming. In association with recent regional warming, moist tundra has become more shrubby, and trees are increasing in density and expanding into tundra near the arctic treeline (Sturm et al., 2001). Shrub tundra and forest have lower albedo (i.e. they absorb more radiation) and greater sensible heat flux than does the moist tundra, thus transferring more energy to the atmosphere than does the moist tundra, which they are replacing. The increase in atmospheric heating caused by these vegetation changes (4–5 W m–2) is similar, on a unit area basis, to the heating associated with two atmospheric forcings known to have large climatic impacts: (1) a 2 % change in solar constant, such as occurred between glacial and interglacial periods; and (2) a doubling of atmospheric CO2 (Chapin et al., 2000a). The total effect of the vegetation change on regional warming depends on how extensive it might be. Simulations with a regional climate model suggest that if the entire tundra of northern Alaska changed from moist to shrub tundra, this would increase July mean air temperature by 1·5–3·5 °C, and this warming effect would extend well south into the boreal forest. The recent increases in shrubbiness observed in Alaska in the past 50 years (Sturm et al., 2001) may therefore have contributed to the recent summer warming observed in the region.
Vegetation changes associated with fire in the boreal forest can have a cooling effect on climate. Late‐successional conifers, which dominate the landscape in the absence of fire, have a low albedo and stomatal conductance, and therefore transfer large amounts of sensible heat to the atmosphere. Post‐fire deciduous forests, in contrast, absorb less energy due to their high albedo, and transmit more of this energy to the atmosphere as latent rather than sensible heat, resulting in less immediate warming of the atmosphere and more moisture available to support precipitation (Chapin et al., 2000b). If these vegetation changes were widespread, they could have a negative feedback to high‐latitude warming and reduce the probability of fire. This is one of the few negative feedbacks to regional warming that has been identified at high latitudes.
CONCLUSION
The effects of species traits on ecosystem and regional processes are sufficiently well understood that they can be incorporated into regional and global models that link changes in vegetation with changes in ecosystem processes. In many cases, the traits of dominant species that determine ecosystem properties, such as total leaf area, can be sensed remotely and mapped at regional and global scales. This provides the basis for incorporating our current understanding of species effects into large‐scale ecosystem and climate models to provide a more informed basis for developing policies that mitigate the undesirable effects of human activities on the global environment.
ACKNOWLEDGEMENTS
Work leading to these ideas was funded by grants from the US Forest Service and the National Science Foundation to the University of Alaska in support of the Bonanza Creek Long‐Term Ecological Research Program (PNW01‐JV11261952‐231 and DEB‐0080609) and the Arctic System Science programme in Arctic Transitions in the Land‐Atmosphere System (ATLAS) (OPP‐9732126). I thank Jason Beringer, Donie Bret‐Harte, John Bryant, Scott Chambers, Melissa Chapin, Catherine Copass, Joe Craine, Werner Eugster, Valerie Eviner, Paul Grogan, Sarah Hobbie, Dave Hooper, Bruce Hungate, Michelle Mack, Joe McFadden and Heather Reynolds for helpful discussions that led to the development of these ideas.
Table 2.
Energy budget feedbacks to regional summer climate in arctic tundra
| Feedback from vegetation change | Energy budget (W m –2) |
| Conversion of moist tundra to shrub tundra | 3·9 |
| Conversion of moist tundra to forest | 5·0 |
| 2 % change in solar constant (glacial to interglacial) | 4·6 |
| Doubling of atmospheric CO2 concentration | 4·4 |
Data from Chapin et al. (2000a) and Beringer et al. (2001).
Supplementary Material
Received: 9 April 2002; Returned for revision: 9 July 2002; Accepted: 27 November 2002
References
- AertsR, Chapin FS, III.2000. The mineral nutrition of wild plants revisited: a re‐evaluation of processes and patterns. Advances in Ecological Research 30: 1–67. [Google Scholar]
- ArmstrongW, Justin SHFW, Beckett PM, Lythe S.1991. Root adaptation to soil waterlogging. Aquatic Botany 39: 57–73. [Google Scholar]
- BaldocchiD, Kelliher FM, Black TA, Jarvis PG.2000. Climate and vegetation controls on boreal zone energy exchange. Global Change Biology 6 (suppl. 1): 69–83. [DOI] [PubMed] [Google Scholar]
- BerendseF, Oudhof H, Bol J.1987. A comparative study on nutrient cycling in wet heathland ecosystems. I. Litter production and nutrient losses from the plant. Oecologia 74: 174–184. [DOI] [PubMed] [Google Scholar]
- BeringerJ, Tapper NJ, Chapin FS, III, McHugh I, Lynch AH, Serreze M, Slater A.2001. Impact of arctic treeline on synoptic climate. Geophysical Research Letters 28: 4247. [Google Scholar]
- BradshawAD.1983. The reconstruction of ecosystems. Journal of Ecology 20: 1–17. [Google Scholar]
- BryantJP, Chapin FS, III, Klein DR.1983. Carbon/nutrient balance of boreal plants in relation to vertebrate herbivory. Oikos 40: 357–368. [Google Scholar]
- CarpenterSR, Kitchell JF.1993. The Trophic Cascade in Lakes. Cambridge: Cambridge University Press. [Google Scholar]
- CaterTC, Chapin FS, III.2000. Differential species effects on boreal tree seedling establishment after fire: Resource competition or modification of microenvironment. Ecology 81: 1086–1099. [Google Scholar]
- ChapinFS, III.1980. The mineral nutrition of wild plants. Annual Review of Ecology and Systematics 11: 233–260. [Google Scholar]
- ChapinFS, III.1993. Functional role of growth forms in ecosystem and global processes. In: Ehleringer JR, Field CB eds. Scaling physiological processes: leaf to globe San Diego: Academic Press, 287–312. [Google Scholar]
- ChapinFS, III, Kedrowski RA.1983. Seasonal changes in nitrogen and phosphorus fractions and autumn retranslocation in evergreen and deciduous taiga trees. Ecology 64: 376–391. [Google Scholar]
- ChapinFS, III, Autumn K, Pugnaire F.1993. Evolution of suites of traits in response to environmental stress. American Naturalist 142: S78–S92. [Google Scholar]
- ChapinFS, III, Matson PA, Mooney HA.2002. Principles of terrestrial ecosystem ecology. New York: Springer‐Verlag. [Google Scholar]
- ChapinFS, III, Torn MS, Tateno M.1996. Principles of ecosystem sustainability. American Naturalist 148: 1016–1037. [Google Scholar]
- ChapinFS, III, Walker LR, Fastie CL, Sharman LC.1994. Mechanisms of primary succession following deglaciation at Glacier Bay, Alaska. Ecological Monographs 64: 149–175. [Google Scholar]
- ChapinFS, III, Eugster W, McFadden JP, Lynch AH, Walker DA.2000a Summer differences among arctic ecosystems in regional climate forcing. Journal of Climate 13: 2002–2010. [Google Scholar]
- ChapinFS, IIIet al.2000b Arctic and boreal ecosystems of western North America as components of the climate system. Global Change Biology 6 (Suppl. 1): 1–13. [DOI] [PubMed] [Google Scholar]
- ChapinFS, IIIet al.2000c Consequences of changing biotic diversity. Nature 405: 234–242. [DOI] [PubMed] [Google Scholar]
- CornelissenJHC.1996. An experimental comparison of leaf decomposition rates in a wide range of temperate plant species and types. Journal of Ecology 84: 573–582. [Google Scholar]
- CraineJM, Froehle J, Tilman DG, Wedin DA, Chapin FS, III.2001. The relationships among root and leaf traits of 76 grassland species and relative abundance along fertility and disturbance gradients. Oikos 93: 274–285. [Google Scholar]
- D’AntonioCM, Vitousek PM.1992. Biological invasions by exotic grasses, the grass‐fire cycle, and global change. Annual Review of Ecology and Systematics 23: 63–87. [Google Scholar]
- DiamondJ.1999. Guns, germs, and steel: the fates of human societies. New York: W.W. Norton and Company. [Google Scholar]
- DokuchaevVV.1879. Abridged historical account and critical examination of the principal soil classifications existing. Transactions of the Petersburg Society of Naturalists 1: 64–67. [Google Scholar]
- EvinerVT, Chapin FS, III.2001. Plant species provide vital ecosystem functions for sustainable agriculture, rangeland management and restoration. California Agriculture 55: 54–59. [Google Scholar]
- EvinerVT, Chapin FS, III.2003a Biogeochemical interactions and biodiversity. In: Melillo JM, Field CB, Moldan M, eds. Element interactions: rapid assessment project of SCOPE. Washington: Island Press. [Google Scholar]
- EvinerVT, Chapin FS, III.2003b Gopher‐plant‐fungal interactions affect establishment of an invasive grass. Ecology (in press). [Google Scholar]
- FastieCL.1995. Causes and ecosystem consequences of multiple pathways of primary succession at Glacier Bay, Alaska. Ecology 76: 1899–1916. [Google Scholar]
- FieldC, Chapin FS, III, Matson PA, Mooney HA.1992. Responses of terrestrial ecosystems to the changing atmosphere: a resource‐based approach. Annual Review of Ecology and Systematics 23: 201–235. [Google Scholar]
- FlanaganPW, Van Cleve K.1983. Nutrient cycling in relation to decomposition and organic matter quality in taiga ecosystems. Canadian Journal of Forest Research 13: 795–817. [Google Scholar]
- GowerST.2002. Productivity of terrestrial ecosystems. In: Mooney HA, Canadell J, eds. Encyclopedia of Global Change Oxford: Blackwell Scientific, 516–521. [Google Scholar]
- HobbieSE.1992. Effects of plant species on nutrient cycling. Trends in Ecology and Evolution 7: 336–339. [DOI] [PubMed] [Google Scholar]
- HobbieSE.1995. Direct and indirect effects of plant species on biogeochemical processes in arctic ecosystems. In: Chapin FS, III, Körner C, eds. Arctic and alpine biodiversity: patterns, causes and ecosystem consequences Berlin: Springer‐Verlag, 213–224. [Google Scholar]
- JennyH.1941. Factors of soil formation. New York: McGraw‐Hill. [Google Scholar]
- JohnsonEA.1992. Fire and vegetation dynamics. Studies from the North American boreal forest. Cambridge: Cambridge University Press. [Google Scholar]
- KelliherFM, Leuning R, Raupach MR, Schulze E‐D.1995. Maximum conductances for evaporation from global vegetation types. Agricultural and Forest Meteorology 73: 1–16. [Google Scholar]
- KiellandK, Bryant J.1998. Moose herbivory in taiga: effects on biogeochemistry and vegetation dynamics in primary succession. Oikos 82: 377–383. [Google Scholar]
- LambersH, Poorter H.1992. Inherent variation in growth rate between higher plants: a search for physiological causes and ecological consequences. Advances in Ecological Research 23: 187–261. [Google Scholar]
- LiethH.1975. Modeling the primary productivity of the world. In: Lieth H, Whittaker RH, eds. Primary productivity of the biosphere Berlin: Springer‐Verlag, 237–263. [Google Scholar]
- LynchJA, Clark JS, Bigelow NH, Edwards ME, Finney BP.2003. Spatial and temporal variations in fire history in boreal ecosystems of Alaska. Journal of Geophysical Research (in press). [Google Scholar]
- MelilloJM, Aber JD, Muratore JF.1982. Nitrogen and lignin control of hardwood leaf litter decomposition dynamics. Ecology 63: 621–626. [Google Scholar]
- PaulEA, Clark FE.1996. Soil microbiology and biochemistry, 2nd edn. San Diego: Academic Press. [Google Scholar]
- Perez‐HarguindeguyN, Diaz S, Cornelissen JHC, Vendramini F, Cabido M, Castellanos A.2000. Chemistry and toughness predict leaf litter decomposition rates over a wide spectrum of functional types and taxa in central Argentina. Plant and Soil 218: 21–30. [Google Scholar]
- PimmSL, Russell GJ, Gittleman JL, Brooks TM.1995. The future of biodiversity. Science 269: 347–350. [DOI] [PubMed] [Google Scholar]
- ReadDJ.1991. Mycorrhizas in ecosystems. Experientia 47: 376–391. [Google Scholar]
- ReichPB, Walters MB, Ellsworth DS.1997. From tropics to tundra: global convergence in plant functioning. Proceedings of the National Academy of Sciences of the USA 94: 13730–13734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RoblesM, Chapin FS, III.1995. Comparison of the influence of two exotic species on ecosystem processes in the Berkeley Hills. Madroño 42: 349–357. [Google Scholar]
- SalaOEet al.2000. Global biodiversity scenarios for the year 2100. Science 287: 1770–1776. [DOI] [PubMed] [Google Scholar]
- SaugierB, Roy J, Mooney HA.2001. Estimations of global terrestrial productivity: Converging toward a single number? In: Roy J, Saugier B, Mooney HA, eds. Terrestrial global productivity San Diego: Academic Press, 543–557. [Google Scholar]
- SchulzeE‐D, Kelliher FM, Körner C, Lloyd J, Leuning R.1994. Relationship among maximum stomatal conductance, ecosystem surface conductance, carbon assimilation rate, and plant nitrogen nutrition: a global ecology scaling exercise. Annual Review of Ecology and Systematics 25: 629–660. [Google Scholar]
- ShuklaJ, Nobre C, Sellers P.1990. Amazon deforestation and climate change. Science 247: 1322–1325. [DOI] [PubMed] [Google Scholar]
- SturmM, Racine C, Tape K.2001. Increasing shrub abundance in the Arctic. Nature 411: 546–547. [DOI] [PubMed] [Google Scholar]
- TatenoM, Chapin FS, III.1997. The logic of carbon and nitrogen interactions in terrestrial ecosystems. American Naturalist 149: 723–744. [Google Scholar]
- Van CleveK, Chapin FS, III, Dyrness CT, Viereck LA.1991. Element cycling in taiga forest: state‐factor control. BioScience 41: 78–88. [Google Scholar]
- VervilleJH, Hobbie SE, Chapin FS, III, Hooper DU.1998. Response of tundra CH4 and CO2 flux to manipulation of temperature and vegetation. Biogeochemistry 41: 215–235. [Google Scholar]
- VitousekPM, Howarth RW.1991. Nitrogen limitation on land and in the sea: how can it occur? Biogeochemistry 13: 87–115. [Google Scholar]
- VitousekPM, Reiners WA.1975. Ecosystem succession and nutrient retention: a hypothesis. BioScience 25: 376–381. [Google Scholar]
- VitousekPM, Mooney HA, Lubchenco J, Melillo JM.1997. Human domination of Earth’s ecosystems. Science 277: 494–499. [Google Scholar]
- VitousekPM, Walker LR, Whiteaker LD, Mueller‐Dombois D, Matson PA.1987. Biological invasion by Myrica faya alters ecosystem development in Hawaii. Science 238: 802–804. [DOI] [PubMed] [Google Scholar]
- WalkerB, Kinzig A, Langridge J.1999. Plant attribute diversity, resilience, and ecosystem function: the nature and significance of dominant and minor species. Ecosystems 2: 95–113. [Google Scholar]
- WedinDA, Tilman D.1990. Species effects on nitrogen cycling: a test with perennial grasses. Oecologia 84: 433–441. [DOI] [PubMed] [Google Scholar]
- WilsonJB, Agnew DQ.1992. Positive‐feedback switches in plant communities. Advances in Ecological Research 23: 263–336. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.