Abstract
Background and Purpose
EEG studies show that 5-HT is involved in regulation of sleep–wake state and modulates cortical oscillations. Vortioxetine is a 5-HT3, 5-HT7, and 5-HT1D receptor antagonist, 5-HT1B partial agonist, 5-HT1A agonist, and 5-HT transporter inhibitor. Preclinical (animal) and clinical studies with vortioxetine show positive impact on cognitive metrics involving cortical function. Here we assess vortioxetine's effect on cortical neuronal oscillations in actively awake rats.
Experimental Approach
Telemetric EEG recordings were obtained with the following treatments (mg·kg−1, s.c.): vehicle, vortioxetine (0.1, 1.0, 3.0, 10), 5-HT1A agonist flesinoxan (2.5), 5-HT3 antagonist ondansetron (0.30), 5-HT7 antagonist SB-269970-A (10), escitalopram (2.0), duloxetine (10) and vortioxetine plus flesinoxan. Target occupancies were determined by ex vivo autoradiography.
Key Results
Vortioxetine dose-dependently increased wakefulness. Flesinoxan, duloxetine, ondansetron, but not escitalopram or SB-269970-A increased wakefulness. Quantitative spectral analyses showed vortioxetine alone and with flesinoxan increased θ (4–8 Hz), α (8–12 Hz) and γ (30–50 Hz) power. Duloxetine had no effect on θ and γ, but decreased α power, while escitalopram produced no changes. Ondansetron and SB-269970 (≈31–35% occupancy) increased θ power. Flesinoxan (≈41% occupancy) increased θ and γ power.
Conclusions and Implications
Vortioxetine increased wakefulness and increased frontal cortical activity, most likely because of its 5-HT7 and 5-HT3 antagonism and 5-HT1A agonism. Vortioxetine differs from escitalopram and duloxetine by increasing cortical θ, α and γ oscillations. These preclinical findings suggest a role of vortioxetine in modulating cortical circuits known to be recruited during cognitive behaviours and warrant further investigation as to their clinical impact.
Table of Links
| TARGETS | LIGANDS |
|---|---|
| SERT, 5-HT transporter | ACh |
| 5-HT1A receptors | Dopamine |
| 5-HT1B receptors | Glutamate |
| 5-HT1D receptors | Histamine |
| 5-HT3 receptors | Noradrenaline |
| 5-HT7 receptors | Vortioxetine |
| Escitalopram | |
| Flesinoxan | |
| Duloxetine | |
| Ondansetron | |
| 8-OH-DPAT | |
| SB-269970-A |
This Table lists protein targets and ligands in this article which are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Pawson et al., 2014) and the Concise Guide to PHARMACOLOGY 2013/14 (Alexander et al., 2013a, Alexander et al., 2013b).
Introduction
Treatment response with current antidepressant medications is limited (Rush et al., 2006) and calls for new drugs with improved response rates (Fatemi et al., 1999; Artigas et al., 2006). Among the significant unmet medical needs in depression is adequate treatment of deficits in cognitive function such as memory, executive function and speed of processing. Furthermore, deficits in cognitive function may persist beyond clinical recovery from the depressive symptoms (Bhardwaj et al., 2010; Hasselbalch et al., 2011). Thus ameliorating such deficits is important in future treatments (Clark et al., 2009; Marazziti et al., 2010).
The 5-HT system, with ascending projections from the raphe nuclei throughout the cortex, is implicated in regulating mood, attention, and learning and memory (Roth, 1994; Roth et al., 2004; Enge et al., 2011; Chandler et al., 2013; Sumiyoshi and Higuchi, 2013). Furthermore, during wakefulness, this transmitter system is in close interaction with other neurotransmitter systems, including ACh, glutamate, dopamine, noradrenaline, histamine and orexin (hypocretin), and is involved in the regulation of circadian and cognitive processes (Sebban et al., 1999; Monti, 2011; Miyamoto et al., 2012; Wright et al., 2012). These neuromodulatory functions of 5-HT are mediated through various receptor subtypes.
The multimodal antidepressant vortioxetine {Lu AA21004; 1-[2-(2,4-dimethylphenyl-sulfanyl)-phenyl]-piperazine} is an antagonist at the 5-HT3 receptor ligand-gated ion channel, a 5-HT7 and 5-HT1D receptor antagonist, 5-HT1B receptor partial agonist, 5-HT1A receptor agonist and 5-HT transporter (SERT) inhibitor in vitro (Bang-Andersen et al., 2011; Westrich et al., 2012). Microdialysis studies in rats have shown that vortioxetine enhances extracellular levels of 5-HT, ACh, noradrenaline, dopamine, and histamine in brain regions involved in the regulation of emotional and cognitive functions, such as the medial prefrontal cortex (mPFC) and ventral hippocampus (Leiser et al., 2009; Bang-Andersen et al., 2011; Mørk et al., 2012; 2013; Pehrson et al., 2013). Furthermore, the cellular localization of the receptors upon which vortioxetine acts suggest that it may also have a modulatory role on GABA and glutamate function, and through these mechanisms may mediate antidepressant and pro-cognitive effects (Pehrson and Sanchez, 2014). Preclinical studies have demonstrated the antidepressant potential of vortioxetine (Bang-Andersen et al., 2011; Li et al., 2012; Mørk et al., 2012; Westrich et al., 2012; Bétry et al., 2013; Pehrson et al., 2013), and its antidepressant efficacy has been demonstrated in clinical studies (Adell, 2010; Alvarez et al., 2012; Baldwin et al., 2012a,b,c; Boulenger et al., 2012; Henigsberg et al., 2012; Katona et al., 2012; McIntyre et al., 2013). In rats, vortioxetine prolongs acquisition and retention of time-dependent contextual fear memory and time-dependent object recognition memory in rats (Mørk et al., 2013). Furthermore, clinical studies of depressed patients with cognitive dysfunction, vortioxetine has shown beneficial effects on several cognitive domains compared with placebo either as a pre-specified secondary outcome measure (Katona et al., 2012), or as the primary outcome measure (McIntyre et al., 2013).
Yet, there is an interest in linking target engagement, receptor localization and neurotransmission to such antidepressive and pro-cognitive end points. EEG might help bridge this gap. Quantitative EEG (qEEG) enables characterization of defined cellular and cerebral circuitries and has been used by others to study EEG changes during cognitive behaviours and depression (Başar et al., 1999; 2000; Başar, 2008; Kucewicz et al., 2011; Millan et al., 2012; Harmony, 2013; Harvey et al., 2013). Moreover, specific EEG patterns have emerged linking the ascending arousal system of the hypothalamus and brainstem to the corticothalamic system thought to play an important role in mood and depression (Robinson et al., 2011). Specifically, although neocortical activation is maintained by multiple, parallel neural systems through indirect pathways (e.g. amygdala, locus coeruleus, superior colliculus and orbitofrontal cortex), involving noradrenaline, dopamine, histamine and glutamate, it is the direct cholinergic inputs from the basal forebrain and 5-HT inputs from the midbrain raphe pathways that are essential for cortical activation (Vanderwolf, 1988; Dringenberg and Vanderwolf, 1998). Furthermore, substantial evidence shows oscillations act coherently as resonant communication networks through large populations of neurons and, as such, play a role in mood, memory and integrative (cognitive) processes (Başar et al., 2000). The full breadth of the association between neural oscillations and cognition is outside the scope of this paper. However, as reviewed by Ward, specific oscillations, at least in humans, can be identified with particular cognitive processes: theta and gamma rhythms with memory encoding and retrieval, alpha and gamma rhythms with attention or focusing, and gamma synchronization with conscious awareness (Ward, 2003).
Although there has yet to be a consensus whether rat and human EEG spectral oscillations are directly or indirectly comparable, evidence does suggest the cellular substrate as well as neuroanatomical projections are relatively conserved. Importantly, there is increasing evidence that EEG methodologies have high translational value from rodent to human (Ruigt, 2002; Drinkenburg and Ahnaou, 2004; Paterson et al., 2007; Javitt et al., 2008; 2011; Day et al., 2011; Leiser et al., 2011; Wilson et al., 2013). For example, citalopram decreased δ and β power in Møll-Wistar rats (Neckelmann et al., 1996) and healthy human subjects (Lader et al., 1986). Fluoxetine decreased delta, but elicited no change in theta, alpha, beta or gamma in Long-Evans rats (Dringenberg et al., 2000) or in healthy subjects (Saletu, 1982; Saletu and Grünberger, 1985). Paroxetine and fluvoxamine decreased power in delta, theta, alpha, beta and gamma in Fisher rats (Dimpfel, 2003) as well as healthy subjects (Knott et al., 2002; Saletu et al., 1996). Lastly, similar effects on rapid eye movement (REM) sleep and wakefulness were also found for rat and healthy subjects for each of these drugs and other antidepressants (Staner et al., 1999; Rijnbeek et al., 2003).
Given the location of the receptors upon which vortioxetine acts, we hypothesized that vortioxetine would function differently than a selective 5-HT (serotonin) reuptake inhibitor (SSRI) or 5-HT/noradrenaline reuptake inhibitor (SNRI) antidepressant by increasing frontal cortical oscillations. In the present study, the effect of vortioxetine on frontal cortical activity was explored via qEEG in awake, freely behaving rats in their home-cages and related to escitalopram (SSRI) and duloxetine (SNRI). Furthermore, vortioxetine's receptor mechanisms were investigated by studying flesinoxan (5-HT1A receptor agonist), ondansetron (5-HT3 receptor antagonist) and SB 269970-A (5-HT7 receptor antagonist). Ex vivo autoradiography was used to determine levels of target occupancy.
Methods
Animals
All animal care and experimental procedures complied with guidance on the care and use of laboratory animals by Lundbeck and the National Research Council (2011). All studies involving animals are reported in accordance with the ARRIVE guidelines for reporting experiments involving animals (Kilkenny et al., 2010; McGrath et al., 2010). A total of 32 animals were used in the experiments described here. Male Sprague-Dawley rats (250–300 g) from Charles River were individually housed under a 12 h light/dark cycle and temperature- (21 ± 2°C) and humidity- (60 ± 10%) control with chow and water ad libitum.
Ex vivo target occupancy
Determination of target occupancies using ex vivo autoradiography was undertaken, as previously described and reported (du Jardin et al., 2013; Pehrson et al., 2013), in a group of satellite animals. Additionally, 5-HT1A and 5-HT7 occupancies were determined as described below. In the previously published data animals were dosed with the appropriate vehicle or vortioxetine at 0.001, 0.01, 0.1, 1, 3 or 10 mg·kg−1; escitalopram at 0.16, 0.49 or 1.6 mg·kg−1; duloxetine at 1 or 10 mg·kg−1; flesinoxan at 2.5 mg·kg−1; ondansetron at 0.3 mg·kg−1; or SB269970 at 10 mg·kg−1 (du Jardin et al., 2013; Pehrson et al., 2013). In all studies, three animals were used per dose. One hour after drug administration, rats were anaesthetized using CO2, decapitated and their brains quickly harvested, flash-frozen, then sectioned coronally 20 μm thick and mounted on slides. A minimum of three replicate slices were used for each brain per experiment. Tissue was sectioned beginning at approximately +1.2 mm anterior from Bregma for SERT and 5-HT1B receptors, −2.1 mm posterior from Bregma for 5-HT7 receptors, and −4.8 mm posterior from Bregma for 5-HT1A and 5-HT3 receptors. The region of interest (ROI) for each assay was selected a priori by receptor mapping studies (Table 1), showing that the ROI had a reliable specific binding signal for the relevant radioligand. Although subcortical regions were used to estimate target occupancy, these estimates are thought to represent receptors throughout the brain, including in cortical regions. This assumption is based on two principles: (i) according to the law of mass action, fractional receptor occupancy depends only on the concentration of the drug in the biophase and its affinity for the target receptor, and (ii) drugs that penetrate the blood–brain barrier (BBB) reach an equilibrium concentration in the biophase that is similar everywhere within the BBB.
Table 1.
Receptor and SERT occupancy assay conditions for each target
| 5-HT3 | 5-HT7 | 5-HT1B | 5-HT1A | SERT | |
|---|---|---|---|---|---|
| Preincubation buffer | 50 mM Tris HCl 150 mM NaCl | 50 mM Tris HCl 4 mM CaCl2 0.5 μM L-ascorbic acid | 170 mM Tris HCl 4 mM CaCl2 5.67 mM L-ascorbic acid | None | None |
| Preincubation time | 1 × 5 min | 1 × 3 min | 1 × 3 min | None | None |
| Assay buffer | 50 mM Tris HCl 150 mM NaCl 4 mM CaCl2 | 170 mM Tris HCl 4 mM CaCl2 0.5 uM L-ascorbic acid 100 μM pargyline | 170 mM Tris HCl 4 mM CaCl2 5.67 mM L-ascorbic acid 10 μM pargyline | 170 mM Tris HCl 4 mM CaCl2 5.67 mM L-ascorbic acid 10 μM pargyline | 50 mM Tris HCl 150 mM NaCl 5 mM KCl |
| Radioligand | [3H]LY278584 2 nM | [3H]SB269970a 5.9 nM | [3H]GR125743 1 nM | [3H]8-OH-DPATb 3 nM | [3H]escitalopram 4.5 nM |
| Non-specific binding agent | ondansetron 10 μM | SB269970 1 μM | SB216641 1 μM | WAY-100635 1 uM | Paroxetine 1 uM |
| Incubation time | 1 h | 1 h | 1 h | 1 h | 1 h |
| Region of Interest | amygdala, piriform cortex | Anterior paraventricular thalamic nucleus interanteromedial thalamic nucleus, other medial thalamic regions | Caudate, putamen, nucleus accumbens | Hippocampus | Lateral septum, medial septum, olfactory tubercle |
Specific details of each assay for each target are provided. See also du Jardin et al., 2013 and Pehrson et al., 2013.
[3H]SB269970 was supplied by Amersham (Piscataway, NJ, USA), and has a specific activity of 33 Ci/mmol and radioactivity concentration of 0.2 mCi/mL. Given a counting efficiency of 44%, the cpm/mL equivalent of 5.9 nM [3H]SB269970 is approximately 190 000.
[3H]8-OH-DPAT was supplied by Perkin Elmer (Waltham, MA, USA) and has a specific activity of 154.2 Ci/mmol and radioactivity concentration of 1.0 mCi/mL. Given a counting efficiency of 44%, the cpm/mL equivalent of 3 nM [3H]8-OH-DPAT is approximately 452 000.
Autoradiography
Slides were defrosted, briefly preincubated in appropriate buffer (see below), allowed to dry then incubated in an assay buffer that included the appropriate tritiated radioligand at a concentration determined a priori by saturation binding experiments. Non-specific binding was determined by incubating slices from a vehicle-treated animal in assay buffer that contained the appropriate radioligand and a high concentration of a non-radioactive competitor for the target. After incubation, slides were washed twice for 5 min in cold (4°C) assay buffer. Finally, slides were transferred to a vacuum desiccator for at least 1 h before being exposed in a Beta-imager (Biospace Labs) for 15–24 h. Specific details for each assay are noted in Table 1. Surface radioactivity (counts per minute per mm2) was measured and averaged from three replicate brain slices from each rat using Beta-vision plus software (Biospace Labs). Specific binding was ascertained by subtracting nonspecific binding from total binding. For each brain, specific binding was normalized to the average specific binding from brains in the vehicle-treated group. These values were expressed as a percentage of vehicle-specific binding levels, and were finally subtracted from 100 to obtain the percentage of occupancy and determine ED50 values. Where appropriate, doses were log-transformed and a non-linear regression analysis was applied using a sigmoidal dose–response curve. Values were constrained to 0–100, while the Hill coefficient was not constrained.
5-HT1A receptor occupancy
Slides were incubated for 1 h in assay buffer consisting of 170 mM Tris HCl (pH = 7.4), 4 mM CaCl2, 5.67 mM L-ascorbic acid and 10 μM of pargyline that contained 3 nM of the 5-HT1A receptor agonist [3H]8-OH-DPAT. Although [3H] 8-OH-DPAT has some affinity at 5-HT7 receptors, empirical in vitro competition experiments performed in this laboratory suggest that 5-HT7 receptor-specific bound radioactivity is a negligible proportion of the total binding observed under the assay conditions defined earlier.
5-HT7 receptor occupancy
Defrosted slides were preincubated for 3 min at 4°C in a buffer containing 50 mM Tris HCl (pH = 7.4), 4 mM CaCl2, and 0.5 μM L-ascorbic acid. After drying, slides were incubated for 1 h in assay buffer consisting of 50 mM Tris HCl (pH = 7.4), 4 mM CaCl2, 0.5 μM L-ascorbic acid and 100 μM of pargyline that contained 5.9 nM of the 5-HT7 receptor-selective radioligand [3H]SB269970.
EEG
Stainless-steel screw electrodes for EEG and wire electrodes for EMG of dorsal neck muscles were implanted in each animal under anaesthesia as described previously (Vogel et al., 2002; Bastlund et al., 2004). Bipolar (differential) EEG screw electrodes were placed supradural approximately 2.0 mm anterior and 2.0 mm lateral to Bregma bilaterally for frontal cortical EEG and intrahemispheric at 4.0 mm posterior and 2.0 mm lateral to Bregma for fronto-parietal EEG. Electrodes were connected to a sterile multi-channel telemetric device (TL10M3-F50-EEE; Data Sciences International, DSI) that was implanted s.c. on the flank. These transmitters also digitally monitor locomotor activity (LMA). Data from 16 rats were recorded simultaneously. To accomplish the multiple drug arm comparison presented herein, two sets of animals were subjected to a cross-over study wherein each rat would receive each compound in a random order, with a minimum of 3 days wash-out between dosing. The results presented combine these data since all animals were housed, handled, dosed, and recorded from identically. In support of combining the data there was no statistical significant difference between vehicle groups across the two studies (anova). Recordings were started 90 min before injection (∼10:00 h; 4 h into light cycle) and for up to 4 h post-injection using Dataquest A.R.T software (DSI) at a sampling rate of 500 Hz.
Offline, using NeuroScore (DSI), artefacts were removed from the data and sleep stages assigned manually for every 10 s epoch using EEG, EMG and LMA by conventional methods as previously described (Ivarsson et al., 2005; Parmentier-Batteur et al., 2012) using the fronto-parietal EEG, LMA and EMG: active wake (less regular, low-amplitude EEG with high EMG and LMA activity); quiet wake (less regular, low-amplitude EEG, with low EMG and no LMA activity); slow wave sleep (or non-REM, NREM, sleep), consisting of high-amplitude waves with predominant delta (1–4 Hz), low EMG and no LMA; paradoxical or REM sleep exhibited stable, low-amplitude waves dominated by theta (4–8 Hz) with near absent EMG and no LMA. All data were scored into these stages however only Active Wake is shown in this paper in order to maximize the likelihood of translatability (Maire et al., 2013). The amount of time in each stage was calculated as a percentage change for each animal and used to compare versus vehicle (treatment × stage anova).
Spectral frequencies (1–50 Hz) for the active wake state of frontal cortical EEG only were calculated in NeuroScore (DSI) with 1 Hz resolution. Frequencies were binned into delta (1–4 Hz), theta (4–8 Hz), alpha (8–12 Hz), beta-I (12–18 Hz), beta-II (18–30 Hz), and gamma (30–50 Hz) power bands. Pharmacologically induced changes were evaluated by averaging the 10-s bins constituting 15–75 min before (‘Baseline’) and 45–90 min post-treatment. This post-dose time was chosen to match the time receptor occupancy was measured (Figure 1). Using each rat as its own control, the percentage of change from baseline was calculated for each spectral bin from the pre- and post-dose averages using the formula: [(MeanPost − MeanPre)/MeanPre × 100%]. This percentage of change allowed for standardization of power changes for comparisons across all rats without the need for normalizing to total or computing relative power. A one-way anova with Fisher's least significant difference (LSD) post hoc test (Statistica, StatSoft, Inc., Plymouth Meeting, PA, USA) was used to determine differences of each power band for each treatment group versus vehicle. This post hoc test was chosen because it is well suited for testing multiple hypotheses and performing specific multiple comparisons in a large dataset. The anova for timeseries data was computed as Treatment × Epoch (15 min) for each power band; post hoc LSD test was used to compare each treatment versus vehicle at each time epoch for each power band.
Figure 1.
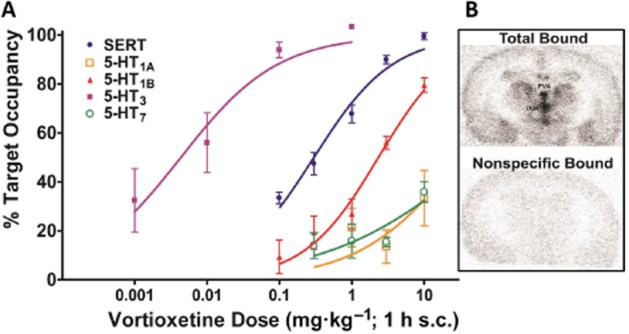
Ex vivo occupancies of vortioxetine. Occupancies of the multimodal drug vortioxetine for each of its targets (A) and the regions of interest used in the 5-HT7 receptor assay (B) are shown. The percentage of target occupancies for the 5-HT3, 5-HT7, 5-HT1B, 5-HT1A receptors and SERT elicited by vortioxetine are graphed as a function of dose. PVA, paraventricular thalamus; IAM, interanteromedial nucleus.
Materials
Vortioxetine, escitalopram, duloxetine and flesinoxan were synthesized by Lundbeck. Ondansetron and SB-269970-A were purchased from Sigma-Aldrich (St. Louis, MO, USA). All drugs were dissolved in 20% aqueous β-cyclodextrin and administered s.c. in a volume of 2.0 mL·kg−1. Doses were chosen based on target occupancies measured by ex vivo autoradiography. Doses expressed as mg·kg−1 of the base, and number of rats in parentheses were: vehicle (n = 12), vortioxetine 0.1 (n = 9), 1.0 (n = 8), 3.0 (n = 8), 5.0 (n = 8), and 10.0 (n = 8) mg·kg−1; SB-269970-A 10.0 mg·kg−1 (n = 8); ondansetron 0.3 mg·kg−1 (n = 8); escitalopram 2.0 mg·kg−1 (n = 8); duloxetine 10.0 mg·kg−1 (n = 8); and flesinoxan 2.5 mg·kg−1 (n = 9). As vortioxetine has lower affinity at the rat than the human 5-HT1A receptor (Ki 15 vs. 230 nM), a combination of vortioxetine and the 5-HT1A receptor agonist flesinoxan (2.5 mg·kg−1, s.c.) was chosen to mimic the estimated occupancy level in humans at this receptor in the rat: vortioxetine 1.0 (n = 8), 3.0 (n = 8), 5.0 (n = 9) and 10.0 (n = 9) mg·kg−1 in combination with flesinoxan.
Results
Ex vivo target occupancy
Acute vortioxetine administration engendered dose-dependent increases in occupancies at each of its receptor targets that were in line with the rank order of in vitro affinities (Figure 1). ED50 values with 95% confidence intervals are shown in Table 2. Time course data for vortioxetine (not shown) suggests that receptor occupancies are at a relative maximum from 1–4 h and thus the values reported earlier are a good estimate of target engagement over the course of the EEG data described later. At 1 hour post s.c. dose, escitalopram administration at 0.16, 0.49 and 1.6 mg·kg−1 engendered mean SERT occupancies of 83 ± 3, 87 ± 4 and 95 ± 1%, respectively, while 1 and 10 mg·kg−1 duloxetine corresponded to 86 ± 3% and 97 ± 1%, and 2.5 mg·kg−1 flesinoxan lead to 41 ± 9% 5-HT1A receptor occupancy, while 10 mg·kg−1 SB-269970 caused 31 ± 3% 5-HT7 receptor occupancy, and 0.3 mg·kg−1 ondansetron engendered 12 ± 5% 5-HT3 receptor occupancy.
Table 2.
Summary of ex vivo occupancies in the rat and in vitro binding affinities of vortioxetine
| Target | Ex vivo occupancy in the rat ED50 (mg·kg−1) [95% CI] | In vitro binding affinity, Ki (nM) | |
|---|---|---|---|
| Rat | Human | ||
| 5-HT3 | 0.004 [0.0016–0.011] | 1.1 | 3.7 |
| 5-HT7 | NC | 190 | 19 |
| 5-HT1D | ND | 3.7 | 54 |
| 5-HT1B | 2.3 [1.7–3.2] | 16 | 33 |
| 5-HT1A | NC | 230 | 15 |
| SERT | 0.3 [0.24–0.37] | 8.6 | 1.6 |
The ex vivo occupancy ED50 values in the rat were derived based on occupancy–dose curves of vortioxetine for the 5-HT3, 5-HT7, 5-HT1B, 5-HT1A receptors and the SERT as shown in Figure 1. The previously published in vitro binding affinities (Bang-Andersen et al., 2011; Mørk et al., 2012) are also shown in order to highlight the species differences for some of the targets. NC, not calculated due to limited range; ND, not determined.
Wakefulness
Sleep staging was performed in order to obtain active wake periods for spectral analyses. Results of the sleep staging analyses are not included as it is outside the scope of this paper. Analysis revealed a significant treatment by stage effect [F(48, 544) = 51.8, P < 0.001, anova]. Focusing on active wake only, post hoc comparisons versus vehicle showed a significant dose-dependent increase in the time spent in active wake with vortioxetine (3, 5 and 10 mg·kg−1, P < 0.05, Figure 2). As vortioxetine has a considerably lower affinity for rat, compared with human, 5-HT1A receptors (Table 2), combined treatments with flesinoxan (2.5 mg·kg−1) were included to mimic 5-HT1A occupancy in clinical settings. With flesinoxan, all doses of vortioxetine (1, 3, 5 and 10 mg·kg−1) resulted in a significant increase in active wake (P < 0.05). The increase in active wake reached a physiological maximum, as flesinoxan alone produced the same magnitude increase (P < 0.05). Additionally, the 5-HT3 receptor antagonist ondansetron (0.3 mg·kg−1) and the SNRI duloxetine (10 mg·kg−1), but not the 5-HT7 receptor antagonist SB-269970-A (10 mg·kg−1) or the SSRI escitalopram (2 mg·kg−1), increased active wake time (P < 0.05), although these magnitudes were smaller.
Figure 2.
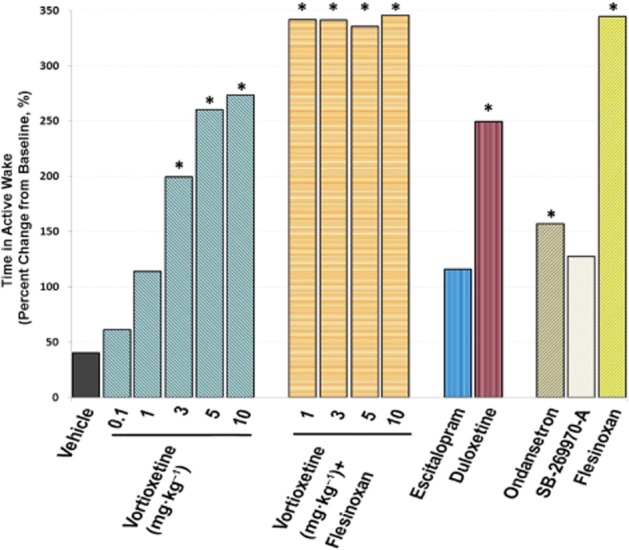
Comparison of vortioxetine and other treatments on impacting wakefulness. The effects on active wake in the rat by different treatment regimens measured by EEG are shown: vehicle, vortioxetine 0.1–10 mg·kg−1, vortioxetine 1–10 mg·kg−1 plus flesinoxan 2.5 mg·kg−1, SB-269970-A 10 mg·kg−1, ondansetron 0.3 mg·kg−1, escitalopram 2 mg·kg−1, duloxetine 10 mg·kg−1 and flesinoxan 2.5 mg·kg−1. Data are expressed as percentage of change from baseline. *P < 0.05, post-dose versus vehicle, one-way ANOVA with LSD post hoc comparison.
Active wake state-specific quantitative EEG
Quantitative analyses of the spectral frequencies on frontal cortical EEG were performed when the animal was in the active wake state. Post hoc comparisons were made to vehicle. Table 3 summarizes the effects across all power bands. The effects on theta, alpha, and gamma power are shown in Figures 3, 5 and 7. Bar graphs show mean changes during 45–90 min post-treatment with respect to pretreatment baseline; this time matches when receptor occupancy was measured. Time series data for up to 240 min post-dose is also presented for these spectra for comparisons of vortioxetine with and without flesinoxan to vehicle, escitalopram, and duloxetine (Figures 4, 6, and 8).
Table 3.
Summary of changes in EEG power spectra induced by drug treatment
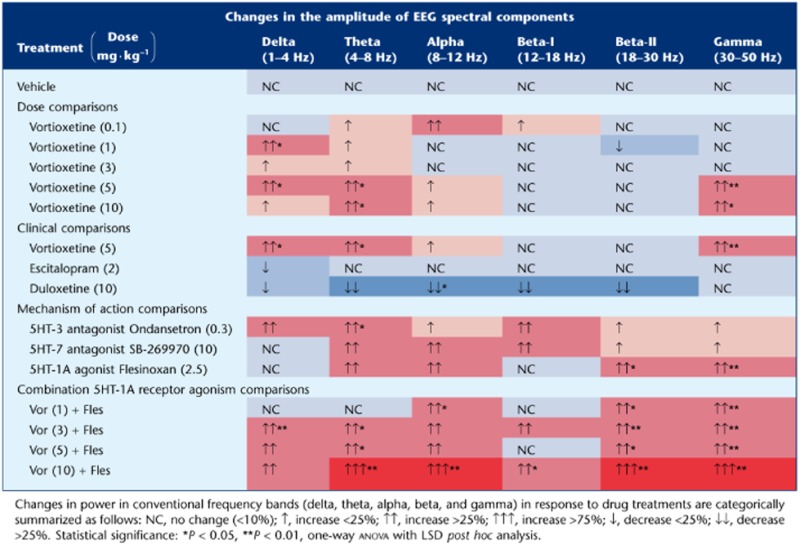 |
Figure 3.
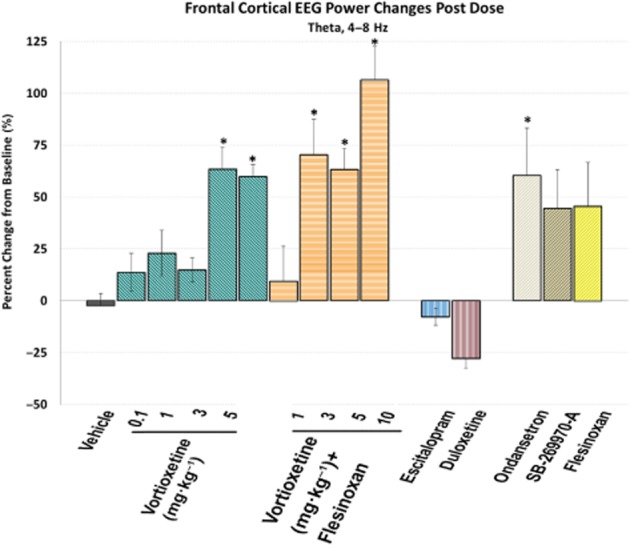
Vortioxetine increases frontal cortical theta power. Frontal cortical theta power during active wake, expressed as the percentage of change from baseline, is shown for each different treatment. A negative value indicates a decrease in EEG power from baseline. *P < 0.05, post-dose versus vehicle, one-way ANOVA with LSD post hoc comparison.
Figure 5.
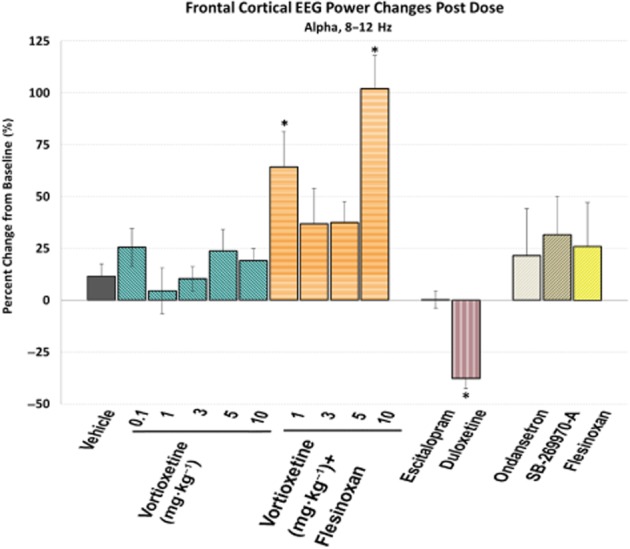
Vortioxetine increased frontal cortical alpha power. Frontal cortical alpha power during active wake, expressed as the percentage of change from baseline, is shown for each different treatment. A negative value indicates a decrease in EEG power from baseline. *P < 0.05, post-dose versus vehicle, one-way anova with LSD post hoc comparison.
Figure 7.
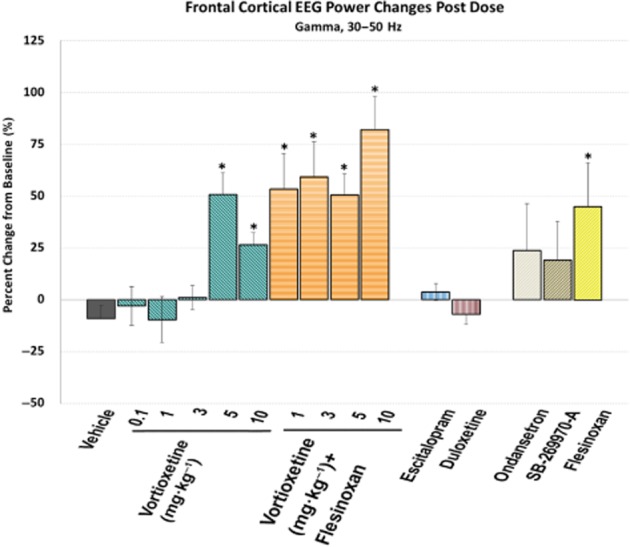
Vortioxetine increases frontal cortical gamma power. Frontal cortical gamma power during active wake, expressed as the percentage of change from baseline, is shown for each different treatment. A negative value indicates a decrease in EEG power from baseline. *P < 0.05, post-dose versus vehicle, one-way anova with LSD post hoc comparison.
Figure 4.
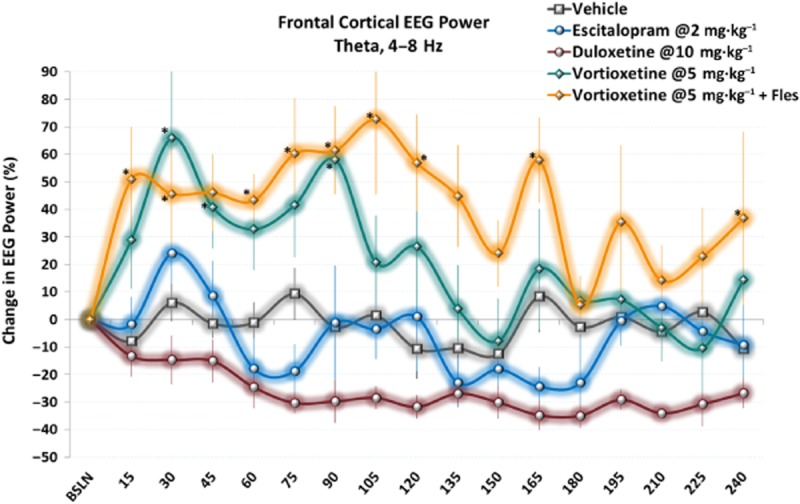
Vortioxetine increases frontal cortical theta power unlike escitalopram and duloxetine. Time-series data show elevated theta power immediately following acute doses of vortioxetine alone or with flesinoxan. Only data during active wake is shown. *P < 0.05 versus vehicle, LSD post hoc comparison.
Figure 6.
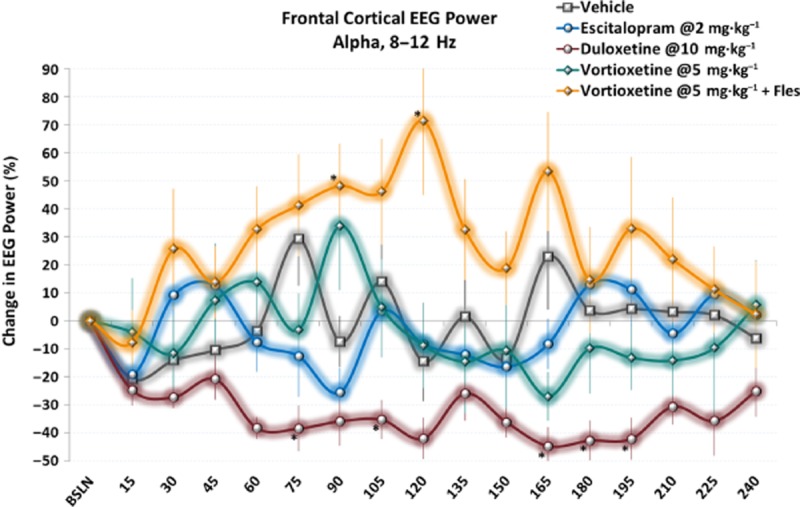
Time-series data show elevated alpha power following acute doses of vortioxetine with flesinoxan. Effects are more transient than other spectra; however, clear differences can still be seen between vortioxetine with flesinoxan and escitalopram and duloxetine. Only data during active wake is shown. *P < 0.05 versus vehicle, LSD post hoc comparison.
Figure 8.
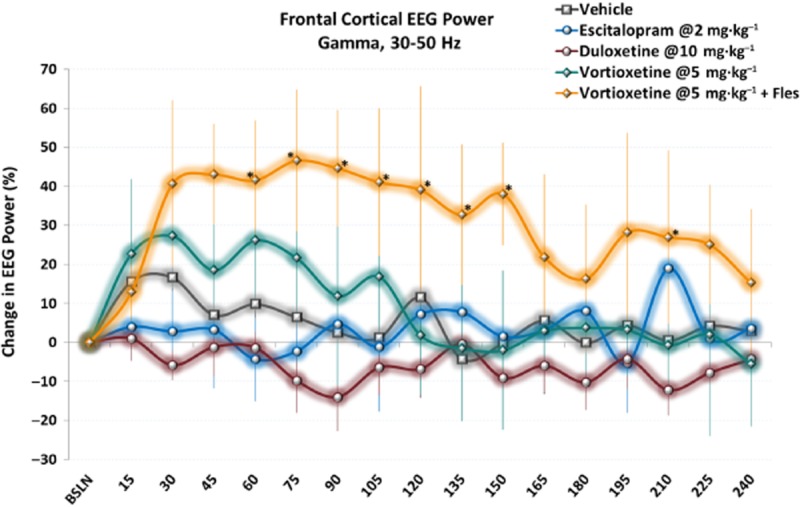
Vortioxetine with flesinoxan elicits a sustained effect on frontal cortical gamma power unlike escitalopram and duloxetine. Timeseries data show elevated gamma power immediately following acute doses of vortioxetine alone (non-significant trend) or with flesinoxan (statistically significant). Only data during active wake is shown. *P < 0.05 versus vehicle, LSD post hoc comparison.
There was a significant treatment effect for frontal cortical theta power [F(14, 113) = 3.0, P < 0.001, one-way anova] (Figure 3). Vortioxetine (5 and 10 mg·kg−1) significantly increased theta power alone and with flesinoxan (P < 0.05), whereas SB-269970-A and flesinoxan produced an increase but did not reach statistical significance (P = 0.096 and P = 0.079, respectively). Ondansetron significantly increased theta power (P = 0.026). In contrast, whereas escitalopram had little effect, duloxetine caused a modest but not significant decrease. These effects can be seen throughout the timecourse as well (Figure 4).
There was also a significant treatment effect for frontal cortical alpha power [F(14, 113) = 3.0, P < 0.001, one-way anova] (Figure 5). All doses of vortioxetine non-significantly increased alpha power (>3 times vehicle) with vortioxetine (1 and 10 mg·kg−1) plus flesinoxan significantly increasing alpha power (P < 0.05). Interestingly, flesinoxan alone also non-significantly increased alpha, suggesting an additive effect with vortioxetine's other 5-HT targets. Ondansetron and SB-269970-A modestly increased alpha power. Escitalopram had little effect, whereas duloxetine elicited a robust and significant decrease (P < 0.05). These changes can be seen throughout the time course (Figure 6).
Finally, there was a significant treatment effect for frontal cortical gamma power [F(14, 113) = 5.2, P < 0.001, one-way anova] (Figure 7). Vortioxetine (5 and 10 mg·kg−1) significantly increased gamma power alone and at all doses in combination with flesinoxan (P < 0.05). Flesinoxan alone significantly increased gamma power (P < 0.05), while SB-269970-A and ondansetron non-significantly elevated gamma (P = 0.11 and 0.06 respectively). Escitalopram and duloxetine elicited no change. These changes are observable throughout the time course with a clear increase in gamma oscillations by vortioxetine plus flesinoxan (Figure 8).
Discussion
There is considerable evidence linking selective attention, memory and cognitive efficiency to arousal (Hebb, 1955; Dickman, 2002; Mair et al., 2011; Mather and Sutherland, 2011). Thus, the first step in investigating vortioxetine's role in modulating cortical oscillations was to assess its effect on arousal, here measured as increased wakefulness. Cognitive and affective processes vary over the 24 h day, with peak performance occurring when the ascending brainstem, basal forebrain and hypothalamic arousal systems are activated, producing enhanced wakefulness. The sleep–wake cycle is regulated by complex neurobiological circuits; waking is regulated via ACh, dopamine, noradrenaline, 5-HT, histamine and orexin (hypocretin) systems (Datta and Maclean, 2007), while sleep is regulated via GABAergic and cholinergic systems within select brain regions, including the cortex (Murillo-Rodriguez et al., 2009; Brown et al., 2012). As vortioxetine has been shown to enhance extracellular levels of ACh, dopamine,noradrenaline, 5-HT and histamine in the prefrontal cortex (Mørk et al., 2013; Pehrson et al., 2013), it appeared reasonable to hypothesize that vortioxetine can increase frontal cortical oscillations in a manner that may act to prime the cortex to respond better to incoming afferent information.
We found vortioxetine significantly and dose-dependently increased wakefulness, as measured as increased time spent in active wake (Figure 2). From the results shown in Figures 1 and 2, it is apparent that active doses of vortioxetine in our study in rats were in clinically equivalent range corresponding to 50–90% SERT occupancy as seen in clinical PET studies (Areberg et al., 2012). Taking into consideration the lower affinity of vortioxetine for rat, compared with human, 5-HT1A receptors (Table 2), results of combined dosing of vortioxetine and flesinoxan indicate that 5-HT1A agonism plays a critical role in mediating the effects of vortioxetine on wakefulness. Flesinoxan administered alone or with vortioxetine, produced a maximal increase in wakefulness. The flesinoxan dose corresponded to ≈40% occupancy, which based on extrapolations from in vitro human and rat affinities and ex vivo occupancy measurements in rats, in principle should be attainable by vortioxetine in clinical settings. The effects of flesinoxan are in line with published studies where the full 5-HT1A receptor agonist, 8-OH-DPAT, was found to increase wakefulness (Wilson et al., 2005). The 5-HT7 receptor antagonist SB-269970, at dose corresponding ≈31% occupancy, increased active wake moderately to a level corresponding to vortioxetine at 1 mg·kg−1. The time course of EEG results suggests that the 1 h time point used for 5-HT3 receptor occupancy determinations was after the time of maximal brain exposure for ondansetron because 1 h after administration 5-HT3 receptor occupancy was only about 12%, yet 0.3 mg·kg−1 ondansetron also increased active wake to a level similar to that seen with vortioxetine. Thus, it is plausible that not only 5-HT1A receptor agonism, but also 5-HT3 and 5-HT7 receptor antagonism may contribute to vortioxetine's increase of wakefulness and thereby increase attention and vigilance. In addition, vortioxetine has lower in vitro affinity for the rat versus human 5-HT7 receptor (Table 2) making it likely that the effects of vortioxetine mediated by 5-HT7 receptor antagonism may be greater in humans.
In line with previous studies (Katoh et al., 1995; Sanchez et al., 2007), our data show that duloxetine, 10 mg·kg−1 corresponding to >80% SERT occupancy, increased wakefulness significantly. The effect size was comparable with that of vortioxetine at 5 mg·kg−1. We further showed that escitalopram, 2 mg·kg−1 corresponding to >80% SERT occupancy, only moderately increased wakefulness similarly to vortioxetine at 1 mg·kg−1. These results are consistent with previous findings and the difference between duloxetine and escitalopram is ascribed to the increased noradrenergic neurotransmission elicited by duloxetine (Sanchez et al., 2007). Clinical literature has substantially provided evidence that SERT occupancy of ∼80% is necessary to achieve therapeutic effects of SSRI and SNRI (Meyer et al., 2004; Takano et al., 2006; Shang et al., 2007; Voineskos et al., 2007; Kasper et al., 2009). Thus, the doses used in our studies are clinically equivalent doses.
The next step was to explore vortioxetine's effects on spectral frequency domains suggested to play important roles in cognitive function. Although complex and still evolving, some fundamental understandings about each brain rhythm have emerged (Başar et al., 1999; 2000; 2001; Klimesch, 1999; 2012; Hajós et al., 2003a; Başar, 2008; Millan et al., 2012). Vortioxetine, unlike escitalopram and duloxetine, elicited an increased power in key spectra, demonstrating the multimodal mechanism of action of vortioxetine involving modulation of 5-HT receptors and SERT inhibition results in a distinct profile compared with antidepressants acting through inhibition of monoamine transporters (Nutt et al., 2010). Although in this study, EEG from rats was not recorded during a cognitive task, but rather innate exploratory behaviour, we believe our findings set a framework for cortical activation that warrants further study both in animals and in humans.
Delta waves are believed to be generated by the summation of long-lasting after hyper-polarizations (AHPs) in pyramidal neurons (layers II–III or V) and increases reflect more cells exhibiting AHPs (inhibition of pyramidal neurons by local-circuit cells) (Steriade, 2005). Further, cortical delta activity has been shown to increase during concentration demand (Harmony, 2013). Our data show vortioxetine produced an increase in delta power, alone and in combination with flesinoxan, whereas duloxetine and escitalopram produced a non-significant decrease. The 5-HT3 receptor antagonism of vortioxetine appears to contribute to this effect as ondansetron did increase delta activity significantly (P < 0.05, Fisher's LSD), as seen previously (Bo et al., 1993), while neither SB-269970-A nor flesinoxan elicited any change.
Theta oscillations have been associated with cognitive functions. For example, preclinical and clinical data suggest that theta oscillations tend to increase during memory tasks, especially during encoding (Klimesch, 1996; 1999; Başar et al., 2000). Theta oscillations are predominantly driven via hippocampal–entorhinal–cortical projections. Extensive coupling of theta oscillations throughout the midline cortices and hippocampus has been demonstrated, and this coherence is believed to reflect binding of cortical and hippocampal pathways into functional units by behavioural demands (Young and McNaughton, 2009). Moreover, hippocampal theta power is linked to memory, particularly spatial memory in rats, while in humans, an increase in theta correlated with improved performance on working memory tasks (Caplan et al., 2001; Raghavachari et al., 2001; 2006). Cortical theta power is proposed to reflect coordinating neural networks involved in monitoring behaviour and the environment as well as facilitating task-specific adaptive changes in performance. Modulation by 5-HT is known to play an important role in the generation and regulation of theta power (Stäubli and Xu, 1995; Vertes and Kocsis, 1997; Bland et al., 1999; Dragoi et al., 1999; Buzsaki, 2002; Hajós et al., 2003b; McNaughton et al., 2007; Sörman et al., 2011). Our data show vortioxetine (5 and 10 mg·kg−1) significantly increased frontal cortical theta power. While flesinoxan (≈35% 5-HT1A receptor occupancy) alone only modestly increased theta power, vortioxetine (3, 5, and 10 mg·kg−1) plus flesinoxan significantly increased theta power, indicating an additive effect. Similarly, 8-OH-DPAT, a 5-HT1A receptor agonist, has increased theta power in hippocampal EEG in cats (Bjorvatn et al., 1997). Ondansetron significantly increased theta power in the present study, consistent with previously published data on the augmentation of theta power in hippocampal EEG recordings from freely-moving rats (Stäubli and Xu, 1995). Similarly, ondansetron dose-dependently increased hippocampal theta rhythm as well as the magnitude and duration of CA1 long-term potentiation in rat (Stäubli and Xu, 1995). The 5-HT7 receptor antagonist SB-269970-A increased theta power significantly even at a low level of occupancy. In contrast, escitalopram and duloxetine produce non-statistically significant decreases of theta power. In a previous study, escitalopram was found to decrease theta (5–9 Hz) after acute treatment during active wake in rats (Vas et al., 2013). This further differentiates vortioxetine from escitalopram and duloxetine. Thus, our results suggest that 5-HT3 and 5-HT7 receptor antagonism and 5-HT1A receptor agonism may collectively contribute to the enhancement of both the coordination and synchronization of neuronal networks, and this is likely a functional mechanistic component in mediating the actions of vortioxetine in humans.
Here we show vortioxetine in combination with flesinoxan significantly increased alpha power. The numerical yet not significant increases in alpha power following treatment of vortioxetine at 5 and 10 mg·kg−1, SB-269970-A, ondansetron, and flesinoxan, suggest that converging action at multiple 5-HT receptors is necessary and sufficient to elicit changes in alpha wave generation as caused by vortioxetine plus flesinoxan, at a dose that should mimic clinical levels of 5-HT1A occupancy. Of further interest is that duloxetine caused a significant decrease in alpha power, while escitalopram had no effect. Although, interpretation and identification of alpha power and its generators are still a matter of debate (Klimesch et al., 1999; Shaw, 2003), there is some consensus that alpha waves reflect synchronization of neurons for sensorimotor integration (coordinated cortical output to afferent input) and is generated by thalamocortical feedback loops (excitatory and inhibitory) and cortico-cortical networks involving layer V pyramidal neurons (Steriade et al., 1990; Lopes da Silva, 1991) and cognitive tasks elicit increases in alpha power (Klimesch et al., 1999; Bastiaansen et al., 2002; Schack and Klimesch, 2002; Meltzer et al., 2007). In conclusion, the implication of vortioxetine's effects on alpha power and in general the role of alpha power in cognition remains to be studied further.
Gamma synchronization occurs across neuronal networks that represent related features of an object (‘feature binding’) to generate a coherent percept via networks of inhibitory interneurons acting at pyramidal neurons. Gamma power is a near-ubiquitous feature of ongoing cortical activity and is involved in multiple aspects of cognitive computation, sensory representation and short-term memory (Engel et al., 2001; Kaiser and Lutzenberger, 2005; Kaiser et al., 2008; Ainsworth et al., 2011). Moreover, gamma power is associated with transient coupling of brain areas during memory refreshment, memory formation and experience-dependent plasticity (Ward, 2003; Jutras and Buffalo, 2010; Fell and Axmacher, 2011; Headley and Weinberger, 2011). There is also a putative link between gamma power and the speed of processing in a neural network, where reduced gamma power was linked to age-related cognitive and behavioural slowing (Insel et al., 2012). Given that the neural basis of gamma oscillations have been associated with the GABAergic interneuronal system (Shin et al., 2011) and 5-HT1A, 5-HT3 and 5-HT7 receptors are present on GABAergic interneurons, we hypothesized gamma power would be modulated by vortioxetine.
We showed a robust and significant increase in gamma power following vortioxetine at 5 and 10 mg·kg−1, as well as at 1, 3, 5 and 10 mg·kg−1 when in combination with flesinoxan as well as flesinoxan alone. Conversely, both escitalopram and duloxetine elicited no change in gamma power. Further, SB-269970-A and ondansetron trended to increase gamma power, but both elicited submaximal effects. Thus, a combined effect of 5-HT3 and 5-HT7 receptor antagonism and 5-HT1A receptor agonism is likely to contribute to vortioxetine's increase of gamma power. That gamma oscillations increased in frontal cortex is substantiated by previous work showing that vortioxetine, but not escitalopram, disinhibits pyramidal cell function by blocking the 5-HT- and mCPBG (5-HT3 receptor agonist)-induced increases in GABA transmission and enhanced theta-burst LTP in the rat hippocampus (Dale et al., 2013) and in a separate study dose-dependently increased the discharge rate of rat mPFC pyramidal neurons projecting to midbrain (Riga et al., 2013).
As mentioned, a blockade of 5-HT3 receptors may contribute to the modulation of cortical activation via the cholinergic system. Previous data suggested beneficial effects of 5-HT3 receptor antagonists on memory function in preclinical studies (Brambilla et al., 1993; Fontana et al., 1995; Pitsikas and Borsini, 1996; Arnsten et al., 1997; Roychoudhury and Kulkarni, 1997). In clinical settings, ondansetron has shown promise in treating cognitive impairment in schizophrenia (Zhang et al., 2006; Akhondzadeh et al., 2009; Khodaie-Ardakani et al., 2013). Previous preclinical studies also demonstrate the memory-enhancing effects of 5-HT7 receptor antagonists, probably through modulation of the glutamatergic or GABAergic systems (Meneses, 2004; McLean et al., 2009; Horiguchi et al., 2011; Horisawa et al., 2011; Nolan and Roman, 2012; Dale et al., 2013). Activation at 5-HT1A receptors, which serves as both a somatodendritic autoreceptor and postsynaptic heteroceptor in cognition-associated brain regions such as the dorsal raphe, entorhinal cortex, hippocampus and central amygdala (Chalmers and Watson, 1991; Polter and Li, 2010), has been linked to memory-enhancing effects (Meneses and Hong, 1999; Meeter et al., 2006; Newman-Tancredi et al., 2009; Depoortere et al., 2010).
Study limitations
We did not measure oscillatory power during a cognitive task and thus we cannot directly tie our observations to cognitive function. Rather, given that vortioxetine does improve cognition in animal models and in clinical trials, we suggest via our EEG data that it may do so via frontal cortical activation and through its action on specific 5-HT receptor subtypes. Also that the acute dose was administered approximately 2 h into the light cycle when rats are typically quiescent may have an impact on performance as different tasks and different task parameters may depend upon circadian phase (Goel et al., 2010). We further propose that the underlying cellular mechanisms generating the oscillations are similar to those recruited during cognitive tasks and that these would be relevant in humans, yet we understand and note that data is lacking to conclude on how reliably EEG can directly translate between rat and human. In example, the generators for θ and α remain enigmatic and thus it is unclear whether the increases in rat cortical θ and α power observed can be directly correlated to human cortical θ and α power, yet it is clear that these increases were elicited by vortioxetine and have not been observed for SSRIs or SNRIs to our knowledge, and thus warrants further investigation.
Conclusions
Our data of vortioxetine shows a distinct profile compared with escitalopram and duloxetine. Unlike vortioxetine, escitalopram had no effect on frontal cortical delta, theta, alpha, beta or gamma power. Consistently, a meta-analysis reported that low doses of SSRIs had virtually no effect on delta and theta and in 60% of studies caused small decreases in alpha and increases in beta, while high doses increased low frequencies with no change in alpha and beta power (Dumont et al., 2005). The authors further report ‘disappearance of cognitive stimulation’ for increasing SSRI doses (Dumont et al., 2005). Meanwhile, increased noradrenergic activity decreases spectral power (Sebban et al., 1999). This is consistent with our findings with duloxetine.
Collectively, these data argue that 5-HT1A receptor agonism, and 5-HT3 and 5-HT7 receptor antagonism, in addition to SERT inhibition and possibly other actions not investigated here (e.g. 5-HT1D receptor antagonism and 5-HT1B receptor partial agonism) are likely all important elements in the actions of vortioxetine on modulating cortical oscillations. Given the impact of vortioxetine on cortical oscillations and the putative connection between these oscillations and cognitive function, these observations warrant further investigation of vortioxetine's effects on cognitive functioning in preclinical and clinical settings. Specifically, implementing integrated EEG recordings and behavioural assessments using methods that translate between rodent and humans would be helpful to understand the value of our preclinical data in predicting EEG changes in human and also the relationship of cortical oscillations to cognitive function.
Acknowledgments
The authors would like to thank Drs. David Simpson (Lundbeck) and Huailing Zhong (U-Pharm Laboratories LLC, Parsippany, NJ, USA) for helpful insights and comments, and Huailing Zhong for assistance in preparing the paper.
Glossary
- AHP
after hyper-polarization SB-269970-A
- BBB
blood–brain barrier
- DSI
Data Sciences International
- LMA
locomotor activity
- qEEG
quantitative EEG
- REM
rapid eye movement sleep
- ROI
region of interest
- SERT
5-HT (serotonin) transporter
- SNRI
5-HT (serotonin) / noradrenaline reuptake inhibitor
- SSRI
5-HT (serotonin) reuptake inhibitor
Author contributions
S. C. L. and C. S. wrote the paper; A. L. P. performed the ex vivo autoradiography; P. J. R. performed the EEG recordings and sleep staging; S. C. L. performed the qEEG analyses.
Conflict of interest
The work by all authors was performed as full-time employees or consultants of Lundbeck at the time of the study. This study was funded by H. Lundbeck A/S.
References
- Adell A. Lu-AA21004, a multimodal serotonergic agent, for the potential treatment of depression and anxiety. IDrugs. 2010;13:900–910. [PubMed] [Google Scholar]
- Ainsworth M, Lee S, Cunningham MO, Roopun AK, Traub RD, Kopell NJ, et al. Dual gamma rhythm generators control interlaminar synchrony in auditory cortex. J Neurosci. 2011;31:17040–17051. doi: 10.1523/JNEUROSCI.2209-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akhondzadeh S, Mohammadi N, Noroozian M, Karamghadiri N, Ghoreishi A, Jamshidi AH, et al. Added ondansetron for stable schizophrenia: a double blind, placebo controlled trial. Schizophr Res. 2009;107:206–212. doi: 10.1016/j.schres.2008.08.004. [DOI] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: Transporters. British Journal of Pharmacology. 2013a;170:1706–1796. doi: 10.1111/bph.12450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: G Protein-Coupled Receptors. Br J Pharmacol. 2013b;170:1459–1581. doi: 10.1111/bph.12445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez E, Perez V, Dragheim M, Loft H, Artigas F. A double-blind, randomized, placebo-controlled, active reference study of Lu AA21004 in patients with major depressive disorder. Int J Neuropsychopharmacol. 2012;15:589–600. doi: 10.1017/S1461145711001027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Areberg J, Luntang-Jensen M, Sogaard B, Nilausen DO. Occupancy of the serotonin transporter after administration of Lu AA21004 and its relation to plasma concentration in healthy subjects. Basic Clin Pharmacol Toxicol. 2012;110:401–404. doi: 10.1111/j.1742-7843.2011.00810.x. [DOI] [PubMed] [Google Scholar]
- Arnsten AF, Lin CH, Van Dyck CH, Stanhope KJ. The effects of 5-HT3 receptor antagonists on cognitive performance in aged monkeys. Neurobiol Aging. 1997;18:21–28. doi: 10.1016/s0197-4580(96)00162-5. [DOI] [PubMed] [Google Scholar]
- Artigas F, Adell A, Celada P. Pindolol augmentation of antidepressant response. Curr Drug Targets. 2006;7:139–147. doi: 10.2174/138945006775515446. [DOI] [PubMed] [Google Scholar]
- Baldwin DS, Hansen T, Florea I. Vortioxetine (Lu AA21004) in the long-term open-label treatment of major depressive disorder. Curr Med Res Opin. 2012a;28:1717–1724. doi: 10.1185/03007995.2012.725035. [DOI] [PubMed] [Google Scholar]
- Baldwin DS, Loft H, Dragheim M. A randomised, double-blind, placebo controlled, duloxetine-referenced, fixed-dose study of three dosages of Lu AA21004 in acute treatment of major depressive disorder (MDD) Eur Neuropsychopharmacol. 2012b;22:482–491. doi: 10.1016/j.euroneuro.2011.11.008. [DOI] [PubMed] [Google Scholar]
- Baldwin DS, Loft H, Florea I. Lu AA21004, a multimodal psychotropic agent, in the prevention of relapse in adult patients with generalized anxiety disorder. Int Clin Psychopharmacol. 2012c;27:197–207. doi: 10.1097/YIC.0b013e3283530ad7. [DOI] [PubMed] [Google Scholar]
- Bang-Andersen B, Ruhland T, Jorgensen M, Smith G, Frederiksen K, Jensen KG, et al. Discovery of 1-[2-(2,4-dimethylphenylsulfanyl)phenyl]piperazine (Lu AA21004): a novel multimodal compound for the treatment of major depressive disorder. J Med Chem. 2011;54:3206–3221. doi: 10.1021/jm101459g. [DOI] [PubMed] [Google Scholar]
- Bastiaansen MC, Posthuma D, Groot PF, de Geus EJ. Event-related alpha and theta responses in a visuo-spatial working memory task. Clin Neurophysiol. 2002;113:1882–1893. doi: 10.1016/s1388-2457(02)00303-6. [DOI] [PubMed] [Google Scholar]
- Bastlund JF, Jennum P, Mohapel P, Vogel V, Watson WP. Measurement of cortical and hippocampal epileptiform activity in freely moving rats by means of implantable radiotelemetry. J Neurosci Methods. 2004;138:65–72. doi: 10.1016/j.jneumeth.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Başar E. Oscillations in ‘brain-body-mind’ – a holistic view including the autonomous system. Brain Res. 2008;1235:2–11. doi: 10.1016/j.brainres.2008.06.102. [DOI] [PubMed] [Google Scholar]
- Başar E, Başar-Eroglu C, Karakas S, Schurmann M. Are cognitive processes manifested in event-related gamma, alpha, theta and delta oscillations in the EEG? Neurosci Lett. 1999;259:165–168. doi: 10.1016/s0304-3940(98)00934-3. [DOI] [PubMed] [Google Scholar]
- Başar E, Başar-Eroglu C, Karakas S, Schurmann M. Brain oscillations in perception and memory. Int J Psychophysiol. 2000;35:95–124. doi: 10.1016/s0167-8760(99)00047-1. [DOI] [PubMed] [Google Scholar]
- Başar E, Başar-Eroglu C, Karakas S, Schurmann M. Gamma, alpha, delta, and theta oscillations govern cognitive processes. Int J Psychophysiol. 2001;39:241–248. doi: 10.1016/s0167-8760(00)00145-8. [DOI] [PubMed] [Google Scholar]
- Bétry C, Pehrson AL, Etievant A, Ebert B, Sanchez C, Haddjeri N. The rapid recovery of 5-HT cell firing induced by the antidepressant vortioxetine involves 5-HT3 receptor antagonism. Int J Neuropsychopharmacol. 2013;16:1115–1127. doi: 10.1017/S1461145712001058. [DOI] [PubMed] [Google Scholar]
- Bhardwaj A, Wilkinson P, Srivastava C, Sharma M. Cognitive deficits in euthymic patients with recurrent depression. J Nerv Ment Dis. 2010;198:513–515. doi: 10.1097/NMD.0b013e3181e4c5ba. [DOI] [PubMed] [Google Scholar]
- Bjorvatn B, Fagerland S, Eid T, Ursin R. Sleep/waking effects of a selective 5-HT1A receptor agonist given systemically as well as perfused in the dorsal raphe nucleus in rats. Brain Res. 1997;770:81–88. doi: 10.1016/s0006-8993(97)00758-0. [DOI] [PubMed] [Google Scholar]
- Bland BH, Oddie SD, Colom LV. Mechanisms of neural synchrony in the septohippocampal pathways underlying hippocampal theta generation. J Neurosci. 1999;19:3223–3237. doi: 10.1523/JNEUROSCI.19-08-03223.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bo P, Marchioni E, Soragna D, Murelli R, Savoldi F. Eegraphic and behavioural effects of ondansetron, a 5HT3 antagonist, in rabbits. Prog Neuropsychopharmacol Biol Psychiatry. 1993;17:801–823. doi: 10.1016/0278-5846(93)90062-w. [DOI] [PubMed] [Google Scholar]
- Boulenger JP, Loft H, Florea I. A randomized clinical study of Lu AA21004 in the prevention of relapse in patients with major depressive disorder. J Psychopharmacol. 2012;26:1408–1416. doi: 10.1177/0269881112441866. [DOI] [PubMed] [Google Scholar]
- Brambilla A, Ghiorzi A, Pitsikas N, Borsini F. DAU 6215, a novel 5-HT3-receptor antagonist, selectively antagonizes scopolamine-induced deficit in a passive-avoidance task, but not scopolamine-induced hypermotility in rats. J Pharm Pharmacol. 1993;45:841–843. doi: 10.1111/j.2042-7158.1993.tb05698.x. [DOI] [PubMed] [Google Scholar]
- Brown RE, Basheer R, McKenna JT, Strecker RE, McCarley RW. Control of sleep and wakefulness. Physiol Rev. 2012;92:1087–1187. doi: 10.1152/physrev.00032.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsaki G. Theta oscillations in the hippocampus. Neuron. 2002;33:325–340. doi: 10.1016/s0896-6273(02)00586-x. [DOI] [PubMed] [Google Scholar]
- Caplan JB, Madsen JR, Raghavachari S, Kahana MJ. Distinct patterns of brain oscillations underlie two basic parameters of human maze learning. J Neurophysiol. 2001;86:368–380. doi: 10.1152/jn.2001.86.1.368. [DOI] [PubMed] [Google Scholar]
- Chalmers DT, Watson SJ. Comparative anatomical distribution of 5-HT1A receptor mRNA and 5-HT1A binding in rat brain – a combined in situ hybridisation/in vitro receptor autoradiographic study. Brain Res. 1991;561:51–60. doi: 10.1016/0006-8993(91)90748-k. [DOI] [PubMed] [Google Scholar]
- Chandler DJ, Lamperski CS, Waterhouse BD. Identification and distribution of projections from monoaminergic and cholinergic nuclei to functionally differentiated subregions of prefrontal cortex. Brain Res. 2013;1522:38–58. doi: 10.1016/j.brainres.2013.04.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark L, Chamberlain SR, Sahakian BJ. Neurocognitive mechanisms in depression: implications for treatment. Annu Rev Neurosci. 2009;32:57–74. doi: 10.1146/annurev.neuro.31.060407.125618. [DOI] [PubMed] [Google Scholar]
- Dale E, Zhang H, Leiser SC, Chao Y, Yang C, Plath N, et al. Vortioxetine (Lu AA21004) disinhibits pyramidal cell output and enhances theta rhythms and long-term plasticity in the hippocampus. Eur Neuropsychopharmacol. 2013;23(S2):S394. [Google Scholar]
- Datta S, Maclean RR. Neurobiological mechanisms for the regulation of mammalian sleep–wake behavior: reinterpretation of historical evidence and inclusion of contemporary cellular and molecular evidence. Neurosci Biobehav Rev. 2007;31:775–824. doi: 10.1016/j.neubiorev.2007.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day M, Fox GB, Marek GJ. Translational medicine special issue. Biochem Pharmacol. 2011;81:1353–1355. doi: 10.1016/j.bcp.2011.01.018. [DOI] [PubMed] [Google Scholar]
- Depoortere R, Auclair AL, Bardin L, Colpaert FC, Vacher B, Newman-Tancredi A. F15599, a preferential post-synaptic 5-HT1A receptor agonist: activity in models of cognition in comparison with reference 5-HT1A receptor agonists. Eur Neuropsychopharmacol. 2010;20:641–654. doi: 10.1016/j.euroneuro.2010.04.005. [DOI] [PubMed] [Google Scholar]
- Dickman SJ. Dimensions of arousal: wakefulness and vigor. Hum Factors. 2002;44:429–442. doi: 10.1518/0018720024497673. [DOI] [PubMed] [Google Scholar]
- Dimpfel W. Preclinical data base of pharmaco-specific rat EEG fingerprints (tele-stereo-EEG) Eur J Med Res. 2003;8:199–207. [PubMed] [Google Scholar]
- Dragoi G, Carpi D, Recce M, Csicsvari J, Buzsaki G. Interactions between hippocampus and medial septum during sharp waves and theta oscillation in the behaving rat. J Neurosci. 1999;19:6191–6199. doi: 10.1523/JNEUROSCI.19-14-06191.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dringenberg HC, Vanderwolf CH. Involvement of direct and indirect pathways in electrocorticographic activation. Neurosci Biobehav Rev. 1998;22:243–257. doi: 10.1016/s0149-7634(97)00012-2. [DOI] [PubMed] [Google Scholar]
- Dringenberg HC, Diavolitsis P, Noseworthy PA. Effect of tacrine on EEG slowing in the rat: enhancement by concurrent monoamine therapy. Neurobiol Aging. 2000;21:135–143. doi: 10.1016/s0197-4580(00)00108-1. [DOI] [PubMed] [Google Scholar]
- Drinkenburg WH, Ahnaou A. The use of pharmaco-electroencephalography in preclinical models in drug discovery. In: Drinkenburg WHIM, Ruigt GSF, Jobert M, editors. Essentials and Applications of EEG Research in Preclinical and Clinical Pharmacology: Textbook for the Training Course of the 13th Biennal International Pharmaco-EEG Society Symposium (IPEG), September (2004), Antwerp, Belgium. Berlin: Unipublish Verlag; 2004. pp. 131–148. [Google Scholar]
- Dumont GJ, de Visser SJ, Cohen AF, van Gerven JM. Biomarkers for the effects of selective serotonin reuptake inhibitors (SSRIs) in healthy subjects. Br J Clin Pharmacol. 2005;59:495–510. doi: 10.1111/j.1365-2125.2005.02342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enge S, Fleischhauer M, Lesch KP, Strobel A. On the role of serotonin and effort in voluntary attention: evidence of genetic variation in N1 modulation. Behav Brain Res. 2011;216:122–128. doi: 10.1016/j.bbr.2010.07.021. [DOI] [PubMed] [Google Scholar]
- Engel AK, Fries P, Singer W. Dynamic predictions: oscillations and synchrony in top-down processing. Nat Rev Neurosci. 2001;2:704–716. doi: 10.1038/35094565. [DOI] [PubMed] [Google Scholar]
- Fatemi SH, Emamian ES, Kist DA. Venlafaxine and bupropion combination therapy in a case of treatment-resistant depression. Ann Pharmacother. 1999;33:701–703. doi: 10.1345/aph.18249. [DOI] [PubMed] [Google Scholar]
- Fell J, Axmacher N. The role of phase synchronization in memory processes. Nat Rev Neurosci. 2011;12:105–118. doi: 10.1038/nrn2979. [DOI] [PubMed] [Google Scholar]
- Fontana DJ, Daniels SE, Henderson C, Eglen RM, Wong EH. Ondansetron improves cognitive performance in the Morris water maze spatial navigation task. Psychopharmacology (Berl) 1995;120:409–417. doi: 10.1007/BF02245812. [DOI] [PubMed] [Google Scholar]
- Goel N, Van Dongen HPA, Dinges DF. Circadian rhythms in sleepiness, alertness, and performance. In: Kryger MH, Roth T, Dement WC, editors. Principles and Practice of Sleep Medicine. 5th edn. St. Louis, MI: Elsevier Saunders; 2010. pp. 445–455. [Google Scholar]
- Hajós M, Hoffmann WE, Robinson DD, Yu JH, Hajós-Korcsok E. Norepinephrine but not serotonin reuptake inhibitors enhance theta and gamma activity of the septo-hippocampal system. Neuropsychopharmacology. 2003a;28:857–864. doi: 10.1038/sj.npp.1300116. [DOI] [PubMed] [Google Scholar]
- Hajós M, Hoffmann WE, Weaver RJ. Regulation of septo-hippocampal activity by 5-hydroxytryptamine(2C) receptors. J Pharmacol Exp Ther. 2003b;306:605–615. doi: 10.1124/jpet.103.051169. [DOI] [PubMed] [Google Scholar]
- Harmony T. The functional significance of delta oscillations in cognitive processing. Front Integr Neurosci. 2013;7:83. doi: 10.3389/fnint.2013.00083. eCollection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey BD, Siok CJ, Kiss T, Volfson D, Grimwood S, Shaffer CL, et al. Neurophysiological signals as potential translatable biomarkers for modulation of metabotropic glutamate 5 receptors. Neuropharmacology. 2013;75:19–30. doi: 10.1016/j.neuropharm.2013.06.020. [DOI] [PubMed] [Google Scholar]
- Hasselbalch BJ, Knorr U, Kessing LV. Cognitive impairment in the remitted state of unipolar depressive disorder: a systematic review. J Affect Disord. 2011;134:20–31. doi: 10.1016/j.jad.2010.11.011. [DOI] [PubMed] [Google Scholar]
- Headley DB, Weinberger NM. Gamma-band activation predicts both associative memory and cortical plasticity. J Neurosci. 2011;31:12748–12758. doi: 10.1523/JNEUROSCI.2528-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebb DO. Drives and the C.N.S. (conceptual nervous system) Psychol Rev. 1955;62:243–254. doi: 10.1037/h0041823. [DOI] [PubMed] [Google Scholar]
- Henigsberg N, Mahableshwarkar AR, Jacobsen P, Chen Y, Thase ME. A randomized, double-blind, placebo-controlled 8-week trial of the efficacy and tolerability of multiple doses of Lu AA21004 in adults with major depressive disorder. J Clin Psychiatry. 2012;73:953–959. doi: 10.4088/JCP.11m07470. [DOI] [PubMed] [Google Scholar]
- Horiguchi M, Huang M, Meltzer HY. The role of 5-hydroxytryptamine 7 receptors in the phencyclidine-induced novel object recognition deficit in rats. J Pharmacol Exp Ther. 2011;338:605–614. doi: 10.1124/jpet.111.180638. [DOI] [PubMed] [Google Scholar]
- Horisawa T, Ishibashi T, Nishikawa H, Enomoto T, Toma S, Ishiyama T, et al. The effects of selective antagonists of serotonin 5-HT7 and 5-HT1A receptors on MK-801-induced impairment of learning and memory in the passive avoidance and Morris water maze tests in rats: mechanistic implications for the beneficial effects of the novel atypical antipsychotic lurasidone. Behav Brain Res. 2011;220:83–90. doi: 10.1016/j.bbr.2011.01.034. [DOI] [PubMed] [Google Scholar]
- Insel N, Patron LA, Hoang LT, Nematollahi S, Schimanski LA, Lipa P, et al. Reduced gamma frequency in the medial frontal cortex of aged rats during behavior and rest: implications for age-related behavioral slowing. J Neurosci. 2012;32:16331–16344. doi: 10.1523/JNEUROSCI.1577-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivarsson M, Paterson LM, Hutson PH. Antidepressants and REM sleep in Wistar–Kyoto and Sprague–Dawley rats. Eur J Pharmacol. 2005;522:63–71. doi: 10.1016/j.ejphar.2005.08.050. [DOI] [PubMed] [Google Scholar]
- du Jardin KG, Jensen JB, Sanchez C, Pehrson AL. Vortioxetine dose-dependently reverses 5-HT depletion-induced deficits in spatial working and object recognition memory: a potential role for 5-HT1A receptor agonism and 5-HT3 receptor antagonism. Eur Neuropsychopharmacol. 2013;24:160–171. doi: 10.1016/j.euroneuro.2013.07.001. [DOI] [PubMed] [Google Scholar]
- Javitt DC, Spencer KM, Thaker GK, Winterer G, Hajós M. Neurophysiological biomarkers for drug development in schizophrenia. Nat Rev Drug Discov. 2008;7:68–83. doi: 10.1038/nrd2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javitt DC, Schoepp D, Kalivas PW, Volkow ND, Zarate C, Merchant K, et al. Translating glutamate: from pathophysiology to treatment. Sci Transl Med. 2011;3:102mr102. doi: 10.1126/scitranslmed.3002804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jutras MJ, Buffalo EA. Synchronous neural activity and memory formation. Curr Opin Neurobiol. 2010;20:150–155. doi: 10.1016/j.conb.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser J, Lutzenberger W. Cortical oscillatory activity and the dynamics of auditory memory processing. Rev Neurosci. 2005;16:239–254. doi: 10.1515/revneuro.2005.16.3.239. [DOI] [PubMed] [Google Scholar]
- Kaiser J, Heidegger T, Wibral M, Altmann CF, Lutzenberger W. Distinct gamma-band components reflect the short-term memory maintenance of different sound lateralization angles. Cereb Cortex. 2008;18:2286–2295. doi: 10.1093/cercor/bhm251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasper S, Sacher J, Klein N, Mossaheb N, Attarbaschi-Steiner T, Lanzenberger R, et al. Differences in the dynamics of serotonin reuptake transporter occupancy may explain superior clinical efficacy of escitalopram versus citalopram. Int Clin Psychopharmacol. 2009;24:119–125. doi: 10.1097/YIC.0b013e32832a8ec8. [DOI] [PubMed] [Google Scholar]
- Katoh A, Eigyo M, Ishibashi C, Naitoh Y, Takeuchi M, Ibii N, et al. Behavioral and electroencephalographic properties of duloxetine (LY248686), a reuptake inhibitor of norepinephrine and serotonin, in mice and rats. J Pharmacol Exp Ther. 1995;272:1067–1075. [PubMed] [Google Scholar]
- Katona C, Hansen T, Olsen CK. A randomized, double-blind, placebo-controlled, duloxetine-referenced, fixed-dose study comparing the efficacy and safety of Lu AA21004 in elderly patients with major depressive disorder. Int Clin Psychopharmacol. 2012;27:215–223. doi: 10.1097/YIC.0b013e3283542457. [DOI] [PubMed] [Google Scholar]
- Khodaie-Ardakani MR, Seddighi S, Modabbernia A, Rezaei F, Salehi B, Ashrafi M, et al. Granisetron as an add-on to risperidone for treatment of negative symptoms in patients with stable schizophrenia: randomized double-blind placebo-controlled study. J Psychiatr Res. 2013;47:472–478. doi: 10.1016/j.jpsychires.2013.01.011. [DOI] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG. Animal research: reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1577–1579. doi: 10.1111/j.1476-5381.2010.00872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimesch W. Memory processes, brain oscillations and EEG synchronization. Int J Psychophysiol. 1996;24:61–100. doi: 10.1016/s0167-8760(96)00057-8. [DOI] [PubMed] [Google Scholar]
- Klimesch W. EEG alpha and theta oscillations reflect cognitive and memory performance: a review and analysis. Brain Res Brain Res Rev. 1999;29:169–195. doi: 10.1016/s0165-0173(98)00056-3. [DOI] [PubMed] [Google Scholar]
- Klimesch W. Alpha-band oscillations, attention, and controlled access to stored information. Trends Cogn Sci. 2012;16:606–617. doi: 10.1016/j.tics.2012.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimesch W, Doppelmayr M, Schwaiger J, Auinger P, Winkler T. ‘Paradoxical’ alpha synchronization in a memory task. Brain Res Cogn Brain Res. 1999;7:493–501. doi: 10.1016/s0926-6410(98)00056-1. [DOI] [PubMed] [Google Scholar]
- Knott V, Mahoney C, Kennedy S, Evans K. EEG correlates of acute and chronic paroxetine treatment in depression. J Affect Disord. 2002;69:241–249. doi: 10.1016/s0165-0327(01)00308-1. [DOI] [PubMed] [Google Scholar]
- Kucewicz MT, Tricklebank MD, Bogacz R, Jones MW. Dysfunctional prefrontal cortical network activity and interactions following cannabinoid receptor activation. J Neurosci. 2011;31:15560–15568. doi: 10.1523/JNEUROSCI.2970-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lader M, Melhuish A, Frcka G, Fredricson Overø K, Christensen V. The effects of citalopram in single and repeated doses and with alcohol on physiological and psychological measures in healthy subjects. Eur J Clin Pharmacol. 1986;31:183–190. doi: 10.1007/BF00606656. [DOI] [PubMed] [Google Scholar]
- Leiser SC, Bowlby MR, Comery TA, Dunlop J. A cog in cognition: how the alpha 7 nicotinic acetylcholine receptor is geared towards improving cognitive deficits. Pharmacol Ther. 2009;122:302–311. doi: 10.1016/j.pharmthera.2009.03.009. [DOI] [PubMed] [Google Scholar]
- Leiser SC, Dunlop J, Bowlby MR, Devilbiss DM. Aligning strategies for using EEG as a surrogate biomarker: a review of preclinical and clinical research. Biochem Pharmacol. 2011;81:1408–1421. doi: 10.1016/j.bcp.2010.10.002. [DOI] [PubMed] [Google Scholar]
- Li Y, Pehrson AL, Budac DP, Sanchez C, Gulinello M. A rodent model of premenstrual dysphoria: progesterone withdrawal induces depression-like behavior that is differentially sensitive to classes of antidepressants. Behav Brain Res. 2012;234:238–247. doi: 10.1016/j.bbr.2012.06.034. [DOI] [PubMed] [Google Scholar]
- Lopes da Silva F. Neural mechanisms underlying brain waves: from neural membranes to networks. Electroencephalogr Clin Neurophysiol. 1991;79:81–93. doi: 10.1016/0013-4694(91)90044-5. [DOI] [PubMed] [Google Scholar]
- Mair RG, Onos KD, Hembrook JR. Cognitive activation by central thalamic stimulation: the Yerkes–Dodson law revisited. Dose Response. 2011;9:313–331. doi: 10.2203/dose-response.10-017.Mair. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maire M, Reichert CF, Schmidt C. Sleep–wake rhythms and cognition. J Cogn Behav Psychother. 2013;13(1a):133–170. [Google Scholar]
- Marazziti D, Consoli G, Picchetti M, Carlini M, Faravelli L. Cognitive impairment in major depression. Eur J Pharmacol. 2010;626:83–86. doi: 10.1016/j.ejphar.2009.08.046. [DOI] [PubMed] [Google Scholar]
- Mather M, Sutherland MR. Arousal-biased competition in perception and memory. Perspect Psychol Sci. 2011;6:114–133. doi: 10.1177/1745691611400234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath J, Drummond G, McLachlan E, Kilkenny C, Wainwright C. Guidelines for reporting experiments involving animals: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1573–1576. doi: 10.1111/j.1476-5381.2010.00873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre RS, Lophaven S, Olsen CK. Randomized, double-blind, placebo-controlled study of the efficacy of vortioxetine on cognitive dysfunction in adult patients with major depressive disorder (MDD) Neuropsychopharmacology. 2013;38:S380–S381. [Google Scholar]
- McLean SL, Woolley ML, Thomas D, Neill JC. Role of 5-HT receptor mechanisms in sub-chronic PCP-induced reversal learning deficits in the rat. Psychopharmacology (Berl) 2009;206:403–414. doi: 10.1007/s00213-009-1618-0. [DOI] [PubMed] [Google Scholar]
- McNaughton N, Kocsis B, Hajós M. Elicited hippocampal theta rhythm: a screen for anxiolytic and procognitive drugs through changes in hippocampal function? Behav Pharmacol. 2007;18:329–346. doi: 10.1097/FBP.0b013e3282ee82e3. [DOI] [PubMed] [Google Scholar]
- Meeter M, Talamini L, Schmitt JA, Riedel WJ. Effects of 5-HT on memory and the hippocampus: model and data. Neuropsychopharmacology. 2006;31:712–720. doi: 10.1038/sj.npp.1300869. [DOI] [PubMed] [Google Scholar]
- Meltzer JA, Negishi M, Mayes LC, Constable RT. Individual differences in EEG theta and alpha dynamics during working memory correlate with fMRI responses across subjects. Clin Neurophysiol. 2007;118:2419–2436. doi: 10.1016/j.clinph.2007.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meneses A. Effects of the 5-HT7 receptor antagonists SB-269970 and DR 4004 in autoshaping Pavlovian/instrumental learning task. Behav Brain Res. 2004;155:275–282. doi: 10.1016/j.bbr.2004.04.026. [DOI] [PubMed] [Google Scholar]
- Meneses A, Hong E. 5-HT1A receptors modulate the consolidation of learning in normal and cognitively impaired rats. Neurobiol Learn Mem. 1999;71:207–218. doi: 10.1006/nlme.1998.3866. [DOI] [PubMed] [Google Scholar]
- Meyer JH, Wilson AA, Sagrati S, Hussey D, Carella A, Potter WZ, et al. Serotonin transporter occupancy of five selective serotonin reuptake inhibitors at different doses: an [11C]DASB positron emission tomography study. Am J Psychiatry. 2004;161:826–835. doi: 10.1176/appi.ajp.161.5.826. [DOI] [PubMed] [Google Scholar]
- Millan MJ, Agid Y, Brune M, Bullmore ET, Carter CS, Clayton NS, et al. Cognitive dysfunction in psychiatric disorders: characteristics, causes and the quest for improved therapy. Nat Rev Drug Discov. 2012;11:141–168. doi: 10.1038/nrd3628. [DOI] [PubMed] [Google Scholar]
- Miyamoto H, Nakamaru-Ogiso E, Hamada K, Hensch TK. Serotonergic integration of circadian clock and ultradian sleep–wake cycles. J Neurosci. 2012;32:14794–14803. doi: 10.1523/JNEUROSCI.0793-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monti JM. Serotonin control of sleep–wake behavior. Sleep Med Rev. 2011;15:269–281. doi: 10.1016/j.smrv.2010.11.003. [DOI] [PubMed] [Google Scholar]
- Mørk A, Pehrson A, Tottrup BLT, Moller NS, Zhong H, Lassen AB, et al. Pharmacological effects of Lu AA21004: a novel multimodal compound for the treatment of major depressive disorder. J Pharmacol Exp Ther. 2012;340:666–675. doi: 10.1124/jpet.111.189068. [DOI] [PubMed] [Google Scholar]
- Mørk A, Montezinho LP, Miller S, Trippodi-Murphy C, Plath N, Li Y, et al. Vortioxetine (Lu AA21004), a novel multimodal antidepressant, enhances memory in rats. Pharmacol Biochem Behav. 2013;105C:41–50. doi: 10.1016/j.pbb.2013.01.019. [DOI] [PubMed] [Google Scholar]
- Murillo-Rodriguez E, Arias-Carrion O, Sanguino-Rodriguez K, Gonzalez-Arias M, Haro R. Mechanisms of sleep–wake cycle modulation. CNS Neurol Disord Drug Targets. 2009;8:245–253. doi: 10.2174/187152709788921654. [DOI] [PubMed] [Google Scholar]
- National Research Council. Guide for the Care and Use of Laboratory Animals. 8th edn. Washington, DC: The National Academies Press; 2011. [PubMed] [Google Scholar]
- Neckelmann D, Bjorvatn B, Bjørkum AA, Ursin R. Citalopram: differential sleep/wake and EEG power spectrum effects after single dose and chronic administration. Behav Brain Res. 1996;79:183–192. doi: 10.1016/0166-4328(96)00013-7. [DOI] [PubMed] [Google Scholar]
- Newman-Tancredi A, Martel JC, Assie MB, Buritova J, Lauressergues E, Cosi C, et al. Signal transduction and functional selectivity of F15599, a preferential post-synaptic 5-HT1A receptor agonist. Br J Pharmacol. 2009;156:338–353. doi: 10.1111/j.1476-5381.2008.00001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan SF, Roman MW. Lurasidone (LATUDA®): an atypical antipsychotic. Issues Ment Health Nurs. 2012;33:342–343. doi: 10.3109/01612840.2012.669025. [DOI] [PubMed] [Google Scholar]
- Nutt DJ, Davidson JR, Gelenberg AJ, Higuchi T, Kanba S, Karamustafalioglu O, et al. International consensus statement on major depressive disorder. J Clin Psychiatry. 2010;71(Suppl. E1):e08. doi: 10.4088/JCP.9058se1c.08gry. doi: 10.4088/JCP.9058se1c.08gry. [DOI] [PubMed] [Google Scholar]
- Parmentier-Batteur S, O'Brien JA, Doran S, Nguyen SJ, Flick RB, Uslaner JM, et al. Differential effects of the mGluR5 positive allosteric modulator CDPPB in the cortex and striatum following repeated administration. Neuropharmacology. 2012;62:1453–1460. doi: 10.1016/j.neuropharm.2010.11.013. [DOI] [PubMed] [Google Scholar]
- Paterson LM, Wilson SJ, Nutt DJ, Hutson PH, Ivarsson M. A translational, caffeine-induced model of onset insomnia in rats and healthy volunteers. Psychopharmacology (Berl) 2007;191:943–950. doi: 10.1007/s00213-006-0672-0. [DOI] [PubMed] [Google Scholar]
- Pawson AJ, Sharman JL, Benson HE, Faccenda E, Alexander SP, Buneman OP, et al. The IUPHAR/BPS Guide to PHARMACOLOGY: an expert-driven knowledge base of drug targets and their ligands. Nucleic Acids Research. 2014;42(Database Issue):D1098–1106. doi: 10.1093/nar/gkt1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pehrson AL, Sanchez C. Serotonergic modulation of glutamate neurotransmission as a strategy for treating depression and cognitive dysfunction. CNS Spectr. 2014;19:121–133. doi: 10.1017/S1092852913000540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pehrson AL, Cremers T, Betry C, Van Der Hart MG, Jorgensen L, Madsen M, et al. Lu AA21004, a novel multimodal antidepressant, produces regionally selective increases of multiple neurotransmitters – a rat microdialysis and electrophysiology study. Eur Neuropsychopharmacol. 2013;23:133–145. doi: 10.1016/j.euroneuro.2012.04.006. [DOI] [PubMed] [Google Scholar]
- Pitsikas N, Borsini F. Itasetron (DAU 6215) prevents age-related memory deficits in the rat in a multiple choice avoidance task. Eur J Pharmacol. 1996;311:115–119. doi: 10.1016/0014-2999(96)00586-9. [DOI] [PubMed] [Google Scholar]
- Polter AM, Li X. 5-HT1A receptor-regulated signal transduction pathways in brain. Cell Signal. 2010;22:1406–1412. doi: 10.1016/j.cellsig.2010.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghavachari S, Kahana MJ, Rizzuto DS, Caplan JB, Kirschen MP, Bourgeois B, et al. Gating of human theta oscillations by a working memory task. J Neurosci. 2001;21:3175–3183. doi: 10.1523/JNEUROSCI.21-09-03175.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghavachari S, Lisman JE, Tully M, Madsen JR, Bromfield EB, Kahana MJ. Theta oscillations in human cortex during a working-memory task: evidence for local generators. J Neurophysiol. 2006;95:1630–1638. doi: 10.1152/jn.00409.2005. [DOI] [PubMed] [Google Scholar]
- Riga MS, Celada P, Sanchez C, Artigas F. Role of 5-HT3 receptors in the mechanism of action of the investigational antidepressant vortioxetine. Eur Neuropsychopharmacol. 2013;23(S2):S393–S394. [Google Scholar]
- Rijnbeek B, de Visser SJ, Franson KL, Cohen AF, van Gerven JM. REM sleep effects as a biomarker for the effects of antidepressants in healthy volunteers. J Psychopharmacol. 2003;17:196–203. doi: 10.1177/0269881103017002008. [DOI] [PubMed] [Google Scholar]
- Robinson PA, Phillips AJ, Fulcher BD, Puckeridge M, Roberts JA. Quantitative modelling of sleep dynamics. Philos Trans A Math Phys Eng Sci. 2011;369:3840–3854. doi: 10.1098/rsta.2011.0120. [DOI] [PubMed] [Google Scholar]
- Roth BL. Multiple serotonin receptors: clinical and experimental aspects. Ann Clin Psychiatry. 1994;6:67–78. doi: 10.3109/10401239409148985. [DOI] [PubMed] [Google Scholar]
- Roth BL, Hanizavareh SM, Blum AE. Serotonin receptors represent highly favorable molecular targets for cognitive enhancement in schizophrenia and other disorders. Psychopharmacology (Berl) 2004;174:17–24. doi: 10.1007/s00213-003-1683-8. [DOI] [PubMed] [Google Scholar]
- Roychoudhury M, Kulkarni SK. Effects of ondansetron on short-term memory retrieval in mice. Methods Find Exp Clin Pharmacol. 1997;19:43–46. [PubMed] [Google Scholar]
- Ruigt GS. Animal pharmaco-EEG: models, facts and fallacies. Methods Find Exp Clin Pharmacol. 2002;24(Suppl. D):59–61. [PubMed] [Google Scholar]
- Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163:1905–1917. doi: 10.1176/ajp.2006.163.11.1905. [DOI] [PubMed] [Google Scholar]
- Saletu B. Pharmaco-EEG profiles of typical and atypical antidepressants. Adv Biochem Psychopharmacol. 1982;32:257–268. [PubMed] [Google Scholar]
- Saletu B, Grünberger J. Classification and determination of cerebral bioavailability of fluoxetine: pharmacokinetic, pharmaco-EEG and psychometric analyses. J Clin Psychiatry. 1985;46:45–52. [PubMed] [Google Scholar]
- Saletu B, Grünberger J, Anderer P, Linzmayer L, Zyhlarz G. Comparative pharmacodynamic studies with the novel serotonin uptake-enhancing tianeptine and -inhibiting fluvoxamine utilizing EEG mapping and psychometry. J Neural Transm. 1996;103:191–216. doi: 10.1007/BF01292627. [DOI] [PubMed] [Google Scholar]
- Sanchez C, Brennum LT, Storustovu S, Kreilgard M, Mørk A. Depression and poor sleep: the effect of monoaminergic antidepressants in a pre-clinical model in rats. Pharmacol Biochem Behav. 2007;86:468–476. doi: 10.1016/j.pbb.2007.01.006. [DOI] [PubMed] [Google Scholar]
- Schack B, Klimesch W. Frequency characteristics of evoked and oscillatory electroencephalic activity in a human memory scanning task. Neurosci Lett. 2002;331:107–110. doi: 10.1016/s0304-3940(02)00846-7. [DOI] [PubMed] [Google Scholar]
- Sebban C, Zhang XQ, Tesolin-Decros B, Millan MJ, Spedding M. Changes in EEG spectral power in the prefrontal cortex of conscious rats elicited by drugs interacting with dopaminergic and noradrenergic transmission. Br J Pharmacol. 1999;128:1045–1054. doi: 10.1038/sj.bjp.0702894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang Y, Gibbs MA, Marek GJ, Stiger T, Burstein AH, Marek K, et al. Displacement of serotonin and dopamine transporters by venlafaxine extended release capsule at steady state: a [123I]2beta-carbomethoxy-3beta-(4-iodophenyl)-tropane single photon emission computed tomography imaging study. J Clin Psychopharmacol. 2007;27:71–75. doi: 10.1097/JCP.0b013e31802e0017. [DOI] [PubMed] [Google Scholar]
- Shaw JC. Brain's Alpha Rhythm and the Mind. Amsterdam: Elsevier; 2003. [Google Scholar]
- Shin YW, O'Donnell BF, Youn S, Kwon JS. Gamma oscillation in schizophrenia. Psychiatry Investig. 2011;8:288–296. doi: 10.4306/pi.2011.8.4.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sörman E, Wang D, Hajós M, Kocsis B. Control of hippocampal theta rhythm by serotonin: role of 5-HT2c receptors. Neuropharmacology. 2011;61:489–494. doi: 10.1016/j.neuropharm.2011.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staner L, Luthringer R, Macher JP. Effects of antidepressant drugs on sleep EEG in patients with major depression. CNS Drugs. 1999;11:49–60. [Google Scholar]
- Stäubli U, Xu FB. Effects of 5-HT3 receptor antagonism on hippocampal theta rhythm, memory, and LTP induction in the freely moving rat. J Neurosci. 1995;15:2445–2452. doi: 10.1523/JNEUROSCI.15-03-02445.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M. Cellular substrates of brain rhythms. In: Niedermeyer E, Lopes Da Silva F, editors. Electroencephalography: Basic Principles, Clinical Applications, and Related Fields. 5th edn. Philadelphia: Lippincott Williams & Wilkins; 2005. pp. 31–83. [Google Scholar]
- Steriade M, Gloor P, Llinas RR, Lopes de Silva FH, Mesulam MM. Report of IFCN Committee on basic mechanisms. Basic mechanisms of cerebral rhythmic activities. Electroencephalogr Clin Neurophysiol. 1990;76:481–508. doi: 10.1016/0013-4694(90)90001-z. [DOI] [PubMed] [Google Scholar]
- Sumiyoshi T, Higuchi Y. Facilitative effect of serotonin(1A) receptor agonists on cognition in patients with schizophrenia. Curr Med Chem. 2013;20:357–362. [PubMed] [Google Scholar]
- Takano A, Suzuki K, Kosaka J, Ota M, Nozaki S, Ikoma Y, et al. A dose-finding study of duloxetine based on serotonin transporter occupancy. Psychopharmacology (Berl) 2006;185:395–399. doi: 10.1007/s00213-005-0304-0. [DOI] [PubMed] [Google Scholar]
- Vanderwolf CH. Cerebral activity and behavior: control by central cholinergic and serotonergic systems. Int Rev Neurobiol. 1988;30:225–340. doi: 10.1016/s0074-7742(08)60050-1. [DOI] [PubMed] [Google Scholar]
- Vas S, Katai Z, Kostyalik D, Pap D, Molnar E, Petschner P, et al. Differential adaptation of REM sleep latency, intermediate stage and theta power effects of escitalopram after chronic treatment. J Neural Transm. 2013;120:169–176. doi: 10.1007/s00702-012-0847-2. [DOI] [PubMed] [Google Scholar]
- Vertes RP, Kocsis B. Brainstem-diencephalo-septohippocampal systems controlling the theta rhythm of the hippocampus. Neuroscience. 1997;81:893–926. doi: 10.1016/s0306-4522(97)00239-x. [DOI] [PubMed] [Google Scholar]
- Vogel V, Sanchez C, Jennum P. EEG measurements by means of radiotelemetry after intracerebroventricular (ICV) cannulation in rodents. J Neurosci Methods. 2002;118:89–96. doi: 10.1016/s0165-0270(02)00148-6. [DOI] [PubMed] [Google Scholar]
- Voineskos AN, Wilson AA, Boovariwala A, Sagrati S, Houle S, Rusjan P, et al. Serotonin transporter occupancy of high-dose selective serotonin reuptake inhibitors during major depressive disorder measured with [11C]DASB positron emission tomography. Psychopharmacology (Berl) 2007;193:539–545. doi: 10.1007/s00213-007-0806-z. [DOI] [PubMed] [Google Scholar]
- Ward LM. Synchronous neural oscillations and cognitive processes. Trends Cogn Sci. 2003;7:553–559. doi: 10.1016/j.tics.2003.10.012. [DOI] [PubMed] [Google Scholar]
- Westrich L, Pehrson A, Zhong H, Nielsen SM, Frederiksen K, Stensbøl TB, et al. In vitro and in vivo effects of the multimodal antidepressant vortioxetine (Lu AA21004) at human and rat targets. Int J Psychiatry Clin Pract. 2012;5(Suppl. 1):47. [Google Scholar]
- Wilson FJ, Leiser SC, Ivarsson M, Christensen SR, Bastlund JF. Can pharmaco-electroencephalography help improve survival of central nervous system drugs in early clinical development? Drug Discov Today. 2013;19:282–288. doi: 10.1016/j.drudis.2013.08.001. [DOI] [PubMed] [Google Scholar]
- Wilson SJ, Bailey JE, Rich AS, Nash J, Adrover M, Tournoux A, et al. The use of sleep measures to compare a new 5HT1A agonist with buspirone in humans. J Psychopharmacol. 2005;19:609–613. doi: 10.1177/0269881105058775. [DOI] [PubMed] [Google Scholar]
- Wright KP, Lowry CA, Lebourgeois MK. Circadian and wakefulness–sleep modulation of cognition in humans. Front Mol Neurosci. 2012;5:50. doi: 10.3389/fnmol.2012.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young CK, McNaughton N. Coupling of theta oscillations between anterior and posterior midline cortex and with the hippocampus in freely behaving rats. Cereb Cortex. 2009;19:24–40. doi: 10.1093/cercor/bhn055. [DOI] [PubMed] [Google Scholar]
- Zhang ZJ, Kang WH, Li Q, Wang XY, Yao SM, Ma AQ. Beneficial effects of ondansetron as an adjunct to haloperidol for chronic, treatment-resistant schizophrenia: a double-blind, randomized, placebo-controlled study. Schizophr Res. 2006;88:102–110. doi: 10.1016/j.schres.2006.07.010. [DOI] [PubMed] [Google Scholar]


