Abstract
• Background and Aims Previous work has shown that Borszczowia aralocaspica (Chenopodiaceae) accomplishes C4 photosynthesis in a unique, polarized single‐cell system in leaves. Mature cotyledons have the same structure as leaves, with chlorenchyma cells having biochemical polarization of dimorphic chloroplasts and C4 functions at opposite ends of the cell.
• Key Results Development of the single‐celled C4 syndrome in cotyledons was characterized. In mature seeds, all cell layers are already present in the cotyledons, which contain mostly lipids and little starch. The incipient chlorenchyma cells have a few plastids towards the centre of the cell. Eight days after germination and growth in the dark, small plastids are evenly distributed around the periphery of the expanding cells. Immunolocalization studies show slight labelling of Rubisco in plastids in seeds, including chlorenchyma, hypodermal and water storage, but not epidermal, cells. After imbibition and 8 d of growth in the dark labelling for Rubisco progressively increased, being most prominent in chlorenchyma cells. There was no immunolabelling for the plastid C4 enzyme pyruvate, Pi dikinase under these conditions. Cotyledons developing in light show formation of chlorenchyma tissue, induction of the cytosolic enzyme phosphoenolpyruvate carboxylase and development of dimorphic chloroplasts at opposite ends of the cells. Proximal chloroplasts have well‐developed grana, store starch and contain Rubisco; those located distally have reduced grana, lack starch and contain pyruvate, Pi dikinase.
• Conclusions The results show cotyledons developing in the dark have a single structural plastid type which expresses Rubisco, while light induces formation of dimorphic chloroplasts from the single plastid pool, synthesis of C4 enzymes, and biochemical and structural polarization leading to the single‐cell C4 syndrome.
Key words: Borszczowia aralocaspica, C4‐plants, Chenopodiaceae, chloroplast, cotyledon development, immunolocalization, photosynthetic enzymes
INTRODUCTION
The highly efficient C4 system of photosynthesis is generally associated with differentiation of two cell types, bundle sheath and mesophyll, that are coupled in an anatomical arrangement referred to as Kranz anatomy. Because C4 photosynthesis has advantages over C3 photosynthesis under certain conditions where CO2 can be limiting (higher temperatures, drought, salinity), there is much interest in the possibility of introducing this feature into crop species, the majority of which exhibit the less efficient C3 photosynthesis. However, this will require a better understanding of the anatomical, developmental and biochemical features of the various types of C4 anatomies. The Chenopodiaceae may have the greatest structural and biochemical diversity in evolution of types of C4 photosynthesis of any plant family. It has five types of Kranz anatomy (atriplicoid, salsoloid, kochioid, conospermoid and suaedoid) if arrangement of mesophyll and bundle sheath cells versus other tissues (vascular and water storage, if present) is considered (Carolin et al., 1975; Freitag and Stichler, 2000), and two ultrastructural types of chlorenchyma cells related to two biochemical C4 cycle subtypes, NAD–malic enzyme (NAD–ME) and NADP–malic enzyme (NADP–ME) (Gamaley and Voznesenskaya, 1986; Voznesenskaya and Gamaley, 1986; Voznesenskaya et al., 1999; Pyankov et al., 2000). Thus, this family provides an excellent system for the study of the integration of anatomical and biochemical features of the C4 syndrome, as well as questions on evolution of various features.
While comparison of the C4 syndrome in related and unrelated species has provided valuable information about the range of features used in C4 photosynthesis, comparison of leaves and cotyledons of the same species can also shed light on development of the C4 syndrome. Leaves develop chlorenchyma from initially undifferentiated meristematic cells, while cotyledons that become photosynthetic generally must transform storage cells into chlorenchyma, which entails a complete shift in biochemical functions. There are dicotyledon C4 species, which have the same type of Kranz anatomy in leaves and cotyledons, including species of Chenopodiaceae (Butnik, 1974; Pyankov et al., 1999, 2000; Voznesenskaya et al., 1999), Amaranthaceae (Wang et al., 1993) and Asteraceae (Shu et al., 1999). However, there are also chenopod species which have C4 photosynthesis in leaves, but have a completely different type of anatomy or photosynthesis in cotyledons. For example, there are species in the tribe Salsoleae having leaves with C4 salsoloid‐type Kranz anatomy, but cotyledons with either C3‐type photosynthesis and anatomy, or C4‐type atriplicoid anatomy (Butnik, 1974; Pyankov et al., 1999, 2000; Voznesenskaya et al., 1999). Identifying factors controlling this differential expression of photosynthetic anatomies between leaf and cotyledon will be useful to our understanding of the C4 syndrome and its potential integration into C3 species.
Recently, it was shown that two species of the family Chenopodiaceae, Borszczowia aralocaspica Bunge and Bienertia cycloptera Bunge, each perform C4 photosynthesis in leaves through unique single‐cell systems, which has broken a paradigm that Kranz anatomy is required for function of C4 photosynthesis in terrestrial plants (Voznesenskaya et al., 2001b; Voznesenskaya et al., 2002). In the present study, it is shown that mature cotyledons of Borszczowia have the same structural and biochemical features as previously reported in mature leaves, with chlorenchyma having dimorphic chloroplasts located at opposite ends of elongated cells. The structure and compartmentation, and expression of photosynthetic enzymes in cotyledons in seeds, and in cotyledons following germination, were evaluated to determine how the single‐cell C4 system develops. The results show a light‐ and developmentally dependent transition from C3‐like chlorenchyma cells to C4‐like chlorenchyma cells having specialized cytoplasmic compartments with dimorphic chloroplasts. This unique development of C4 photosynthesis in cotyledons of Borszczowia is discussed relative to that in species having Kranz anatomy, including another species in the family Chenopodiaceae which has been characterized recently, Salsola richteri (Moq.) Kar. Ex Litw. (Voznesenskaya et al., 2003b).
MATERIALS AND METHODS
Plant material
Seeds of Borszczowia aralocaspica Bunge were stored at 3–5 °C prior to use. Like many annual species of the family Chenopodiaceae, Borszczowia is characterized by heterocarpy and has two types of seeds, one with light‐brown, thin coats and the other with thick, black coats (Fig. 1A; see also Butnik, 1991). Both types of seeds in this species are rather close in size. This type of heterocarpy in chenopods was classified based on differences in seeds and secondary seed coats (Levina, 1987). Both types of the seeds were sectioned for microscopy to determine the structure of cotyledons. Seeds were germinated on moist paper in Petri dishes at room temperature. When the radical emerged, the seed coats were removed and fixation of material for various studies was made after 2–3 h of exposure to light. This point in time was designated day 0 (Fig. 2A). In thick‐coated seeds the process of imbibition can take a long time (up to several weeks), but we needed to have uniform material comparable with that from thin‐coated seeds. Thus, the covers of thick‐coated seeds were gently scarified after 2 d of imbibition and the coats were removed the next day. Seeds were germinated in either the dark or light to determine the effect of light on cotyledon anatomy relative to the development of the photosynthetic system.
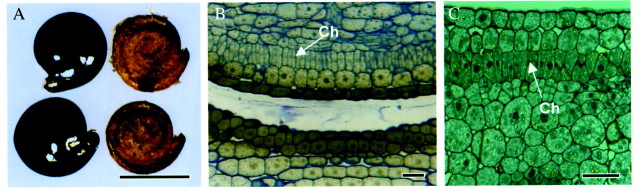
Fig. 1. General Borszczowia aralocaspica seed structure. (A) Heterocarpous seeds with light, thin coats and dark, thick coats. Bar = 0·2 cm. (B) Cross‐section of cotyledon in seed. All the cell layers of the mature cotyledon are already present. Bar = 20 µm. (C) Immunolocalization of Rubisco in cotyledon in seed (label appears as yellow dots). Some Rubisco is already present in plastids throughout the seed tissues. Bar = 20 µm. Ch, chlorenchyma tissue.
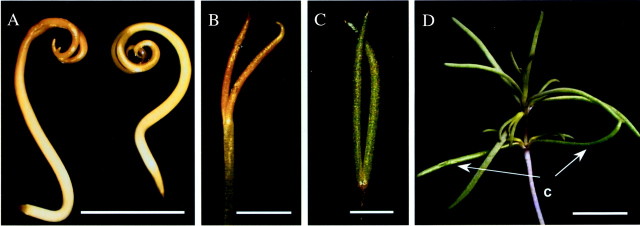
Fig. 2. General view of seedlings/cotyledons of Borszczowia aralocaspica. (A) Seedling, 0 day. Bar = 0·5 cm. (B) Cotyledons, 8 d, dark. Bar = 0·25 cm. (C) Cotyledon, 8 d, light. Bar = 0·2 cm. (D) One‐month‐old seedling with mature cotyledons still attached. Bar = 1·5 cm. c, cotyledon.
For dark treatment, seeds were germinated in a darkroom at 25 °C. These plants were transferred to soil on day 1 and maintained in the dark. Samples of cotyledons were taken under a green safe light on day 8 (Fig. 2B) and processed as described below for microscopy analysis.
For growth in the light, seeds were first maintained at room temperature in Petri dishes for 1 d at 20 µmol photosynthetic quanta m–2 s–1 (PPFD). They were then transplanted to 10‐cm pots, containing a soil mixture of 3 parts clay soil, 3 parts sand, 1 part commercial potting soil and 15 g dolomite powder, and grown for 3 d under 30 PPFD and 30/25 °C day/night temperature regime. Beginning on day 4, plants were transferred to a growth chamber (model GC‐16; Enconair Ecological Chambers Inc., Winnipeg, Canada) under approx. 400 PPFD with a 16 h/8 h light/dark photoperiod and 25/15 °C day/night temperature regime. Samples of cotyledons were taken from 8‐d‐old seedlings (Fig. 2C) and from mature cotyledons of seedlings approx. 1 month old (Fig. 2D) grown in the light. When mature, cotyledons of this species are terete and succulent and achieve up to 3 cm in length and 0·4 cm in width.
Light and electron microscopy
Samples for structural study were fixed at 4 °C in 2 % (v/v) paraformaldehyde and 2 % (v/v) glutaraldehyde in 0·1 m phosphate buffer (pH 7·2), post‐fixed in 4 % (w/v) OsO4, and then, after a standard acetone dehydration procedure, embedded in Spurr’s epoxy resin. Cross‐sections were made on a Reichert Ultracut R ultramicrotome (Reichert‐Jung GmbH, Heidelburg, Germany). For light microscopy, semi‐thin sections were stained with 1 % (w/v) Toluidine blue O in 1 % (w/v) Na2B4O7. Ultra‐thin sections were stained for transmission electron microscopy (TEM) with 2 % (w/v) lead citrate or 1 : 2 dilution of 1 % (w/v) KMnO4:4 % (w/v) uranyl acetate. Hitachi H‐600 and JEOL JEM‐1200 EX transmission electron microscopes were used for observation and photography.
In situ immunolocalization
Leaf samples were fixed at 4 °C in 2 % (v/v) paraformaldehyde and 1·25 % (v/v) glutaraldehyde in 0·05 m phosphate buffer, pH 7·2. The samples were dehydrated with a graded ethanol series and embedded in London Resin White (LR White, Electron Microscopy Sciences, Fort Washington, PA, USA) acrylic resin. Antibodies used (all raised in rabbit) were anti‐Spinacea oleracea L Rubisco (LSU) IgG (courtesy of B. McFadden), commercially available anti‐Zea mays L. phosphoenolpyruvate carboxylase (PEPC) IgG (Chemicon, Temecula, CA, USA), anti‐Amaranthus hypochondriacus L. mitochondrial NAD–ME IgG, which was prepared against the 65‐KDa α subunit (courtesy of J. Berry; Long and Berry 1996), anti‐Zea mays L. 62‐KDa NADP–ME IgG (courtesy of C. Andreo; Maurino et al., 1996), anti‐Zea mays L. pyruvate, Pi dikinase (PPDK) IgG (courtesy of T. Sugiyama) and anti‐Pisum sativum L. glycine decarboxylase (GDC) (courtesy of D. Oliver). Procedures for immunolabelling of thick sections for light microscopy have been described previously (Voznesenskaya et al., 2003a). Pre‐immune serum was used in all cases for controls.
Cross‐sections, 0·8–1 µm thick, were dried from a drop of water onto gelatin‐coated slides and blocked for 1 h with TBST + BSA (10 mm Tris–HCl, 150 mm NaCl, 0·1 % v/v Tween 20, 1 % w/v bovine serum albumin, pH 7·2). They were then incubated for 3 h with either the pre‐immune serum diluted in TBST + BSA (1 : 100), anti‐Rubisco (1 : 500 dilution), anti‐PEPC (1 : 200 dilution), anti‐NAD–ME (1 : 100), anti‐PPDK (1 : 100) or anti‐GDC (1 : 600) antibodies. The slides were washed with TBST + BSA and then treated for 1 h with protein A‐gold (10 nm particles diluted 1 : 100 with TBST + BSA). After washing, the sections were exposed to a silver enhancement reagent for 20 min according to the manufacturer’s directions (Amersham, Arlington Heights, IL, USA), stained with 0·5 % (w/v) Safranin O and imaged in a reflected/transmitted mode using a BioRad 1024 confocal system with Nikon Eclipse TE 300 inverted microscope and Lasergraph image program 3·10. The background labelling with pre‐immune serum was very low, although some infrequent labelling occurred in areas where the sections were wrinkled due to trapping of antibodies/label (results not shown, but see Edwards et al. (2001) as example with the same antibodies).
For TEM immunolabelling, thin sections on Formvar‐coated nickel grids were incubated for 1 h in TBST + BSA to block non‐specific protein binding on the sections. They were then incubated for 3 h with the pre‐immune serum diluted in TBST + BSA, anti‐NAD–ME (1 : 50), or anti‐GDC (1 : 400) antibodies. After washing with TBST plus BSA, the sections were incubated for 1 h with Protein A‐gold (10 nm) diluted 1 : 100 with TBST/BSA. The sections were washed sequentially with TBST plus BSA, TBST, and distilled water, and then post‐stained with a 1 : 4 dilution of 1 % (w/v) potassium permanganate and 2 % (w/v) uranyl acetate. Images were collected using a JEOL JEM‐1200 EX transmission electron microscope.
Staining for polysaccharides
The periodic acid–Schiff’s procedure (PAS) was used for staining starch in sectioned materials. Sections, 0·8–1 µm thick, were made from the same samples used for immunolocalization, dried onto gelatin‐coated slides, incubated in periodic acid (1 % w/v) for 30 min, washed and then incubated with Schiff’s reagent (Sigma, St Louis, MO, USA) for 1 h. After rinsing, the sections were ready for analysis by light microscopy. Cell walls and starch stained a bright reddish pink, while other elements of the cells (cytoplasm) remained unstained. Controls lacking the periodic acid treatment (required for oxidation of the polysaccharides giving rise to Schiff’s‐reactive groups) showed little or no background staining (not shown).
RESULTS
General anatomy and development
Dark‐coated and light‐coated seeds of Borszczowia (Fig. 1A) contain the same spiral embryo form, indicating the major difference is in seed coat pigments and other structural (wall, cell size) properties of the seed coat. The dry cotyledons already have present all the types and layers of tissues found in the mature germinated cotyledon, including epidermal, hypodermal, incipient chlorenchyma, water storage and vascular tissues, although they are not yet expanded (Fig. 1B). The cotyledons in dry seeds are colourless and the cells are tightly packed but, surprisingly, there is a small amount of Rubisco already expressed in the plastids of most tissues (Fig. 1C). Nuclei are mostly located midway along the length of the incipient chlorenchyma cells, and they are maintained in this position even in the mature cells.
When they first emerge from the seed coats the cotyledons are curled and colourless or light yellow and acquire a yellow‐brown colour after some hours in the light (Fig. 2A). After 8 d growth in the dark, the cotyledons expand and are mostly erect or slightly curved, and maintain a yellow‐brown colour (Fig. 2B). The light‐grown cotyledons become dark green (Fig. 2C), and are about 1·6 times longer than those of the dark‐grown seedlings. The cotyledons are persistent in this species and are maintained for some time as green structures similar in size to the leaves that are formed subsequently (Fig. 2D).
When seeds are imbibed with water, the cells in the cotyledon tissues begin to expand quickly. There appears to be very little cell division occurring subsequent to imbibition in most tissue layers. In thin‐coated seeds, some radicals emerge as early as 2–3 h after imbibition, but most appear by the next day; in thick‐coated seeds this can take from several hours up to some weeks. When the radical emerges and seedlings are separated from the seed coats (designated as 0 d) there is the beginning of formation of an internal air space system between the hypodermal and water storage cells; however all incipient chlorenchyma cells are still tightly packed (Fig. 3A). At 0 d, the length of incipient chlorenchyma cells is approx. 25 µm. The end of the incipient chlorenchyma cells towards the outer surface of the cotyledon abutting the hypodermis will be referred to as the distal end while the end abutting the veins and water storage tissue will be referred to as the proximal end, in keeping with the convention used for mature leaves (Voznesenskaya et al., 2001b). PAS staining shows the presence of a few, large starch grains mainly in hypodermal cells and water storage tissue, with some smaller grains scattered throughout the incipient chlorenchyma cells (Fig. 3B).
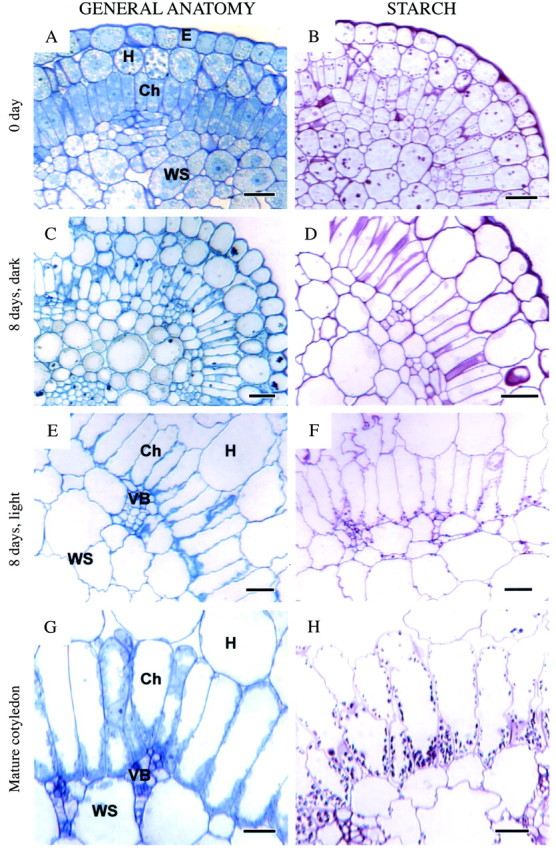
Fig. 3. General anatomy (left) and starch localization (right) in developing cotyledons of Borszczowia aralocaspica. (A, B) Cotyledon, 0 d: (A) general view; (B) PAS staining for polysaccharides. Starch grains are present in hypodermal, chlorenchyma and water storage tissues. (C, D) Cotyledon, 8 d, dark: (C) general anatomy; (D) PAS. Starch is absent. (E, F) Cotyledon, 8 d, light: (E) general view; (F) PAS. Starch has accumulated mostly in proximal part of chlorenchyma cells and in water storage tissue. (G, H) Mature cotyledon: (G) cross‐section of cotyledon showing one layer of chlorenchyma cells with polarization of cytoplasm towards their proximal ends; (H) PAS for carbohydrates. Starch is accumulated abundantly in the proximal part of chlorenchyma cells with some grains in distal part, hypodermal and water storage cells. Scale bars = 20 µm. Ch, chlorenchyma cells; E, epidermis; H, hypodermis; VB, vascular bundle; WS, water storage tissue.
In the 8‐d‐old dark‐grown cotyledons most of the cells, including incipient chlorenchyma, have a well‐developed central vacuole with the cytoplasm displaced to the periphery of the cells (Fig. 3C). The plastids in the incipient chlorenchyma layer are evenly dispersed in the cytoplasm. The length of incipient chlorenchyma cells has increased only slightly, up to approx. 28–30 µm. There are some air spaces between distal ends of incipient chlorenchyma cells, but most cells are still tightly packed. Starch is absent in cotyledons grown under these conditions of development (Fig. 3D).
In 8‐d‐old light‐grown cotyledons, considerable differentiation of cell layers has taken place. Water storage tissues are surrounded by a layer of elongated chlorenchyma cells with some accumulation of organelles towards the proximal end of the cell (Fig. 3E). Starch is located mostly in the proximal end of chlorenchyma cells and in water storage cells (Fig. 3F). Considerable air space has developed around the hypodermal cells and in the water storage tissue, and at the distal end of the chlorenchyma cells.
In chlorenchyma cells of fully mature cotyledons, the layer of cytoplasm is thicker at the proximal end of the cell, partly due to the partitioning of more organelles to this end (Fig. 3G). The chlorenchyma walls remain appressed against each other along most of their length, with air spaces primarily at the distal end. Numerous chloroplasts occur at the proximal end and are fewer in number along the wall in the distal end of the chlorenchyma cells. Starch is observed mainly in the proximal ends of the chlorenchyma cells, though a few small grains are also seen in some of the distal chloroplasts (Fig. 3H).
Electron microscopy
At day 0, cotyledon cells are densely cytoplasmic with very little vacuole space. All tissues are filled with storage materials, mostly with numerous lipid droplets, but there are also protein bodies clearly visible (Fig. 4A and B). Lipids tend to be concentrated towards the periphery of the cells while organelles, including plastids and mitochondria, and protein bodies are located mostly in the middle part of the cell. The plastids have rare, mostly single thylakoids, sometimes with prolamellar body, and a few large starch grains (Fig. 4B). Mitochondria are rather small (the length of the short axis is approx. 0·3–0·4 µm) with some cristae on the periphery and translucent areas in the middle (Fig. 4B).
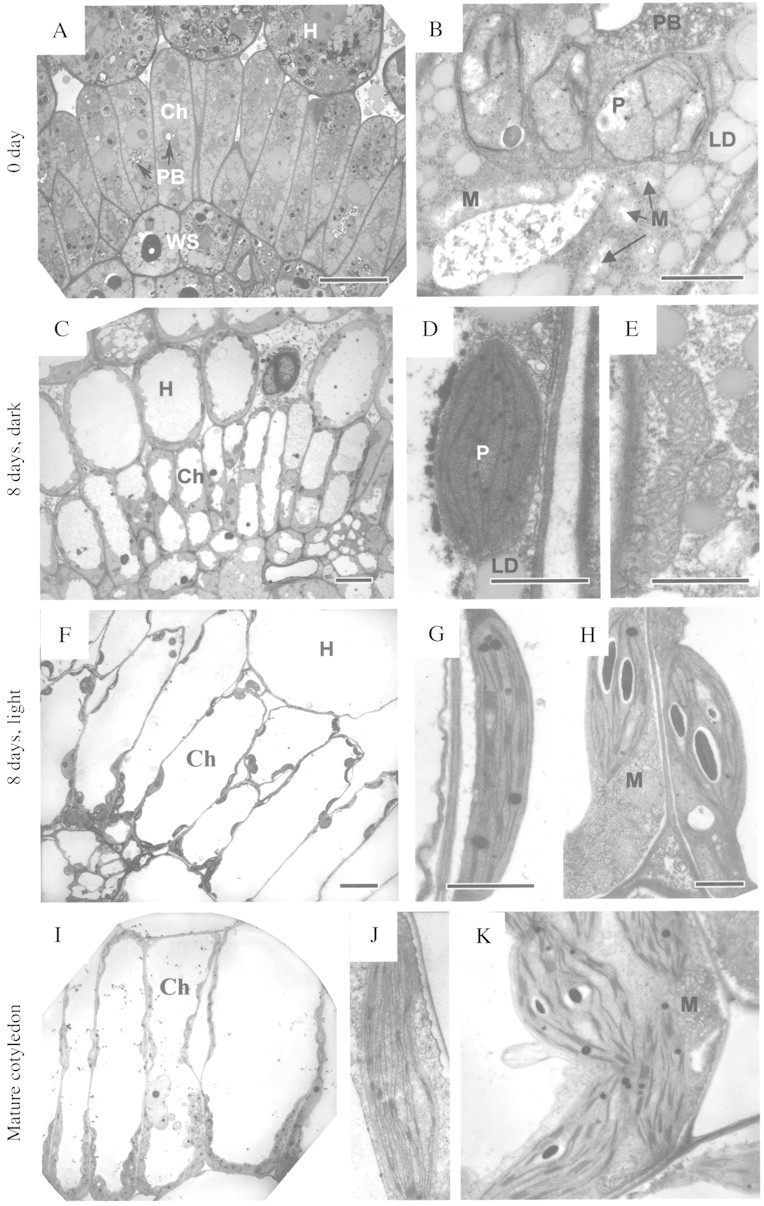
Fig. 4. Electron microscopy of chlorenchyma cells, plastids and mitochondria in developing cotyledons (A–K) of Borszczowia aralocaspica. Cell differentiation and development of thylakoid system is illustrated. (A, B) Cotyledon 0 d. (A) Layer of chlorenchyma cells, filled with storage materials. Scale bar = 10 µm. (B) Plastids and mitochondria. Scale bar = 1 µm. (C–E) 8 d, dark. (C) Chlorenchyma cells. Scale bar = 10 µm. (D) Chlorenchyma cell plastid. (E) Chlorenchyma cell mitochondria. Scale bars = 1 µm. (F–H) 8 d, light. (F) Chlorenchyma cells, 8 d light. Scale bar = 10 µm. (G) Distal chloroplast. (H) Proximal chloroplasts and mitochondria. Scale bars = 1 µm. (I–K) Mature cotyledon. (I) Chlorenchyma cells. Note the more numerous organelles in the centripetal position of the cells. Scale bar = 10 µm. (J) Nearly agranal distal chloroplasts. (K) Proximal chloroplast showing grana and numerous large mitochondria in proximal part of chlorenchyma cell. Scale bars = 1 µm. Ch, chlorenchyma cell; LD, lipid droplets; M, mitochondria; PB, protein bodies; H, hypodermis; P, plastid.
In 8‐d dark‐grown seedlings, all tissues in the cotyledons still contain numerous lipid droplets (Fig. 4C–E), which are now in the thin peripheral layer of cytoplasm surrounding a large vacuole in most cells. There are a few, small plastids with dense stroma which are evenly distributed in the incipient chlorenchyma cells; they are comparable in size to plastids in 0‐d cotyledons. The plastids contain a few thylakoids and rare grana having two or three thylakoids (Fig. 4D); phytoferritin crystals are also rather prominent (not shown). Occasionally, plastids with prolamellar bodies are seen (not shown). At this stage of development, mitochondria are distributed throughout the cell. The length of their short axis is similar to that in 0‐d cotyledons; nevertheless frequently they are very long and have the appearance of being in a very active state, as they have numerous cristae in a rather dense matrix (Fig. 4E).
After 8 d of development in the light, distinct partitioning of organelles in the chlorenchyma cells is seen. The chloroplasts at the distal end of the chlorenchyma cells are not very numerous (Fig. 4F) and have grana consisting of three to seven thylakoids (Fig. 4G). Chloroplasts at the proximal end of the cell also have grana, but with up to nine thylakoids per stack (Fig. 4H). Chloroplasts at the proximal end of the cell contain several starch grains and protein crystals; whereas those in the distal part have only a few, small starch grains along with some protein crystals. Mitochondria are also most abundant at the proximal end of the cell underlying the chloroplasts, or are located between them, and they are quite large (short axis between 0·4 and 0·5 µm, with a mean length of about 0·9 µm) (Fig. 4H). Most of these mitochondria still have a translucent area in the centre but in some the tubular cristae are more extensively developed. There are also some smaller mitochondria between chloroplasts at the distal end of the cell at this stage of cotyledon development (not shown). Peroxisomes are more prominent in the proximal part of the cell but also often could be seen between chloroplasts in the distal end of the cell (not shown).
In fully mature cotyledons, the layer of cytoplasm in the proximal part of the chlorenchyma cell is much thicker and contains more numerous organelles in comparison to the distal part of the cell (Fig. 4I). The proximal part of the cell contains chloroplasts with well‐developed grana (Fig. 4K), peroxisomes and large mitochondria (the length of their short axis is approx. 0·9–1 µm) with numerous cristae (Fig. 4K), whereas in the distal part of the cell chloroplasts are deficient in grana and mitochondria are not found (Fig. 4J).
Immunocytochemistry
As demonstrated in Fig. 1C, the plastids in cotyledons of dry seeds contain small amounts of Rubisco. In imbibed seeds/0‐d cotyledons, the amount of labelling for Rubisco has increased considerably in plastids of incipient chlorenchyma, hypodermal and water storage tissues, but not in epidermal cells (Fig. 5A). In contrast, there is no apparent labelling for PEPC except for some non‐specific labelling in epidermal cells (Fig. 5B). Labelling for PPDK is also essentially absent at this stage (Fig. 5C).
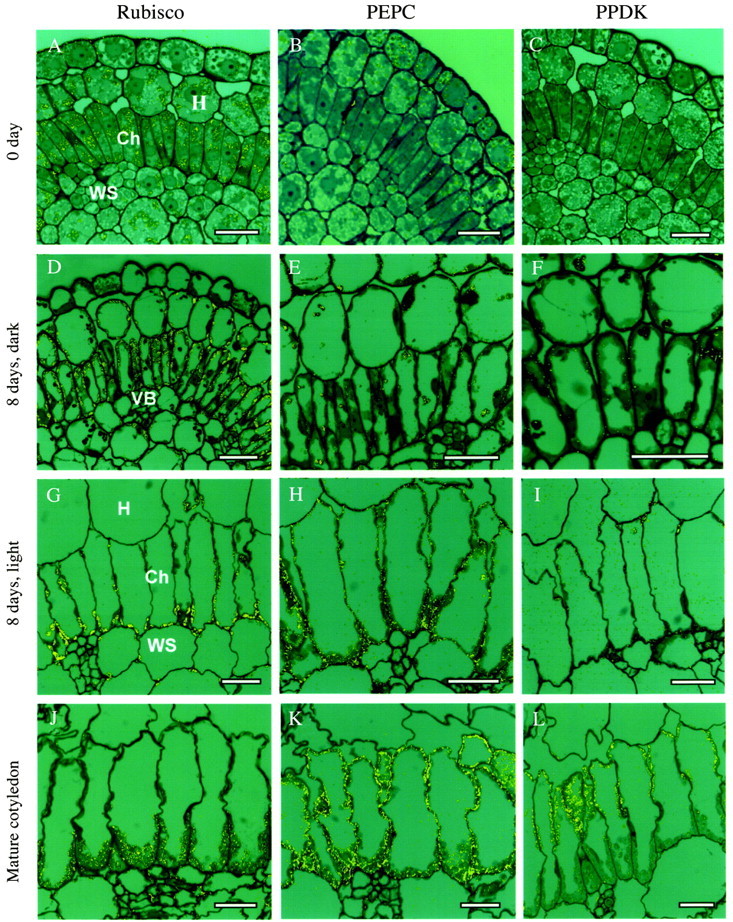
Fig. 5. Reflected/transmitted confocal imaging of in situ immunolocalization of photosynthetic enzymes in cotyledons of Borszczowia aralocaspica at different stages of their development. Label appears as yellow dots. (A–C) Cotyledon, 0 d: (A) Rubisco; (B) PEPC; (C) PPDK. (D–F) Cotyledon, 8 d, dark: (D) Rubisco: (E) PEPC: (F) PPDK. (G–I) Cotyledon, 8 d, light: (G) Rubisco; (H) PEPC; (I) PPDK. (J–L) Mature cotyledon: (J) Rubisco; (K) PEPC; (L) PPDK. Scale bars = 20 µm.
After 8 d growth in the dark, there is strong labelling for Rubisco in all incipient chlorenchyma plastids, with lower labelling in plastids of hypodermal and water storage cells (Fig. 5D). Labelling for PEPC is very low in all tissues at this stage (Fig. 5E), and much of it appears to be non‐specific labelling associated with contents of the vacuole. Labelling for PPDK is present, but very low, in most cell types under these developmental conditions (Fig. 5F).
In 8‐d light‐grown cotyledons, the layer of cytoplasm in the proximal part of the chlorenchyma cells is a little thicker due to partitioning of more chloroplasts and numerous mitochondria to this region. Labelling for Rubisco is primarily in the chloroplasts in this part of the cell, but there is some labelling in the chloroplasts in the distal part of the cell (Fig. 5G). Also, the chloroplasts of hypodermal and water storage cells have some labelling for Rubisco. After 8 d in the light, there is a strong increase of labelling for PEPC in chlorenchyma cells, which is uniformly distributed in the cytoplasm, and there is also some unspecific association with thick cell walls of vascular bundles (Fig. 5H). There is some labelling for PPDK at this stage of cotyledon development, located mostly in chloroplasts in the distal part of chlorenchyma cells, with low labelling in chloroplasts in the proximal part of the cells, hypodermal and water storage tissue (Fig. 5I).
In the mature cotyledons, practically all labelling for Rubisco is concentrated in the chloroplasts in the proximal part of the chlorenchyma cell (Fig. 5J), with low labelling in chloroplasts in the intermediate position (also in water storage cells), but no labelling for Rubisco in chloroplasts in the distal part of the cell. Also, in mature cotyledons there is strong labelling for PEPC in chlorenchyma cells throughout the whole cytoplasm (Fig. 5K). In contrast, PPDK is strongly compartmentalized to chloroplasts in the distal part of the chlorenchyma cells (Fig. 5L). Confocal microscopy and electron microscopy of the localization of GDC and NAD–ME, mitochondrial enzymes, showed both are also localized in the proximal part of chlorenchyma cells, as was found for mature leaves (results not shown; Voznesenskaya et al., 2001b).
DISCUSSION
Anatomy
The mature cotyledons of Borszczowia aralocaspica have anatomy like that of mature leaves including elongated chlorenchyma cells (see Voznesenskaya et al., 2001b). All tissues represented in the mature cotyledon are already well defined in cotyledons of the dry seeds; thus the formation of this unusual C4 cell type in cotyledons does not occur through the initial formation of Kranz anatomy (bundle sheath and mesophyll) with subsequent elimination of the cell walls between the two cell types. The incipient chlorenchyma cells are tightly packed in the cotyledons at 0 d and even after 8 d of growth in the dark. Only as they expand in size in the light do they acquire their characteristic shape, with closely appressed walls at the proximal ends and an intensively developed system of intercellular air spaces between walls at their distal ends. This structure is considered to facilitate the diffusion of atmospheric CO2 into the cells at the distal end and the trapping of CO2 by Rubisco as it is released from C4 acids at the proximal end of the cell (Voznesenskaya et al., 2001b).
Ultrastructure
The ultrastructural investigations indicate the importance of light to the development of part of the single‐cell C4 syndrome. In incipient chlorenchyma cells of the youngest cotyledons, organelles are distributed primarily in the central part of the cells. They do not show any selective structural or biochemical differentiation. These observations indicate that a single pool of plastids gives rise to the dimorphic chloroplasts seen in the mature cotyledons.
In 8‐d light‐grown cotyledons, cells have undergone considerable elongation and there are clear indications of the early stages of organelle partitioning towards opposite ends of the cell. The plastids in both positions are clearly differentiated into chloroplasts, as indicated by the thylakoid/grana abundance and the presence of chlorophyll. There is no sign of chloroplast ultrastructural difference between the two ends of the cell at this stage, except for abundant starch grains in the chloroplasts of the proximal, but not the distal, end of the cell. In contrast, in 8‐d dark‐grown cotyledons cells are only slightly elongated and all organelles are distributed around the periphery of the cell without any special orientation. Thus, in comparing cotyledon development in 8‐d dark‐ versus light‐grown seedlings, it is evident that light is required for chlorenchyma cell expansion, development of an elaborate chloro plast structure and organelle partitioning.
In chlorenchyma cells in mature cotyledons, there is clear compartmentation of two types of chloroplasts, which are nearly agranal in the distal part of the cell and granal in the proximal part, similar to mature chlorenchyma in leaves (Voznesenskaya et al., 2001b, 2003a). Also, large mitochondria are located in the proximal part of mature chlorenchyma cells in cotyledons similar to those in chlorenchyma cells in leaf. The cotyledons thus develop a photosynthetic structure essentially identical to that of mature leaves.
Immunolocalization
Immunolocalization studies on enzymes of the C4 pathway provided interesting information on the regulation of enzyme expression relative to the structural development of the single‐cell C4 syndrome in cotyledons. The small amount of Rubisco in plastids in seeds (chlorenchyma, hypodermal and water storage tissue) might function as a storage protein. However, there is a progressive increase in Rubisco labelling following imbibition and radical emergence (0 day), which becomes very heavy even after 8 d of growth in the dark. In contrast, labelling for the other C4 pathway enzymes, PEPC and PPDK is very low. This suggests that, in the dark, the cotyledons are in a C3 default condition; i.e. they have the incipient condition of developing C3‐type leaves where chlorenchyma tissue expresses Rubisco. In 8‐d light‐grown seedlings there is selective expression of Rubisco in chlorenchyma cells in the proximally located chloroplasts along with starch. This indicates light‐induced differentiation of biochemical polarity, even though at this point there is no obvious ultrastructural difference between chloroplasts located in different parts of the cell. Selective appearance of PEPC in the cytoplasm of chlorenchyma cells is also light‐dependent, as seen by comparing 8‐d dark‐ and light‐grown cotyledons. The biochemical compartmentation of Rubisco into chloroplasts of the proximal part of chlorenchyma cells and development of PPDK in distal chloroplasts was completed only together with full structural differentiation and positioning of chloroplasts and mitochondria in the light. These observations demonstrate that there is expression of Rubisco in Borszczowia cotyledons in the dark; but the differential expression between chlorenchyma and other cell types requires light. In addition, differentiation of two chloroplast biochemical types in the chlorenchyma is coordinated with the partitioning of the chloroplasts to different ends of the cells, and both these processes require light. In the absence of this partitioning, the cells remain in a C3‐like, Rubisco‐containing, default mode.
Comparison to other C4 systems
It is interesting to compare the development of single‐cell C4 photosynthesis in cotyledons of Borszczowia with development of cotyledons of two other species having C4 Kranz‐type anatomy, Salsola richteri (Chenopodiaceae) (Voznesenskaya et al., 2003b) and Amaranthus hypochondriacus L. (Amaranthaceae) (Wang et al., 1993). Borszczowia aralocaspica cotyledons, like leaves (Voznesenskaya et al., 2003a), have NAD–ME‐type C4 photosynthesis. Salsola richteri is an NADP–ME‐type species which has salsoloid‐type Kranz anatomy in both leaves and cotyledons (Voznesenskaya et al., 2003b). Unlike Borszczowia, S. richteri has green cotyledons in seeds, with well‐developed plastids containing extensive grana stacking. However, as in Borszczowia, some Rubisco is present in all plastids in 0‐d cotyledons, with strong labelling in plastids of both mesophyll and bundle sheath cells after 15 d in the dark. Also, like Borszczowia, strong and selective labelling for C4 enzymes like PPDK and PEPC in S. richteri is dependent on light. Thus, as in Borszczowia, the chloroplasts in the dark are in the C3‐like default, Rubisco‐containing mode, with light required for development of the C4 system, including structurally dimorphic chloroplasts.
In comparison to Borszczowia, the development of C4 photosynthesis is much faster in cotyledons of Amaranthus hypochondriacus (atriplicoid‐type Kranz anatomy), in which Rubisco is specifically localized in bundle sheath and PEPC in mesophyll cells after 3 d in the light (Wang et al., 1993). While light enhances development in amaranth, it is not absolutely required since expression of PEPC and selective compartmentation of Rubisco occurs in cotyledons grown for 7 d in the dark (Wang et al., 1993).
In C4 plants photosynthetic enzymes of the C3 and C4 cycles are nuclear encoded, with the exception of the large subunit of Rubisco (Sheen, 1999; Matsuoka et al., 2001). Studies on development of C4 photosynthesis and Kranz anatomy in maize, amaranth and Flaveria trinervia (Spreng.) C. Mohr indicate that selective, cell‐specific production of photosynthetic enzymes, like Rubisco, PEPC and PPDK, occurs through evolution of multiple levels of control of gene expression, including strong evidence for transcriptional regulation (Berry et al., 1997; Sheen, 1999; Shu et al., 1999). A common feature in development of C4 photosynthesis in Kranz‐type plants appears to be conversion from an initial C3‐like default position, with expression of Rubisco in all plastids, followed by biochemical and structural differentiation to develop C4 photosynthesis. This same pattern is observed in development of the single‐cell C4 photosynthesis in Borszczowia. Thus, early in development in Borszczowia, Rubisco is synthesized while control is exerted at some level to prevent synthesis of proteins encoded by C4 genes. However, control of the development of dimorphic chloroplasts in Borszczowia in a single cell must be very different from that reported in Kranz‐type C4 plants. While differential control of transcription of nuclear encoded photosynthetic genes is important in development of cell‐specific functions in Kranz‐type anatomy, post‐transcriptional control must be required in the single‐cell Borszczowia aralocaspica, e.g. through mRNA targeting to specific cytoplasmic compartments, an area that is being investigated currently.
In summary, chlorenchyma of Borszczowia cotyledons has the same single‐cell C4 structural features and partitioning of photosynthetic enzymes as in leaves (Voznesenskaya et al., 2003a). The current study of Borszczowia shows that cotyledons in seeds have all the cell layers of the mature organ, and that they are transformed from a storage function to a photosynthetic function without formation of new cells, unlike what happens in leaves during development. This transformation requires ultrastructural and biochemical changes in the organelles, partitioning of organelles in different compartments within an individual cell, phased expression of selected C4 genes, and targeting of the mRNA from these genes or the gene product to the correct end of the cell. This is clearly demonstrated by our comparison of dark‐ and light‐grown cotyledons. After germination, the development of cotyledons in the dark follows a C3‐like default pattern with increased expression of Rubisco, but not of C4 enzymes, in incipient chlorenchyma cells. Light is required for greening of the cotyledons, the development of dimorphic chloroplasts and strong expression of C4 proteins in the chlorenchyma cells. This demonstrates the incredible plasticity that is inherent in the plant cell and provides an unusual example of the exquisite control of subcellular processes that can be exerted to accomplish very complicated biochemical functions.
ACKNOWLEDGEMENTS
We thank the Electron Microscopy Center of Washington State University for use of their facilities and staff assistance and acknowledge support of this work by NSF Grant IBN‐0131098 and NSF Grant IBN‐0236959.
Supplementary Material
Received: 21 July 2003;; Returned for revision: 15 September 2003; Accepted: 27 October 2003: Published electronically: 5 January 2004
References
- BerryJ, McCormac D, Long J, Boinski JJ, Corey A.1997. Photosynthetic gene expression in amaranth, an NAD‐ME type C4 dicot. Australian Journal of Plant Physiology 24: 423–428. [Google Scholar]
- ButnikAA.1974. Structural types of cotyledons in chenopods (Chenopodiaceae). Morphobiological features of wild‐growing plants of Uzbekistan Tashkent: FAN, 43–49 [in Russian]. [Google Scholar]
- ButnikAA.1991. Family Chenopodiaceae. In: Tachtadjan AL, ed. Comparative seed anatomy. Dicotyledonous. Caryophyllidae‐Dilleniidae, Vol. 3 Leningrad: Nauka, 77–82 [in Russian]. [Google Scholar]
- CarolinRC, Jacobs SWL, Vesk M.1975. Leaf structure in Chenopodiaceae. Botanische Jahrbücher für Systematische Pflanzengeschichte und Pflanzengeographie 95: 226–255. [Google Scholar]
- EdwardsGE, Franceschi VR, Ku MSB, Voznesenskaya EV, Pyankov VI, Andreo CS.2001. Compartmentation of photosynthesis in cells and tissues of C4 plants. Journal of Experimental Botany 52: 577–590. [PubMed] [Google Scholar]
- FreitagH, Stichler W.2000. A remarkable new leaf type with unusual photosynthetic tissue in a Central Asiatic genus of Chenopodiaceae. Plant Biology 2: 154–160. [Google Scholar]
- GamaleyYV, Voznesenskaya EV.1986. Structural and biochemical types of C4‐plants. Fiziologiya Rastenii 33: 802–819 [in Russian]. [Google Scholar]
- LevinaRE.1987. Morphology and anatomy of seeds. In: Tzvelev NN, ed. Leningrad: Nauka, 160 [in Russian]. [Google Scholar]
- LongJJ, Berry JO.1996. Tissue‐specific and light‐mediated expression of the C4 photosynthetic NAD‐dependent malic enzyme of amaranth mitohondria. Plant Physiology 112: 473–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MatsuokaM, Furbank RT, Fukayama H, Miyao M.2001. Molecular engineering of C4 photosynthesis. Annual Review of Plant Physiology and Plant Molecular Biology 52: 297–314. [DOI] [PubMed] [Google Scholar]
- MaurinoVG, Drincovich MF, Andreo CS.1996. NADP‐malic enzyme isoforms in maize leaves. Biochemistry and Molecular Biology International 38: 239–250. [PubMed] [Google Scholar]
- PyankovVI, Black CC, Jr, Artyusheva EG, Voznesenskaya EV, Ku MSB, Edwards GE.1999. Features of photosynthesis in Haloxylon species of Chenopodiaceae that are dominant plants in Central Asian deserts. Plant and Cell Physiology 40: 125–134. [Google Scholar]
- PyankovVI, Voznesenskaya EV, Kuz’min AN, Ku MSB, Ganko E, Franceschi VR, Black CC, Jr, Edwards GE.2000. Occurrence of C3 and C4 photosynthesis in cotyledons and leaves of Salsola species (Chenopodiaceae). Photosynthesis Research 63: 69–84. [DOI] [PubMed] [Google Scholar]
- SheenJ.1999. C4 gene expression. Annual Review of Plant Physiology and Plant Molecular Biology 50: 187–217. [DOI] [PubMed] [Google Scholar]
- ShuG, Pontieri V, Dengler NG, Mets LJ.1999. Light induction of cell type differentiation and cell‐type‐specific gene expression in cotyledons of a C4 plant, Flaveria trinervia Plant Physiology 121: 731–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VoznesenskayaEV, Gamaley YV.1986. The ultrastructural characteristics of leaf types with Kranz‐anatomy. Botanicheskii Zhurnal 71: 1291–1307 [in Russian]. [Google Scholar]
- VoznesenskayaEV, Artyusheva EG, Franceschi VR, Pyankov VI, Kiirats O, Ku MSB, Edwards GE.2001a.Salsola arbusculiformis, a C3‐C4 intermediate in Salsoleae (Chenopodiaceae). Annals of Botany 88: 337–348. [Google Scholar]
- VoznesenskayaEV, Edwards GE, Kiirats O, Artyusheva EG, Franceschi VR.2003a. Development of biochemical specialization and organelle partitioning in the single celled C4 system in leaves of Borszczowia aralocaspica (Chenopodiaceae). American Journal of Botany 90: 1669–1680. [DOI] [PubMed] [Google Scholar]
- VoznesenskayaEV, Franceschi VR, Artyusheva EG, Black CC, Jr, Pyankov VI, Edwards GE.2003b. Development of the C4 photosynthetic apparatus in cotyledons and leaves of Salsola richteri (Chenopodiaceae). International Journal of Plant Sciences 164: 471–487. [Google Scholar]
- VoznesenskayaEV, Franceschi VR, Kiirats O, Artyusheva EG, Freitag H, Edwards GE.2002. Proof of C4 photosynthesis without Kranz anatomy in Bienertia cycloptera (Chenopodiaceae). Plant Journal 31: 649–662. [DOI] [PubMed] [Google Scholar]
- VoznesenskayaEV, Franceschi VR, Kiirats O, Freitag H, Edwards GE.2001b. Kranz anatomy is not essential for terrestrial C4 plant photosynthesis. Nature 414: 543–546. [DOI] [PubMed] [Google Scholar]
- VoznesenskayaEV, Franceschi VR, Pyankov VI, Edwards GE.1999. Anatomy, chloroplast structure and compartmentation of enzymes relative to photosynthetic mechanisms in leaves and cotyledons of species in the tribe Salsoleae (Chenopodiaceae). Journal of Experimental Botany 50: 1779–1795. [Google Scholar]
- WangJ‐L, Long JJ, Hotchkiss T, Berry JO.1993. C4 photosynthetic gene expression in light‐ and dark‐grown amaranth cotyledons. Plant Physiology 102: 1085–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


