Abstract
• Background and Aims Border cells are released from the root tips of many plant species, and can remain viable in the rhizosphere for 1 week. Whether border cells are capable of controlled glucose exchange with their environment was investigated.
• Methods Border cells were removed from Zea mays L. root tips, and immersed in 14C‐labelled d‐glucose. In one experiment, the hexose transport inhibitor, phlorizin, was used to investigate active glucose uptake from a range of glucose concentrations. In another experiment, glucose efflux from border cells was monitored over time.
• Key Results Glucose uptake by the border cells increased with increasing glucose concentration from 0·2 to 20 mm. At 0·2 mm glucose, uptake was mainly active, as evidenced by the approx. 60 % inhibition with phlorizin. At 2 and 20 mm glucose, however, uptake was mainly via diffusion, as phlorizin inhibition was negligible. Glucose efflux increased with time for live border cells in both 2 and 20 mm glucose. There was no clear efflux/time pattern for heat‐killed border cells.
• Conclusions Border cells actively take up glucose, and also release it. Under our experimental conditions, glucose uptake and efflux were of similar order of magnitude. In the rhizosphere net glucose exchange will almost certainly depend on local soil conditions.
Key words: glucose exchange, carbon flow, border cells, glucose uptake, sugar efflux
Introduction
A layer of detached, living cells surrounds the root tips of many plant species (Hawes and Pueppke, 1986; Arriola et al., 1997). These ‘root border cells’ (Hawes and Lin, 1990) originate in the root cap meristem. They migrate through the cap, performing a variety of functions en route including starch synthesis, gravitropism and mucilage secretion, before becoming detached from the root cap surface due to enzyme action (Hawes and Lin, 1990; Stephenson and Hawes, 1994; Brigham et al., 1995; Wen et al., 1999). As the root grows, border cells and the associated root cap mucilage become distributed up to several centimetres behind the root cap. Border cells have intact peripheral cytoplasm and ‘normal’ organelles (Vermeer and McCully, 1982). They can remain viable for up to 3 months on agar (Guinel and McCully, 1987) and for 1 week or more in the rhizosphere (Vermeer and McCully, 1982), and thus have the potential for interaction with rhizosphere microorganisms (Sherwood, 1987; for a review, see Hawes et al., 1998).
Mucilage is released by peripheral root cap cells, but there is little evidence that border cells produce mucilage after detachment. Guinel and McCully (1987) observed that dictyosomes are smaller in border cells than in the mucilage‐secreting layers of the root cap, and Clowes and Juniper (1968) noticed a change in the appearance of root cap cell Golgi bodies after border cell detachment: Golgi bodies in cells in the periphery of the root cap are surrounded by hypertrophied Golgi vesicles, whereas those in border cells are not. Gochnauer et al. (1990) suggest that detached border cells must import fixed carbon from surrounding mucilage, as they apparently have negligible starch or lipid reserves. It is conceivable, then, that border cells could even compete with rhizosphere micro‐organisms for nutrients from root exudates, so interfering with microbe colonization of the root tip.
In vitro studies have shown that root protoplasts favour glucose uptake over sucrose (Lin et al., 1984; Getz et al., 1987). This uptake may be passive or active (Felle et al., 1983) depending on the concentration of glucose in the external medium, with active proton co‐transport occurring at low external glucose concentrations and passive uptake at high external concentrations (Getz et al., 1987; Stanzel et al., 1988a, b). Efflux of sugars from whole root tips is mainly via passive diffusion and does not require metabolic energy (Jones and Darrah, 1996). Measurement of glucose exchange in border cells has not been reported previously. In this study, glucose uptake and efflux from border cells was measured in vitro. Glucose uptake was compared with and without the hexose co‐transport inhibitor, phlorizin (Felle et al., 1983), to determine whether uptake is an active process.
MATERIALS AND METHODS
Border cell collection
Zea mays L. caryopses were surface‐sterilized using 10 mg L–1 tetracyline hydrochloride then 0·1 % silver nitrate (Speakman and Kruger, 1983), and germinated at 25 °C between sheets of sterile, moist filter paper in 14‐cm Petri dishes placed at 40° to the vertical. Border cells were collected from 2–3 d‐old‐seedlings (primary root 25–35 mm long). The root tips were hydrated for 15–20 min in 1 mL sterile distilled water in 1·5‐mL microcentrifuge tubes and wiped along the inside of the tubes to release the cells.
Glucose uptake
Border cells were collected from 850 Zea mays ‘KXO141’ roots, pooled, and washed onto 5‐µm nylon mesh in a sterile vacuum filter apparatus to remove root cap mucilage. The mesh was immersed in buffer solution [25 mm MES‐KOH to pH 6, and 20 mm CaCl2, with 400 mm mannitol (Wyse et al., 1986)], and the cells starved overnight (14 h) in an orbital incubator at 25 °C, with 200 r.p.m. agitation for increased aeration.
Sterile glucose solutions of 0·2, 2 and 20 mm in buffer (as above) were prepared with and without 2 mm phlorizin hydrate, a specific inhibitor of hexose carriers in the plasmalemma (Felle et al., 1983). The pH of these incubation solutions was adjusted to 5·5 (optimum for activity of the cell proton pumps; Getz et al., 1987), then 1‐mL aliquots were pipetted into sterile 1·5 mL microcentrifuge tubes. A glucose‐free buffer‐only solution was included as a control.
A sterile mini‐vacuum‐filter apparatus was used to wash the border cells from 1‐mL aliquots of vortexed starvation medium on to discs of 5‐µm nylon mesh. One mesh disc was shaken for 20 s in each 1 mL of incubation solution to release the border cells, and 10 µl suspension per tube was removed for cell counts. 14C (20 KBq) in labelled d‐glucose (20 µl) (Sigma‐Aldrich, Poole, Dorset, UK) was added to each replicate of the ‘+ glucose’ treatments immediately after the addition of cells, and the suspensions incubated for 2 h at 22 °C. The remaining cell suspension was pipetted onto nutrient agar for a sterility check.
After incubation, cells were washed from the incubation solutions on to fresh sterile 5‐µm mesh discs using buffer solution. The washings were collected for scintillation counts (Minaxi Tri‐Carb 4000 Series; Packard Bioscience, Pangbourne, Berkshire, UK), for confirmation of the removal of all 14C not closely associated with the border cells. The mesh discs with border cells were placed into McCartney bottles and covered with 5 mL chromic acid, and a glass vial containing 1 mL of 2 m sodium hydroxide was placed into each bottle. Bottles were sealed, autoclaved, and scintillation counts were obtained from the sodium hydroxide. Scintillation counts were used to calculate the glucose uptake as follows:
Gup= Bq × (14CG + G)/(Bqtot × ctot)
where Gup is glucose uptake in µg C cell–1, Bq is activity in bequerels, 14CG is µg glucose in labelled carbon, G is µg glucose, Bqtot is total activity of incubation solution in bequerels and ctot is number of border cells.
Glucose efflux
Border cells from Zea mays ‘Renard’ were twice washed free of excess mucilage in a bench‐top centrifuge (Minispin plus; Eppendorf, Hamburg, Germany) at 14000 g for 5 min, and pooled into tubes of 2 or 20 mm glucose solutions (20 mL per replicate). Cells in half of the replicates from each glucose concentration were killed by placing the tubes into boiling water for 10 min, then 40 KBq 14C‐labelled glucose was added to each replicate. Control treatments without border cells were also included.
Border cells were incubated at 18 °C in the dark overnight (16 h) to allow glucose uptake, then washed through sterile 5‐µm nylon mesh three times with sterile cold (unlabelled) glucose until scintillation readings from the final washings indicated the removal of all external 14C. The border cells from each replicate were then transferred into a further 20 mL sterile cold glucose for efflux. The glucose concentrations used in glucose uptake, washing and efflux solutions remained constant for each treatment throughout. Aliquots (500 µl) of the efflux solutions were taken for scintillation readings immediately after set‐up, and again after 2, 20 and 30 h. Glucose efflux was calculated from these scintillation counts, after subtracting the counts for the cell‐free treatments, using the same formula as for glucose uptake. Scintillation counts were also done on the final cell suspension and chromic acid digestion done (as before) to obtain a 14C mass balance and confirm that all of the 14C had been accounted for.
Measurement of total organic carbon (TOC)
Border cells were obtained and washed clean of excess mucilage as in the efflux experiment. Cells were incubated in sterile distilled water at 15 °C for 24 h. Border cells were removed with centrifugation and retained for cell counts, and the supernatant filter‐sterilized using a 2‐µm filter (Nalgene, Hereford, UK). This ‘border cell exudate’ was tested for TOC, measured by UV oxidation and IR gas analysis using a Labtoc analyser (Pollution and Process Monitoring, Sevenoaks, UK), in three separate experiments.
Statistical analysis
Data were analysed using the statistical software package Genstat, version 6·1 (Lawes Agricultural Trust). Square root transformation of the glucose uptake data was necessary for normalization prior to analysis of variance. Repeated measures analysis was performed on the glucose efflux data. The standard error of difference between means (s.e.d.) is included in the table and figures for both experiments.
Results
Glucose uptake
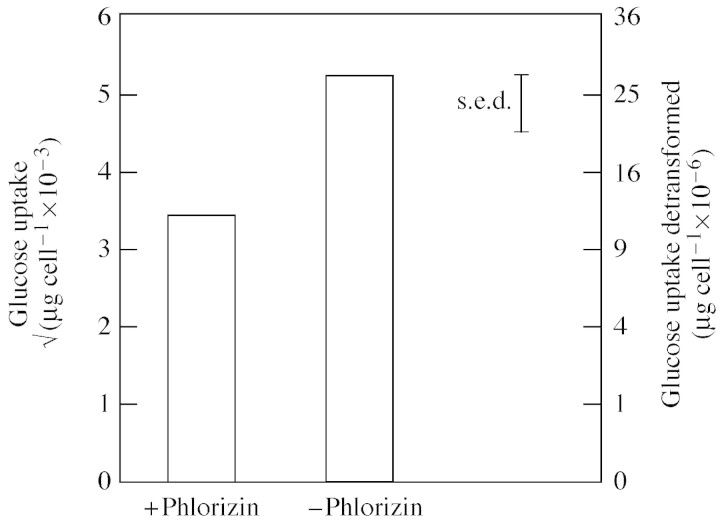
Glucose uptake by border cells increased (P < 0·001) with increasing glucose concentration in the incubation solution (Table 1). At the lowest glucose treatment (0·2 mm), phlorizin significantly inhibited glucose uptake (P < 0·05). This inhibition was almost 30 % of the non‐phlorizin control (Fig. 1). Phlorizin did not cause any significant difference in glucose uptake from 2 or 20 mm glucose (data not presented). Starved border cells plated on to nutrient agar demonstrated no sign of contamination after 48 h incubation.
Table 1.
Glucose uptake by detached border cells, calculated from [14C]glucose in the cells after 2 h incubation
| Glucose concentration in incubation solution (mm) | Glucose uptake (square root transformed × 10–3) | Glucose uptake (detransformed) (µg cell–1 × 10–6) |
| 0·2 | 4·34 | (18·8) |
| 2 | 7·93 | (62·9) |
| 20 | 16·79 | (281·9) |
| s.e.d. | 1·622 | |
| d.f. | 18 |
Data are means of five replicates.
Fig. 1. Phlorizin (2 mm) inhibition of 0·2 mm glucose uptake by border cells after 2 h. Data are means of five replicates.
Glucose efflux
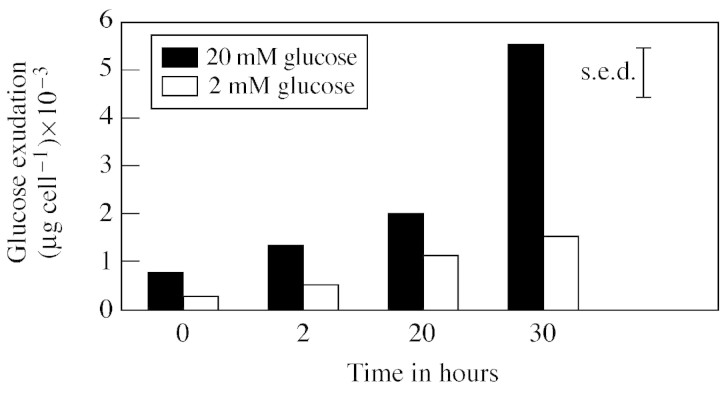
Glucose efflux from live root border cells increased (P < 0·05) over time (Fig. 2), with a more marked effect in 20 mm glucose than in 2 mm glucose. There was no such trend with the dead cells (data not presented). A mass balance, from scintillation counts from the final cell suspension and chromic acid digestion, indicated that all of the 14C had been accounted for.
Fig. 2. Glucose efflux from live border cells over time, calculated from radioactivity efflux into cold glucose after approx. 16 h incubation in [14C]glucose of the same concentration. Data are the means of five replicates.
Total organic carbon (TOC)
The mean organic carbon content of the border cell exudates was 4·9 µg C root–1 (s.e. = 0·54), calculated from three similar experiments. The mean border cell count from these experiments was approx. 2000 border cells per root, so that about 2·5 × 10–3 µg C would have been released per cell.
Discussion
Border cells are clearly capable of glucose uptake. The demonstrated effect of phlorizin on uptake from 0·2 mm glucose implies a blocking of the active proton transport mechanism used for hexose movement in and out of plant cells. The degree of inhibition caused by phlorizin was comparable with previously published data (Felle et al., 1983; Getz et al., 1987) for other plant cells. However, no phlorizin inhibition of glucose uptake was observed from 2 or 20 mm solutions, indicating that at these concentrations the glucose was entering the cells mainly via diffusion. This is also in accordance with the literature (Getz et al., 1987; Stanzel et al., 1988a, b). The mean uptake of 2·8 × 10–4 µg glucose per cell from 20 mm glucose can also be expressed as approx. 25 µmoles glucose g f. wt–1 h–1, using fresh weight estimates from border cell dimensions in Iijima et al. (2000). This figure is similar to the approx. 22 µmoles glucose g f. wt–1 h–1 uptake in Stanzel et al. (1988b) for Streptanthus suspension cells.
The quantity of glucose released from border cells increased with time for live cells, but there was no clear pattern for the heat‐killed cells, probably due to membrane damage. Net glucose loss from live cells would have been limited by the intact plasmalemma, cell metabolism and simultaneous glucose uptake. According to Jones and Darrah (1996), efflux of sugars from the root is not an active process, as it does not require metabolic energy, and it is likely that efflux of glucose from border cells is also passive. The relatively large efflux from border cells in 20 mm glucose, compared with those in 2 mm glucose, is almost certainly due to the increase in the rate of diffusive exchange between external cold glucose and internal labelled glucose (Gogarten and Bentrup, 1989). The relatively large increase in glucose efflux at 20 mm towards 30 h may also have been partly due to a deterioration in membrane integrity of border cells, whereas at 0·2 mm glucose, osmoregulation between the cell and the cell wall may have reduced the loss of small molecular weight solutes into solution (Wyse et al., 1986). In addition, border cells in the lower glucose concentration would probably have been metabolizing a greater proportion of the sugars taken up from both incubation and efflux solutions, reducing their net release into the efflux medium. Characterization of 14C within the cells would elucidate its fate but this was beyond the scope of this experiment.
A mean estimate of 1·5 × 10–4 µg glucose cell–1 h–1 (5·8 × 10–5 µg C cell–1 h–1) can be obtained from the data for efflux into 20 mm glucose in the 14C efflux experiment. This is in line with the TOC figure of 2·5 × 10–3 µg C cell–1 from 24 h efflux, particularly as the TOC measurements would have included amino acids (Brigham et al., 1995) and other forms of carbon as well as sugars, while the 14C efflux experiment measured glucose efflux only.
To relate glucose uptake to efflux, the duration of the labelled glucose uptake phase must be taken into account. An estimated mean 1·5 × 10–4 µg glucose cell–1 h–1 was released in the 24 h during the second 14C experiment, with an uptake phase of 16 h. The maximum glucose uptake was 1·4 × 10–4 µg glucose cell–1 h–1 from 20 mm glucose. If it is assumed that glucose uptake is linear with time (Lin et al., 1984; Getz et al., 1987) due to glucose metabolism, it is estimated that glucose uptake would have totalled 2·3 × 10–3 µg glucose cell–1 (0·9 × 10–3 µg C cell–1) after the 16‐h uptake period. The estimates made here for influx and efflux of carbon for border cells are therefore of a similar order of magnitude. The number of border cells present [in turn dependent on genotype (Hawes and Pueppke, 1986; V. E. C. Stubbs, unpub. res.) and soil compaction (Iijima et al., 2000)], would also be important in determining the direction of net carbon flow. Additional carbon would also be released into the rhizosphere with border cell senescence and death.
In conclusion, border cells can actively take up glucose. Efflux of glucose from border cells was of a similar order of magnitude to glucose uptake under the experimental conditions described here, and no evidence was found that border cells are specially adapted for glucose exudation. Net carbon flow in the rhizosphere is therefore likely to depend on local soil conditions, including the presence of soil biota.
Acknowledgements
We thank Prof. John Farrar and Dr Ian Bingham for helpful discussions, and Dr Sara Preston, Dr Jules Dawson and Dr Dave Genney for their assistance in the laboratory. The Scottish Crop Research Institute receives grant‐in‐aid from the Scottish Executive Environment and Rural Affairs Department. This work was funded by the Biotechnology and Biological Sciences Research Council.
Supplementary Material
Received: 6 August 2003;; Returned for revision: 3 October 2003; Accepted: 16 October 2003
References
- Arriola, L, Niemira, BA, Safir, GR.1997. Border cells and arbuscular mycorrhizae in four Amaranthus species. Phytopathology 87: 1240–1242. [DOI] [PubMed] [Google Scholar]
- Brigham, LA, Woo, H‐H, Nicoll, M, Hawes, MC.1995. Differential expression of proteins and mRNA’s from border cells and root tips of pea. Plant Physiology 109: 457–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clowes, FAL, Juniper BE.1968.Plant cells. Oxford and Edinburgh: Blackwell Scientific Publications. [Google Scholar]
- Felle, H, Gogarten, JP, Bentrup, F‐W.1983. Phlorizin inhibits hexose transport across the plasmalemma of Riccia fluitans Planta 157: 267–270. [DOI] [PubMed] [Google Scholar]
- Getz, H‐P, Knauer, D, Willenbrink, J.1987. Transport of sugars across the plasma membrane of beetroot protoplasts. Planta 171: 185–196. [DOI] [PubMed] [Google Scholar]
- Gochnauer, MB, Sealey, LJ, McCully, ME.1990. Do detached root‐cap cells influence bacteria associated with maize roots? Plant, Cell and Environment 13: 793–801. [Google Scholar]
- Gogarten, JP, Bentrup, F‐W.1989. Substrate specifity of the hexose carrier in the plasmalemma of Chenopodium suspension cells probed by transmembrane exchange diffusion. Planta 178: 52–60. [DOI] [PubMed] [Google Scholar]
- Guinel, FC, and McCully, ME.1987. The cells shed by the root cap of Zea: their origin and some structural and physiological properties. Plant, Cell and Environment 10: 565–578. [Google Scholar]
- Hawes, MC, Lin, H‐J.1990. Correlation of pectolytic enzyme activity with the programmed release of cells from root caps of pea (Pisum sativum). Plant Physiology 94: 1855–1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawes, MC, Pueppke, SG.1986. Sloughed peripheral root cap cells: yield from different species and callus formation from single cells. American Journal of Botany 73: 1466–1473. [Google Scholar]
- Hawes, MC, Brigham, LA, Wen, F, Woo, HH, Zhu, Y.1998. Function of root border cells in plant health: pioneers in the rhizosphere. Annual Review of Phytopathology 36: 311–327. [DOI] [PubMed] [Google Scholar]
- Iijima, M, Griffiths, B, Bengough, AG.2000. Sloughing of cap cells and carbon exudation from maize seedling roots in compacted sand. New Phytologist 145: 477–482. [DOI] [PubMed] [Google Scholar]
- Jones, DL, Darrah, PR.1996. Re‐sorption of organic compounds by roots of Zea mays L. and its consequences in the rhizosphere. 3. Characteristics of sugar influx and efflux. Plant and Soil 178: 153–160. [Google Scholar]
- Lin, W, Schmitt, MR, Hitz, WD, Giaquinta, RT.1984. Sugar transport in isolated corn root protoplasts. Plant Physiology 76: 894–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn, HMI, Lochey, F, Mosley, L, Read, D.1999. Analysis of polysaccharides and monosaccharides in the root mucilage of maize (Zea mays L.) by gas chromatography. Journal of Chromatography 831: 267–276. [Google Scholar]
- Sherwood, RT.1987. Papilla formation in corn root‐cap cells and leaves inoculated with Colletotrichum graminicola Phytopathology 77: 930–934. [Google Scholar]
- Speakman, JB, Kruger, W.1983. A comparision of methods to surface sterilize wheat seeds. Transactions of the British Mycological Society 80: 374–376 [Google Scholar]
- Stanzel, M, Sjolund, RD, Komor, E.1988a. Transport of glucose, fructose and sucrose by Streptanthus tortuosus suspension cells. I. Uptake at low sugar concentration. Planta 174: 201–209. [DOI] [PubMed] [Google Scholar]
- Stanzel, M, Sjolund, RD, Komor, E.1988b. Transport of glucose, fructose and sucrose by Streptanthus tortuosus suspension cells. II. Uptake at high sugar concentration. Planta 174: 210–216. [DOI] [PubMed] [Google Scholar]
- Stephenson, M, Hawes, MC.1994. Correlation of pectin methylesterase activity in root caps of pea with root border cell separation. Plant Physiology 106: 739–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermeer, J, McCully, ME.1982. The rhizosphere in Zea: new insight into its structure and development. Planta 156: 45–61. [DOI] [PubMed] [Google Scholar]
- Wen, F, Zhu, Y, Hawes, MC.1999. Effect of pectin methylesterase gene expression on pea root development. Plant Cell 11: 1129–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyse, RE, Zamski, E, Tomos, AD.1986. Turgor regulation of sucrose transport in sugar beet tissue. Plant Physiology 81: 478–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.