Abstract
Clematis flammula var. maritima is a woody lianoid plant that grows on coastal sand dunes in the Mediterranean region. Older perennial stems are present as extensive underground axes. These generate surface growth of shorter‐lived stems producing monospecific trellises above the surface of the sand. Despite its sand dune habitat and shortage of host support plants, this variety of Clematis shows mechanical characteristics during growth that are closely comparable with those of scandent woody lianas. A significant decrease in the value of structural Young’s modulus is observed from the aerial trellis‐forming shoots (1·619 ± 0·492 GN m–2) to emergent axes (0·855 ± 0·253 GN m–2) and underground woody stems (0·470 ± 0·113 GN m–2). Biomechanical and evelopmental observations indicate that most emergent branches are optimized geometrically and mechanically in relation to their points of emergence from the sand, with increases in structural Young’s modulus and the second moment of area around the surface of the sand. Lianoid plants, physiologically capable of withstanding sand dune environments, might represent acceptable natural or introduced species for dune stabilization and conservation.
Key words: Biomechanics, Clematis flammula var. maritima L., growth habit, liana, sand dune
INTRODUCTION
Climbing plants differ from self‐supporting plants, such as shrubs and trees, in a range of characteristics, most notably the mechanical properties of the stem. Biomechanical approaches are increasingly being used to study ecological processes and mechanisms underlying the mechanical architecture of different growth forms, such as lianas, shrubs and trees (Putz and Holbrook, 1991; Speck, 1991; Niklas, 1992; Rowe and Speck, 1996; Speck and Rowe, 1999; Gallenmüller et al., 2001). Previous biomechanical studies on plant stems have shown that different growth forms can be characterized by changes in mechanical properties of the stem during ontogeny (Speck, 1991, 1994a). Such changes can be observed in mechanical parameters, such as flexural stiffness (EI) (the tangible resistance of a structure to bending forces) and structural Young’s modulus (E) (a measure of the stiffness of a material), which represents a value independent of the actual size or geometry of the tested material. As such, structural Young’s modulus is an effective parameter for comparing mechanical properties of plant stems of different shapes and sizes. Recent biomechanical studies indicate that self‐supporting plants show an increase in structural Young’s modulus during ontogeny, and differ from lianoid plants in which structural Young’s modulus decreases during ontogeny from young to old flexible stems (Speck and Rowe, 1999). A third broad category of plant growth forms has been quantitatively delimited, termed semi‐self‐supporting, in which the structural Young’s modulus of the stems remains unchanged during ontogeny (Speck and Rowe, 1999). In general, two biomechanical strategies are seen for the majority of lianas during their growth trajectory: a juvenile self‐supporting phase and a compliant adult climbing phase. A self‐supporting strategy observed in young phases of growth is consistent with young individuals and young branches acting as searchers, ideally comprising stiff materials for traversing open spaces and reaching potential mechanical supports. Greater compliance in older stems might be interpreted as a strategy whereby the slender plant stems are protected from dangerous levels of stress building up as a result of movement, displacement or collapse of the host support (Dobbins and Fisher, 1986; Fisher and Ewers, 1989; Fisher and Ewers, 1991; Putz and Holbrook, 1991).
In lianas, the shift from self‐supporting to non‐self‐supporting growth is accompanied by remarkable changes in anatomical development (Bhambie, 1972; Caballé, 1993, 1998; Speck, 1994a, b; Rowe and Speck, 1996). As well as being free from mechanical provisioning of a self‐supporting architecture, lianas are often believed to gain advantage from directing biomass into extension growth and stem length rather than stem stiffness (Darwin, 1867; Putz, 1984; Niklas, 1994). Studies based on a wide variety of plant families indicate an extreme diversity of anatomical and developmental organizations among lianas (Obaton, 1960; Caballé, 1993), and a dramatic shift in anatomical and mechanical properties during growth can be dependent on the availability of supports. If a support is unavailable, the growth trajectory may be modified according to the constraints and opportunities within the immediate environment (Putz, 1984; Gartner, 1991a; Den Dubbelden and Oosterbeek, 1995; Caballé, 1998). For example, juvenile phases of liana growth with stiff stems can develop in stages of development from seedlings to treelet‐sized stages. In certain conditions, such as low light or absence of climbing supports, the juvenile phase can amount to a ‘waiting’ period where a self‐supporting phase is maintained with little elongation growth until a change in light conditions or physical contact with a nearby support (Caballé, 1998). Other studies have noted changes in resource allocation with reference to the availability of supports as well as rates of development, leaves, stoloniferous axes and other climbing aids (Jaffe, 1973; Peñalosa, 1983; Gartner, 1991b; Den Dubbelden and Oosterbeek, 1995).
Quantitative changes in the mechanical properties of climbing plant stems, and their correlation with development and habitat have only recently been studied in detail (Speck, 1994a; Speck et al., 1994; Rowe and Speck, 1996, 1997; Speck and Rowe, 1999; Gallenmüller et al., 2001). Other studies have indicated that different climbing strategies are reflected in dissimilar biomechanical signals and underlying anatomy, which are related to varying types of attachment to the host (Rowe and Speck, 1998). Variable biomechanical behaviour might also be shown by sympatric individuals of the same species (Gallenmüller et al., 2001). Furthermore, biomechanical studies aimed at different branches of the same individual can show how mechanical features can vary according to precise extrinsic and intrinsic changes, such as before and after attachment to a support (Rowe and Speck, 1996).
Recent quantitative studies have focused mainly on large‐bodied climbing plants, including tropical woody lianas, but relatively little is known about the biomechanical variation of smaller‐bodied climbing, scrambling and creeping plants, and how mechanical properties and anatomy change during different climbing and other growth strategies. One example of a climbing vine found in temperate to Mediterranean habitats is Clematis flammula L., which is relatively common in hedges, scrublands, shrubs and woodland margins (Bonnier, 1912). This species can grow as a relatively small‐bodied climbing plant producing straight searchers and climbing axes which develop relatively compliant older stems with an exfoliating outer periderm tissue typical of some other species of Clematis, e.g. Clematis vitalba. Clematis flammula var. maritima L. is a common sub‐species in the Mediterranean area (Bonnier, 1912), which establishes itself on coastal sand dunes and either climbs on other dune vegetation or forms monospecific trellises (Fig. 1A). The trellises are mechanically complex and consist mostly of interconnected annual axes produced from perennial stems beneath the sand (Fig. 1). The overall mechanical growth form of C. maritima is apparently a modification of that of many viny climbing plants, and shows a number of differences from the hedge or marginal habitats of the main species C. flammula in terms of the edaphic, sand dune conditions and extremes of light, heat, water and wind exposure of its habitat.

Fig. 1.Clematis maritima. A, Typical habit of aerial trellis comprising spreading axes that colonize above‐ground areas of coastal sand dunes; B, creeping axes form early stage of trellis: young axes of stage I and II emerge from the sand and form upright members of the trellis or else arch over and continue a creeping growth along the sand surface.
The main aims of this paper are to report on the biomechanical properties and underlying anatomical and developmental changes of a climbing plant that has a modified growth strategy for open sand dune habitats. Clematis flammula var. maritima is an example of a lianoid, smaller‐bodied viny plant that has adapted to open conditions where there are few climbing opportunities. We wished to establish whether the trellis‐forming parts of the plant exhibit similar mechanical and anatomical strategies to those of young stages of other climbing plants, and to quantify changes in mechanics and anatomy in stems that survive annual senescence and remain to recolonize areas of sand dune. The analysis was carried out in three stages: (1) bending mechanical properties of stem segments from all stages of growth were measured; (2) shifts in mechanical properties along the stem from younger to older stages were characterized, linking developmental and anatomical features with position and orientation of individual axes; and (3) distributions and changes in geometry of specific tissues that influence the mechanics of the stem during the development of the stem system on the dune environment were measured. Finally, this work represents part of a long‐term research effort in which mechanical architectures are being investigated in terms of the evolution of architectures of trees, shrubs, lianas and procumbent growth forms. Ongoing studies aim to present these types of architectural change in terms of phylogenetic patterns within plant groups and in terms of the underlying development.
MATERIALS AND METHODS
Habitat and growth form of Clematis flammula var. maritima
Clematis flammula is a woody climbing species that occurs in coastal areas of the Mediterranean (Coste, 1901; Bonnier, 1912). Climbing stems are narrow and can reach up to 3–5 m in length. They bear bipinnate leaves with small, oval to lanceolate leaflets (Tutin et al., 1964). Both the leaf structure and habitat distinguish C. flammula var. maritima from C. flammula. The former is a colonizer of coastal sand dunes, forming more or less prostrate or underground stems, from which emerging annual leaders (Fig. 1A) or searchers climb on nearby vegetation or form monospecific trellises. The leaves of C. flammula var. maritima differ markedly from those of C. flammula, being slender and bi‐ or tripinnate with linear leaflets (Bonnier, 1912). Young stems of the developing trellis are interconnected via touch‐sensitive leaf petioles, which curve around supports, so that a single axis can be attached very securely along numerous points of attachment (Fig. 2A).
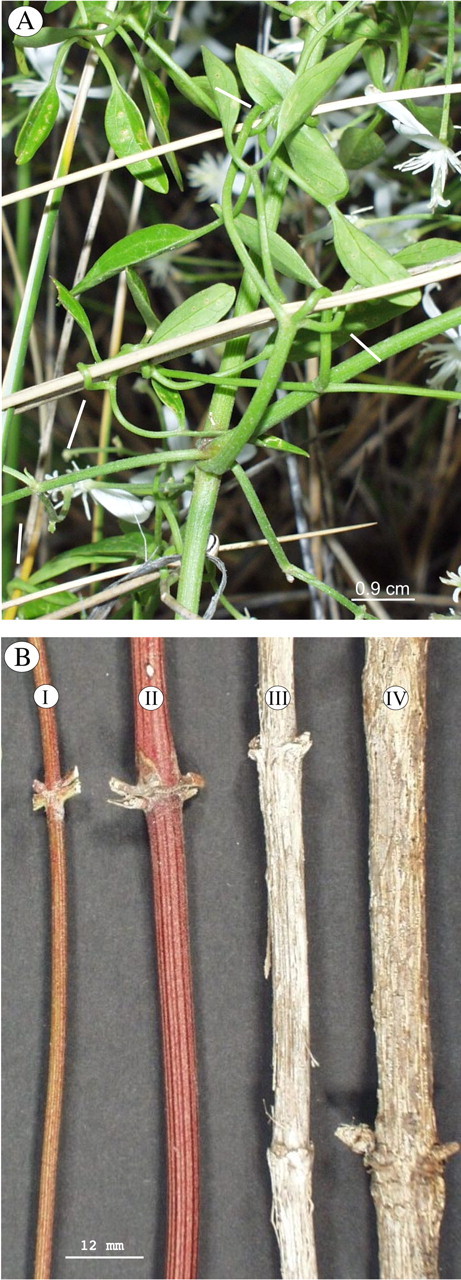
Fig. 2.Clematis maritima. A, Mechanically sensitive petioles forming attachment with neighbouring branches and associated plants. The central axis is attached via four sensitive leaves over a distance of 10 cm. B, Ontogenetic stages I–IV of C. maritima. Young stages of development are stiffened by longitudinal bands of fibres which are visible in stages I and II. Stage III stems exhibit early stages of secondary growth; fibres begin to slough off from the outer part of the stem. The stage IV stem illustrated here shows an outer covering of periderm with fine strips of fibres formed from the secondary phloem.
Biomechanics and quantitative morphology and anatomy
All plants studied were collected from a sand dune system approx. 100 m wide that extends for 9 km between Carnon and La Grande Motte (Languedoc–Roussillon) on the Mediterranean coast of France. Two sampling methods were employed in which over 100 segments were pruned from more than 13 stem systems of 5‐ to 6‐year‐old plants. The first sample concentrated on collecting a general data set of all available types of stems produced from aerial trellises, intermediate stems emerging from the sand and older axes either partially or completely buried in the sand. A second sample focused on bending measurements of stem segments taken from along the length of six axes with reference to the point of departure from the sand surface. Sampling was confined to healthy branches showing no signs of browsing or trampling.
Bending tests were carried out on freshly collected material or on specimens kept fresh and hydrated for a maximum of 3 d before testing. Three‐ and four‐point bending tests (Fig. 3) were carried out on stem segments of all developmental stages, and the flexural stiffness (EI) of each segment was calculated (Vincent, 1990; Rowe and Speck, 1996). Four‐point bending tests were used preferentially as they measure resistance to bending without including the influence of shear, which can occur in more simple three‐ and two‐point bending tests and can lead to an underestimation of flexural stiffness if the test specimen has a relatively low span to depth ratio. Three‐ and two‐point bending measurements therefore require a sequence of ‘span tests’ to determine the necessary length/diameter ratio for each segment in order to avoid measuring shear. Four‐point bending tests were carried out on a manual bending apparatus in which a metal pannier was suspended from the plant stem at two precisely predetermined points. Up to six or eight weights were placed manually on the pannier at 1‐min intervals and the deflection of the plant stem was measured using a binocular microscope fitted with a graduated objective; flexural stiffness was calculated from the resulting force/deflection curve (Rowe and Speck, 1996; Gallenmüller et al., 2001). The axial second moment of area (I) of the tested axes was established from measurements of the diameters of the tested segments in three to five positions along the segment, and a simple elliptical model was used to determine the second moment of area of the tested segment in relation to the force applied (Vincent, 1990; Niklas, 1992). Structural Young’s modulus (Estr) was calculated from experimental measurements of flexural stiffness and the mean value of axial second moment of area for each segment tested. In this paper the terminology follows that used in recent investigations where the term ‘structural Young’s modulus’ is employed for this quantity in plant stems, which are clearly composite structures rather than homogeneous materials (Gallenmüller et al., 2001). Following bending experiments, segments of stem were preserved in FAA (250 ml 40 % formalin, 10 ml 80 % acetic acid, 2175 ml 50 % ethanol) for quantitative anatomical studies. Transverse and longitudinal sections were hand cut, soaked in 35 % HCl and stained with a solution of 3 % phloroglucinol in 92 % ethanol, which stains lignified tissues red. Stem segments were reorientated and digitized using a macro‐photographic system, and component tissues were studied using Optimas software (Media Cybernetics, Inc., Silver Spring, MD, USA). Digitized surfaces permitted percentage contributions of component tissues, such as fibres, cortex and wood, to be calculated in terms of area (mm2) and axial second moment of area (mm4) of the entire stem cross‐section.
Fig. 3. Schematic representation of the four‐point bending test. Arrows represent the direction of the force applied in bending to the tested stem. The deflection is measured via a graduated scale in a binocular microscope.
Developmental stages of the plant
Prior to carrying out bending tests, four developmental stages were defined, based on morphological and architectural characters, to distinguish growth stages of the plant (Fig. 2B).
Stage I.
Young axes forming herbaceous aerial axes that produce monospecific trellises or occasionally scramble over other plants. Green stems are interconnected by sensitive petioles and bear pinnate leaves. The height of the trellis structure does not generally exceed 40 cm.
Stage II.
Axes that connect directly with underground axes and that emerge from the sand. The stems are no longer green and bear small scales rather than pinnate leaves.
Stage III.
Perennial axes that are mostly underground but that are occasionally exposed by dune movements. These stems show the first external evidence of secondary growth and are produced from older axes buried in the sand.
Stage IV.
Underground stems that show significant degrees of secondary growth of wood; the outer surface has exfoliating strips of periderm and secondary phloem.
RESULTS
Trends in geometry and bending mechanics of the stem
Second moment of area (I).
The second moment of area of stems varied from 1·37 to 779 mm4, reflecting the relatively small size of the plant (Fig. 4). The majority of segments tested had values between 1·37 and 30 mm4, and included axes from the first three ontogenetic stages which show a modest increase in size from stage I to stage III (Table 1). By stage III, the increase is attributable to a significant onset of secondary growth, which takes place in underground stems (Fig. 5A). Segments of the first three growth stages are often produced from meristems issuing from buds on older axes of ontogenetic stage IV, which explains the progressive increase in size of the axes. However, certain axes of stage III develop necrotic apices, and young stems of stages I and II develop from axial buds. Older, underground stems show a significant increase in size and second moment of area, resulting in stems having an average I = 152 mm4 (Table 1).
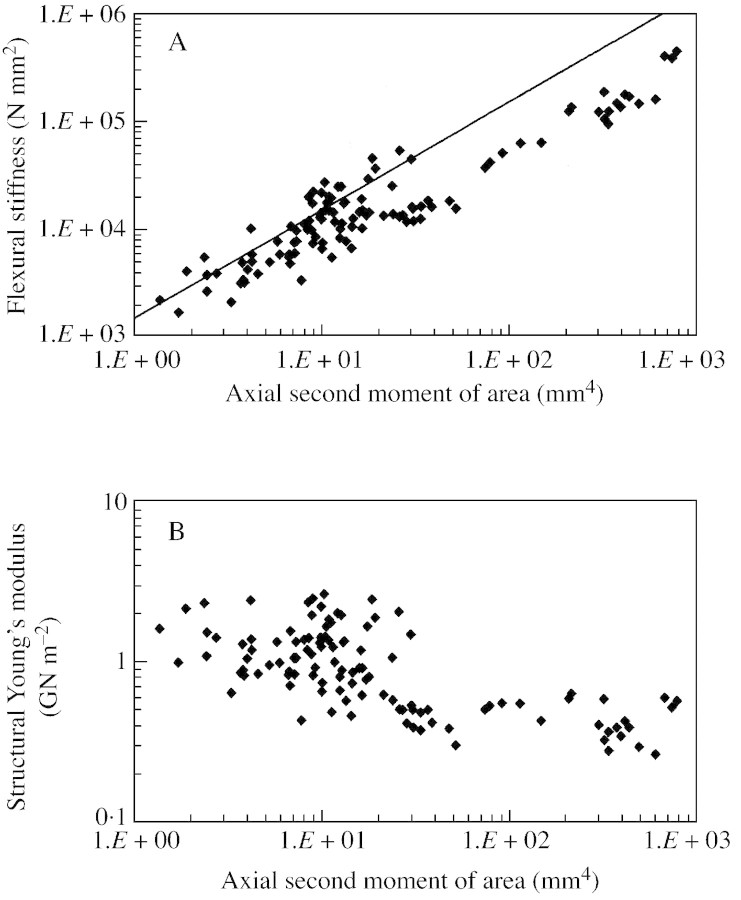
Fig. 4. Biomechanical results of entire data set including unbiased samples from over 13 plant stems. The entire data set includes all types of axes of all stages of growth and ontogeny. A, Double logarithmic plot of flexural stiffness (EI) against axial second moment of area (I) (see text for explanation of neutral line). B, Double logarithmic plot of structural Young’s modulus (Estr) against axial second moment of area (I).
Table 1.
Mean values and standard deviations of area (A), second moment of area (I), structural Young’s modulus (Estr) and flexural stiffness (EI) for the four ontogenetic stages of Clematis maritima
| Stage I (n = 21) | Stage II (n = 10) | Stage III (n = 13) | Stage IV (n = 10) | |
| A (mm2) | 10·2 ± 3·2 | 12·9 ± 3·2 | 17·6 ± 4·1 | 41·6 ± 16·4 |
| I (mm4) | 8·8 ± 4·9 | 13·8 ± 6·6 | 25·5 ± 11·3 | 152·3 ± 116·6 |
| Estr (GN m–2) | 1·619 ± 0·492 | 0·855 ± 0·253 | 0·568 ± 0·142 | 0·470 ± 0·113 |
| EI (N mm–2) | 14 536 ± 10393 | 10 683 ± 4894 | 12 607 ± 3792 | 69 957 ± 46 935 |
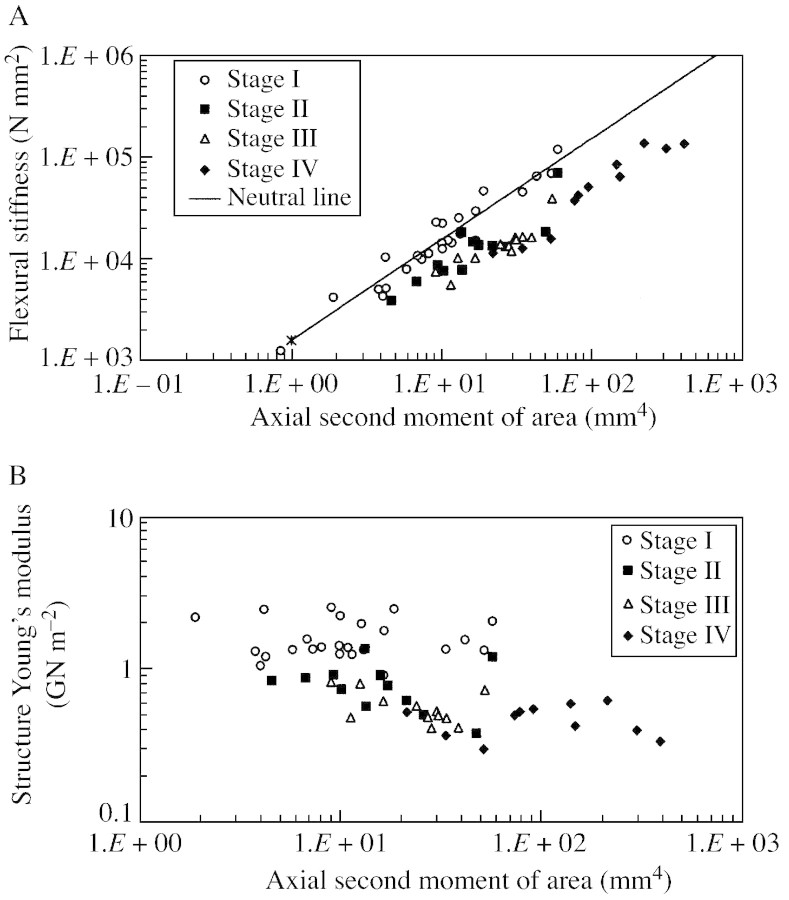
Fig. 5. Biomechanical results of different ontogenetic stages (see Fig. 2B), based on representative stages of growth from individuals in the total data set (Fig. 4). A, Double logarithmic plot of flexural stiffness (EI) against axial second moment of area (I). B, Double logarithmic plot of structural Young’s modulus (Estr) against axial second moment of area (I).
Flexural stiffness (EI).
Values of flexural stiffness varied from 1731 to 444 162 N mm–2 during ontogeny (Fig. 4A). There was an increase in flexural stiffness from young to older stages, concomitant with an increase in stem axial second moment of area (Fig. 4A). The neutral line depicted here (Speck et al., 1992; Speck, 1994b; Speck and Rowe, 1999) is based on a mean value of structural Young’s modulus calculated for young stems belonging to stage I. This graph of flexural stiffness plotted against ontogenetic stage (Fig. 4A) clearly indicates that the larger stems are positioned beneath the neutral line, which is typical of non‐self‐supporting plants, and lianas in particular (Speck, 1994b; Speck et al., 1994; Speck and Rowe, 1999). Older ontogenetic stages, from stage II to stage IV, showed relatively low values of flexural stiffness compared with those found for younger stems comprising the trellis (Fig. 5A). The first three ontogenetic stages were not clearly distinguished by external stem size as represented by second moment of area (Fig. 5A).
Structural Young’s modulus (Estr).
The structural Young’s modulus fell from 1·619 ± 0·492 GN m–2 in stage I to 0·470 ± 0·113 GN m–2 in stage IV (Fig. 4B; Table 1). This represents a reduction during ontogeny to 29 % of the initial value in stage I. The decrease in structural Young’s modulus is typical of non‐self‐supporting plants and lianas. There was significant variation in the structural Young’s modulus for stems with values of I between 1·37 and 30 mm4 (2·678–0·437 GN m–2), but in stems larger than this the modulus remained relatively stable (0·634–0·265 GN m–2) (Fig. 4B). There was a marked transition from the first to second developmental stage, where there was a significant drop in mean structural Young’s modulus from 1·619 ± 0·492 to 0·855 ± 0·253 GN m–2 (F = 17·06, P < 0·01) without significant variation in size or geometry, as seen in the relatively similar second moments of area of the two stages (Table 1; Fig. 5B). Apart from this transition, the structural Young’s modulus decreased with increasing second moment of area during stage III, this process beginning in older stems of stage II. The transition from stage III to the last stage of development was marked by an abrupt increase in second moment of area from 25·5 ± 11·3 mm4 in stage III to 152 ± 116 mm4 in stage IV, but without a drastic change in structural Young’s modulus (0·568 ± 0·142 GN m–2 in stage III compared with 0·470 ± 0·113 GN m–2 in stage IV) (Fig. 5B).
Geometrical and mechanical variation along the stem
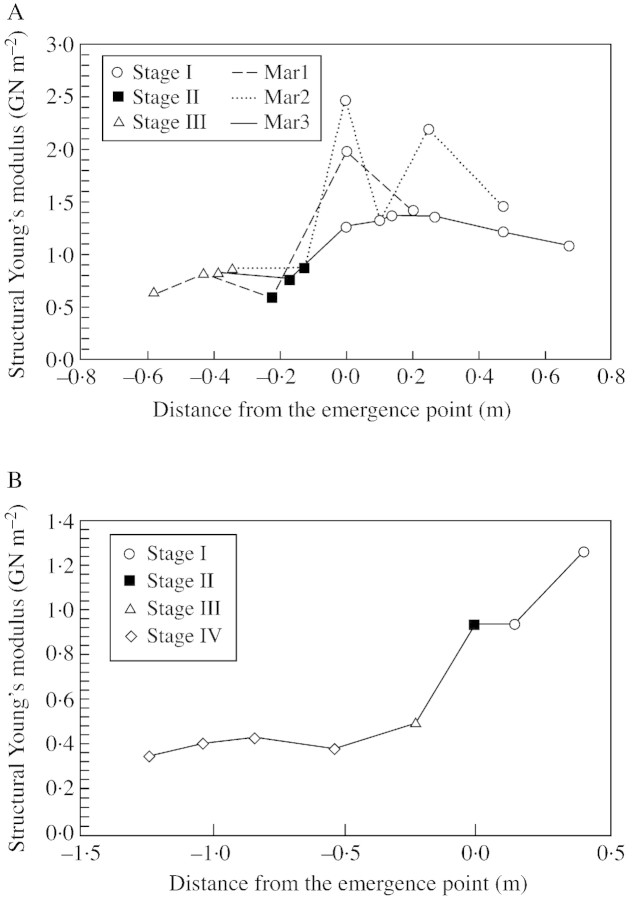
Geometrical and mechanical parameters were measured along six selected axes with respect to the point of emergence of the axis from the sand surface (Fig. 6). Values of the second moment of area tended to decrease from the most basal parts of the underground stem segment towards the sand surface. Just below the surface of the sand, values of the second moment of area either levelled off or increased slightly (Fig. 6A). An increase in size was seen most clearly along older stems (developing stage IV), characterized by significant levels of secondary growth (Fig. 6A). Values of flexural stiffness were strongly influenced by variations in I, especially in older segments, and the highest values were seen in stage IV stems, with the largest diameters (Fig. 6A and B). A peak in EI was clearly visible in all stem segments at the point of emergence from the sand surface (Fig. 6B). The increase in EI and its subsequent decrease just below ground level resulted from a basipetal increase and then either a levelling off in I (Fig. 6A) or a basipetal decrease in structural Young’s modulus (Fig. 6C). The structural Young’s modulus fell rapidly and significantly at ground level in all segments tested. The main decrease in structural Young’s modulus occurred in stage II (Fig. 7A), where there was a shift from 1·987 to 0·579 GN m–2 over 22 cm (Mar1), from 2·463 to 0·853 GN m–2 over 13 cm (Mar2) and from 1·253 to 0·747 GN m–2 over 17 cm (Mar3). A levelling off or only a slight increase in the modulus in stage III axes can be explained by reiteration of the stem where young axes issue directly from stems of stage III and so the shift from young to older axis is not linear. In one case (Fig. 7B, Mar5), there was a fall in structural Young’s modulus in stage III and then a levelling off of material properties during stage IV.
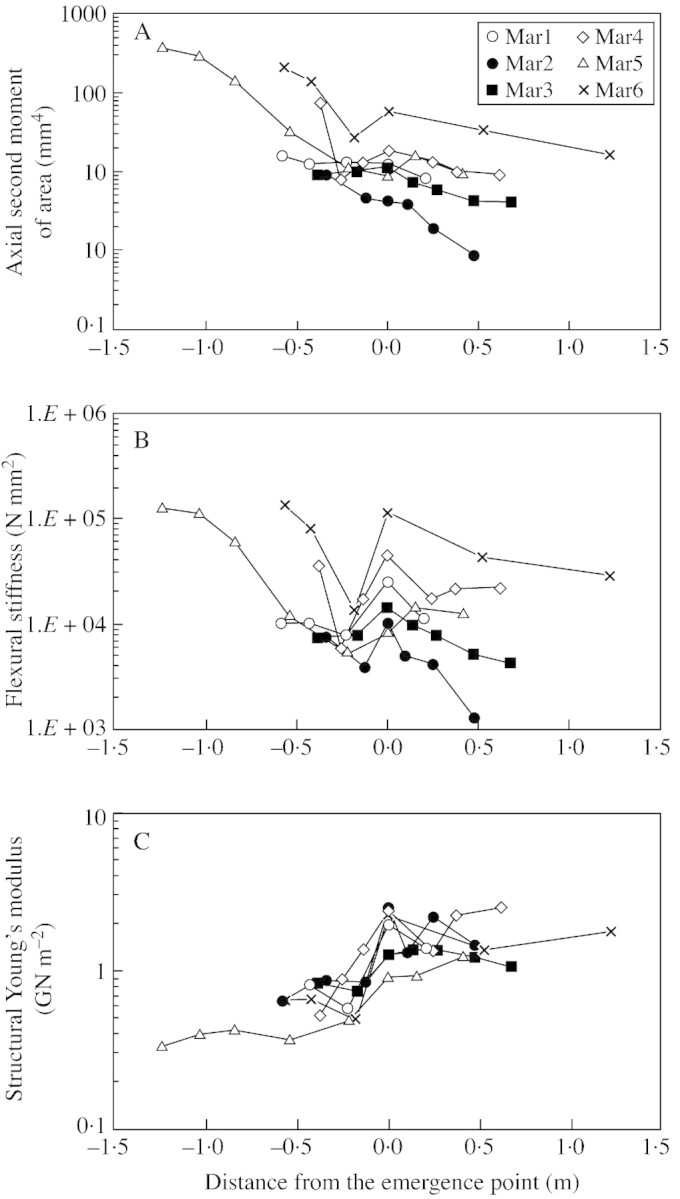
Fig. 6. Geometrical and biomechanical measurements along six representative stems (Mar1–Mar6) from underground stages to aerial stems of the trellis (0 = sand surface). A, Axial second moment of area (I); B, flexural stiffness (EI); and C, structural Young’s modulus (Estr).
Fig. 7. Structural Young’s modulus (E) and ontogenetic stages along four representative axes from underground rhizomes to aerial stems (0 = sand surface). A, Stems (Mar1–Mar3); B, Mar4.
In the majority of segments examined in this way the transition from stage I to stage II represented an important modification in material bending properties, but sometimes the decrease in E occurred in stage III segments. Interestingly, and in all cases examined, the decrease in structural Young’s modulus occurred at sand level. Further above the surface, the variation in structural Young’s modulus was random and did not exceed values of stem segments immediately around ground level. Finally, despite wide variations in E between the aerial portions of tested stems, flexural stiffness does diminish distally, closely mirroring the trend in I and the tapering of the stem.
Anatomical changes during ontogeny and their bearing on biomechanical properties of the stem
The youngest stems of ontogenetic stage I possess a parenchymatous cortex and a vascular cylinder (Fig. 8A). Collenchyma is present as more or less isolated bundles beneath the angular extensions of the stem. Primary phloem fibres are present towards the outside of the primary phloem forming dense bundles. In some cases these are implicated in the onset of secondary growth where phloem fibres are produced alongside secondary phloem (Fig. 8A). In stems of ontogenetic stage II, the initial periderm (Esau, 1958) appears in the first year of growth and is initiated in the primary phloem. Development of this periderm tissue is linked with the death and collapse of the parenchymatous cortical cells. Primary phloem fibres and the collenchyma are displaced outwards by the development of periderm and this is believed to disrupt their mechanical integrity (Fig. 8B). Secondary phloem fibres develop from the vascular cambium although secondary vascular growth remains limited.
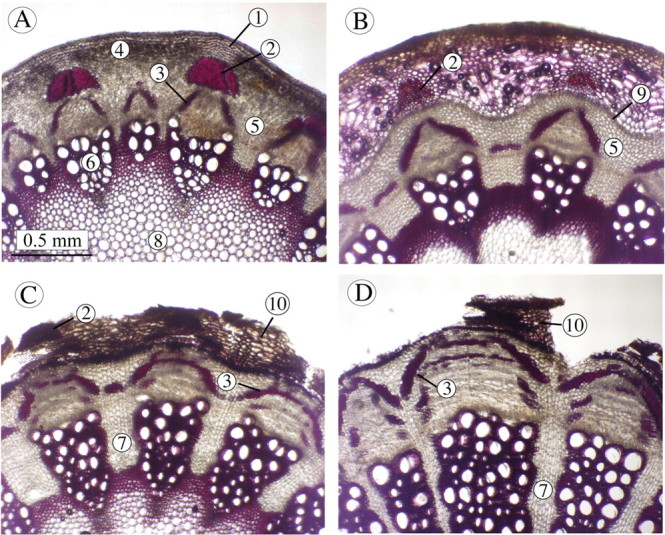
Fig. 8. Anatomy and development of four defined ontogenetic stages: A, Stage I; B, Stage II; C, Stage III; D, Stage IV. 1, Collenchyma; 2, primary fibres; 3, secondary fibres; 4, cortex; 5, cortical parenchyma; 6, xylem; 7, rays; 8, pith; 9, initial periderm; 10, sequent periderm. Sections are hand‐cut and lignified tissues are stained red/pink in HCl and phloroglucinol solution.
During stage III (Fig. 8C), sequent periderms are initiated deeper within the secondary phloem. The outer part of the cortex as well as the primary phloem detaches in a more or less continuous segment or ringbark (Esau, 1958). The cambium forms increasing amounts of ray‐like parenchymatous tissue, wood containing large vessels, and phloem tissue containing increasing amounts of secondary phloem fibres. In the oldest stages, represented by stage IV (Fig. 8D), the sequent periderm is produced in progressively deeper layers in the secondary phloem and the outer part of the periderm is detached after forming loosely attached strips of partially decorticated tissue.
The first major change in material properties of the stem occurs towards the end of stage I with the development of a phellogen within the primary phloem. This process effectively separates the mechanically important collenchyma from the rest of the stem, and severely reduces the mechanical role of this tissue in stage II (Fig. 8B). Quantitative measurements of the contribution of primary fibres to the second moment of area of the stem indicate that the reduction in contribution of primary fibres between stage I and stage II is concomitant with the reduction in structural Young’s modulus (Fig. 9A), although their contribution to I is low. This decrease is gradual throughout stage I but is more marked in stage II (Fig. 9A).
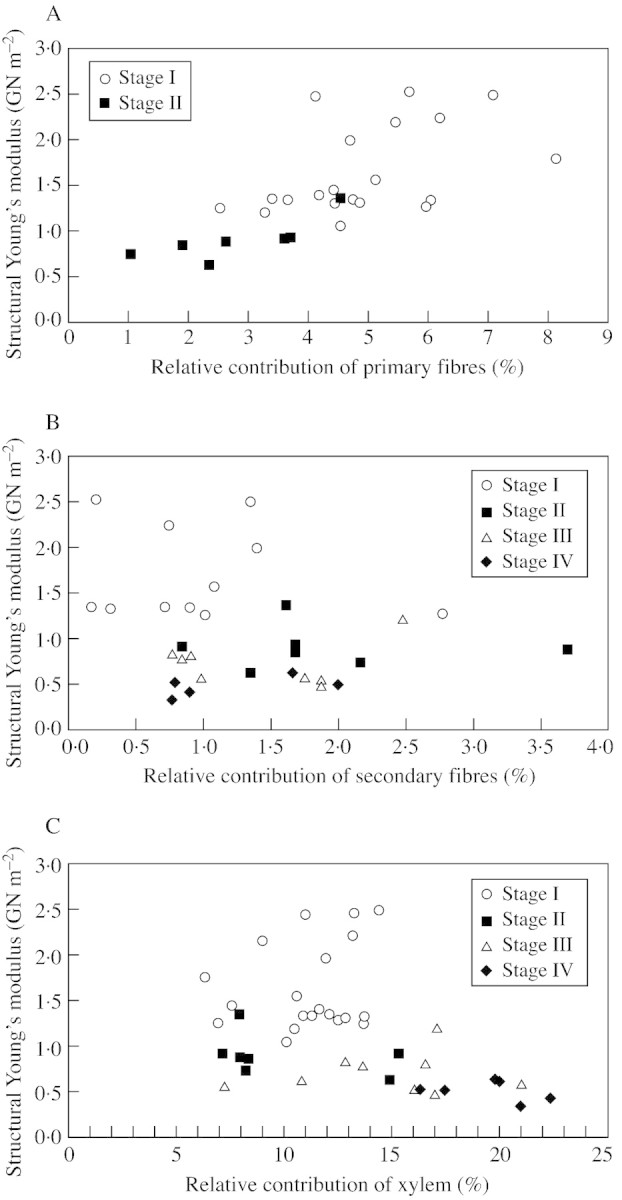
Fig. 9. Biomechanical properties (structural Young’s modulus) plotted against relative contribution of major mechanical tissues to second moment of area of the stem for each ontogenetic stage. A, Relative contribution of primary fibres in the first and second ontogenetic stages; B, relative contribution of secondary fibres; C, relative contribution of xylem.
In most cases, the total removal of fibres by stage III does not appear to influence significantly the value of the structural Young’s modulus (Fig. 7A) which, by this point, has reached almost its lowest value. This might be explained by the very low contribution of the primary fibres by stage II (approx. 2·8 %), as well as by their organization. After stage II the fibres become loosened with respect to the surrounding tissues. They are also partially disrupted from the interior part of the stem (Fig. 8B) after the development of the initial periderm, and are probably less efficient in resisting bending forces. By this stage of development the fibres probably no longer contribute significantly towards the stiffness of the stem, although they are still present in the stem cross‐section. In some cases (Fig. 7B), the decrease in structural Young’s modulus does not occur before stage III. It appears that the bands of fibres can remain intact and functional right up to, or near to, the point at which they are sloughed away with the remaining periderm.
Fibres of the secondary phloem contribute little to the second moment of area (mean percentage contribution to I = 1·37, n = 17), and variation in the contribution to I appears to be independent from that to the structural Young’s modulus (Fig. 9B). Quantitative studies show low contributions for both primary and secondary fibres, but the correlation of their contribution with changes in structural Young’s modulus emphasizes the mechanical role of fibres produced by the primary phloem. This difference in mechanical influence is probably explained by the organization in compact strands of primary fibres.
The contribution of secondary xylem to the second moment of area is relatively low and constant (average = 10·5 %) in young ontogenetic stages (Fig. 9C). However, during young ontogenetic stages there is, nevertheless, a significant drop in structural Young’s modulus of the stem, from approx. 2·7 to 1·0 GN m–2. In older axes (stages III and IV), there is a further drop in structural Young’s modulus, which is accompanied by an increase in the contribution of xylem to 15 % (stage III) and, finally, to 20·2 % (stage IV) (Fig. 9C). Xylem tissue observed in young ontogenetic stages is not dense and contains relatively thin‐walled elements and large vessels (Fig. 8A and B). This tissue probably does not contribute significantly to stem stiffness in young stages of growth. The anatomical changes contributing to the fall in structural Young’s modulus are probably the result of: (1) the reduced relative contribution and reduced bonding of primary fibres by stages III and IV; (2) the development of a compliant periderm near the periphery of the stem; and (3) the development and increase in contribution of compliant wood, including parenchymatous infrafascicular ray‐like tissue.
DISCUSSION
Biomechanics of a ‘lianoid’ sand dune‐adapted plant
Based on all the general biomechanical data, the trend in mechanical properties in Clematis maritima during development follows that of other non‐self‐supporting plants, such as woody lianas, in which a decrease in structural Young’s modulus is seen during ontogeny (Speck, 1991, 1994b; Rowe and Speck, 1996, 1997, 1998; Speck et al., 1996; Speck and Rowe, 1999). Although old stages of C. maritima show similar properties to those of most other lianas studied, in that they have a very low structural Young’s modulus, the young stages of this species are not especially stiff and have lower values of Estr compared with those of many other lianas tested (Table 2). As a result, the developmental fall in structural Young’s modulus of about 70 % is somewhat less than that of many other lianas in which the value of the structural Young’s modulus of the oldest ontogenetic stages is less than 15 % of that of the youngest stage (Table 2) (Speck, 1991; Rowe and Speck, 1996; Speck et al., 1996; Gallenmüller et al., 2001).
Table 2.
Values of structural Young’s modulus (Estr) for the youngest and oldest stages and the factor of decrease of structural Young’s modulus during ontogeny for eight climbing species (Speck et al., 1996) compared with that for Clematis maritima
| Structural Young’s modulus (GN m–2) | |||
| Species | Youngest ontogenetic stage | Oldest ontogenetic stage | Relative change |
| Aristolochia macrophylla | 2·47 ± 0·48 | 0·5 ± 0·39 | 0·20 |
| Clematis vitalba | 3·46 ± 0·6 | 0·56 ± 0·09 | 0·16 |
| Fallopia aubertii | 2·74 ± 0·29 | 0·73 ± 0·15 | 0·27 |
| Maripa scandens | 4·11 ± 0·51 | 0·39 ± 0·03 | 0·09 |
| Passiflora glandulifera | 4·54 ± 0·62 | 0·63 ± 0·13 | 0·14 |
| Condylocarpon guianense | 2·72 ± 0·9 | 0·31 ± 0·05 | 0·11 |
| Bauhinia guianensis | 8·48 ± 2·82 | 0·35 ± 0·1 | 0·04 |
| Doliocarpus sp. | 6·87 ± 0·98 | 0·29 ± 0·03 | 0·04 |
| Clematis maritima | 1·61 ± 0·46 | 0·47 ± 0·14 | 0·29 |
As in many woody plants, the increase in flexural stiffness (EI) in C. maritima follows that of many growth forms, and results largely from an increase in total second moment of area (I). As a result of an increase in compliant tissues and a decrease in stiff tissues during ontogeny, the structural Young’s modulus decreases during development. As in many other lianas tested, such stems are considerably less stiff than those of similar diameters characterizing typical self‐supporting plants in which a basipetal increase in EI comprises an increase in I as well as an increase in Estr (Speck, 1994a; Speck and Rowe, 1999). Among climbing lianas, a relatively low value of EI in older stems reflects a number of properties by which the plant avoids mechanical stresses that result from movement and breakage of the host vegetation, and optimizes hydraulic supply rather than developing mechanical stiffness (Dobbins and Fisher, 1986; Fisher and Ewers, 1989; Fisher and Ewers, 1991; Putz and Holbrook, 1991). In the case of C. maritima, older ontogenetic stages of the liana are usually buried in sand and effectively function as a rhizome.
In young growth stages of many lianas the axes must be stiff to reach host supports or traverse spaces to colonize new surfaces. This mechanical strategy is also deployed in C. maritima for the development of aerial trellises. The positional data described above indicate that flexural stiffness is increased or optimized via changes in both I and Estr at the level of emergence from the sand surface. Self‐supporting trellis members, or at least temporarily self‐supporting trellis members, are advantageous for this geophytic mode of life where emergent monospecific or clonal trellises are developed in the absence of host supports, and where relatively rigid young axes supply mutual support and climbing surfaces. The mechanical structure and variation of young axes constituting the trellises are complex, with wide variations in mechanical and geometric properties in ontogenetic stages I and II. It is possible that some axes serve as relatively stiffer support members of the structure, whereas others, which perhaps develop later, are able to climb and bind on to these, each showing different geometric and mechanical properties. Similar variation has been observed among twining tropical lianas (Rowe and Speck, 1996) where similarly aged young stems might show variable mechanical properties according to their precise growth mode—host or climber—within the trellis structure.
Tissue development and biomechanics of the stem/‘rhizome’ system
Qualitative and quantitative assessments of changes in anatomy indicate that primary fibres are important for maintaining stiff mechanical properties during the early phase of development. Young axes have higher values of structural Young’s modulus than do older stages and this is reflected by the presence of fibre tissues, primary phloem fibres and collenchyma placed near the periphery of young axes.
The decrease in the contribution of fibres to the second moment of area in young stems during ontogeny is broadly coherent with the decrease in structural Young’s modulus from 1·619 ± 0·492 GN m–2 in stage I to 0·855 ± 0·253 GN m–2 in stage II. The relatively wide variation observed could be due to the interaction of other tissues contributing to mechanical variation, such as relative proportions of wood and ray tissue, or smaller scale variation of the fibre tissue itself, such as ultrastructural or biochemical variation (Speck et al., 1996; Köhler et al., 2000). The main mechanical shift during development takes place in the second ontogenetic stage with the development of an initial periderm tissue in the phloem combined with the disruption of fibre bundles and sloughing off of the outer peripheral areas of collenchyma. This complex anatomical process underlies the decrease in the contribution of the primary fibres and collenchyma to the second moment of area and, therefore, the initial fall in structural Young’s modulus in stage II. The sloughing off of fibre bundles and the development of compliant wood in the third ontogenetic stage contribute to the further decrease in structural Young’s modulus characterizing the underground axes of older stages. As in many other lianas, secondary growth eliminates the stiff primary‐formed fibre tissue and produces a compliant type of wood, which is primarily responsible for further decreases in structural Young’s modulus of the stem. Modifications in the material properties of stems apparently occur rapidly during the first year of growth. The mechanical role of the secondary fibre tissue appears to be negligible in contributing to the stiffness of the stem. However, secondary‐formed fibres associated with the phloem tissue persist for the rest of the development of the plant, possibly as a protective sheath enclosing the phloem tissue.
CONCLUSION
The establishment of Clematis flammula var. maritima on sand dunes is an example of a lianoid growth form occupying an ecological niche radically different from that which characterizes other lianescent species of the genus. The mechanical signal characterizing its growth form does not show a large degree of modification compared with that of lianoid forms. Older aerial axes characteristic of C. flammula var. maritima have become underground rhizome‐like stems, and the variety has retained the bending properties of aerial stems of climbing lianas. Reiterative growth from underground axes produces a large number of stiff, upright annual axes, with mechanical properties that allow the development of an aerial trellis in an unstable environment, whilst being effectively ‘rooted’ just below the surface of the sand. The increase in flexural stiffness towards the base of aerial axes results from an increase in size as well as an increase in structural Young’s modulus. However, the structural Young’s modulus of young axes of this species is somewhat lower than that recorded for many other lianas, but it is sufficient for a trellis up to 40 cm high to be formed in the absence of other host plants.
Lianoid biomechanical properties in this species are effectively deployed and adapted for a ‘mobile’ substrate and in the absence of large or extensive host plants. One of the problems that sand dune plants have to accommodate is the movement of sand dunes and the risk of becoming buried or exposed, either after short‐term storms or long‐term sand movement. In Clematis, tendrils are formed from modified leaves and are responsible for attaching to host plants. In C. maritima, these structures form essential connecting devices for forming trellises when other host species are unavailable and where a self‐supporting phase of growth can be curtailed. Climbing growth habits have evolved independently in many groups of plants, suggesting a strong selective pressure for non‐self‐supporting growth habits. Despite the tendency for evolution towards climbing strategies, the reversal or evolution back to self‐supporting growth forms is less well documented, and the possibility exists that the development of lianescent growth forms involves a high developmental burden which is difficult to change or escape from (Speck et al., 1997). Clematis maritima is a striking example of a plant with a lianoid biomechanical architecture that is apparently modified only slightly but which allows the plant to develop successfully in a quite different habitat where there is little available support.
Lianoid plants might be suited to sand dune environments and may be of value as sand dune‐stabilizing species. Their perennial underground stems can withstand the mobile sand dune environment and can provide an extensive and protected source of future surface growth from underground buds, whilst their aerial trellises can form extensive mats on the sand surface, helping to stabilize sand dunes.
ACKNOWLEDGEMENTS
This work was carried out during receipt of travelling and maintenance grants awarded by the OFAJ (France) and the DAAD (Germany) to S.I. and by a PROCOPE exchange grant awarded to N.R. and T.S. This financial aid is gratefully acknowledged. We thank Guy Caballé, Karl Niklas and Julian Vincent for helpful comments.
Supplementary Material
Received: 25 July 2002; Returned for revision: 27 September 2002; Accepted: 15 November 2002 Published electronically: 16 January 2003
References
- BhambieS.1972. Correlation between form, structure and habit in some lianas. Proceedings of the Indian Academy of Sciences 75: 246–256. [Google Scholar]
- BonnierG.1912. Flore compléte de France, Suisse et Belgique, I. Paris: Orlhac. [Google Scholar]
- CaballéG.1993. Liana structure, function and selection: a comparative study of xylem cylinders of tropical rainforest species in Africa and America. Botanical Journal of the Linnean Society 113: 41–60. [Google Scholar]
- CaballéG.1998. Le port autoportant des lianes tropicales: une synthèse des stratégies de croissance. Canadian Journal of Botany 76: 1703–1716. [Google Scholar]
- CosteH.1901. Flore de la France, I. Paris: Librairie des Sciences Naturelles. [Google Scholar]
- DarwinC 1867. On the movements and habits of climbing plants. Journal of the Linnean Society 9: 1–118. [Google Scholar]
- Den DubbeldenKC, Oosterbeek B.1995. The availability of external support affects allocation pattern and morphology of herbaceous climbing plants. Functional Ecology 9: 628–634. [Google Scholar]
- DobbinsDR, Fisher JB.1986. Wound responses in girdled stems of lianas. Botanical Gazette 147: 278–289. [Google Scholar]
- EsauK 1958. Plant anatomy. New York: John Wiley and Sons, Inc. [Google Scholar]
- FisherJB, Ewers FW.1989. Wound healing in stems of lianas after twisting and girdling. Botanical Gazette 150: 251–265. [Google Scholar]
- FisherJB, Ewers FW.1991. Structural responses to stem injury in vines. In: Putz FE, Holbrook NM, eds. The biology of vines Cambridge: Cambridge University Press, 99–124. [Google Scholar]
- GallenmüllerF, Müller U, Rowe NP, Speck T.2001. The growth form of Croton pullei (Euphorbiaceae) – functional morphology and biomechanics of a neotropical liana. Plant Biology 3: 50–61. [Google Scholar]
- GartnerBL.1991a Is the climbing habit of poison oak ecotypic? Functional Ecology 5: 696–704. [Google Scholar]
- GartnerBL.1991b Relative growth rates of vines and shrubs of western poison oak, Toxicodendron diversilobum (Anacardiaceae). American Journal of Botany 78: 1345–1353. [Google Scholar]
- JaffeMJ.1973. Thigmomorphogenesis: the response of plant growth and development to mechanical stimulation. Planta 114: 143–157. [DOI] [PubMed] [Google Scholar]
- KöhlerL, Speck T, Spatz HC.2000. Micromechanical and anatomical changes during early ontogeny of two lianescent Aristolochia species. Planta 210: 691–700. [DOI] [PubMed] [Google Scholar]
- NiklasKJ.1992. Plant biomechanics: an engineering approach to plant form and function. Chicago: University of Chicago Press. [Google Scholar]
- NiklasKJ.1994. Comparison among biomass allocation and spatial distribution patterns of some vine, pteridophyte, and gymnosperm shoots. American Journal of Botany 81: 1416–1421. [Google Scholar]
- ObatonM.1960. Les lianes ligneuses à structure anormale des forêts denses d‘Afrique occidentale. Paris: Masson et Cie. [Google Scholar]
- PeñalosaJ.1983. Shoot dynamics and adaptive morphology of Ipomoea phillomega (Vell.) House (Convolvulaceae), a tropical rainforest liana. Annals of Botany 52: 737–754. [Google Scholar]
- PutzFE.1984. The natural history of lianas on Barro Colorado Island, Panama. Ecology 65: 1713–1724. [Google Scholar]
- PutzFE, Holbrook NM.1991. Biomechanical studies of vines. In: Putz FE, Mooney HA, eds. The biology of vines Cambridge: Cambridge University Press, 73–97. [Google Scholar]
- RoweNP, Speck T.1996. Biomechanical characteristics of the ontogeny and growth habit of the tropical liana Condylocarpon guianense (Apocynaceae). International Journal of Plant Science 157: 406–417. [Google Scholar]
- RoweNP, Speck T.1997. Biomechanics of Lycopodiella cernua and Huperzia squarrosa: implications for inferring growth habits of fossil small‐bodied lycopsids. Mededelingen Nederlands Instituut voor Toegepaste Geowetenschappen TNO 58: 293–302. [Google Scholar]
- RoweNP, Speck T.1998. Biomechanics of plant growth forms: the trouble with fossil plants. Review of Palaeobotany and Palynology 102: 43–62. [Google Scholar]
- SpeckT.1991. Changes of the bending mechanics of lianas and self‐supporting taxa during ontogeny. Natural structures. Principles, strategies and models in architecture and nature. Proceedings of the II International Symposium Sonderforschungsbereich 230, part I. Mitteilungen des SFB 230 Heft 6: 89–95. [Google Scholar]
- SpeckT.1994a Bending stability of plant stems: ontogenetical, ecological, and phylogenetical aspects. Biomimetics 2: 109–127. [Google Scholar]
- SpeckT 1994b A biomechanical method to distinguish between self‐supporting and non self‐supporting fossil plants. Review of Paleobotany and Palynology 81: 65–82. [Google Scholar]
- SpeckT, Rowe NP.1999. A quantitative approach for analytically defining size, growth form and habit in living and fossil plants. In: Kurmann MH, Hemsley AR, eds. The evolution of plant architecture Kew: Royal Botanic Gardens, 447–479. [Google Scholar]
- SpeckT, Rowe N, Vogellehner D.1994. Growth habits in plants and their correlation with stem’s functional anatomy and biomechanics. II: Fossil plants with secondary growth. Architecture, structure, mécanique de l’arbre. Cinquième séminaire interne, Paris 5. [Google Scholar]
- SpeckT, Neinhuis C, Gallenmüller F, Rowe NP.1997. Trees and shrubs in the mainly lianescent genus Aristolochia s.l.: secondary evolution of the self‐supporting habit? In: Jeronimidis G, Vincent JFV, eds. Plant Biomechanics Reading: University of Reading, 201–208. [Google Scholar]
- SpeckT, Rowe NP, Brüchert F, Haberer W, Gallenmüller F, Spatz HC.1996. How plants adjust the ‘material properties’ of their stems according to differing mechanical constraints during growth: an example of smart design in nature. ESDA (Proceeding of the Engineering Systems Design and Analysis Conference) 77: 233–241. [Google Scholar]
- TutinTG, Heywood VH, Burges NA, Valentine DH, Walters SM, Webb DA.1964. Flora Europaea, I – Lycopodiaceae to Platanaceae. Cambridge: Cambridge University Press. [Google Scholar]
- VincentJFV.1990. Structural biomaterials. Princeton: Princeton University Press. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.