Abstract
Migraine pain represents sensations arising from the activation of trigeminal afferents, which innervate the meningeal vasculature and project to the trigeminal nucleus caudalis (TNC). Pain secondary to meningeal input is referred to extracranial regions innervated by somatic afferents that project to homologous regions in the TNC. Such viscerosomatic convergence accounts for referral of migraine pain arising from meningeal afferents to particular extracranial dermatomes. Botulinum toxins (BoNTs) delivered into extracranial dermatomes are effective in and approved for treating chronic migraine pain. Aside from their well-described effect upon motor endplates, BoNTs are also taken up in local afferent nerve terminals where they cleave soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) proteins, and prevent local terminal release. However, a local extracranial effect of BoNT cannot account for allthe effects of BoNT upon migraine. We now know that peripherally delivered BoNTs are taken up in sensory afferents and transported to cleave SNARE proteins in the ganglion and TNC, prevent evoked afferent release and downstream activation. Such effects upon somatic input (as from the face) likewise would not alone account for block of input from converging meningeal afferents. This current work suggests that BoNTs may undergo transcytosis to cleave SNAREs in second-order neurons or in adjacent afferent terminals. Finally, while SNAREs mediate exocytotic release, they are also involved in transport of channels and receptors involved in facilitated pain states. The role of such post-synaptic effects of BoNT action in migraine remains to be determined.
Table of Links
| TARGETS | LIGANDS |
|---|---|
| AMPA (GluA2) receptors | CGRP |
| NK1 receptors | PACAP |
| NMDA (GluN2C) receptors | Substance P |
| TRPV1 channels |
This Table lists the protein targets and ligands in this article which are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Pawson et al., 2014) and the Concise Guide to PHARMACOLOGY 2013/14 (Alexander et al., 2013a, b, c).
Introduction
Migraine is characterized by recurrent episodes of unilateral throbbing head pain lasting for 4–72 h accompanied by aura and symptoms like nausea, photophobia and phonophobia (Olesen, 2013). Broadly speaking, migraine headache occurring with a frequency of less than 15 attacks per month is referred to as episodic migraine, and headache that occurs more frequently that is more than 15 attacks per month for more than 3 months, is referred to as chronic migraine (Olesen, 2013). An estimated 14% of the general population suffer from migraine, with the prevalence ratio of 1:3 (male : female) (Russell et al., 1995; Lipton et al., 2007) making it one of the most common debilitating neurological disorders. Migraine costs more than $20 billion each year in the USA and thus represents a heavy socio-economic burden to the society primarily because of decreased working efficiency and workdays lost (Serrano et al., 2013). Most of the prophylactic drugs currently available have some ameliorating effect on headache with frequent treatment-limiting side effects (Evers, 2008). Initial anecdotal reports in patients receiving botulinum toxin (BoNT) for facial cosmetic purposes (Binder et al., 1998) noted the effects of these injections on headache (Wheeler, 1998) and trigger point-initiated pain syndromes (Acquadro and Borodic, 1994; Cheshire et al., 1994), which appeared to be independent of its effects upon muscle tone. This led to clinical trials that resulted in the approval of a BoNT-A (BoTox®) injected into superficial cranial musculature as a treatment for migraine (Diener et al., 2010; Dodick et al., 2010). BoNT-A is currently approved for the prophylactic treatment of adult chronic migraine in approximately 67 countries, including the USA, all countries in the European Economic Area as well as Australia, Brazil, Canada, India, Korea and Russia (http://www.sec.gov/Archives/edgar/data/850693/000085069314000002/agn10-k2013.htm).
This reported efficacy of extracranial BoNT in treating migraine is surprising given the current thinking that migraine pain may not be the result of increased tone in cranial musculature nor does it represent a cutaneous trigger point-initiated syndrome. As will be reviewed later, current thinking is that migraine pain results from activation of intracranial meningeal perivascular afferents (Strassman et al., 1994; 1996; Burstein et al., 1998) with some studies suggesting the role of extracranial afferents (Schueler et al., 2013; Burstein et al., 2014). In this review, we will consider the literature reflecting the clinical efficacy of BoNT in migraine; outline the anatomical organization believed to underlie migraine; and then review the actions of BoNTs that appear to underlie the ability of extracranially applied BoNT to block nociceptive inputs arising from peripheral afferents.
Clinical history of BoNT in migraine
The A serotype of BoNT (BoNT-A: onabotulinumtoxin A: BoTox) is approved for cosmetic delivery to the face. Such injections lead to a local relaxation of the musculature secondary to the local block of ACh release at the neuromuscular junction. As reviewed later, this block reflects the persistent cleavage of soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) proteins complexes which includes synaptobrevin/vesicle-associated membrane proteins (VAMP), synaptosomal-associated protein 25 (SNAP-25) and syntaxin, that are the key components involved in vesicular fusion during exocytosis) (Rothman, 1994) with the recovery of releasing function and re-occurrence of muscle tone typically occurring over a period of 3–6 months (Flynn, 2010). A variety of early studies addressed the proposition that BoNT would have ameliorative effects on headache (as suggested by the potential role of aberrant muscular contraction in the cranial pain) and this speculation was extended to include migraine.
Systematic clinical trials using BoNT-A (BoTox) as a prophylaxis for migraine have resulted in a mix of positive and negative findings (Table 1). These systematic studies were double-blind, placebo-controlled randomized trials where participants suffered either from episodic or chronic migraine. The injection paradigm differed from one study to another. Some employed a standard fixed injection site approach, with typically the therapeutic dose (155U) divided into 31 spaced injections over the forehead, while others used a ‘follow-the-pain’ treatment (e.g. inject the total dose into divided doses into the cutaneous region to which the pain is referred); and few, a combination of both (Elkind et al., 2006; Hamdy et al., 2009; Lipton et al., 2011). Out of 11 trials performed with episodic migraine, only two studies showed a significant effect of BoNT-A in reducing the frequency of episodic migraine as compared with the placebo (Barrientos and Chana, 2003). A meta-analysis performed on these studies concluded that BoNT-A had no effect on episodic migraine (Jackson et al., 2012). In contrast, BoNT was associated with a greater likelihood of a 50% or greater improvement in the patient suffering from chronic migraine (Jackson et al., 2012). From the pooled analysis of two phase III trials (Research Evaluating Migraine Prophylaxis Therapy studies of BoNT-A in chronic migraineurs), it emerged that efficacy increased over time (up to 56 weeks), and this was paralleled by a self-reported improvement in quality of life (Lipton et al., 2011). A study also suggested that the effectiveness of BoNT was associated with perception of headache and that imploding/ocular migraine headache is more likely to be prevented by prophylactic BoNT than exploding headache (Jakubowski et al., 2006). In general, based on the trials thus far reported (see Table 1), the properties of the therapeutic effects of local BoTox in chronic migraneurs may be summarized as follows.
Table 1.
A summary of the clinical findings of BoNT in acute migraine, chronic migraine and chronic migraine with medication overuse
| Number | Study | Dose of BoNT | Number of participants | Length of the study (including follow ups) | Results | Comments | |
|---|---|---|---|---|---|---|---|
| Primary end points | Secondary endpoints | ||||||
| Acute migraine (<15 attacks per month) | |||||||
| 1 | Silberstein et al., 2000 | 25 U or 75 U | 123 (vehicle = 41, BoNT = 82) | 3 months | Positive | NA | Both doses showed greater reduction of migraine severity |
| 2 | Barrientos and Chana, 2003 | 50 U | 30 (BonT = 15, placebo = 15) | 3 months | Positive | Positive | Greater reductions in headache frequencies and attack on day 90 |
| 3 | Evers et al., 2004 | 16 U or 100 U | 60 (placebo = 20, 16 U BoNT = 20, 100 U BoNT = 20) | 3 months | Negative | Negative | Only sum score of all accompanying symptoms showed some difference |
| 4 | Anand et al., 2006 | 50 U | 32 (placebo = 16, BoNT = 16) | 3 months | Positive | Positive | 75 % patients reported complete relief to mild headache |
| 5 | Elkind et al., 2006 | 7.5 U–50 U | 182 (placebo = 100, BoNT = 82) | 120 days | Negative | Negative | No differences was observed in any efficacy variable |
| 6 | Relja et al., 2007 | 75 U–225 U | n = 515 | 270 days | Negative | Negative | Both placebo and BoNT group showed improvement |
| 7 | Saper et al., 2007 | 25 U | 232 (placebo = 45, BoNT = 187) | 3 months | Negative | Negative | No significant effect of BoNT was observed |
| 9 | Vo et al., 2007 | 205 U | 32 (placebo = 17, BoNT = 15) | 3 months | Negative | Negative | Headache pattern index suggested a protective effect of BoNT against headache severity. |
| 10 | Petri et al., 2009 | 80 U–120 U | 127 (placebo = 63, BoNT = 64) | 3 months | Negative | Negative | A trend in reduction of headache however not significant |
| 11 | Chankrachang et al., 2011 | 120 U–240 U | 128 (placebo = 37, BoNT = 82) | 8–12 weeks | Negative | Positive | BoNT showed significant benefit over placebo at some end points |
| Chronic migraine (>15 attacks per month) | |||||||
| 1 | Mathew et al., 2005 | 105 U–260 U | n = 571 | 11 months | Negative | Positive | Secondary endpoint was met at 180 days |
| 2 | Freitag et al., 2008 | 100 U | n = 86 | 4 months | Positive | Positive | BoNT showed superiority to placebo for both endpoints |
| 3 | Mathew and Jaffri, 2009 | upto 200 U | 60 (topiramate = 29 and BoNT = 26) | 9 months | Positive | Positive | Both BoNT and topiramate showed similar efficacy |
| 4 | Diener et al., 2010 | 155 U–195 U | 679 (BoNT = 341 and placebo = 338) | 32 weeks | Positive | Positive | Favoured all secondary endpoints |
| 5 | Aurora et al., 2010 | 155 U–195 U | 679 (BoNT = 341 and placebo = 338) | 24 weeks | Negative | Positive | PREEMPT 1 trial |
| 6 | Dodick et al., 2010 | 155 U–195 U | 1384 (BoNT = 688 and placebo = 696) | 24 weeks | Positive | Positive | Pooled results of PREEMPT trial |
| 7 | Magalhaes et al., 2010 | 250 U | 72 (BoNT = 35 and amitriptyline = 23) | 90 days | Positive | Positive | BONT was as effective as amitriptyline |
| 8) | Lipton et al., 2011 | 155 U | 1384 (placebo = 696, BoNT = 698) | 56 weeks | Positive | Positive | Significant and clinically meaningful reduction in headache |
| 9) | Aurora et al., 2011 | 155 U–195 U | 1384 (Placebo = 696, BoNT = 698) | 56 weeks | Positive | Positive | Significant reduction in headache days |
| 10) | Cady et al., 2011 | 300 U | 59 (topiramate = 30 and BoNT = 29) | 26 weeks | Positive | Positive | Topiramate and BoNT showed similar efficacy |
| Chronic migraine with medication overuse | |||||||
| 1 | Sandrini et al., 2011 | 100 U | 68 (BoNT = 33 and placebo = 35) | 12 weeks | Negative | Positive | Suggested the tenderness of pericranial muscles in patients with MOH |
| 2 | Silberstein et al., 2013 | 155 U–195 U | 904 (placebo = 459, BoNT = 445) | 24 weeks | Positive | Positive | Change in frequency of acute headache medication intakes was not statistically significant |
| 3 | Grazzi, 2013 | 100 U | n = 10 | 1 year | Negative | Results confirmed the efficacy of BoNT when used at 150 U | |
| 150 U | n = 8 | Positive | |||||
NA, not applicable; MOH, medication overuse headache; PREEMPT, Phase III Research Evaluating Migraine Prophylaxis Therapy.
BoTox doses between 150 U and 195 U are efficacious with limited side effects.
Time of onset of the therapeutic effect on migraine is observed from week 12 with meaningful reduction in headache at 56 weeks.
The duration of action of BoNT reflects the time course of loss of SNARE cleavage activity. This loss is due to the degradation of the intracellular light chain (LC), the component of the toxin responsible for SNARE protein cleavage. Practically, this time course is manifested by the recovery of muscle tone, a marker of neuromuscular junction function. As noted in Table 2, each serotype has a different half-life depending upon the stability of the LC. For BoNT-A the relapse period is in 4–5 months, which in the case of migraine should reflect the time course of the return of the frequency of headache days back to baseline. The PREEMPT trials have used single injections of BOTOX every 12 weeks and has proven to be effective in meeting the primary and secondary endpoints.
Table 2.
Classification of BoNT serotypes A (Schiavo et al., 2000), B (Sloop et al., 1997; Eleopra et al., 1998), C (de Paiva et al., 1999; Jurasinski et al., 2001), D (Yamamoto et al., 2012), E (Foran et al., 2003), F (Kauffman et al., 1985; Ludlow et al., 1992)
| Number | BoNT serotype | Targeted SNARES | Cleavage sites | Half-life (t1/2) | |
|---|---|---|---|---|---|
| Humans | Rodents | ||||
| 1 | BoNT type A (onabotulinumtoxin A) | SNAP-25 | (Gln197-Arg198) 9 residues | >4 months | 1–2 months |
| 2 | BoNT type B (rimabotulinumtoxin B) | Synaptobrevin II or VAMP 2 | Gln59-Phe60 | ∼2 months | 21 days |
| 3 | BoNT type C (C1) | SNAP-25 | Arg198-Ala199 | <3 months | <25 days |
| 4 | BoNT type D | Synaptobrevin II or VAMP 2 | Lys42-Leu43 | NA | NA |
| 5 | BoNT type E | SNAP-25 | (Arg180-lie181) 25 residues | <4 weeks | 4 days |
| 6 | BoNT type F | Synaptobrevin II or VAMP 2 | Gin41-Lys42 | 2 months | 7 days |
| 7 | BoNT type G | Synaptobrevin II or VAMP 2 | NA | NA | NA |
NA, not applicable; SNAP-25, synaptosomal-associated protein 25; SNARE, soluble N-ethylmaleimide-sensitive factor attachment protein receptor.
Even in patients reporting improved pain scores, there are no effects on associated migraine symptomatology (aura, phonophobia, photophobia, nausea).
No difference in efficacy/outcome observed if BoNT is injected in a fixed series of site or into the specific extracranial sites to which the pain is referred.
Regarding comparative efficacy, four clinical trials compared the therapeutic efficacy of BoNT-A with different prophylactic drugs. Although BoNT-A was more effective than steroids, prednisolone (Porta, 2000), it was not better than topiramate, valproate or amitriptyline in reducing the frequency of headache (Blumenfeld et al., 2008; Magalhaes et al., 2010; Cady et al., 2011). According to the International Association for the Study of Pain (IASP), it is presently suggested that the best scientific evidence supports the use of systemic topiramate or local BoNT injections for the prevention of chronic migraine (IASP, 2013).
While the use of BoNT in migraine may only apply to a subpopulation of migraine patients, these clinical studies point to the advantageous use of BoNTs over other prophylactic strategies with respect to reduced side effect, persistent efficacy and tolerability in the treatment of migraine (Samton and Mauskop, 2006).
Migraine: referred pain
Despite many studies, the origin of migraine pain is controversial. However, considerable evidence suggests that the pain sensation in migraine arises from activation of meningeal perivascular afferents (Strassman et al., 1996; Olesen et al., 2009; Zhang et al., 2013).
Meningeal afferents convey nociceptive information
The meningeal vasculature is innervated by small afferents, which project through trigeminal ganglia (TG) to the trigeminal nucleus caudalis (TNC) (Strassman et al., 1986; Levy and Strassman, 2002; Levy et al., 2007; Olesen et al., 2009). These afferents have been shown to display epitopes common to C poly-modal nociceptors, for example TRPV1 receptors, and peptidergic neurotransmitters (Bove and Moskowitz, 1997; Eftekhari et al., 2013). These axons are activated by the terminal application of a variety of proinflammatory substances including PGs, histamine, NO and TNF (Strecker and Messlinger, 2003; Zhang et al., 2007; 2011). In the superficial dorsal horn, these afferents terminate in superficial TNC laminae, where they activate lamina I (marginal) and deep laminae (wide dynamic range) neurons (Strassman et al., 1994; Roch et al., 2007). As with other small-afferent input, this afferent input activates the second-order neurons in a frequency-dependent fashion. Importantly, high-frequency small-afferent traffic can initiate a facilitated state, wherein the second-order neurons will display an enhanced response (wind up) subsequent to afferent input. In humans, mechanical and electrical stimulation of dural and cerebral arteries in patients undergoing open cranium operative procedures, resulted in nausea and headache-like pain sensations referred to specific extracranial regions (Ray and Wolff, 1940; McNaughton and Feindel, 1977). These studies also suggested a possible contribution of extracranial structures like pericranial muscles and arteries in headache generation. While the involvement of extracranial tissues in migraine has been a subject of debate, recent observations argue for this possibility (Jakubowski et al., 2006; Burstein et al., 2014). Neuronal tracing and electrophysiological recordings suggest that the nerve fibres that innervate the extracranial structures (pericranial muscles) may represent functional collaterals passing though cranial sutures from the innervation of intracranial structures (meninges), and that these collaterals can deliver sensory information from the outside of the cranium (Schueler et al., 2013). It should be noted that if this extracranial innervation contributed to the migraine pain states that the actions of topical agents should be effective in treating migraine when delivered at that site only (e.g. over the scalp to which the migraine pain is referred). Given these issues, it is evident to the degree that migrane represents pain originating from the meninges, this referral to extracranial strutures makes this a referred pain state and as with other pain states of a visceral origin, we may consider the potential role of convergence between intracranial and extracranial input.
Viscerosomatic convergence of meningeal and extracranial cutaneous afferents
As noted, an important property of migraine pain is that it is often referred to a specific extracranial region and is an example of referred pain mechanism. In humans, stimulation of the meninges and meningeal vessels results in painful sensation in supraorbital, retrobulbar and occipital region (areas to which the pain component is referred during migraine) (Ray and Wolff, 1940). Such referral of pain of a visceral origin to specific somatic regions is a common property of visceral pain states and is considered to reflect a convergence of the sensory afferents arising from the visceral site (here the meninges) onto second-order neurons, which also receive input from somatic regions (here the face or forehead) (Foreman, 2000; Brumovsky and Gebhart, 2010). Anatomical and electrophysiological studies have indeed reported that nocisponsive neurons in TNC and upper cervical spinal cord dorsal horn (Strassman et al., 1994; Piovesan et al., 2001; Morch et al., 2007) receive convergent input from poly-modal nociceptive afferents that arise from localized regions of the head and face and others from the meninges (Strassman et al., 1994; Burstein et al., 1998; Noma et al., 2008). Thus, as described further later, activation of nociceptive meningeal afferents believed to underlie migraine project to TNC neurons, which also receive trigeminal afferent input from homologous area of skin.
Properties of meningeal and somatic afferent activation
Both the extracranial (somatic) and intracranial originating small poly-modal nociceptive afferents have their respective cell bodies located in the ipsilateral TG. These afferent cell bodies are distributed in the TG according to their respective trigeminal divisions, for example V1 originating from the ipsilateral supraorbital regions and from the meninges overlying the ipsilateral frontal poles are both found in the first division region of the ipsilateral TG (Steiger and Meakin, 1984). These afferents are activated by the exposure of their respective peripheral terminals to a variety of proinflammatory products, such as 5-HT, prostanoids, H+ and bradykinin (Ebersberger, 2001; Strassman and Levy, 2006). Upon activation of their distal terminals, two events transpire. At the local site (in the skin or the meninges), an action potential is generated that travels orthodromically to the spinal terminals (here the nucleus caudalis) and antidromically along collaterals of the parent axon to the local peripheral terminals. At the central and peripheral terminals, the local depolarization opens voltage-gated calcium channels that activate SNARES that mobilize synaptic vesicles to release their contents. These terminals contain and release glutamate and a variety of peptides including calcitonin gene-related peptide (CGRP), pituitary adenylate cyclase activating peptide (PACAP) and substance P (Uddman et al., 1985; 2002; Jansen et al., 1992). Release of these peptides in the periphery (meningeal vasculature) triggers a neurogenic inflammatory response (Markowitz et al., 1988; Ramachandran et al., 2014), for example vasodilatation, plasma protein extravasation and mast cell degranulation (Dimitriadou et al., 1991; Brain and Grant, 2004; Peroutka, 2005). At the central terminals, such release of glutamate and peptides acting upon eponymous excitatory receptors activate second-order neurons.
Visceral afferent sensitization and somatic afferent input
Electrophysiological studies have shown that application of inflammatory products to dural afferents served not only to initiate depolarization, but also to sensitize the peripheral terminals and enhance the response of the second-order TNC neurons to subsequent stimulation (e.g. a central sensitization of the TNC neurons receiving the meningeal input) (Burstein et al., 1998; Bartsch and Goadsby, 2003). Importantly, this activation of meningeal afferents is accompanied by increased sensitivity to stimuli applied to the skin (e.g. a cutaneous tactile allodynia) (Burstein et al., 1998; Oshinsky and Gomonchareonsiri, 2007). The enhanced response to somatic input is believed to reflect the enhanced response of the second-order neuron to the somatic input initiated by the meningeal input into that same pool of second-order neurons. Throbbing pain of migraine is thus likely mediated through both peripheral sensitization of the meningeal afferent axon and the central (TNC) sensitization resulting from the ongoing small-afferent traffic. Such central sensitization and the convergence of somatic upon second-order neurons receiving meningeal afferent traffic may mechanistically account for the migraineurs enhanced sensitivity to light touch applied to the skin (e.g. in effect a secondary tactile allodynia) (Burstein et al., 2000a,b; Jakubowski et al., 2005). Again it should be noted that this motif is similar to that reported in other visceral organ systems where local bowel inflammation will lead to an enlarged area of tactile sensitivity (Laird et al., 2001; Eijkelkamp et al., 2007; Robinson and Gebhart, 2008).
BoNT
BoNTs are synthesized by Clostridium botulinum bacteria (Peck, 2009). There are seven serotypes (A–G). BoNTs are synthesized as single chain sequences and undergo cleavage to generate toxins that consist of a heavy chain (HC) and a LC joined by a single disulphide linkage. The HC (∼100 kDa) is responsible for uptake in the cytosol. The uptake may occur by several mechanisms:
The HC binds the toxin to presynaptic gangliosides on the cell surface and promotes LC (∼50 kDa) translocation into cytosolic endosomes (Fischer and Montal, 2007; Fischer et al., 2009; Montal, 2010). A component of this uptake into neurons is enhanced by membrane depolarization, stimulating BoNT endocytosis by its HC (Keller et al., 2004; Dong et al., 2006; 2007; Rummel et al., 2007).
Internalization by mechanisms that are independent of vesicle recycling involving gangliosides such as synaptic vesicle protein 2 on the plasma membrane (Fotinou et al., 2001; Stenmark et al., 2008).
The acidic environment in the endosome cleaves the disulphide bond and the LC moves into the cytosol. The LC is a zinc (Zn2+) endopeptidase with proteolytic activity located at the N-terminal end, targeting consensus sites on the SNARE proteins (Montecucco and Schiavo, 1994). There are seven different serotypes of BoNT, each acting on different components of SNARE protein. BoNT-A, C and E specifically target SNAP-25, which is largely expressed on plasma membranes (Schiavo et al., 2000), whereas BoNT-B, -D, -F and -G explicitly act on vesicular protein isoforms of synaptobrevin (also called VAMP). BoNT-C also cleaves the plasmalemma protein syntaxin (Rummel, 2013). Cleavage of any one SNARE results in block of vesicle mobilization and release (Figure 1). Types A and B represent the most commonly studied serotypes of this family (Brin et al., 1987; Aoki, 2003; Cui et al., 2004; Huang et al., 2011; Chinnapongse et al., 2012; Marino et al., 2014). Given locally into the musculature the property of BoNT in inhibiting exocytosis of ACh has been strategically used in clinical practice for treating several muscular disorders including cervical dystonia and blepharospasm (Mahant et al., 2000; Dolly et al., 2009). As reviewed earlier, current evidence based on clinical studies now supports the therapeutic potential of BoNT in treatment of chronic migraine (Diener et al., 2010; Dodick et al., 2010; Lipton et al., 2011). Later, we will consider possible mechanisms whereby the peripherally delivered BoNT may alter central processing.
Figure 1.
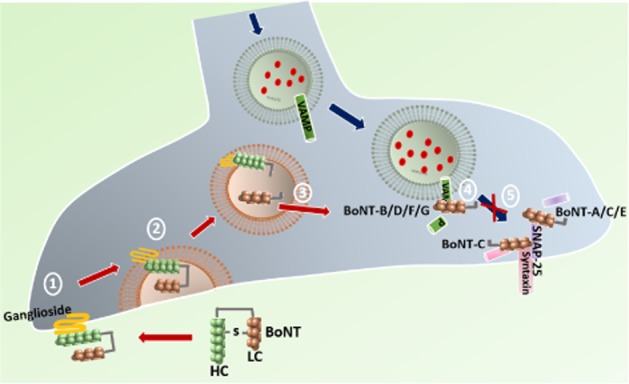
Uptake mechanism and targets of BoNT. HC of BoNT binds to the presynaptic ganglioside present on the plasma membrane (1) that promotes translocation of LC into endosomes (2). Acidic milieu in the endosome cleaves the disulphide linkage between the HC and LC releasing LC into the cytosol (3). The LC of BoNT-B/D/F/G specifically cleaves VAMP on the vesicle, BoNT-A/C/E cleaves SNAP-25 and BoNT-C also cleaves syntaxin on plasma membrane (4), thus inhibiting the vesicular fusion and blocking the neurotransmitter release (5).
Mechanisms of actions of BoNT in migraine
Several hypotheses have been tendered and will be considered later.
Muscle tone
Not unreasonably, it was earlier proposed that the effectiveness of BoNT in treating pain conditions was due to the relaxation of pathological muscle tension, an effect suggested by its potent and efficacious ability to block local neuromuscular transmission. This hypothesis, however, does not garner support as: (i) Pain alleviation occurred in conditions of heightened muscle tone, like cervical dystonia prior to muscle relaxation (Brin et al., 1987); and (ii) The effectiveness of BoNT in migraine was observed in the areas with no reduction in muscle tension (Brin et al., 1987), thus ruling out a primary role of aberrant muscle tone in the migraine pain.
Anti-inflammatory activity
Following local stimulation, the peripheral terminals of unmyelinated sensory afferents display calcium-dependent vesicularly mediated release of local transmitters such as substance P and CGRP (Gupta et al., 2010). These products can lead to a cascade of local events including plasma extravasation, mast cell degranulation and chemotaxis of inflammatory cells (Markowitz et al., 1988; Kowalski et al., 1990; Dimitriadou et al., 1991; Peroutka, 2005) that may jointly change the local chemical milieu and activate the peripheral nerve terminal (Zhang et al., 2007)’. Not surprisingly, this local terminal release of peptide transmitters and the consequence of this release (e.g. vasodilatation and increased capillary permeability), typically imitated by the local delivery of the TRPV1 channel agonist capsaicin, can be blocked by the local (peripheral) delivery of BoNTs in humans (Kramer et al., 2003; Voller et al., 2003; Gazerani et al., 2006; Tugnoli et al., 2007) and in animals (Bach-Rojecky et al., 2008; Carmichael et al., 2010; Marino et al., 2014). This effect occurs in visceral afferent as well, as indicated by the effects of BoNTs on peptide release for example from bladder afferents (Rapp et al., 2006; Lucioni et al., 2008). The local block by local BoNTs typically occurs with a short latency, within hours (see Marino, et al., 2014), and is achieved at low doses, resulting in an action limited to the tissue close to the site of delivery (e.g. ipsilateral and not a result of a systemic redistribution). Similar local effects have been observed in humans examining the evoked flare after BoNT (Gazerani et al., 2006; 2009a,b; Tugnoli et al., 2007). These effects are consistent with the ability of toxins to prevent afferent transmitter release, as measured by effects on evoked release in dorsal root ganglion cell culture systems (Meng et al., 2007; 2009; Dolly and O'Connell, 2012). While there is little doubt that local BoNTs can alter release from local nerve terminals, the role of such a local effect in the skin and superficial musculature to changes in migraine activity is unclear. It should be noted that, while controversial, the onset of migraine does not typically present itself with a change in extracranial blood flow or with signs of local peripheral inflammation (Zwetsloot et al., 1991).
Central (spinopetal) afferent transport
The paralytic effect of local BoNTs emphasizes that local BoNT uptake occurs in motor neuron terminals, while the effects upon neurogenic antidromic vasodilatation emphasizes that peripheral uptake also occurs in sensory afferents, certainly in C poly-modal peptidergic nociceptors.
The current work increasingly emphasizes that the local sensory (and motor) terminal will not only take up local BoNTs, but move them in an active form via fast axonal transport to central terminals. Such transport can be demonstrated by movement of radiolabelled toxins as shown in early studies (Habermann, 1974; Wiegand et al., 1976; Black and Dolly, 1986); and by the cleavage of the respective SNARE protein in the dorsal root ganglion (DRG) and TG or motor neurons in ventral horn (Aoki, 2003; Antonucci et al., 2008; Matak et al., 2011; Restani et al., 2011; 2012; Lawrence et al., 2012; Simpson, 2013; Marino et al., 2014). Thus, in the mouse, ipsilateral intraplantar (IPLT) delivery of BoNT-B into the hind paw cleaves VAMP in ipsi- but not in contralateral DRG within 24 h after IPLT delivery (Marino et al., 2014). For the BoNT-A serotype, truncated (cleaved) SNAP-25 products were observed in the ipsilateral TNC at most by day 3 following the injection of BoNT-A into the whisker pad. Importantly, such central effects can be blocked by treatment of the nerve with blockers of axon transport (Matak et al., 2012; Marino et al., 2014). These results showing homotopic cleavage in the respective sensory ganglia argue that the peripherally delivered BoNT-A and B serotypes in an active form had gained access to and were cleaving SNARES in the DRGs of axons that were innervating the injected skin region. While early work suggested that the transported toxin may be non-functional (Lawrence et al., 2012), the physiological relevance of this cleavage is indeed suggested by three observations. (i) IPLT BoNT blocked not only the release of substance P from the ipsilateral primary C fibre afferent, but prevented the ipsilateral constitutive Finkel-osteogenic sarcoma (cFOS) expression (marker of neuronal activation) otherwise evoked by unilateral IPLT formalin (Marino et al., 2014). In the trigeminal system, activation of trigeminal sensory neurons by capsaicin evokes CGRP release, which is attenuated by BoNT (Durham et al., 2004; Meng et al., 2009). (ii) IPLT BoNT-B prevented the dorsal horn release of substance P and cFOS activation otherwise evoked by the intrathecal delivery of capsaicin (e.g. direct stimulation of the spinal terminals of the TRPV bearing, substance P positive, C fibres, emphasizing that the block of central release by the peripheral BoNT was not due to a block by the peripheral BoNT of afferent activation by the formalin) (Marino et al., 2014). (iii) BoNTs (A and B) delivered unilaterally in the paw and/or the whisker pad will produce a homotopic anti hyperpathic (defined as ‘a painful syndrome characterized by an abnormally painful reaction to a stimulus, especially a repetitive stimulus, as well as an increased threshold’) effect in mouse and rat models such as Phase 2 flinching evoked by formalin (Cui et al., 2004; Aoki, 2005; Marino et al., 2014) and after inflammation (Bach-Rojecky and Lackovic, 2005; Bach-Rojecky et al., 2008; Matak et al., 2011; 2013) and in mouse and/or rats with mononeuropathies (nerve ligation) or poly neuropathies (diabetes, chemotherapeutics (Bach-Rojecky and Lackovic, 2005; 2009; Park et al., 2006; Luvisetto et al., 2007; Ma et al., 2012; Marinelli et al., 2012). Moreover, the block of formalin-evoked flinching produced by pretreatment with BoNT in the whisker pad was prevented by block of axonal transport using colchicine applied to the infraorbital nerve (Matak et al., 2011).
Importantly, comparable results have been reported in human studies where local BoNTs reduce hyperesthesia (defined as ‘increased sensitivity to stimulation, excluding the special senses’) in post-herpetic neuralgia (Liu et al., 2006; Ruiz and Bermejo, 2008; Xiao et al., 2010), diabetic neuropathy (Yuan et al., 2009), nerve injury (Piovesan et al., 2005; Ranoux et al., 2008; Fabregat et al., 2013) and as reviewed in certain forms of migraine (Diener et al., 2010; Dodick et al., 2010). Such results suggest that the peripheral BoNT is taken up in the afferent axon and transported in an active form to the DRG of the axons innervating the injection site (Dolly and O'Connell, 2012).
While the earlier commentary emphasizes that peripherally delivered BoNTs can indeed gain access to the homolateral sensory ganglion and dorsal horn and cleave SNARE proteins, preventing release from that terminal and downstream activation otherwise produced by that cleavage, this central action on the presynaptic afferent terminal does not appear to provide a tenable explanation for the ability of BoNT transported spinopetally in somatic afferents (arising from the injection site in the extracranial skin and musculature) to alter the input from the intracranial meninges.
Trans-synaptic movement of BoNT
As reviewed earlier, it is now certain that BoNTs are taken up and undergo fast axonal transport in brain to the spinal cord after peripheral application. It has long been considered that such transport, in contrast to that displayed by tetanus toxin, if occurred, was largely limited to the axon in which the uptake occurs (Rossetto and Montecucco, 2008). This was initially based on the classic observations that intramuscular tetanus toxin would induce spasticity, a sign that the toxin had moved from the motor neurons to a local inhibitory (glycinergic) interneurons whereas BoNT yielded only a flaccid paralysis (Rossetto and Montecucco, 2008). However, it seems clear now that BoNT induces an immediate flaccidity whereas the local tetanus paralytic action is delayed. Central transport occurs quickly, but the potential central, trans-synaptic, effects of BoNT are obscured by the peripheral block of muscle function (Restani et al., 2012). There is now evidence that BoNTs may in fact also undergo a transcytotic movement in neurons (Lalli et al., 2003; Antonucci et al., 2008; Restani et al., 2011; Torii et al., 2011; Akaike et al., 2013; Marchand-Pauvert et al., 2013) and glia (Marinelli et al., 2012). Several specific studies may be cited to show such transport in the afferent systems. (i) In recent work, we observed that dorsal horn cFOS activation otherwise initiated bilaterally by intrathecally delivered substance P is reduced in the dorsal horn, ipsilateral to the paw that received IPLT BoNT-B (Marino et al., 2014). As the receptors for substance P (NK1) are largely postsynaptic to the primary afferent (Littlewood et al., 1995) and because the ipsilateral block of bilateral activation by IPLT BoNT is considered to represent an effect postsynaptic to the primary afferent terminal, these findings suggest that BoNT is not only transported to the central terminal, but may also undergo trans-synaptic movement from the central afferent terminal to second-order neurons. (ii) The presence of cleaved SNARE (Filipovic et al., 2012) (specifically SNAP-25) has been found in dorsal horn astrocytes after IPLT delivery, suggesting a transcytotic movement of the BoNT from the afferent to the proximal glial membrane (Marinelli et al., 2012). (iii) In an elegant study, it was found that unilateral injection of BoNT in to the vibrissae pad reduced dural extravasation (Filipovic et al., 2012), suggesting that BoNT-A in the somatic afferent reached the meningeal afferents responsible for the local release of pro-inflammatory substances leading to dural extravasation.
Other studies have provided support for transcytotic movement of BoNTs in motor axons (Akaike et al., 2013). Similar studies in animals (Antonucci et al., 2008; Torii et al., 2011; Restani et al., 2012) and humans (Marchand-Pauvert et al., 2013) support this hypothesized trans-synaptic action.
The mechanisms of the transcytotic BoNT movement are not certain and controversial. Several uptake pathways have been identified including one mediated by local vesicle recycling and a distal retrograde endosome-based trafficking pathway. Different fractions of the BoNT being taken up may enter either pathway with the predominant fraction thought to be in the local pool. It has been speculated that the intact BoNT may be transported, depending on its HC component, and the transport of active toxin depends upon a lack of exposure of the transported BoNT to an acidic environment (which serves to cleave the heavy and light chains) (Restani et al., 2012). While there are no direct data supporting the role of such trans-synaptic movement in mediating the effects of extracranial BoNT on the migraine phenomena, the ability of BoNT to undergo central transport along primary afferents and its apparent ability to move to a second-order membrane to reduce SNARE mediated functions, presents an intriguing if speculative hypothesis for how BoNT moving in the somatic afferent can act upon the input arising from the meningeal afferent. It can either be by transport between adjacent cell bodies in the TG or between spatially contiguous terminals in the nucleus caudalis or between the somatic afferent and the second-order nucleus caudalis neurons receiving convergent input from the trigeminovascular and somatic afferents. Accordingly, we hypothesize that BoNT injected into cranial dermatomes will be transported retrogradely in the primary afferents and cleave its target (SNAREs) in TG and further, will alter by a presynaptic effect upon the meningeal afferent or the convergent second-order neurons excitation arising from the meningeal afferent and underlying the aversive component of the migraine state (Figure 2).
Figure 2.
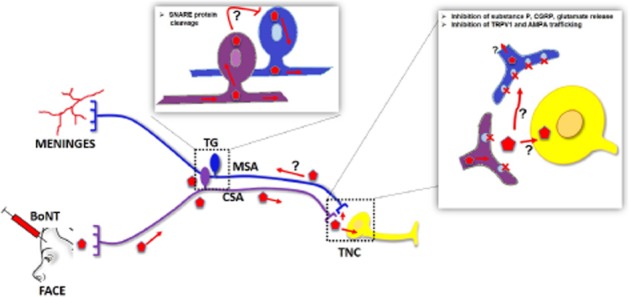
Speculative mechanism(s) of BoNT action. BoNT injected into cranial dermatomes will be taken up by the cutaneous sensory afferents (CSA) and transported retrogradely to the TG where it cleaves SNAREs and is then transported centrally to TNC. Transported BoNT may undergo a trans-synaptic movement either at the second-order neuron (which receives convergent input from the meningeal afferent) or the terminal of the converging activated meningeal afferent. Such a trancytosis may also hypothetically occur in TG sensory neurons and block the activated meningeal afferent release. Future experiments are required to address these options.
Pre- versus postsynaptic effects of BoNTs
As reviewed, BoNTs exert their principal effect through the cleavage of SNARES. Accordingly, the effects of BoNTs reflect upon the role played by SNAREs in cellular function.
Regulation of exocytotic release
The principal discussion in the preceding sections has been based on the defined relevance of SNARES to the mobilization of neurotransmitter vesicles. As reviewed above BoNTs block evoked release from sensory and motor systems as well as from central excitatory and inhibitory neurons (see Marino et al., 2014). However, such effects have been reported in virtually every exocytotic release system studied including parasympathetic axons (Ikeda et al., 2012; Shan et al., 2013), post-ganglionic sympathetics (Smyth et al., 2006), chromaffin cells (Lawrence et al., 2002) enterocromaffin cells (Zanner et al., 2002), pancreatic Islet cells (He et al., 2008) pituitary hormone secretion (Leggett et al., 2013) and mast cells (Bottinger et al., 1987; Park, 2013). In the present context, one might speculate that the analgesic effects of afferent transported BoNT would be represented by this well-defined effect upon afferent terminal release. It is interesting to note, however, that peripheral BoNTs are largely ineffective in models of acute nociception, for example lack of effect upon acute thermal threshold in both animals (Marino et al., 2014) and humans (Gazerani et al., 2009a); but are most efficacious in models where there is the activation of a facilitated states, for example the second, but not first phase of the formalin flinching model for both paw and whisker injections (Matak et al., 2013; Marino et al., 2014). Were the principal effects of BoNTs on mechanisms of primary afferent transmission, we would anticipate a potent effect upon acute thresholds (much as agents such as morphine) which act presynaptically on the primary afferent and are well known to alter acute threshold response (Yaksh et al., 1999). Other actions of SNAREs may thus be in play to account for the antihyperalgesic effects of peripheral BoNTs.
Intracellular transport
An alternative BoNT target relates to the appreciation of the role of SNAREs in the trafficking of several ionotropic and metabotropic receptors, such as the subunits of TRPV1 channels (Montell, 2004) and AMPA (Steinberg et al., 2004) and NMDA receptors (Lau et al., 2010), that are actively involved in the process of spinal sensitization. Several specific examples will be noted.
Central sensitization process involves rapid trafficking of the glutamate receptors to the membrane. During the phase of repetitive afferent stimulation, there is an increase in the transport and insertion of subunits into the membrane, for the calcium permeable AMPA receptor (Schenk et al., 2003; Steinberg et al., 2004; Lau et al., 2010; Ahmad et al., 2012) and block of these spinal receptors has a powerful anti-hyperpathic action (Choi et al., 2010; Tao, 2012). Strong evidence suggests that SNAREs play a mediating role in this vesicular transport of AMPA receptors to the membrane. BoNTs can alter such trafficking, although this has been primarily assessed in cerebellum (Kakegawa and Yuzaki, 2005). Interventions by BoNT in glutamate ionophore trafficking would unquestionably influence facilitated processing thereby having profound effects upon facilitated states in migraine.
The TRPV1 channel is expressed both centrally and peripherally in the trigeminal afferent system. In trigeminal models, activation of TRPV1 channels evoke CGRP release from trigeminal afferents (Akerman et al., 2003). Inhibition of TRPV1 channels can both prevent and reverse established allodynia (http://clinicaltrials.gov, 2014). Subcutaneous BoNT-A into the face decreased TRPV1 positive neurons in the ophthalmic division of the rat TG, secondary to an inhibition of their trafficking to the plasma membrane (Shimizu et al., 2012). This property of BoNT is likely to hold true for most of the ionotropic and metabotropic receptors involved in pain facilitation.
Finally, while there is only limited data thus far, we suggest that the potential role of BoNTs in regulating cell surface expression of a variety of receptors and channels is extensive. Most examples of intracellular protein trafficking involves formation of a coated vesicle that displays tethering and fusion proteins and is moved as cargo via actin- or tubulin-based filaments. Upon approximation to the target compartment, the vesicle tethering proteins utilize SNAREs to initiate binding and fusion to deliver the contents to the target membrane (Angers and Merz, 2011; Juliano et al., 2013). It is thus likely that BoNTs can regulate trafficking of a wide range of targets, including catalytic receptors, such as receptor tyrosine kinases and GPCRs. Importantly, SNARES are greatly enriched in lipid rafts (Lang, 2007) and this organization may be important for membrane trafficking of these receptor and channel proteins as well as the spatial organization of the secretory machinery required for exocytosis (Chamberlain et al., 2001). Given the ubiquitous role of SNARES in cell function, the relative specificity and lack of evident toxicity suggests a high degree of targeting, which is only just beginning to be appreciated.
Therapeutic potential of other BoNT serotypes
The work outlined earlier characterizing the action of BoNTs in pain processing has typically been limited and restricted to one or two BoNT serotypes (typically A and B). As noted, the seven different serotypes of BoNT have a similar function of inhibiting neurotransmitter release, but by targeting different machineries involved in the process of exocytosis (Table 2). However, the individuality of each BoNT is in part defined by the activity of the LC-protease. For example, the LC-protease of BoNT-A cleaves only nine residues from the C-terminus of SNARE protein SNAP-25 and thus partially blocks its participation in the exocytosis process (Molgo et al., 1990; Meng et al., 2009). BoNT-E, also targeting SNAP-25, cleaves a total of 26 residues from the C-terminus, completely blocking neuroexocytosis (Wang et al., 2011; Lawrence et al., 2012). However, BoNT-A/LC has a longer half-life of 3–4 months as compared with the very short half-life of BoNT-E (Keller et al., 1999; Foran et al., 2003). This therapeutic disadvantage was overcome by engineering the toxins to form a recombinant molecule providing relief in inflammatory pain models (Dolly et al., 2009). A study reported that BoNT-A showed only limited inhibition of capsaicin-induced CGRP release as compared with K+ and bradykinin-evoked release. As capsaicin was found to require 180–197 residues of SNAP-25 for exocytosis (Meng et al., 2009), BoNT-A/LC that targets only nine residues was unable to completely abolish the capsaicin-induced CGRP release. This property may contribute to the mixed findings observed in clinical trials for BoTox. However, recombinant chimeras of BoNT-A and BoNT-E successfully inhibited capsaicin-induced CGRP release from trigeminal sensory neurons, highlighting the potential of other serotypes (Meng et al., 2009). Another study generated an active catalytic conjugate by coupling BoNT-A to lectin from Erythrina crystagalli. This derivative shows selectivity for nociceptive afferents neurons with little effect on neighbouring spinal neurons in vitro suggesting that the properties of BoNT can be tailored to selectively target the cells of interest (Duggan et al., 2002). Furthermore, in rat TG cultures, BoNT-C1 incompletely cleaves both SNAP-25 and syntaxin I and partially inhibits Ca2+-dependent CGRP release evoked by capsaicin, K+ and bradykinin (Meng et al., 2007). BoNT–C-sensitive syntaxins 2 and 3 (Schiavo et al., 1995) were present in low levels in TG, potentially accounting for the minimal reduction in CGRP release. Treatment of cells with BoNT-D cleaved all the isoforms of synaptobrevin and completely abolished the capsaicin and K+-induced CGRP release. BoNT-D also affected bradykinin induced release, however, with a lower potency (Meng et al., 2007). Effectiveness of BoNT-F in treating torticollis and oromandibular dystonia has been reported, however, with reduced length of benefit and side effects (Ludlow et al., 1992). As the serotypes differ in catalytic targets potential duration of action, and importantly, terminal uptake and transport, future studies should focus on characterization of these properties and manipulate them for the development of pain pharmaceuticals targeted for chronic pain conditions in general and migraine (Borodic et al., 2001; Pellett, 2012).
Concluding remarks
The triptans are currently considered to be the gold standard for the treatment of migraine as it is the only class of specific anti-migraine drugs in clinical use (Buzzi et al., 1991; Nilsson et al., 1997; Burstein and Jakubowski, 2004; Jakubowski et al., 2005; Olesen and Ashina, 2011). Evidence suggests that, triptans act on 5HT1B/1D/1F receptors on the peripheral and central terminals of the meningeal afferents blocking, respectively, peptide neurotransmitter release and thereby reducing the local neuro-inflammatory event in the meninges and attenuating the signals transmitted from primary afferents to second-order neurons (Burstein and Jakubowski, 2004; Burstein et al., 2004; Ramachandran et al., 2012). This property of triptans in migraine correlates with the mechanism of BoNT that is blocking the neurotransmitter release from primary afferents, although each acts by different targeting mechanisms. This property of BoNT if therapeutically modified by improving the pharmacological properties and reducing the unwanted side effects can be a promising approach towards the development of therapies for migraine as well as other debilitating craniofacial disorders including trigeminal neuralgia and temperomandibular joint syndrome.
Acknowledgments
The review was prepared when the senior author was supported as a fellow by the Migraine Research Foundation and DA02110. Some of the studies cited herein were supported in part by funds from DA02110 and Solstice Neurosciences.
Glossary
- BoNT
botulinum toxin
- DRG
dorsal root ganglion
- HC
heavy chain
- IPLT
ipsilateral intraplantar
- LC
light chain
- TG
trigeminal ganglion
Conflict of interest
We declare that there is no conflict of financial interest with regard to our paper.
References
- Acquadro MA, Borodic GE. Treatment of myofascial pain with botulinum A toxin. Anesthesiology. 1994;80:705–706. doi: 10.1097/00000542-199403000-00041. [DOI] [PubMed] [Google Scholar]
- Ahmad M, Polepalli JS, Goswami D, Yang X, Kaeser-Woo YJ, Sudhof TC, et al. Postsynaptic complexin controls AMPA receptor exocytosis during LTP. Neuron. 2012;73:260–267. doi: 10.1016/j.neuron.2011.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akaike N, Shin MC, Wakita M, Torii Y, Harakawa T, Ginnaga A, et al. Transsynaptic inhibition of spinal transmission by A2 botulinum toxin. J Physiol. 2013;591:1031–1043. doi: 10.1113/jphysiol.2012.242131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akerman S, Kaube H, Goadsby PJ. Vanilloid type 1 receptors (VR1) on trigeminal sensory nerve fibres play a minor role in neurogenic dural vasodilatation, and are involved in capsaicin-induced dural dilation. Br J Pharmacol. 2003;140:718–724. doi: 10.1038/sj.bjp.0705486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Catterall WA, et al. The Concise Guide to PHARMACOLOGY 2013/14: Ligand-Gated Ion Channels. Br J Pharmacol. 2013a;170:1582–1606. doi: 10.1111/bph.12446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL. Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: G Protein-Coupled Receptors. Br J Pharmacol. 2013b;170:1459–1581. doi: 10.1111/bph.12445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Catterall WA, et al. The Concise Guide to PHARMACOLOGY 2013/14: Ion Channels. Br J Pharmacol. 2013c;170:1607–1651. doi: 10.1111/bph.12447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand KS, Prasad A, Singh MM, Sharma S, Bala K. Botulinum toxin type A in prophylactic treatment of migraine. Am J Ther. 2006;13:183–187. doi: 10.1097/01.mjt.0000212705.79248.74. [DOI] [PubMed] [Google Scholar]
- Angers CG, Merz AJ. New links between vesicle coats and Rab-mediated vesicle targeting. Semin Cell Dev Biol. 2011;22:18–26. doi: 10.1016/j.semcdb.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonucci F, Rossi C, Gianfranceschi L, Rossetto O, Caleo M. Long-distance retrograde effects of botulinum neurotoxin A. J Neurosci. 2008;28:3689–3696. doi: 10.1523/JNEUROSCI.0375-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki KR. Evidence for antinociceptive activity of botulinum toxin type A in pain management. Headache. 2003;43(Suppl. 1):S9–S15. doi: 10.1046/j.1526-4610.43.7s.3.x. [DOI] [PubMed] [Google Scholar]
- Aoki KR. Review of a proposed mechanism for the antinociceptive action of botulinum toxin type A. Neurotoxicology. 2005;26:785–793. doi: 10.1016/j.neuro.2005.01.017. [DOI] [PubMed] [Google Scholar]
- Aurora SK, Dodick DW, Turkel CC, DeGryse RE, Silberstein SD, Lipton RB, et al. OnabotulinumtoxinA for treatment of chronic migraine: results from the double-blind, randomized, placebo-controlled phase of the PREEMPT 1 trial. Cephalalgia. 2010;30:793–803. doi: 10.1177/0333102410364676. [DOI] [PubMed] [Google Scholar]
- Aurora SK, Winner P, Freeman MC, Spierings EL, Heiring JO, DeGryse RE, et al. Onabotulinumtoxin A for treatment of chronic migraine: pooled analyses of the 56-week PREEMPT clinical program. Headache. 2011;51:1358–1373. doi: 10.1111/j.1526-4610.2011.01990.x. [DOI] [PubMed] [Google Scholar]
- Bach-Rojecky L, Lackovic Z. Antinociceptive effect of botulinum toxin type a in rat model of carrageenan and capsaicin induced pain. Croat Med J. 2005;46:201–208. [PubMed] [Google Scholar]
- Bach-Rojecky L, Lackovic Z. Central origin of the antinociceptive action of botulinum toxin type A. Pharmacol Biochem Behav. 2009;94:234–238. doi: 10.1016/j.pbb.2009.08.012. [DOI] [PubMed] [Google Scholar]
- Bach-Rojecky L, Dominis M, Lackovic Z. Lack of anti-inflammatory effect of botulinum toxin type A in experimental models of inflammation. Fundam Clin Pharmacol. 2008;22:503–509. doi: 10.1111/j.1472-8206.2008.00615.x. [DOI] [PubMed] [Google Scholar]
- Barrientos N, Chana P. Botulinum toxin type A in prophylactic treatment of migraine headaches: a preliminary study. J Headache Pain. 2003;4:146–151. [Google Scholar]
- Bartsch T, Goadsby PJ. The trigeminocervical complex and migraine: current concepts and synthesis. Curr Pain Headache Rep. 2003;7:371–376. doi: 10.1007/s11916-003-0036-y. [DOI] [PubMed] [Google Scholar]
- Binder WJ, Blitzer A, Brin MF. Treatment of hyperfunctional lines of the face with botulinum toxin A. Dermatol Surg. 1998;24:1198–1205. doi: 10.1111/j.1524-4725.1998.tb04098.x. [DOI] [PubMed] [Google Scholar]
- Black JD, Dolly JO. Interaction of 125I-labeled botulinum neurotoxins with nerve terminals. I. Ultrastructural autoradiographic localization and quantitation of distinct membrane acceptors for types A and B on motor nerves. J Cell Biol. 1986;103:521–534. doi: 10.1083/jcb.103.2.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenfeld AM, Schim JD, Chippendale TJ. Botulinum toxin type A and divalproex sodium for prophylactic treatment of episodic or chronic migraine. Headache. 2008;48:210–220. doi: 10.1111/j.1526-4610.2007.00949.x. [DOI] [PubMed] [Google Scholar]
- Borodic GE, Acquadro M, Johnson EA. Botulinum toxin therapy for pain and inflammatory disorders: mechanisms and therapeutic effects. Expert Opin Investig Drugs. 2001;10:1531–1544. doi: 10.1517/13543784.10.8.1531. [DOI] [PubMed] [Google Scholar]
- Bottinger H, Reuner KH, Aktories K. Inhibition of histamine release from rat mast cells by botulinum C2 toxin. Int Arch Allergy Appl Immunol. 1987;84:380–384. doi: 10.1159/000234453. [DOI] [PubMed] [Google Scholar]
- Bove GM, Moskowitz MA. Primary afferent neurons innervating guinea pig dura. J Neurophysiol. 1997;77:299–308. doi: 10.1152/jn.1997.77.1.299. [DOI] [PubMed] [Google Scholar]
- Brain SD, Grant AD. Vascular actions of calcitonin gene-related peptide and adrenomedullin. Physiol Rev. 2004;84:903–934. doi: 10.1152/physrev.00037.2003. [DOI] [PubMed] [Google Scholar]
- Brin MF, Fahn S, Moskowitz C, Friedman A, Shale HM, Greene PE, et al. Localized injections of botulinum toxin for the treatment of focal dystonia and hemifacial spasm. Mov Disord. 1987;2:237–254. doi: 10.1002/mds.870020402. [DOI] [PubMed] [Google Scholar]
- Brumovsky PR, Gebhart GF. Visceral organ cross-sensitization – an integrated perspective. Auton Neurosci. 2010;153:106–115. doi: 10.1016/j.autneu.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burstein R, Jakubowski M. Analgesic triptan action in an animal model of intracranial pain: a race against the development of central sensitization. Ann Neurol. 2004;55:27–36. doi: 10.1002/ana.10785. [DOI] [PubMed] [Google Scholar]
- Burstein R, Yamamura H, Malick A, Strassman AM. Chemical stimulation of the intracranial dura induces enhanced responses to facial stimulation in brain stem trigeminal neurons. J Neurophysiol. 1998;79:964–982. doi: 10.1152/jn.1998.79.2.964. [DOI] [PubMed] [Google Scholar]
- Burstein R, Cutrer MF, Yarnitsky D. The development of cutaneous allodynia during a migraine attack clinical evidence for the sequential recruitment of spinal and supraspinal nociceptive neurons in migraine. Brain. 2000a;123(Pt 8):1703–1709. doi: 10.1093/brain/123.8.1703. [DOI] [PubMed] [Google Scholar]
- Burstein R, Cutrer MF, Yarnitsky D. The development of cutaneous allodynia during a migraine attack clinical evidence for the sequential recruitment of spinal and supraspinal nociceptive neurons in migraine. Brain. 2000b;123(Pt 8):1703–1709. doi: 10.1093/brain/123.8.1703. [DOI] [PubMed] [Google Scholar]
- Burstein R, Collins B, Jakubowski M. Defeating migraine pain with triptans: a race against the development of cutaneous allodynia. Ann Neurol. 2004;55:19–26. doi: 10.1002/ana.10786. [DOI] [PubMed] [Google Scholar]
- Burstein R, Zhang X, Levy D, Aoki KR, Brin MF. Selective inhibition of meningeal nociceptors by botulinum neurotoxin type A: therapeutic implications for migraine and other pains. Cephalalgia. 2014 doi: 10.1177/0333102414527648. doi: 10.1177/0333102414527648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzzi MG, Carter WB, Shimizu T, Heath H, III, Moskowitz MA. Dihydroergotamine and sumatriptan attenuate levels of CGRP in plasma in rat superior sagittal sinus during electrical stimulation of the trigeminal ganglion. Neuropharmacology. 1991;30:1193–1200. doi: 10.1016/0028-3908(91)90165-8. [DOI] [PubMed] [Google Scholar]
- clinicaltrials.gov. 2014. Use of SB-705498 in the acute treatment of migraine. http://clinicaltrials.gov/ct2/show/study/NCT00269022.
- Cady RK, Schreiber CP, Porter JA, Blumenfeld AM, Farmer KU. A multi-center double-blind pilot comparison of onabotulinumtoxinA and topiramate for the prophylactic treatment of chronic migraine. Headache. 2011;51:21–32. doi: 10.1111/j.1526-4610.2010.01796.x. [DOI] [PubMed] [Google Scholar]
- Carmichael NM, Dostrovsky JO, Charlton MP. Peptide-mediated transdermal delivery of botulinum neurotoxin type A reduces neurogenic inflammation in the skin. Pain. 2010;149:316–324. doi: 10.1016/j.pain.2010.02.024. [DOI] [PubMed] [Google Scholar]
- Chamberlain LH, Burgoyne RD, Gould GW. SNARE proteins are highly enriched in lipid rafts in PC12 cells: implications for the spatial control of exocytosis. Proc Natl Acad Sci U S A. 2001;98:5619–5624. doi: 10.1073/pnas.091502398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chankrachang S, Arayawichanont A, Poungvarin N, Nidhinandana S, Boonkongchuen P, Towanabut S, et al. Prophylactic botulinum type A toxin complex (Dysport®) for migraine without aura. Headache. 2011;51:52–63. doi: 10.1111/j.1526-4610.2010.01807.x. [DOI] [PubMed] [Google Scholar]
- Cheshire WP, Abashian SW, Mann JD. Botulinum toxin in the treatment of myofascial pain syndrome. Pain. 1994;59:65–69. doi: 10.1016/0304-3959(94)90048-5. [DOI] [PubMed] [Google Scholar]
- Chinnapongse RB, Lew MF, Ferreira JJ, Gullo KL, Nemeth PR, Zhang Y. Immunogenicity and long-term efficacy of botulinum toxin type B in the treatment of cervical dystonia: report of 4 prospective, multicenter trials. Clin Neuropharmacol. 2012;35:215–223. doi: 10.1097/WNF.0b013e318263163c. [DOI] [PubMed] [Google Scholar]
- Choi JI, Svensson CI, Koehrn FJ, Bhuskute A, Sorkin LS. Peripheral inflammation induces tumor necrosis factor dependent AMPA receptor trafficking and Akt phosphorylation in spinal cord in addition to pain behavior. Pain. 2010;149:243–253. doi: 10.1016/j.pain.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui M, Khanijou S, Rubino J, Aoki KR. Subcutaneous administration of botulinum toxin A reduces formalin-induced pain. Pain. 2004;107:125–133. doi: 10.1016/j.pain.2003.10.008. [DOI] [PubMed] [Google Scholar]
- Diener HC, Dodick DW, Aurora SK, Turkel CC, DeGryse RE, Lipton RB, et al. OnabotulinumtoxinA for treatment of chronic migraine: results from the double-blind, randomized, placebo-controlled phase of the PREEMPT 2 trial. Cephalalgia. 2010;30:804–814. doi: 10.1177/0333102410364677. [DOI] [PubMed] [Google Scholar]
- Dimitriadou V, Buzzi MG, Moskowitz MA, Theoharides TC. Trigeminal sensory fiber stimulation induces morphological changes reflecting secretion in rat dura mater mast cells. Neuroscience. 1991;44:97–112. doi: 10.1016/0306-4522(91)90253-k. [DOI] [PubMed] [Google Scholar]
- Dodick DW, Turkel CC, DeGryse RE, Aurora SK, Silberstein SD, Lipton RB, et al. OnabotulinumtoxinA for treatment of chronic migraine: pooled results from the double-blind, randomized, placebo-controlled phases of the PREEMPT clinical program. Headache. 2010;50:921–936. doi: 10.1111/j.1526-4610.2010.01678.x. [DOI] [PubMed] [Google Scholar]
- Dolly JO, O'Connell MA. Neurotherapeutics to inhibit exocytosis from sensory neurons for the control of chronic pain. Curr Opin Pharmacol. 2012;12:100–108. doi: 10.1016/j.coph.2011.11.001. [DOI] [PubMed] [Google Scholar]
- Dolly JO, Lawrence GW, Meng J, Wang J, Ovsepian SV. Neuro-exocytosis: botulinum toxins as inhibitory probes and versatile therapeutics. Curr Opin Pharmacol. 2009;9:326–335. doi: 10.1016/j.coph.2009.03.004. [DOI] [PubMed] [Google Scholar]
- Dong M, Yeh F, Tepp WH, Dean C, Johnson EA, Janz R, et al. SV2 is the protein receptor for botulinum neurotoxin A. Science. 2006;312:592–596. doi: 10.1126/science.1123654. [DOI] [PubMed] [Google Scholar]
- Dong M, Tepp WH, Liu H, Johnson EA, Chapman ER. Mechanism of botulinum neurotoxin B and G entry into hippocampal neurons. J Cell Biol. 2007;179:1511–1522. doi: 10.1083/jcb.200707184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duggan MJ, Quinn CP, Chaddock JA, Purkiss JR, Alexander FC, Doward S, et al. Inhibition of release of neurotransmitters from rat dorsal root ganglia by a novel conjugate of a Clostridium botulinum toxin A endopeptidase fragment and Erythrina cristagalli lectin. J Biol Chem. 2002;277:34846–34852. doi: 10.1074/jbc.M202902200. [DOI] [PubMed] [Google Scholar]
- Durham PL, Cady R, Cady R. Regulation of calcitonin gene-related peptide secretion from trigeminal nerve cells by botulinum toxin type A: implications for migraine therapy. Headache. 2004;44:35–42. doi: 10.1111/j.1526-4610.2004.04007.x. [DOI] [PubMed] [Google Scholar]
- Ebersberger A. Physiology of meningeal innervation: aspects and consequences of chemosensitivity of meningeal nociceptors. Microsc Res Tech. 2001;53:138–146. doi: 10.1002/jemt.1078. [DOI] [PubMed] [Google Scholar]
- Eftekhari S, Warfvinge K, Blixt FW, Edvinsson L. Differentiation of nerve fibers storing CGRP and CGRP receptors in the peripheral trigeminovascular system. J Pain. 2013;14:1289–1303. doi: 10.1016/j.jpain.2013.03.010. [DOI] [PubMed] [Google Scholar]
- Eijkelkamp N, Kavelaars A, Elsenbruch S, Schedlowski M, Holtmann G, Heijnen CJ. Increased visceral sensitivity to capsaicin after DSS-induced colitis in mice: spinal cord c-Fos expression and behavior. Am J Physiol Gastrointest Liver Physiol. 2007;293:G749–G757. doi: 10.1152/ajpgi.00114.2007. [DOI] [PubMed] [Google Scholar]
- Eleopra R, Tugnoli V, Rossetto O, De GD, Montecucco C. Different time courses of recovery after poisoning with botulinum neurotoxin serotypes A and E in humans. Neurosci Lett. 1998;256:135–138. doi: 10.1016/s0304-3940(98)00775-7. [DOI] [PubMed] [Google Scholar]
- Elkind AH, O'Carroll P, Blumenfeld A, DeGryse R, Dimitrova R. A series of three sequential, randomized, controlled studies of repeated treatments with botulinum toxin type A for migraine prophylaxis. J Pain. 2006;7:688–696. doi: 10.1016/j.jpain.2006.03.002. [DOI] [PubMed] [Google Scholar]
- Evers S. Treatment of migraine with prophylactic drugs. Expert Opin Pharmacother. 2008;9:2565–2573. doi: 10.1517/14656566.9.15.2565. [DOI] [PubMed] [Google Scholar]
- Evers S, Vollmer-Haase J, Schwaag S, Rahmann A, Husstedt IW, Frese A. Botulinum toxin A in the prophylactic treatment of migraine–a randomized, double-blind, placebo-controlled study. Cephalalgia. 2004;24:838–843. doi: 10.1111/j.1468-2982.2004.00754.x. [DOI] [PubMed] [Google Scholar]
- Fabregat G, De AJ, Villanueva-Perez VL, Asensio-Samper JM. Subcutaneous and perineural botulinum toxin type a for neuropathic pain: a descriptive review. Clin J Pain. 2013;29:1006–1012. doi: 10.1097/AJP.0b013e31827eafff. [DOI] [PubMed] [Google Scholar]
- Filipovic B, Matak I, Bach-Rojecky L, Lackovic Z. Central action of peripherally applied botulinum toxin type A on pain and dural protein extravasation in rat model of trigeminal neuropathy. PLoS ONE. 2012;7:e29803. doi: 10.1371/journal.pone.0029803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer A, Montal M. Single molecule detection of intermediates during botulinum neurotoxin translocation across membranes. Proc Natl Acad Sci U S A. 2007;104:10447–10452. doi: 10.1073/pnas.0700046104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer A, Nakai Y, Eubanks LM, Clancy CM, Tepp WH, Pellett S, et al. Bimodal modulation of the botulinum neurotoxin protein-conducting channel. Proc Natl Acad Sci U S A. 2009;106:1330–1335. doi: 10.1073/pnas.0812839106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn TC. Botulinum toxin: examining duration of effect in facial aesthetic applications. Am J Clin Dermatol. 2010;11:183–199. doi: 10.2165/11530110-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Foran PG, Mohammed N, Lisk GO, Nagwaney S, Lawrence GW, Johnson E, et al. Evaluation of the therapeutic usefulness of botulinum neurotoxin B, C1, E, and F compared with the long lasting type A. Basis for distinct durations of inhibition of exocytosis in central neurons. J Biol Chem. 2003;278:1363–1371. doi: 10.1074/jbc.M209821200. [DOI] [PubMed] [Google Scholar]
- Foreman RD. Integration of viscerosomatic sensory input at the spinal level. Prog Brain Res. 2000;122:209–221. doi: 10.1016/s0079-6123(08)62140-8. [DOI] [PubMed] [Google Scholar]
- Fotinou C, Emsley P, Black I, Ando H, Ishida H, Kiso M, et al. The crystal structure of tetanus toxin Hc fragment complexed with a synthetic GT1b analogue suggests cross-linking between ganglioside receptors and the toxin. J Biol Chem. 2001;276:32274–32281. doi: 10.1074/jbc.M103285200. [DOI] [PubMed] [Google Scholar]
- Freitag FG, Diamond S, Diamond M, Urban G. Botulinum toxin type A in the treatment of chronic migraine without medication overuse. Headache. 2008;48:201–209. doi: 10.1111/j.1526-4610.2007.00963.x. [DOI] [PubMed] [Google Scholar]
- Gazerani P, Staahl C, Drewes AM, Arendt-Nielsen L. The effects of botulinum toxin type A on capsaicin-evoked pain, flare, and secondary hyperalgesia in an experimental human model of trigeminal sensitization. Pain. 2006;122:315–325. doi: 10.1016/j.pain.2006.04.014. [DOI] [PubMed] [Google Scholar]
- Gazerani P, Pedersen NS, Drewes AM, Arendt-Nielsen L. Botulinum toxin type A reduces histamine-induced itch and vasomotor responses in human skin. Br J Dermatol. 2009a;161:737–745. doi: 10.1111/j.1365-2133.2009.09305.x. [DOI] [PubMed] [Google Scholar]
- Gazerani P, Pedersen NS, Staahl C, Drewes AM, Arendt-Nielsen L. Subcutaneous botulinum toxin type A reduces capsaicin-induced trigeminal pain and vasomotor reactions in human skin. Pain. 2009b;141:60–69. doi: 10.1016/j.pain.2008.10.005. [DOI] [PubMed] [Google Scholar]
- Grazzi L. Onabotulinum toxin A for treatment of chronic migraine with medication overuse. Neurol Sci. 2013;34(Suppl. 1):S27–S28. doi: 10.1007/s10072-013-1381-1. [DOI] [PubMed] [Google Scholar]
- Gupta S, Amrutkar DV, Mataji A, Salmasi H, Hay-Schmidt A, Sheykhzade M, et al. Evidence for CGRP re-uptake in rat dura mater encephali. Br J Pharmacol. 2010;161:1885–1898. doi: 10.1111/j.1476-5381.2010.01012.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habermann E. 125I-labeled neurotoxin from Clostridium botulinum A: preparation, binding to synaptosomes and ascent to the spinal cord. Naunyn Schmiedebergs Arch Pharmacol. 1974;281:47–56. doi: 10.1007/BF00500611. [DOI] [PubMed] [Google Scholar]
- Hamdy SM, Samir H, El-Sayed M, Adel N, Hasan R. Botulinum toxin: could it be an effective treatment for chronic tension-type headache? J Headache Pain. 2009;10:27–34. doi: 10.1007/s10194-008-0082-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, Elias CL, Huang YC, Gao X, Leung YM, Kang Y, et al. Botulinum neurotoxin A and neurotoxin E cleavage products of synaptosome-associated protein of 25 kd exhibit distinct actions on pancreatic islet beta-cell Kv2.1 channel gating. Pancreas. 2008;36:10–17. doi: 10.1097/mpa.0b013e31812eee28. [DOI] [PubMed] [Google Scholar]
- Huang PP, Khan I, Suhail MS, Malkmus S, Yaksh TL. Spinal botulinum neurotoxin B: effects on afferent transmitter release and nociceptive processing. PLoS ONE. 2011;6:e19126. doi: 10.1371/journal.pone.0019126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IASP. 2013. International Association for the Study of Pain. Global Year Against Headache-Migraine. IASP. Available at: http://www.iasp-pain.org/AM/Template.cfm?Section=Fact_Sheets4&Template=/CM/ContentDisplay.cfm&ContentID=14453. (accessed 1/31/2013)
- Ikeda Y, Zabbarova IV, Birder LA, de Groat WC, McCarthy CJ, Hanna-Mitchell AT, et al. Botulinum neurotoxin serotype A suppresses neurotransmitter release from afferent as well as efferent nerves in the urinary bladder. Eur Urol. 2012;62:1157–1164. doi: 10.1016/j.eururo.2012.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson JL, Kuriyama A, Hayashino Y. Botulinum toxin A for prophylactic treatment of migraine and tension headaches in adults: a meta-analysis. JAMA. 2012;307:1736–1745. doi: 10.1001/jama.2012.505. [DOI] [PubMed] [Google Scholar]
- Jakubowski M, Levy D, Goor-Aryeh I, Collins B, Bajwa Z, Burstein R. Terminating migraine with allodynia and ongoing central sensitization using parenteral administration of COX1/COX2 inhibitors. Headache. 2005;45:850–861. doi: 10.1111/j.1526-4610.2005.05153.x. [DOI] [PubMed] [Google Scholar]
- Jakubowski M, McAllister PJ, Bajwa ZH, Ward TN, Smith P, Burstein R. Exploding vs. imploding headache in migraine prophylaxis with botulinum toxin A. Pain. 2006;125:286–295. doi: 10.1016/j.pain.2006.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen I, Uddman R, Ekman R, Olesen J, Ottosson A, Edvinsson L. Distribution and effects of neuropeptide Y, vasoactive intestinal peptide, substance P, and calcitonin gene-related peptide in human middle meningeal arteries: comparison with cerebral and temporal arteries. Peptides. 1992;13:527–536. doi: 10.1016/0196-9781(92)90084-g. [DOI] [PubMed] [Google Scholar]
- Juliano RL, Carver K, Cao C, Ming X. Receptors, endocytosis, and trafficking: the biological basis of targeted delivery of antisense and siRNA oligonucleotides. J Drug Target. 2013;21:27–43. doi: 10.3109/1061186X.2012.740674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurasinski CV, Lieth E, Dang Do AN, Schengrund CL. Correlation of cleavage of SNAP-25 with muscle function in a rat model of botulinum neurotoxin type A induced paralysis. Toxicon. 2001;39:1309–1315. doi: 10.1016/s0041-0101(01)00082-4. [DOI] [PubMed] [Google Scholar]
- Kakegawa W, Yuzaki M. A mechanism underlying AMPA receptor trafficking during cerebellar long-term potentiation. Proc Natl Acad Sci U S A. 2005;102:17846–17851. doi: 10.1073/pnas.0508910102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauffman JA, Way JF, Jr, Siegel LS, Sellin LC. Comparison of the action of types A and F botulinum toxin at the rat neuromuscular junction. Toxicol Appl Pharmacol. 1985;79:211–217. doi: 10.1016/0041-008x(85)90342-4. [DOI] [PubMed] [Google Scholar]
- Keller JE, Neale EA, Oyler G, Adler M. Persistence of botulinum neurotoxin action in cultured spinal cord cells. FEBS Lett. 1999;456:137–142. doi: 10.1016/s0014-5793(99)00948-5. [DOI] [PubMed] [Google Scholar]
- Keller JE, Cai F, Neale EA. Uptake of botulinum neurotoxin into cultured neurons. Biochemistry. 2004;43:526–532. doi: 10.1021/bi0356698. [DOI] [PubMed] [Google Scholar]
- Kowalski ML, Sliwinska-Kowalska M, Kaliner MA. Neurogenic inflammation, vascular permeability, and mast cells. II. Additional evidence indicating that mast cells are not involved in neurogenic inflammation. J Immunol. 1990;145:1214–1221. [PubMed] [Google Scholar]
- Kramer HH, Angerer C, Erbguth F, Schmelz M, Birklein F. Botulinum toxin a reduces neurogenic flare but has almost no effect on pain and hyperalgesia in human skin. J Neurol. 2003;250:188–193. doi: 10.1007/s00415-003-0971-x. [DOI] [PubMed] [Google Scholar]
- Laird JM, Martinez-Caro L, Garcia-Nicas E, Cervero F. A new model of visceral pain and referred hyperalgesia in the mouse. Pain. 2001;92:335–342. doi: 10.1016/S0304-3959(01)00275-5. [DOI] [PubMed] [Google Scholar]
- Lalli G, Bohnert S, Deinhardt K, Verastegui C, Schiavo G. The journey of tetanus and botulinum neurotoxins in neurons. Trends Microbiol. 2003;11:431–437. doi: 10.1016/s0966-842x(03)00210-5. [DOI] [PubMed] [Google Scholar]
- Lang T. SNARE proteins and ‘membrane rafts. J Physiol. 2007;585:693–698. doi: 10.1113/jphysiol.2007.134346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau CG, Takayasu Y, Rodenas-Ruano A, Paternain AV, Lerma J, Bennett MV, et al. SNAP-25 is a target of protein kinase C phosphorylation critical to NMDA receptor trafficking. J Neurosci. 2010;30:242–254. doi: 10.1523/JNEUROSCI.4933-08.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence GW, Foran P, Oliver DJ. Insights into a basis for incomplete inhibition by botulinum toxin A of Ca2+-evoked exocytosis from permeabilised chromaffin cells. Toxicology. 2002;181–182:249–253. doi: 10.1016/s0300-483x(02)00453-5. [DOI] [PubMed] [Google Scholar]
- Lawrence GW, Ovsepian SV, Wang J, Aoki KR, Dolly JO. Extravesicular intraneuronal migration of internalized botulinum neurotoxins without detectable inhibition of distal neurotransmission. Biochem J. 2012;441:443–452. doi: 10.1042/BJ20111117. [DOI] [PubMed] [Google Scholar]
- Leggett J, Harper E, Waite E, Marks P, Martinez A, Lightman S. GHRH receptor-targeted botulinum neurotoxin selectively inhibits pulsatile GH secretion in male rats. Endocrinology. 2013;154:3305–3318. doi: 10.1210/en.2012-2175. [DOI] [PubMed] [Google Scholar]
- Levy D, Strassman AM. Mechanical response properties of A and C primary afferent neurons innervating the rat intracranial dura. J Neurophysiol. 2002;88:3021–3031. doi: 10.1152/jn.00029.2002. [DOI] [PubMed] [Google Scholar]
- Levy D, Burstein R, Kainz V, Jakubowski M, Strassman AM. Mast cell degranulation activates a pain pathway underlying migraine headache. Pain. 2007;130:166–176. doi: 10.1016/j.pain.2007.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton RB, Bigal ME, Diamond M, Freitag F, Reed ML, Stewart WF. Migraine prevalence, disease burden, and the need for preventive therapy. Neurology. 2007;68:343–349. doi: 10.1212/01.wnl.0000252808.97649.21. [DOI] [PubMed] [Google Scholar]
- Lipton RB, Varon SF, Grosberg B, McAllister PJ, Freitag F, Aurora SK, et al. OnabotulinumtoxinA improves quality of life and reduces impact of chronic migraine. Neurology. 2011;77:1465–1472. doi: 10.1212/WNL.0b013e318232ab65. [DOI] [PubMed] [Google Scholar]
- Littlewood NK, Todd AJ, Spike RC, Watt C, Shehab SA. The types of neuron in spinal dorsal horn which possess neurokinin-1 receptors. Neuroscience. 1995;66:597–608. doi: 10.1016/0306-4522(95)00039-l. [DOI] [PubMed] [Google Scholar]
- Liu HT, Tsai SK, Kao MC, Hu JS. Botulinum toxin A relieved neuropathic pain in a case of post-herpetic neuralgia. Pain Med. 2006;7:89–91. doi: 10.1111/j.1526-4637.2006.00100.x. [DOI] [PubMed] [Google Scholar]
- Lucioni A, Bales GT, Lotan TL, McGehee DS, Cook SP, Rapp DE. Botulinum toxin type A inhibits sensory neuropeptide release in rat bladder models of acute injury and chronic inflammation. BJU Int. 2008;101:366–370. doi: 10.1111/j.1464-410X.2007.07312.x. [DOI] [PubMed] [Google Scholar]
- Ludlow CL, Hallett M, Rhew K, Cole R, Shimizu T, Sakaguchi G, et al. Therapeutic use of type F botulinum toxin. N Engl J Med. 1992;326:349–350. doi: 10.1056/NEJM199201303260516. [DOI] [PubMed] [Google Scholar]
- Luvisetto S, Marinelli S, Cobianchi S, Pavone F. Anti-allodynic efficacy of botulinum neurotoxin A in a model of neuropathic pain. Neuroscience. 2007;145:1–4. doi: 10.1016/j.neuroscience.2006.12.004. [DOI] [PubMed] [Google Scholar]
- Ma L, Nagai J, Sekino Y, Goto Y, Nakahira S, Ueda H. Single application of A2 NTX, a botulinum toxin A2 subunit, prevents chronic pain over long periods in both diabetic and spinal cord injury-induced neuropathic pain models. J Pharmacol Sci. 2012;119:282–286. doi: 10.1254/jphs.12080sc. [DOI] [PubMed] [Google Scholar]
- Magalhaes E, Menezes C, Cardeal M, Melo A. Botulinum toxin type A versus amitriptyline for the treatment of chronic daily migraine. Clin Neurol Neurosurg. 2010;112:463–466. doi: 10.1016/j.clineuro.2010.02.004. [DOI] [PubMed] [Google Scholar]
- Mahant N, Clouston PD, Lorentz IT. The current use of botulinum toxin. J Clin Neurosci. 2000;7:389–394. doi: 10.1054/jocn.2000.0684. [DOI] [PubMed] [Google Scholar]
- Marchand-Pauvert V, Aymard C, Giboin LS, Dominici F, Rossi A, Mazzocchio R. Beyond muscular effects: depression of spinal recurrent inhibition after botulinum neurotoxin A. J Physiol. 2013;591:1017–1029. doi: 10.1113/jphysiol.2012.239178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinelli S, Vacca V, Ricordy R, Uggenti C, Tata AM, Luvisetto S, et al. The analgesic effect on neuropathic pain of retrogradely transported botulinum neurotoxin A involves Schwann cells and astrocytes. PLoS ONE. 2012;7:e47977. doi: 10.1371/journal.pone.0047977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marino MJ, Terashima T, Steinauer JJ, Eddinger KA, Yaksh TL, Xu Q. Botulinum toxin B in the sensory afferent: transmitter release, spinal activation, and pain behavior. Pain. 2014;155:674–684. doi: 10.1016/j.pain.2013.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markowitz S, Saito K, Moskowitz MA. Neurogenically mediated plasma extravasation in dura mater: effect of ergot alkaloids. A possible mechanism of action in vascular headache. Cephalalgia. 1988;8:83–91. doi: 10.1046/j.1468-2982.1988.0802083.x. [DOI] [PubMed] [Google Scholar]
- Matak I, Bach-Rojecky L, Filipovic B, Lackovic Z. Behavioral and immunohistochemical evidence for central antinociceptive activity of botulinum toxin A. Neuroscience. 2011;186:201–207. doi: 10.1016/j.neuroscience.2011.04.026. [DOI] [PubMed] [Google Scholar]
- Matak I, Riederer P, Lackovic Z. Botulinum toxin's axonal transport from periphery to the spinal cord. Neurochem Int. 2012;61:236–239. doi: 10.1016/j.neuint.2012.05.001. [DOI] [PubMed] [Google Scholar]
- Matak I, Stracenski I, Lackovic Z. Comparison of analgesic effects of single versus repeated injection of botulinum toxin in orofacial formalin test in rats. J Neural Transm. 2013;120:141–144. doi: 10.1007/s00702-012-0846-3. [DOI] [PubMed] [Google Scholar]
- Mathew NT, Jaffri SF. A double-blind comparison of onabotulinumtoxina (BOTOX) and topiramate (TOPAMAX) for the prophylactic treatment of chronic migraine: a pilot study. Headache. 2009;49:1466–1478. doi: 10.1111/j.1526-4610.2009.01566.x. [DOI] [PubMed] [Google Scholar]
- Mathew NT, Frishberg BM, Gawel M, Dimitrova R, Gibson J, Turkel C. Botulinum toxin type A (BOTOX) for the prophylactic treatment of chronic daily headache: a randomized, double-blind, placebo-controlled trial. Headache. 2005;45:293–307. doi: 10.1111/j.1526-4610.2005.05066.x. [DOI] [PubMed] [Google Scholar]
- McNaughton FL, Feindel WH. Rose FC, editor. Innervation of intracranial structures: a reappraisal. Physiological Aspects of Clinical Neurology. 1977:279–293. Blackwell Scientific Publications: Oxford. [Google Scholar]
- Meng J, Wang J, Lawrence G, Dolly JO. Synaptobrevin I mediates exocytosis of CGRP from sensory neurons and inhibition by botulinum toxins reflects their anti-nociceptive potential. J Cell Sci. 2007;120:2864–2874. doi: 10.1242/jcs.012211. [DOI] [PubMed] [Google Scholar]
- Meng J, Ovsepian SV, Wang J, Pickering M, Sasse A, Aoki KR, et al. Activation of TRPV1 mediates calcitonin gene-related peptide release, which excites trigeminal sensory neurons and is attenuated by a retargeted botulinum toxin with anti-nociceptive potential. J Neurosci. 2009;29:4981–4992. doi: 10.1523/JNEUROSCI.5490-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molgo J, Comella JX, Angaut-Petit D, Pecot-Dechavassine M, Tabti N, Faille L, et al. Presynaptic actions of botulinal neurotoxins at vertebrate neuromuscular junctions. J Physiol (Paris) 1990;84:152–166. [PubMed] [Google Scholar]
- Montal M. Botulinum neurotoxin: a marvel of protein design. Annu Rev Biochem. 2010;79:591–617. doi: 10.1146/annurev.biochem.051908.125345. [DOI] [PubMed] [Google Scholar]
- Montecucco C, Schiavo G. Mechanism of action of tetanus and botulinum neurotoxins. Mol Microbiol. 1994;13:1–8. doi: 10.1111/j.1365-2958.1994.tb00396.x. [DOI] [PubMed] [Google Scholar]
- Montell C. Exciting trips for TRPs. Nat Cell Biol. 2004;6:690–692. doi: 10.1038/ncb0804-690. [DOI] [PubMed] [Google Scholar]
- Morch CD, Hu JW, Arendt-Nielsen L, Sessle BJ. Convergence of cutaneous, musculoskeletal, dural and visceral afferents onto nociceptive neurons in the first cervical dorsal horn. Eur J Neurosci. 2007;26:142–154. doi: 10.1111/j.1460-9568.2007.05608.x. [DOI] [PubMed] [Google Scholar]
- Nilsson F, Nilsson T, Edvinsson L, Bjorkman S, Nordstrom CH. Effects of dihydroergotamine and sumatriptan on isolated human cerebral and peripheral arteries and veins. Acta Anaesthesiol Scand. 1997;41:1257–1262. doi: 10.1111/j.1399-6576.1997.tb04641.x. [DOI] [PubMed] [Google Scholar]
- Noma N, Tsuboi Y, Kondo M, Matsumoto M, Sessle BJ, Kitagawa J, et al. Organization of pERK-immunoreactive cells in trigeminal spinal nucleus caudalis and upper cervical cord following capsaicin injection into oral and craniofacial regions in rats. J Comp Neurol. 2008;507:1428–1440. doi: 10.1002/cne.21620. [DOI] [PubMed] [Google Scholar]
- Olesen J. ICHD-3 beta is published. Use it immediately. Cephalalgia. 2013;33:627–628. doi: 10.1177/0333102413487610. [DOI] [PubMed] [Google Scholar]
- Olesen J, Ashina M. Emerging migraine treatments and drug targets. Trends Pharmacol Sci. 2011;32:352–359. doi: 10.1016/j.tips.2011.02.016. [DOI] [PubMed] [Google Scholar]
- Olesen J, Burstein R, Ashina M, Tfelt-Hansen P. Origin of pain in migraine: evidence for peripheral sensitisation. Lancet Neurol. 2009;8:679–690. doi: 10.1016/S1474-4422(09)70090-0. [DOI] [PubMed] [Google Scholar]
- Oshinsky ML, Gomonchareonsiri S. Episodic dural stimulation in awake rats: a model for recurrent headache. Headache. 2007;47:1026–1036. doi: 10.1111/j.1526-4610.2007.00871.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Paiva A, Meunier FA, Molgo J, Aoki KR, Dolly JO. Functional repair of motor endplates after botulinum neurotoxin type A poisoning: biphasic switch of synaptic activity between nerve sprouts and their parent terminals. Proc Natl Acad Sci U S A. 1999;96:3200–3205. doi: 10.1073/pnas.96.6.3200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park HJ, Lee Y, Lee J, Park C, Moon DE. The effects of botulinum toxin A on mechanical and cold allodynia in a rat model of neuropathic pain. Can J Anaesth. 2006;53:470–477. doi: 10.1007/BF03022619. [DOI] [PubMed] [Google Scholar]
- Park TH. The effects of botulinum toxin A on mast cell activity: preliminary results. Burns. 2013;39:816–817. doi: 10.1016/j.burns.2012.07.031. [DOI] [PubMed] [Google Scholar]
- Pawson AJ, Sharman JL, Benson HE, Faccenda E, Alexander SP, Buneman OP, et al. The IUPHAR/BPS Guide to PHARMACOLOGY: an expert-driven knowledge base of drug targets and their ligands. Nucleic Acids Research. 2014;42(Database Issue):D1098–1106. doi: 10.1093/nar/gkt1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peck MW. Biology and genomic analysis of Clostridium botulinum. Adv Microb Physiol. 2009;55:183–265. doi: 10.1016/S0065-2911(09)05503-9. 320. [DOI] [PubMed] [Google Scholar]
- Pellett S. Learning from the past: historical aspects of bacterial toxins as pharmaceuticals. Curr Opin Microbiol. 2012;15:292–299. doi: 10.1016/j.mib.2012.05.005. [DOI] [PubMed] [Google Scholar]
- Peroutka SJ. Neurogenic inflammation and migraine: implications for the therapeutics. Mol Interv. 2005;5:304–311. doi: 10.1124/mi.5.5.10. [DOI] [PubMed] [Google Scholar]
- Petri S, Tolle T, Straube A, Pfaffenrath V, Stefenelli U, Ceballos-Baumann A. Botulinum toxin as preventive treatment for migraine: a randomized double-blind study. Eur Neurol. 2009;62:204–211. doi: 10.1159/000228987. [DOI] [PubMed] [Google Scholar]
- Piovesan EJ, Kowacs PA, Tatsui CE, Lange MC, Ribas LC, Werneck LC. Referred pain after painful stimulation of the greater occipital nerve in humans: evidence of convergence of cervical afferences on trigeminal nuclei. Cephalalgia. 2001;21:107–109. doi: 10.1046/j.1468-2982.2001.00166.x. [DOI] [PubMed] [Google Scholar]
- Piovesan EJ, Teive HG, Kowacs PA, Della Coletta MV, Werneck LC, Silberstein SD. An open study of botulinum-A toxin treatment of trigeminal neuralgia. Neurology. 2005;65:1306–1308. doi: 10.1212/01.wnl.0000180940.98815.74. [DOI] [PubMed] [Google Scholar]
- Porta M. A comparative trial of botulinum toxin type A and methylprednisolone for the treatment of tension-type headache. Curr Rev Pain. 2000;4:31–35. doi: 10.1007/s11916-000-0007-5. [DOI] [PubMed] [Google Scholar]
- Ramachandran R, Bhatt DK, Ploug KB, Olesen J, Jansen-Olesen I, Hay-Schmidt A, et al. A naturalistic glyceryl trinitrate infusion migraine model in the rat. Cephalalgia. 2012;32:73–84. doi: 10.1177/0333102411430855. [DOI] [PubMed] [Google Scholar]
- Ramachandran R, Bhatt DK, Ploug KB, Gupta S, Hay-Schmidt A, Jansen-Olesen I, et al. Nitric oxide synthase, calcitonin gene-related peptide and NK-1 receptor mechanisms are involved in GTN induced neuronal activation. Cephalalgia. 2014;34:136–147. doi: 10.1177/0333102413502735. [DOI] [PubMed] [Google Scholar]
- Ranoux D, Attal N, Morain F, Bouhassira D. Botulinum toxin type A induces direct analgesic effects in chronic neuropathic pain. Ann Neurol. 2008;64:274–283. doi: 10.1002/ana.21427. [DOI] [PubMed] [Google Scholar]
- Rapp DE, Turk KW, Bales GT, Cook SP. Botulinum toxin type a inhibits calcitonin gene-related peptide release from isolated rat bladder. J Urol. 2006;175:1138–1142. doi: 10.1016/S0022-5347(05)00322-8. [DOI] [PubMed] [Google Scholar]
- Ray BS, Wolff HG. Experimental studies on headache; pain-sensitive structures of the head and their significance in headache. Arch Surg. 1940;41:813–856. [Google Scholar]
- Relja M, Poole AC, Schoenen J, Pascual J, Lei X, Thompson C. A multicentre, double-blind, randomized, placebo-controlled, parallel group study of multiple treatments of botulinum toxin type A (BoNTA) for the prophylaxis of episodic migraine headaches. Cephalalgia. 2007;27:492–503. doi: 10.1111/j.1468-2982.2007.01315.x. [DOI] [PubMed] [Google Scholar]
- Restani L, Antonucci F, Gianfranceschi L, Rossi C, Rossetto O, Caleo M. Evidence for anterograde transport and transcytosis of botulinum neurotoxin A (BoNT/A) J Neurosci. 2011;31:15650–15659. doi: 10.1523/JNEUROSCI.2618-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Restani L, Giribaldi F, Manich M, Bercsenyi K, Menendez G, Rossetto O, et al. Botulinum neurotoxins A and E undergo retrograde axonal transport in primary motor neurons. PLoS Pathog. 2012;8:e1003087. doi: 10.1371/journal.ppat.1003087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson DR, Gebhart GF. Inside information: the unique features of visceral sensation. Mol Interv. 2008;8:242–253. doi: 10.1124/mi.8.5.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roch M, Messlinger K, Kulchitsky V, Tichonovich O, Azev O, Koulchitsky S. Ongoing activity in trigeminal wide-dynamic range neurons is driven from the periphery. Neuroscience. 2007;150:681–691. doi: 10.1016/j.neuroscience.2007.09.032. [DOI] [PubMed] [Google Scholar]
- Rossetto O, Montecucco C. Presynaptic neurotoxins with enzymatic activities. Handb Exp Pharmacol. 2008;184:129–170. doi: 10.1007/978-3-540-74805-2_6. [DOI] [PubMed] [Google Scholar]
- Rothman JE. Mechanisms of intracellular transport. Nature. 1994;372:55–63. doi: 10.1038/372055a0. [DOI] [PubMed] [Google Scholar]
- Ruiz HC, Bermejo P. [Botulinum toxin type A in the treatment of neuropathic pain in a case of postherpetic neuralgia] Neurologia. 2008;23:259–262. [PubMed] [Google Scholar]
- Rummel A. Double receptor anchorage of botulinum neurotoxins accounts for their exquisite neurospecificity. Curr Top Microbiol Immunol. 2013;364:61–90. doi: 10.1007/978-3-642-33570-9_4. [DOI] [PubMed] [Google Scholar]
- Rummel A, Eichner T, Weil T, Karnath T, Gutcaits A, Mahrhold S, et al. Identification of the protein receptor binding site of botulinum neurotoxins B and G proves the double-receptor concept. Proc Natl Acad Sci U S A. 2007;104:359–364. doi: 10.1073/pnas.0609713104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell MB, Rasmussen BK, Thorvaldsen P, Olesen J. Prevalence and sex-ratio of the subtypes of migraine. Int J Epidemiol. 1995;24:612–618. doi: 10.1093/ije/24.3.612. [DOI] [PubMed] [Google Scholar]
- Samton J, Mauskop A. Treatment of headaches with botulinum toxin. Expert Rev Neurother. 2006;6:313–322. doi: 10.1586/14737175.6.3.313. [DOI] [PubMed] [Google Scholar]
- Sandrini G, Perrotta A, Tassorelli C, Torelli P, Brighina F, Sances G, et al. Botulinum toxin type-A in the prophylactic treatment of medication-overuse headache: a multicenter, double-blind, randomized, placebo-controlled, parallel group study. J Headache Pain. 2011;12:427–433. doi: 10.1007/s10194-011-0339-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saper JR, Mathew NT, Loder EW, DeGryse R, Vandenburgh AM. A double-blind, randomized, placebo-controlled comparison of botulinum toxin type a injection sites and doses in the prevention of episodic migraine. Pain Med. 2007;8:478–485. doi: 10.1111/j.1526-4637.2006.00168.x. [DOI] [PubMed] [Google Scholar]
- Schenk U, Verderio C, Benfenati F, Matteoli M. Regulated delivery of AMPA receptor subunits to the presynaptic membrane. EMBO J. 2003;22:558–568. doi: 10.1093/emboj/cdg059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiavo G, Shone CC, Bennett MK, Scheller RH, Montecucco C. Botulinum neurotoxin type C cleaves a single Lys–Ala bond within the carboxyl-terminal region of syntaxins. J Biol Chem. 1995;270:10566–10570. doi: 10.1074/jbc.270.18.10566. [DOI] [PubMed] [Google Scholar]
- Schiavo G, Matteoli M, Montecucco C. Neurotoxins affecting neuroexocytosis. Physiol Rev. 2000;80:717–766. doi: 10.1152/physrev.2000.80.2.717. [DOI] [PubMed] [Google Scholar]
- Schueler M, Messlinger K, Dux M, Neuhuber WL, De CR. Extracranial projections of meningeal afferents and their impact on meningeal nociception and headache. Pain. 2013;154:1622–1631. doi: 10.1016/j.pain.2013.04.040. [DOI] [PubMed] [Google Scholar]
- Serrano D, Manack AN, Reed ML, Buse DC, Varon SF, Lipton RB. Cost and predictors of lost productive time in chronic migraine and episodic migraine: results from the American Migraine Prevalence and Prevention (AMPP) Study. Value Health. 2013;16:31–38. doi: 10.1016/j.jval.2012.08.2212. [DOI] [PubMed] [Google Scholar]
- Shan XF, Xu H, Cai ZG, Wu LL, Yu GY. Botulinum toxin A inhibits salivary secretion of rabbit submandibular gland. Int J Oral Sci. 2013;5:217–223. doi: 10.1038/ijos.2013.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu T, Shibata M, Toriumi H, Iwashita T, Funakubo M, Sato H, et al. Reduction of TRPV1 expression in the trigeminal system by botulinum neurotoxin type-A. Neurobiol Dis. 2012;48:367–378. doi: 10.1016/j.nbd.2012.07.010. [DOI] [PubMed] [Google Scholar]
- Silberstein S, Mathew N, Saper J, Jenkins S. Botulinum toxin type A as a migraine preventive treatment. For the BOTOX Migraine Clinical Research Group. Headache. 2000;40:445–450. doi: 10.1046/j.1526-4610.2000.00066.x. [DOI] [PubMed] [Google Scholar]
- Silberstein SD, Blumenfeld AM, Cady RK, Turner IM, Lipton RB, Diener HC, et al. OnabotulinumtoxinA for treatment of chronic migraine: PREEMPT 24-week pooled subgroup analysis of patients who had acute headache medication overuse at baseline. J Neurol Sci. 2013;331:48–56. doi: 10.1016/j.jns.2013.05.003. [DOI] [PubMed] [Google Scholar]
- Simpson L. The life history of a botulinum toxin molecule. Toxicon. 2013;68:40–59. doi: 10.1016/j.toxicon.2013.02.014. [DOI] [PubMed] [Google Scholar]
- Sloop RR, Cole BA, Escutin RO. Human response to botulinum toxin injection: type B compared with type A. Neurology. 1997;49:189–194. doi: 10.1212/wnl.49.1.189. [DOI] [PubMed] [Google Scholar]
- Smyth LM, Breen LT, Mutafova-Yambolieva VN. Nicotinamide adenine dinucleotide is released from sympathetic nerve terminals via a botulinum neurotoxin A-mediated mechanism in canine mesenteric artery. Am J Physiol Heart Circ Physiol. 2006;290:H1818–H1825. doi: 10.1152/ajpheart.01062.2005. [DOI] [PubMed] [Google Scholar]
- Steiger HJ, Meakin CJ. The meningeal representation in the trigeminal ganglion – an experimental study in the cat. Headache. 1984;24:305–309. doi: 10.1111/j.1526-4610.1984.hed2406305.x. [DOI] [PubMed] [Google Scholar]
- Steinberg JP, Huganir RL, Linden DJ. N-ethylmaleimide-sensitive factor is required for the synaptic incorporation and removal of AMPA receptors during cerebellar long-term depression. Proc Natl Acad Sci U S A. 2004;101:18212–18216. doi: 10.1073/pnas.0408278102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenmark P, Dupuy J, Imamura A, Kiso M, Stevens RC. Crystal structure of botulinum neurotoxin type A in complex with the cell surface co-receptor GT1b-insight into the toxin-neuron interaction. PLoS Pathog. 2008;4:e1000129. doi: 10.1371/journal.ppat.1000129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strassman A, Mason P, Moskowitz M, Maciewicz R. Response of brainstem trigeminal neurons to electrical stimulation of the dura. Brain Res. 1986;379:242–250. doi: 10.1016/0006-8993(86)90777-8. [DOI] [PubMed] [Google Scholar]
- Strassman AM, Levy D. Response properties of dural nociceptors in relation to headache. J Neurophysiol. 2006;95:1298–1306. doi: 10.1152/jn.01293.2005. [DOI] [PubMed] [Google Scholar]
- Strassman AM, Mineta Y, Vos BP. Distribution of fos-like immunoreactivity in the medullary and upper cervical dorsal horn produced by stimulation of dural blood vessels in the rat. J Neurosci. 1994;14:3725–3735. doi: 10.1523/JNEUROSCI.14-06-03725.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strassman AM, Raymond SA, Burstein R. Sensitization of meningeal sensory neurons and the origin of headaches. Nature. 1996;384:560–564. doi: 10.1038/384560a0. [DOI] [PubMed] [Google Scholar]
- Strecker T, Messlinger K. [Neuropeptide release in the dura mater encephali in response to nitric oxide – relevance for the development of vascular headaches?] Schmerz. 2003;17:179–184. doi: 10.1007/s00482-003-0210-5. [DOI] [PubMed] [Google Scholar]
- Tao YX. AMPA receptor trafficking in inflammation-induced dorsal horn central sensitization. Neurosci Bull. 2012;28:111–120. doi: 10.1007/s12264-012-1204-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torii Y, Akaike N, Harakawa T, Kato K, Sugimoto N, Goto Y, et al. Type A1 but not type A2 botulinum toxin decreases the grip strength of the contralateral foreleg through axonal transport from the toxin-treated foreleg of rats. J Pharmacol Sci. 2011;117:275–285. doi: 10.1254/jphs.11121fp. [DOI] [PubMed] [Google Scholar]
- Tugnoli V, Capone JG, Eleopra R, Quatrale R, Sensi M, Gastaldo E, et al. Botulinum toxin type A reduces capsaicin-evoked pain and neurogenic vasodilatation in human skin. Pain. 2007;130:76–83. doi: 10.1016/j.pain.2006.10.030. [DOI] [PubMed] [Google Scholar]
- Uddman R, Edvinsson L, Ekman R, Kingman T, McCulloch J. Innervation of the feline cerebral vasculature by nerve fibers containing calcitonin gene-related peptide: trigeminal origin and co-existence with substance P. Neurosci Lett. 1985;62:131–136. doi: 10.1016/0304-3940(85)90296-4. [DOI] [PubMed] [Google Scholar]
- Uddman R, Tajti J, Hou M, Sundler F, Edvinsson L. Neuropeptide expression in the human trigeminal nucleus caudalis and in the cervical spinal cord C1 and C2. Cephalalgia. 2002;22:112–116. doi: 10.1046/j.1468-2982.2002.00324.x. [DOI] [PubMed] [Google Scholar]
- Vo AH, Satori R, Jabbari B, Green J, Killgore WD, Labutta R, et al. Botulinum toxin type-a in the prevention of migraine: a double-blind controlled trial. Aviat Space Environ Med. 2007;78:B113–B118. [PubMed] [Google Scholar]
- Voller B, Sycha T, Gustorff B, Schmetterer L, Lehr S, Eichler HG, et al. A randomized, double-blind, placebo controlled study on analgesic effects of botulinum toxin A. Neurology. 2003;61:940–944. doi: 10.1212/01.wnl.0000086374.92906.6a. [DOI] [PubMed] [Google Scholar]
- Wang D, Zhang Z, Dong M, Sun S, Chapman ER, Jackson MB. Syntaxin requirement for Ca2+-triggered exocytosis in neurons and endocrine cells demonstrated with an engineered neurotoxin. Biochemistry. 2011;50:2711–2713. doi: 10.1021/bi200290p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler AH. Botulinum toxin A, adjunctive therapy for refractory headaches associated with pericranial muscle tension. Headache. 1998;38:468–471. doi: 10.1046/j.1526-4610.1998.3806468.x. [DOI] [PubMed] [Google Scholar]
- Wiegand H, Erdmann G, Wellhoner HH. 125I-labelled botulinum A neurotoxin: pharmacokinetics in cats after intramuscular injection. Naunyn Schmiedebergs Arch Pharmacol. 1976;292:161–165. doi: 10.1007/BF00498587. [DOI] [PubMed] [Google Scholar]
- Xiao L, Mackey S, Hui H, Xong D, Zhang Q, Zhang D. Subcutaneous injection of botulinum toxin a is beneficial in postherpetic neuralgia. Pain Med. 2010;11:1827–1833. doi: 10.1111/j.1526-4637.2010.01003.x. [DOI] [PubMed] [Google Scholar]
- Yaksh TL, Provencher JC, Rathbun ML, Kohn FR. Pharmacokinetics and efficacy of epidurally delivered sustained-release encapsulated morphine in dogs. Anesthesiology. 1999;90:1402–1412. doi: 10.1097/00000542-199905000-00025. [DOI] [PubMed] [Google Scholar]
- Yamamoto H, Ida T, Tsutsuki H, Mori M, Matsumoto T, Kohda T, et al. Specificity of botulinum protease for human VAMP family proteins. Microbiol Immunol. 2012;56:245–253. doi: 10.1111/j.1348-0421.2012.00434.x. [DOI] [PubMed] [Google Scholar]
- Yuan RY, Sheu JJ, Yu JM, Chen WT, Tseng IJ, Chang HH, et al. Botulinum toxin for diabetic neuropathic pain: a randomized double-blind crossover trial. Neurology. 2009;72:1473–1478. doi: 10.1212/01.wnl.0000345968.05959.cf. [DOI] [PubMed] [Google Scholar]
- Zanner R, Gratzl M, Prinz C. Circle of life of secretory vesicles in gastric enterochromaffin-like cells. Ann N Y Acad Sci. 2002;971:389–396. doi: 10.1111/j.1749-6632.2002.tb04501.x. [DOI] [PubMed] [Google Scholar]
- Zhang X, Kainz V, Zhao J, Strassman AM, Levy D. Vascular ERK mediates migraine-related sensitization of meningeal nociceptors. Ann Neurol. 2013;73:741–750. doi: 10.1002/ana.23873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XC, Strassman AM, Burstein R, Levy D. Sensitization and activation of intracranial meningeal nociceptors by mast cell mediators. J Pharmacol Exp Ther. 2007;322:806–812. doi: 10.1124/jpet.107.123745. [DOI] [PubMed] [Google Scholar]
- Zhang XC, Kainz V, Burstein R, Levy D. Tumor necrosis factor-alpha induces sensitization of meningeal nociceptors mediated via local COX and p38 MAP kinase actions. Pain. 2011;152:140–149. doi: 10.1016/j.pain.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwetsloot CP, Caekebeke JF, Jansen JC, Odink J, Ferrari MD. Blood flow velocity changes in migraine attacks – a transcranial Doppler study. Cephalalgia. 1991;11:103–107. doi: 10.1046/j.1468-2982.1991.1102103.x. [DOI] [PubMed] [Google Scholar]


