Abstract
Background and Purpose
Calcium-activated chloride channels (CaCCs) are key depolarizing mechanisms that have an important role in vascular smooth muscle contraction. Here, we investigated whether these channels are regulated by phosphatidylinositol (4,5) bisphosphate [P(4,5)P2], a known regulator of various ion channels.
Experimental Approach
Calcium-activated Cl− currents (IClCa) were recorded by patch clamp electrophysiology of rat isolated pulmonary artery smooth muscle cells. TMEM16A protein-phosphoinositide interaction was studied by co-immunoprecipitation and phosphoinositide binding arrays on protein lysates from whole pulmonary arteries and HEK293 cells overexpressing TMEM16A, the molecular correlate.
Key Results
PI(4,5)P2 and other phospholipids were shown to bind directly to TMEM16A isolated from whole pulmonary artery (PA) and TMEM16A-eGFP expressed in HEK293 cells. Agents that reduced PI(4,5)P2 levels through different routes [PLC activation, PI4K inhibition, PI(4,5)P2 scavenging and absorption] all increased IClCa evoked by solutions containing clamped-free [Ca2+], whereas enrichment of activating solutions with PI(4,5)P2 inhibited IClca in PA smooth muscle cells with approximately 50% reduction at 1 μM.
Conclusions and Implications
These data are the first to show a negative regulation of TMEM16A-encoded CaCCs by PI(4,5)P2 and propose that control of PI(4,5)P2 levels is a key determinant of arterial physiology.
Introduction
Stimulation of Ca2+-activated Cl− channels (CaCCs) has been implicated in a number of physiological processes. In vascular smooth muscle, CaCCs provide a depolarizing mechanism because these cells actively accumulate Cl− ions through the Na/K/2Cl transporter and the HCO3/Cl− exchanger (Chipperfield and Harper, 2000), resulting in a Cl− equilibrium potential sufficiently more positive than the resting membrane potential to allow Cl− efflux. As vascular smooth muscle contraction is precipitated by a rise in intracellular Ca2+, principally through Ca2+ influx via voltage-dependent Ca2+ channels (VDCCs), then CaCC stimulation will increase the likelihood of the VDCCs being open and the concomitant Ca2+ influx will enhance vascular contractility. Calcium-activated chloride currents (IClCa) have been described in vascular smooth muscle cells from a number of different arteries (Greenwood et al., 2001; Angermann et al., 2006; Sones et al., 2010; Dam et al., 2013), and inhibition of the Cl− accumulation mechanisms or direct block of Cl− channels by non-specific agents such as niflumic acid attenuates contraction in a number of smooth muscles (Criddle et al., 1996; Yamazaki and Kitamura, 2001). However, the lack of a defined molecular identity for CaCCs has prohibited more precise investigations into the role of CaCCs in vascular physiology and pathophysiology.
Recently, expression products of TMEM16A (anoctamin 1) were shown to comprise native CaCCs (Caputo et al., 2008; Schroeder et al., 2008; Yang et al., 2008) and TMEM16A expression has been identified in various rodent and human arteries (Manoury et al., 2010; Sones et al., 2010; Thomas-Gatewood et al., 2011; Bulley et al., 2012; Wang et al., 2012; Davis et al., 2010; 2013). Reduction of TMEM16A activity either through siRNA, targeted antibodies or use of TMEM16A-specifc pharmacological tools such as aminophenylthiazole (T16Ainh-AO1) diminishes IClCa (Manoury et al., 2010; Thomas-Gatewood et al., 2011; Davis et al., 2013) and attenuates vasoconstriction (Dam et al., 2013; Davis et al., 2013), supporting a role for CaCCs in vascular tone. This view is consolidated by the observation that targeted disruption of TMEM16A in vascular smooth muscle cells leads to lower blood pressure and less severe hypertension following angiotensin II infusion (Heinze et al., 2014). Consequently, TMEM16A-encoded CaCCs are key determinants of vascular reactivity, and mechanisms that regulate these channels will have considerable bearing on arterial physiology.
There is growing appreciation that in addition to being a substrate for PLC, phosphatidylinositol (4,5) bisphosphate [PI(4,5)P2] is also a regulator of various ion channels including KCNQ-encoded channels, transient receptor potential channel 1 (TRPC1), Kir and epithelial sodium channels (ENaC) (see Suh and Hille, 2008 for summary; nomenclature conforms to Alexander et al., 2013). However, there is no information about the regulation of CaCCs by PI(4,5)P2. The aim of the present study was to determine, using a combination of different pharmacological reagents, single cell electrophysiology, co-immunoprecipitation and biochemical analysis, whether alterations in PI(4,5)P2 levels affect native IClCa in rat pulmonary artery smooth muscle cells (PASMCs). Our data revealed that PI(4,5)P2 is a powerful negative regulator of CaCC activity that has considerable implications for cellular physiology.
Methods
Cell isolation
PASMCs were isolated from male Wistar rats (200–225 g), killed in accordance with Schedule 1 of the United Kingdom Animal Acts 1986. The cells were isolated by incubating segments of artery in Ca2+-free physiological salt solution (PSS) containing 0.5 mg·mL−1 papain, 1 mM DTT and 2 mg·mL−1 BSA overnight at 4°C, followed by trituration through a wide bore glass pipette. Cells were plated on coverslips in PSS, supplemented with 0.75 mM [Ca2+], and experiments were carried out between 1 and 6 h post-seeding. Following the reviewer's comments, a few experiments were performed on cells isolated by a more acute method (1 h in 1.5 mg·mL−1 papain at 4°C, then 6 min in 1.5 mg·mL−1 papain and 1 mg·mL−1 DTT, before being treated with 1.4 mg·mL−1 collagenase for 5 min, both at 37°C).
Cell culture and transfection
HEK293 cells were seeded in 6-well plates in MEM, supplemented with 10% FBS, 1% penicillin/streptomycin, 1% non-essential amino acids, 1% sodium pyruvate and 1% l-glutamine. HEK293 cells grown to 70% confluence were transfected with mouse TMEM16A-eGFP (‘a’ variant) using lipofectamine 2000. Cells were exposed to the plasmid (0.1 μg·cm−2) in antibiotic-free media for 6 h before the medium was replaced with the standard growth media. Cells were then removed with trypsin-EGTA and protein was extracted.
Western blot analysis
Samples of rat pulmonary arteries were homogenized in ice-cold lysis buffer [in mM: 20 Tris base, 137 NaCl, 2 EDTA, 1% nonidet P-40, 10% glycerol (pH 8) and 10 μL·mL−1 protease inhibitor cocktail], incubated for 15 min and centrifuged (15 min, 4°C, 9400× g). Supernatant was transferred to a separate tube and protein concentration was calculated by the Bradford assay. Knockout samples were a kind gift from Professor Karl Kunzelmann (University of Regensburg, Germany). For each sample, 30 μg of protein was transferred to a PVDF membrane and subjected to analysis using XCell surelock system (Life Technologies, Paisley, UK). Samples were blocked with 5% milk powder in PBS-Tween (0.1%, PBS-T) for 1 h and were probed, overnight at 4°C, with anti-TMEM16A (1:200, ab53212; Abcam, Cambridge, UK), which is the undiluted form of the antibody previously characterized by Davis et al. (2010). Excess primary antibody was removed with PBS-T and HRP-conjugated anti-rabbit secondary (1:25 000; Sigma, Poole, Dorset, UK) for 1 h at room temperature (RT). All antibodies were diluted in the milk buffer. Samples were detected with ECL chemiluminescence, exposed to photographic film (both GE Health care, Little Chalfont, UK).
Immunoprecipitation
Immunoprecipitation and dot blots were carried out as described previously (Shi et al., 2014). Whole rat pulmonary arteries were dissected and treated with either 1 μM U46619 (15 min), 20 μM wortmannin or 3 mg·mL−1 α-cyclodextrin (α-CD) (all 1 h). Tissues were lysed and subjected to the same procedure as that used to prepare samples for Western blot analysis. Immunoprecipitation of the rat pulmonary artery was carried out using a Millipore Catch and Release Kit according to the manufacturer's instructions; 400 μg of protein was used per sample and 4 μg of TMEM16A antibody. TMEM16A protein overexpressed in HEK was immunoprecipitated using GFP-Trap_A Kit (Chromotek, Krailling, Germany) according to the manufacturer's instructions, with 400 μg of protein per sample.
Dot blots
Three microlitres of each sample was added to a PVDF membrane and dried, before being blocked in 5% milk powder in PBS-T for 1 h. It was then probed with anti-PI(4,5)P2 (1:200; Santa Cruz, Heidelberg, Germany) overnight at 4°C, as described previously (Barroso-González et al., 2009; Shi et al., 2014). Excess primary antibody was then removed from the membranes by washing with PBS-T, before the samples were incubated with HRP-conjugated secondary antibody (anti-mouse IgM, 1:2500; Santa Cruz) for 1 h and TMEM16A detected on photographic film as above. Drying the membrane produced dots of various sizes; therefore, the intensities of the dot blots were calculated and normalized to known concentrations of TMEM16A using Image Studio software (LI-COR, Lincoln, NE, USA). Data shown represent findings from at least 3 different animals.
Lipid assay
PIP strips (Life Technologies) were used to test which phospholipids TMEM16A can potentially bind to and experiments were carried out according to the manufacturer's instructions. Briefly, strips were blocked in TBS-Tween (0.1%, TBS-T) and 3% BSA for 1 h. Samples were then diluted in the blocking buffer to 0.5 μg·mL−1 and incubated at room temperature for 3 h, washed three times in blocking buffer before the sample was again incubated for another 3 h. Samples were then probed for TMEM16A similar to the dot blot method, but using TBS-T and 3% BSA blocking buffer and detected using Westar Supernova ECL (Geneflow Limited, Lichfield, UK). It is worth stressing that on the PIP strip, although there is 100 pM of lipid in each spot, we did not make comparisons between dot intensities, as they varied in concentration and location due to their physiological nature.
Whole cell recordings
IClCa was activated by a known amount of ‘free’ [Ca2+] up to 500 nM, which produced a current immediately upon rupture of the membrane. The pipette solution had the following composition (in mM): TEA (20), CsCl (106), HEPES–CsOH (10, pH 7.2), BAPTA (10), ATP·Mg−1 (5) and GTP·diNa−1 (0.2). To this solution, 4.2, 6.8 and 7.08 mM CaCl2 were added to achieve free [Ca2+] of 100, 250 and 500 nM respectively (Greenwood et al., 2001; Angermann et al., 2006). The bathing solution had the following composition (in mM): NaCl (126), HEPES–NaOH (10, pH 7.35), TEA (8.4), glucose (20), MgCl2 (1.2) and CaCl2 (1.8). Experiments were carried out under voltage clamp, using protocols described previously (Greenwood et al., 2001; Angermann et al., 2006). Briefly, the ‘test’ protocol consisted of a step from the holding potential (HP) of −50 to +70 mV and then down to −80 mV before returning to the HP. I–V relationships consisted of stepping from the HP to between −100 and +100 mV in 20 mV increments before returning to the HP. Perforated patch recordings were obtained by inclusion of 600 μg·mL−1 of amphotericin B in a standard pipette solution. All whole cell recordings were carried out at room temperature.
Single channel recordings
To test the potential role of PI(4,5)P2 on IClCa activity, experiments were carried out using the inside-out patch configuration, which enables direct access to intracellular membrane and, therefore, is probably the site of PI(4,5)P2 interactions. The pipette solution contained (in mM) N-methyl-d-glutamine (NMDG-Cl) (126, equimolar addition of NMDG and HCl), MgCl2 (1.2) CaCl2 (10) HEPES (10), pH set at 7.2. The bathing solution (intracellular) contained NMDG-Cl (126), HEPES (10), MgCl2 (1.2), EGTA (0.1) and MgATP (1), pH 7.2 – 64 μM CaCl2 to achieve 500 nM free [Ca2+]. Single channel IClCa was recorded at room temperature with a HP of −100 mV. Recordings were analysed as shown previously (Piper and Large, 2003; Davis et al., 2013). Briefly, single channel current amplitudes were calculated from traces of at least 60 s in duration using the 50% threshold method.
Statistical analysis
All data are shown as the mean of ‘n’ animals ± SEM. After testing for normality, the data were tested for statistical significance using the correct test on GraphPad Prism software (GraphPad Software Inc., San Diego, CA, USA). Whole cell I–V relationships and time-course experiments were compared using a two-way anova, with a Bonferroni post hoc test at each potential or time period. Single channel relative NP0 were tested using a paired t-test or a Wilcoxon test. Dot blot intensities were compared using a one-way anova, with a Bonferoni post hoc test to compare with untreated control.
Materials
T16inh-A01 was from Millipore (Watford, UK) and PI(4,5)P2 was from Echelon Biosciences (Salt Lake City, UT, USA). All other chemicals were purchased from Sigma.
Results
PI(4,5)P2 is bound to native rat PA TMEM16A at rest
Similar to our previous studies on mouse and rat blood vessels (Davis et al., 2010; Forrest et al., 2012), probing with a TMEM16A-antibody produced a band at about 120 kDa in rat PA protein lysates. In HEK293 cells heterologously expressing eGFP-tagged TMEM16A, the band was slightly higher at about 150 kDa, consistent with the extra molecular weight of the eGFP tag, which is separated from the endogenous TMEM16A within HEK293 cells. (Figure 1Ai). TMEM16A was not detected in kidney lysate from TMEM16A knockout mice, but was detected in wild-type control animals (Figure 1Aii). This antibody was then used to precipitate out TMEM16A from non-denatured rat PA protein lysate. Incubation of the TMEM16A-immunoprecipiated protein lysates with PI(4,5)P2 antisera produced positive signals consistent with this phospholipid being associated with the channel in rat PA (Figure 1B). Treatment of whole PA with agents that deplete PI(4,5)P2, namely the thromboxane receptor agonist U46619 (1 μM, 15 min), wortmannin (20 μM, 1 h) and αCD (3 mg·mL−1, 1 h), resulted in a reduction in signal intensity produced by the PI(4,5)P2 antisera (Figure 1Bii). Incubation of TMEM16A-precipitated protein lysate with phosphoinositides stabilized onto membrane arrays (so-called ‘PIP strips’) showed that TMEM16A from the rat PA bound phosphatidic acid and all phosphatidylinositols (PI) including PI(4,5)P2 (Figure 1Ci). We repeated these studies using overexpressed TMEM16A-eGFP protein, which was purified using a GFP-Trap kit (Chromotek) and its lipid binding profile was similar to that of native TMEM16A (Figure 1Cii).
Figure 1.
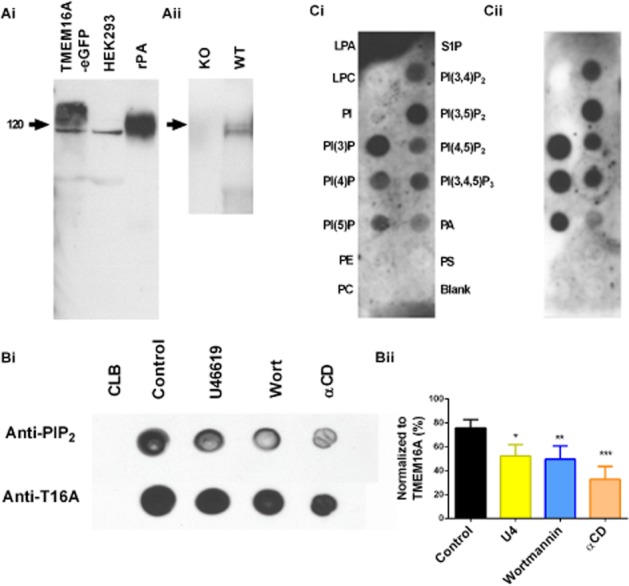
Biochemical analysis of TMEM16A and PI(4,5)P interaction. (A) Western blot analysis of anti-TMEM16A antibody previously characterized by Davis et al. (2010) on lysates isolated from HEK293 cells transfected with TMEM16A-eGFP, untransfected HEK293 cells, rat PA and kidney lysates from knockout (KO) and wild-type (WT) mice. (B) Immunodetection of PI(4,5)P2 in rat PA lysates precipitated using TMEM16A antisera in the absence and presence of U46619 (1 μM, 15 min, *P < 0.05), wortmannin (20 μM, 1 h, **P < 0.01), and αCD (3 mg·mL−1, 1 h, ***P < 0.001) compared using one-way anova versus control. (Bii) The mean intensity of dots produced by the PI(4,5)P2 antisera normalized to the dot intensity when TMEM16A antisera was used (n = 3). (C) PIP strips were used to see the lipid binding potential of purified rat PA TMEM16A. (Ci) Lipids include lysophosphatidic acid (LPA), lysophosphocholine (LPC), phosphatidylinositol (PI), phosphatidylinositol (3) phosphate [PI(3)P], phosphatidylinositol (4) phosphate [PI(4)P], phosphatidylinositol (5) phosphate [PI(5)P], phosphatidylethanolamine (PE), phosphatidylcholine (PC), sphingosine 1-phosphate (S1P), phosphatidylinositol (3,4) bisphosphate [PI(3,4)P2], phosphatidylinositol (3,5) bisphosphate [PI(3,5)P2], phosphatidylinositol (4,5) bisphosphate [PI(4,5)P2], phosphatidylinositol (3,4,5) trisphosphate [PI(3,4,5)P3], phosphatidic acid (PA) and phosphatidylserine (PS). Purified overexpressed TMEM16A-eGFP was tested (Cii), with same lipids as in (Ci).
Enrichment with PI(4,5)P2 attenuated IClca
Having established that TMEM16A and PI(4,5)P2 interact physically, we investigated what effect PI(4,5)P2 had on native Ca2+-dependent Cl− currents (IClca) in rat PA smooth muscle cells. Consistent with previous experiments in this artery (Forrest et al., 2012; Sun et al., 2012) and other vascular smooth muscle cells (Greenwood et al., 2001; Angermann et al., 2006; Sones et al., 2010; Davis et al., 2013), IClCa were evoked immediately after the pipette solutions containing [Ca2+] at fixed levels of 100, 250 or 500 nM gained access to the whole cell, which exhibited distinctive voltage-dependent kinetics (Figure 2Ai,Aii). These currents were inhibited by the TMEM16A-specific blocker T16Ainh-AO1 (Figure 2Aiii; n = 4, P < 0.001) to an extent comparable to that observed in our previous study (Davis et al., 2013). Enriching whole cell pipette solutions with 1 μM diC-8 PI(4,5)P2, a water-soluble form of PI(4,5)P2, attenuated whole cell IClCa compared to same-day controls, (Figure 2B; n = 7, P < 0.001). IClCa generated in cells isolated using a more acute method were also sensitive to 1 μM diC-8 PI(4,5)P2 (8.17 ± 2.4 pA·pF−1 vs. 17.1 ± 2.3 pA·pF−1, in same-day controls P < 0.001, n = 5) (see Supporting Information Fig. S1). To determine the concentration-dependence of this effect, we studied a range of [diC-8 PI(4,5)P2] on single channel IClCa. Application of a bathing solution containing 500 nM free [Ca2+] resulted in robust, low amplitude channel activity similar to previous studies (Piper and Large, 2003; Davis et al., 2013), which was inhibited considerably by application of 10 μM T16inh-A01 (Figure 2C; n = 5, P < 0.01). Application of diC-8 PI(4,5)P2 (100 nM–10 μM) to the cytosolic surface of excised patches, inhibited IClCa, with 1 μM producing approximately 0% reduction (Figure 2Dii). The maximum inhibition of IClCa by diC-8 PI(4,5)P2 was similar to that produced by 10 μM T16inh-A01 (Figure 2Di).
Figure 2.
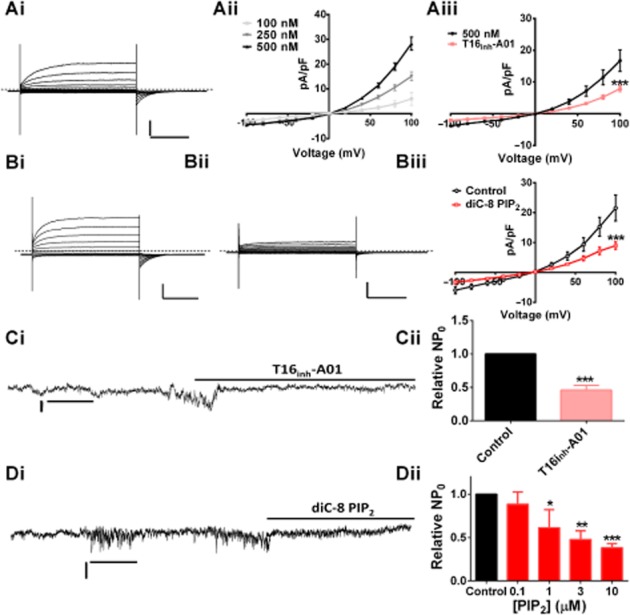
Enrichment of PASMC with diC-8 PI(4,5)P2 attenuated IClCa. (Ai) Representative IClCa activated by 500 nM [Ca2+] within the pipette solution from −100 to +100 mV. (Aii) shows the mean currents evoked using pipette solutions containing different [Ca2+] (n = 4–7). (Aiii) Currents elicited by 500 nM free Ca2+ in the absence (black) and presence of 10 μM T16inh-A01 (pink, ***P < 0.001 ). (Bi and Bii) Currents evoked by 500 nM free Ca2+ alone or after inclusion of 1 μM diC-8 PI(4,5)P2 (Bii). Mean data are shown in (Biii) (n = 7, ***P < 0.001). Whole cell horizontal bars and vertical bars represent 20 pA·pF−1 and 500 ms. Dashed line represents zero current level. (C) Inside-out excised patches activated by 500 nM free [Ca2+] that were sensitive to 10 μM T16inh-A01 (Cii shows mean data, n = 5, ***P < 0.001). (Di) Representative effect of 10 μM diC-PI(4,5)P2 on excised patches and the concentration-dependence of the inhibitory effect of diC-PI(4,5)P2 (100 nM–10 μM) is shown in (Dii) (n = 4–8, *P < 0.05, **P < 0.01, ***P < 0.001). Single channel horizontal bars and vertical bars represent 0.2 pA and 20 s.
Depletion of endogenous PI(4,5)P2 augments IClCa
Intracellular enrichment of PI(4,5)P2 attenuated IClCa so we postulated that depletion of PI(4,5)P2 levels would augment IClCa. Wortmannin, at high concentrations, is a PI4K inhibitor, therefore preventing the synthesis of PI(4,5)P2 and depleting cellular PI(4,5)P2 levels (Saleh et al., 2009). Pretreating PASMC for 1 h with 20 μM wortmannin resulted in currents evoked by 500 nM Ca2+-containing pipette solutions that were considerably larger than same-day controls (Figure 3Ai,Aiii; n = 9, P < 0.001). Application of the α1-adrenoceptor agonist methoxamine to cells incubated in wortmannin produced a further marked increase in current amplitude (Figure 3Aiii; n = 4, P < 0.001) within 5 min. Finally, α-cyclodextrin (α-CD) (3 mg·mL−1), a cell permeable phospholipid acceptor, increased IClCa within 5 min in cells isolated using both the overnight dispersal (Figure 3B; n = 5, P < 0.001) and acute method (7.04 ± 0.8 pA·pF−1 vs. 9.55 ± 0.5 pA·pF−1 at +70 mV; see Supporting information Fig. S1). In single channel experiments, a known PI(4,5)P2 scavenger, poly-l-lysine (50 μg·mL−1) augmented IClCa markedly (Figure 3Ci; n = 9, P < 0.01); this effect was attenuated reversibly by application of 10 μM T16inh-A01 (Figure 4Ci; n = 5). Application of wortmannin for 10 min also increased single channel activity with the NPo increased by twofold (Figure 3Cii; n = 6, P < 0.05).
Figure 3.
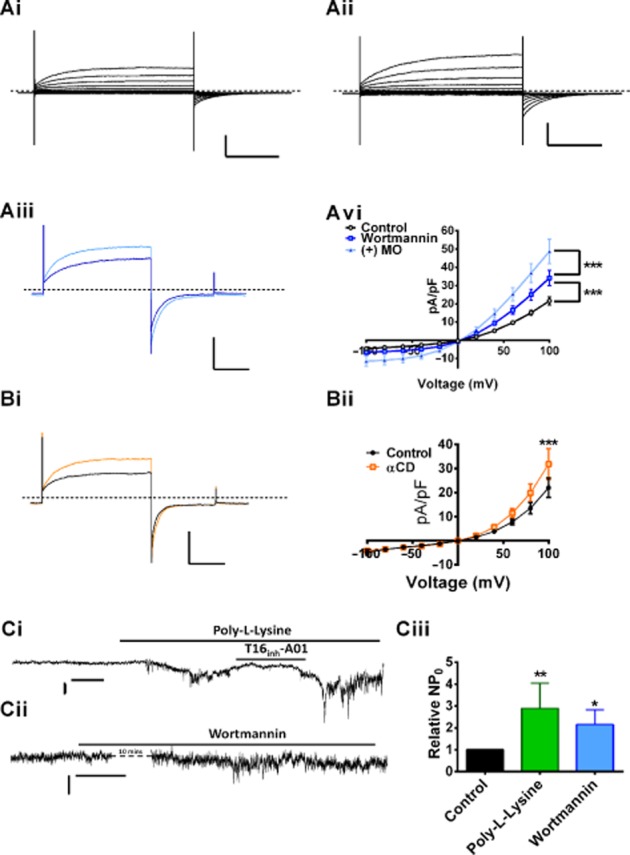
Inhibition of PI(4,5)P2 synthesis and removal of PI(4,5)P2 augments IClCa. Pretreatment of PASMC with wortmannin augmented IClCa (Aii) compared to same-day controls (Ai, n = 9, ***P < 0.001). Further application of 1 μM methoxamine (MO) augmented IClCa in 20 μM wortmannin-treated cells significantly (Aiii, n = 4, P < 0.001). Whole cell horizontal bars and vertical bars represent 20 pA·pF−1 and 500 ms. (B) Application of 3 mg·mL−1 αCD also augmented IClCa, within 5 min (B, n = 5, ***P < 0.001). Horizontal and vertical scale bars represent 500 ms and 200 pA, respectively, dashed line represents 0 pA. (Ci) Application of 50 μg·L−1 poly-l-lysine increased IClCa in excised patches, which was attenuated by subsequent application of 10 μM T16inh-A01 (n = 5). IClCa was also augmented by the application of 20 μM wortmannin. (Ciii) The mean relative NP0 for currents activated by 500 nM Ca2+ under control conditions and in the presence of poly-l-lysine (n = 9, **P < 0.01) and 20 μM wortmannin (n = 6, *P < 0.05). Single channel horizontal bars and vertical bars represent 0.2 pA and 20 s.
Figure 4.
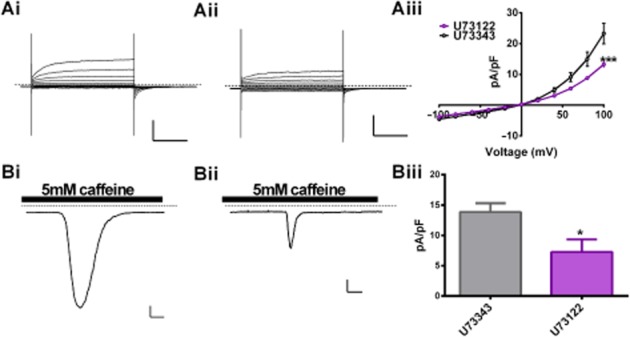
Phospholipase C inhibition attenuated IClCa. (Representative IClCa evoked in PA cells incubated in 2 μM U73343 (Ai) or 2 μM U73122 (Aii) and the mean data are shown in (Aiii) with same-day control (Ai) (n = 7, ***P < 0.001). I–V horizontal bars and vertical bars represent 20 pA·pF−1 and 500 ms. (B) Representative currents recorded with the perforated patch configuration after application of 5 mM caffeine in the presence of U73433 (Bi) or U73122 (Bii) for 10 min. Mean data for four such experiments are shown in (Biii, *P < 0.05). Perforated patch horizontal bars and vertical bars represent 2 pA·pF−1 and 2 s. Dashed line represents 0 pA.
PLC inhibition attenuates IClCa
Having shown that modulation of PI(4,5)P2 levels altered IClCa activity, we then looked at whether PI(4,5)P2 hydrolysis was obligatory for CACC gating per se. To do this, we utilized two different, Gq independent methods to elicit IClCa that should theoretically not involve PLC activation. Pipette solutions containing 500 nM free [Ca2+] generated IClCa that were attenuated by ∼45% after 10 min of incubation with the PLC inhibitor U77312 (2 μM) compared to the inactive analogue U77343 (2 μM) (Figure 4A; n = 6, P < 0.001). In perforated patch recordings, 5 mM caffeine was used to activated ryanodine receptors and increase intracellular Ca2+. This produced robust, transient inward currents at −50 mV that were attenuated by T16inh-A01 and were also sensitive to and reduced by ∼50% on incubation with U73122 (Figure 4B, n = 4, P < 0.05). These data suggest that removal of PI(4,5)P2 may contribute to the inherent gating mechanism of CaCCs in rat PA smooth muscle cells.
Discussion
The present study shows for the first time that PI(4,5)P2 plays an inhibitory role in native CaCC activity. This is based upon many lines of evidence. (i) Agents that reduce PI(4,5)P2 levels through different routes [PLC activation, PI4K inhibition, PI(4,5)P2 scavenging and absorption], all increased IClCa evoked by solutions containing clamped-free [Ca2+]. (ii) Enrichment of activating solutions with PI(4,5)P2 inhibited IClCa in PA smooth muscle cells. (iii) PI(4,5)P2 and other phospholipids were shown to bind directly to TMEM16A isolated from whole PA and TMEM16A-eGFP expressed in HEK cells. The caveats of these studies were that the reagents used indirectly modulate PI(4,5)P2 levels and are likely to alter other intracellular signals. More precise depletion via voltage-sensitive phosphatases used in heterologous expression systems (Falkenburger et al., 2010) would be desirable, but the fact that reagents that alter PI(4,5)P2 through very different mechanisms all produced similar enhancements of the IClCa and supplementation with diC-8 PI(4,5)P2 had opposite effects adds considerable weight to the view that PI(4,5)P2 suppresses CaCC activity. Consequently, localized hydrolysis of this phospholipid within the proximity of the CaCC may be vital for channel activity. The majority of other ion channels regulated by PI(4,5)P2 are stimulated by the phosphoinositide (Suh and Hille, 2008), so the ‘inverted sensitivity’ shown by CaCCs in rat PA is a relatively rare observation. Before this investigation, no interaction of a native CaCC with a phosphoinositide has been shown except for Ins(3,4,5,6)P4-mediated inhibition of a CaMKII-stimulated CaCC in epithelial cells (Xie et al., 1996) and stimulation of TMEM16A currents by two synthetic inositol tetrakisphosphates (Tian et al., 2013). The latter study showed no effect of wortmannin on IClCa in HEK293 cells transfected with TMEM16A, in contrast to our observed binding of TMEM16A-eGFP to concentrated phosphoinositides in ‘PIP strips’. Interestingly, our studies showed that there was no difference in dot blot signals between untransfected HEK293 cells and ones transfected with TMEM16A, consistent with PI(4,5)P2 binding saturating with the endogenous TMEM16A. Consequently, in overexpression studies, the low composition of PI(4,5)P2 in the cell membrane (2%) cannot modulate the high concentration of heterologously expressed TMEM16A protein.
Bioinformatics analysis revealed many potential candidates for PI(4,5)P2 binding sites within the mouse TMEM16A sequence (Figure 5). Being negatively charged, PIP2 would have a favourable interaction for basic residues loosely interspersed by hydrophobic residues. Based upon homology mapping with the KRWRK sequence on TRPV4, which is the PI(4,5)P2 binding site required for channel activation (Garcia-Elias et al., 2013), a PI(4,5)P2 binding motif (KRFRR) is located just after the ‘a’ splice variant domain of TMEM16A (see Figure 5). There is also a potential binding site within the ‘b’ splice variant region, which is known to greatly reduce Ca2+ sensitivity of TMEM16A (Ferrera et al., 2009). Consequently, the suppressive effect of this splice variant may reflect an inhibitory role of PI(4,5)P2 rather than direct Ca2+ binding (Ferrera et al., 2009). While it is beyond the scope of the present study, it will be the focus of future studies to define precisely the molecular architecture of the PI(4,5)P2 binding site on the TMEM16A protein.
Figure 5.
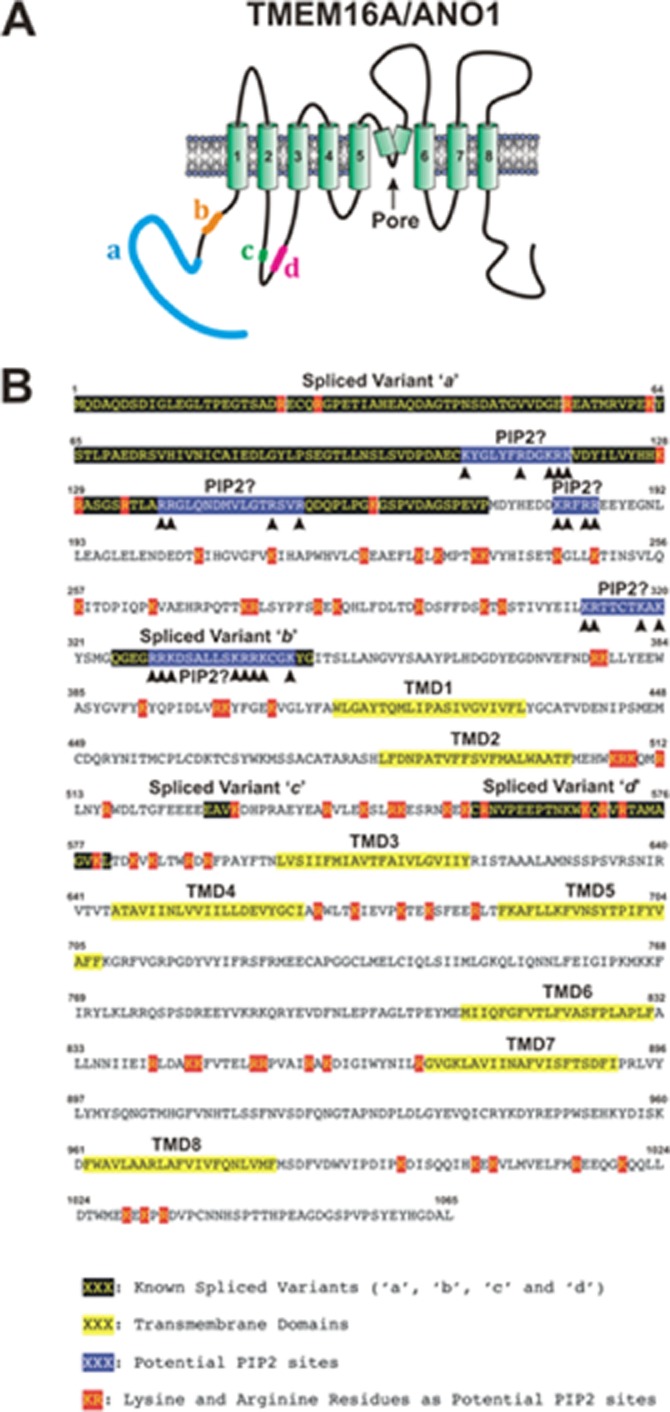
Putative PIP2 binding sites in the mouse TMEM16A full sequence (1065 amino acid; MW: 122 kDa). (A) The predicted secondary structure of the TMEM16A/ANO1 protein. The model highlights the eight transmembrane domains (TMDs), the speculated pore region lying between TMD5 and TMD6, and the position of the four known spliced variants labelled ‘a’, ‘b’, ‘c’ and ‘d’. (B) The full mouse TMEM16A sequence including the four spliced variants, the eight transmembrane domains and sequences (highlighted in blue) that are homologous to sequences previously shown to interact with PIP2 and modulate ion channel activity such as pleckstrin homology (PH) domains (Suh and Hille, 2008) or one recently shown to specifically regulate TRPV4 channels (TRPV4: KRWRK; TMEM16A: K181RFRR185) (Garcia-Elias et al., 2013). Numbers above the sequence indicate amino acid number from the C to the N terminus. Arrows in highlighted candidate PIP2 sequences show the position of arginine (R) and lysine (K) residues. Other R and K residues in all cytoplasmic domains that could potentially represent PIP2 binding sites are also highlighted.
The present study showed that PI(4,5)P2, as low as 1 μM, had a marked effect on IClCa in rat PA smooth muscle cells. This sensitivity is considerably higher than most other ion channels studied. For instance, KCNQ-encoded, voltage-gated Kv7 channels are enhanced by PI(4,5)P2 with EC50s of 2.6–215 μM for KCNQ2, KCNQ3, KCNQ2/3 heteromers and KCNQ4 (Li et al., 2004; Hernandez et al., 2008). In CHO cells, overexpressed KCNQ2 and KCNQ3 homomeric channels have EC50s of 205 and 2.6 μM, respectively, and the KCNQ2/3 heteromer EC50 of 40 μM (Li et al., 2005). PI(4,5)P2 was also required for activation of endogenous TRPC1 in vascular smooth muscle cells with an EC50 of 1–3 μM (Saleh et al., 2009). The [PI(4,5)P2] of mammalian cells is purported to be ∼ 10–210 μM (McLaughlin et al., 2002; Winks et al., 2005), which would result in complete suppression of CaCCs in arterial smooth muscle. Therefore, either [PI(4,5)P2] close to CaCCs is lower than a theoretical bulk concentration or there are local signalling complexes that modulate [PI(4,5)P2] in the proximity of the channel. Previous studies have proposed that TMEM16A proteins reside in lipid raft components of the plasma membrane in vascular smooth muscle (Sones et al., 2010) and dorsal root ganglia (Jin et al., 2013). Our finding that inhibition of PLC reduced the amplitude of IClCa evoked by either caffeine or internal perfusion with pipette solutions of known free [Ca2+], that do not involve IP3 generation, suggests that removal of PI(4,5)P2 suppression may be a fundamental aspect in CaCC gating and may even represent an alternative activation mechanism (see Figure 6). This also raises the possibility of different pools of PI(4,5)P2 and/or PLC isoforms, which are separate from those associated with Gq protein-coupled receptors. Furthermore, our experiments with proprietary phospholipid assays or ‘PIP strips’ revealed that it was not just PIP2 but all forms of phosphoinositide that interacted with TMEM16A. The effect of these other phosphoinositides on endogenous CaCCs will be the focus of future studies, but these findings suggest that regulation of CaCC activity in vascular smooth muscle is likely to be complex and multifactorial.
Figure 6.
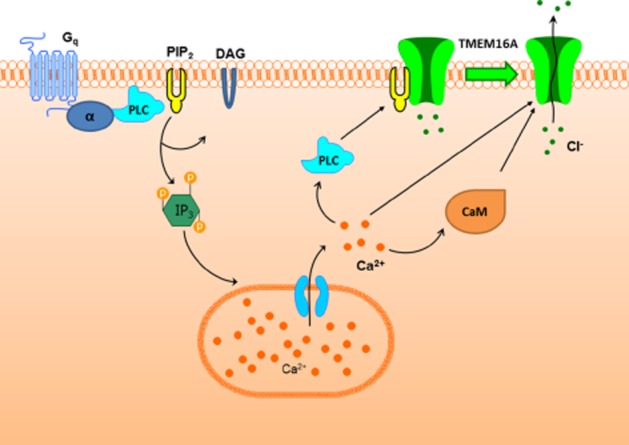
Role of PI(4,5)P2 in TMEM16A regulation. The schematic diagram shows the classical role of PIP2 as a substrate for PLC in the production of diacylglycerol (DAG) and inositol trisphosphate (IP3) via Gq activation pathway. This will lead to an increase in intracellular [Ca2+], which is known to activate TMEM16A. However, we have shown that PI(4,5)P2 is also bound to TMEM16A at rest and subsequently removed when the channel is activated. Therefore, we propose that the increase in intracellular [Ca2+] has two important effects on TMEM16A activity: (i) activates PLC to remove PI(4,5)P2 bound to TMEM16A and (ii) activates the channel either directly or indirectly via calmodulin (CaM).
It is now generally accepted that TMEM16A expression products contribute to native CaCCs in different cell types. However, there are conflicting ideas about the Ca2+-dependent gating mechanism of these channels as there is not a well-established Ca2+ binding site on TMEM16A. Some groups have identified putative Ca2+ binding sites in the first intracellular loop (Xiao et al., 2011), N-terminus (Ferrera et al., 2009) or in TM6-7 intracellular loop (Terashima et al., 2013), whereas others suggest that channel activity is regulated by the binding of Ca2+-calmodulin complex (Tian et al., 2011; Jung et al., 2013; Vocke et al., 2013). It is well established in KCNQ (Delmas and Brown, 2005) and TRPC (Kwon et al., 2007) channels that PI(4,5)P2 and calmodulin play reciprocal roles in channel activity and potentially bind at the same site. If a similar situation exists for TMEM16A, then the debate over the gating of these channels may reflect a variable binding of PI(4,5)P2 that needs to be removed and potentially be replaced by Ca2+-calmodulin. This, in turn, either activates the channel or allows Ca2+ to gate the channel directly. Overall, our data reveal that CaCC activity, certainly in vascular smooth muscle cells, is restrained by a phosphoinositide ‘brake’, and membrane receptor agonists not only provide the trigger for Ca2+, but also allow CaCC activation through PI(4,5)P2 hydrolysis.
Acknowledgments
The original non-eGFP-tagged TMEM16A mouse clone was a kind gift from Professor Lily Jan, University of California, San Francisco. Protein samples from a TMEM16A knockout mouse were kindly provided by Professor Karl Kunzelmann, University of Regensburg, Germany.
Glossary
- CaCC
calcium-activated chloride channel
- PASMC
pulmonary artery smooth muscle cells
- P(4,5)P2
phosphatidylinositol (4,5) bisphosphate
- T16Ainh-AO1
aminophenylthiazole
Author contributions
H. A. T. P.: Acquisition of data, analysis and interpretation of data, and drafting of the manuscript.
N. L., A. P. A. and I. A. G.: Analysis and interpretation of data and drafting of the manuscript.
Conflicts of interest
None.
Supporting information
Additional Supporting Information may be found in the online version of this article at the publisher's web-site:
http://dx.doi.org/10.1111/bph.12778
Figure S1 Acute dispersal of rPASMC is also sensitive to PI(4,5)P2. (A) IClCa was attenuated with inclusion of 1 μM PI(4,5)P2 (Aii) compared to same-day controls (Ai), with mean I–V shown (Aii, n = 5). (B) Application of 3 mg·mL−1 α-cyclodextrin (orange) increased IClCa after 5 min application, with mean data shown at +70 mV (n = 5).
References
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, Peters JA, Harmar AJ CGTP Collaborators. The Concise Guide to PHARMACOLOGY 2013/14: Ion channels. Br J Pharmacol. 2013;170:1607–1651. doi: 10.1111/bph.12447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angermann JE, Sanguinetti AR, Kenyon JL, Leblanc N, Greenwood IA. Mechanism of the inhibition of Ca2+-activated Cl− currents by phosphorylation in pulmonary arterial smooth muscle cells. J Gen Physiol. 2006;128:73–87. doi: 10.1085/jgp.200609507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barroso-González J, Machado JD, García-Expósito L, Valenzuela-Fernández A. Moesin regulates the trafficking of nascent clathrin-coated vesicles. J Biol Chem. 2009;284:2419–2434. doi: 10.1074/jbc.M805311200. [DOI] [PubMed] [Google Scholar]
- Bulley S, Neeb ZP, Burris SK, Bannister JP, Thomas-Gatewood CM, Jangsangthong W, et al. TMEM16A/ANO1 channels contribute to the myogenic response in cerebral arteries. Circ Res. 2012;111:1027–1036. doi: 10.1161/CIRCRESAHA.112.277145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caputo A, Caci E, Ferrera L, Pedemonte N, Barsanti C, Sondo E, et al. TMEM16A, a membrane protein associated with calcium-dependent chloride channel activity. Science. 2008;322:590–594. doi: 10.1126/science.1163518. [DOI] [PubMed] [Google Scholar]
- Chipperfield AR, Harper AA. Chloride in smooth muscle. Prog Biophys Mol Biol. 2000;74:175–221. doi: 10.1016/s0079-6107(00)00024-9. [DOI] [PubMed] [Google Scholar]
- Criddle DN, de Moura RS, Greenwood IA, Large WA. Effect of niflumic acid on noradrenaline-induced contractions of the rat aorta. Br J Pharmacol. 1996;118:1065–1071. doi: 10.1111/j.1476-5381.1996.tb15507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dam VS, Boedtkjer DM, Nyvad J, Aalkjaer C, Matchkov V. TMEM16A knockdown abrogates two different Ca(2+)-activated Cl (−) currents and contractility of smooth muscle in rat mesenteric small arteries. Pflugers Arch. 2013 doi: 10.1007/s00424-013-1382-1. doi: 10.1007/S00424-013-1382-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis AJ, Forrest AS, Jepps TA, Valencik ML, Wiwchar M, Singer CA, et al. Expression profile and protein translation of TMEM16A in murine smooth muscle. Am J Physiol Cell Physiol. 2010;299:C948–C959. doi: 10.1152/ajpcell.00018.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis AJ, Shi J, Pritchard HA, Chadha PS, Leblanc N, Vasilikostas G, et al. Potent vasorelaxant activity of the TMEM16A inhibitor T16A(inh) -A01. Br J Pharmacol. 2013;168:773–784. doi: 10.1111/j.1476-5381.2012.02199.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delmas P, Brown DA. Pathways modulating neural KCNQ/M (Kv7) potassium channels. Nat Rev Neurosci. 2005;6:850–862. doi: 10.1038/nrn1785. [DOI] [PubMed] [Google Scholar]
- Falkenburger BH, Jensen JB, Hille B. Kinetics of PIP2 metabolism and KCNQ2/3 channel regulation studied with a voltage-sensitive phosphatase in living cells. J Gen Physiol. 2010;135:99–114. doi: 10.1085/jgp.200910345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrera L, Caputo A, Ubby I, Bussani E, Zegarra-Moran O, Ravazzolo R, et al. Regulation of TMEM16A chloride channel properties by alternative splicing. J Biol Chem. 2009;284:33360–33368. doi: 10.1074/jbc.M109.046607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrest AS, Joyce TC, Huebner ML, Ayon RJ, Wiwchar M, Joyce J, et al. Increased TMEM16A-encoded calcium-activated chloride channel activity is associated with pulmonary hypertension. Am J Physiol Cell Physiol. 2012;303:C1229–C1243. doi: 10.1152/ajpcell.00044.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Elias A, Mrkonjic S, Pardo-Pastor C, Inada H, Hellmich UA, Rubio-Moscardó F, et al. Phosphatidylinositol-4,5-biphosphate-dependent rearrangement of TRPV4 cytosolic tails enables channel activation by physiological stimuli. PNAS. 2013;110:9553–9558. doi: 10.1073/pnas.1220231110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood IA, Ledoux J, Leblanc N. Differential regulation of Ca(2+)-activated Cl(−) currents in rabbit arterial and portal vein smooth muscle cells by Ca(2+)-calmodulin-dependent kinase. J Physiol. 2001;534:395–408. doi: 10.1111/j.1469-7793.2001.00395.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinze C, Seniuk A, Sokolov MV, Huebner AK, Klementowicz AE, Szijártó IA, et al. Disruption of vascular Ca2+-activated chloride currents lowers blood pressure. J Clin Invest. 2014;124:675–686. doi: 10.1172/JCI70025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez CC, Zaika O, Tolstykh GP, Shapiro MS. Regulation of neural KCNQ channels: signalling pathways, structural motifs and functional implications. J Physiol. 2008;586:1811–1821. doi: 10.1113/jphysiol.2007.148304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin X, Shah S, Liu Y, Zhang H, Lees M, Fu Z, et al. Activation of the Cl− channel ANO1 by localized calcium signals in nociceptive sensory neurons requires coupling with the IP3 receptor. Sci Signal. 2013;6:ra73. doi: 10.1126/scisignal.2004184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung J, Nam JH, Park HW, Oh U, Yoon JH, Lee MG. Dynamic modulation of ANO1/TMEM16A HCO3(-) permeability by Ca2+/calmodulin. PNAS U S A. 2013;110:360–365. doi: 10.1073/pnas.1211594110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon Y, Hofmann T, Montell C. Integration of phosphoinositide- and calmodulin-mediated regulation of TRPC6. Mol Cell. 2007;25:491–493. doi: 10.1016/j.molcel.2007.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Gamper N, Shapiro MS. Single-channel analysis of KCNQ K+ channels reveals the mechanism of augmentation by a cysteine-modifying reagent. J Neurosci. 2004;24:5079–5090. doi: 10.1523/JNEUROSCI.0882-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Gamper N, Hilgemann DW, Shapiro MS. Regulation of Kv7 (KCNQ) K+ channel open probability by phosphatidylinositol 4,5-bisphosphate. J Neurosci. 2005;25:9825–9835. doi: 10.1523/JNEUROSCI.2597-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manoury B, Tamuleviciute A, Tammaro P. TMEM16A/anoctamin 1 protein mediates calcium-activated chloride currents in pulmonary arterial smooth muscle cells. J Physiol. 2010;588((Pt 13)):2305–2314. doi: 10.1113/jphysiol.2010.189506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin S, Wang J, Gambhir A, Murray D. PIP(2) and proteins: interactions, organization, and information flow. Annu Rev Biophys Biomol Struct. 2002;31:151–175. doi: 10.1146/annurev.biophys.31.082901.134259. [DOI] [PubMed] [Google Scholar]
- Piper AS, Large WA. Multiple conductance states of single Ca2+-activated Cl− channels in rabbit pulmonary artery smooth muscle cells. J Physiol. 2003;547:181–196. doi: 10.1113/jphysiol.2002.033688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleh SN, Albert AP, Large WA. Obligatory role for phosphatidylinositol 4,5-bisphosphate in activation of native TRPC1 store-operated channels in vascular myocytes. J Physiol. 2009;587((Pt 3)):531–540. doi: 10.1113/jphysiol.2008.166678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder BC, Cheng T, Jan YN, Jan LY. Expression cloning of TMEM16A as a calcium-activated chloride channel subunit. Cell. 2008;134:1019–1029. doi: 10.1016/j.cell.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J, Birnbaumer L, Large WA, Albert AP. Myristoylated alanine-rich C kinase substrate coordinates native TRPC1 channel activation by phosphatidylinositol 4,5-bisphosphate and protein kinase C in vascular smooth muscle. FASEB J. 2014;28:244–255. doi: 10.1096/fj.13-238022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sones WR, Davis AJ, Leblanc N, Greenwood IA. Cholesterol depletion alters amplitude and pharmacology of vascular calcium-activated chloride channels. Cardiovasc Res. 2010;87:476–484. doi: 10.1093/cvr/cvq057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh BC, Hille B. PIP2 is a necessary cofactor for ion channel function: how and why? Annu Rev Biophys. 2008;37:175–195. doi: 10.1146/annurev.biophys.37.032807.125859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H, Xia Y, Paudel O, Yang XR, Sham JS. Chronic hypoxia-induced upregulation of Ca2+-activated Cl− channel in pulmonary arterial myocytes: a mechanism contributing to enhanced vasoreactivity. J Physiol. 2012;590:3507–3521. doi: 10.1113/jphysiol.2012.232520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terashima H, Picollo A, Accardi A. Purified TMEM16A is sufficient to form Ca2+-activated Cl− channels. PNAS U S A. 2013;110:19354–19359. doi: 10.1073/pnas.1312014110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas-Gatewood C, Neeb ZP, Bulley S, Adebiyi A, Bannister JP, Leo MD, et al. TMEM16A channels generate Ca2+-activated Cl− currents in cerebral artery smooth muscle cells. Am J Physiol Heart Circ Physiol. 2011;301:H1819–H1827. doi: 10.1152/ajpheart.00404.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian Y, Kongsuphol P, Hug M, Ousingsawat J, Witzgall R, Schreiber R, et al. Calmodulin-dependent activation of the epithelial calcium-dependent chloride channel TMEM16A. FASEB J. 2011;25:1058–1068. doi: 10.1096/fj.10-166884. [DOI] [PubMed] [Google Scholar]
- Tian Y, Schreiber R, Wanitchakool P, Kongsuphol P, Sousa M, Uliyakina I, et al. Control of TMEM16A by INO-4995 and other inositolphosphates. Br J Pharmacol. 2013;168:253–265. doi: 10.1111/j.1476-5381.2012.02193.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vocke K, Dauner K, Hahn A, Ulbrich A, Broecker J, Keller S, et al. Calmodulin-dependent activation and inactivation of anoctamin calcium-gated chloride channels. J Gen Physiol. 2013;142:381–404. doi: 10.1085/jgp.201311015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Yang H, Zheng LY, Zhang Z, Tang YB, Wang GL, et al. Downregulation of TMEM16A calcium-activated chloride channel contributes to cerebrovascular remodeling during hypertension by promoting basilar smooth muscle cell proliferation. Circulation. 2012;125:697–707. doi: 10.1161/CIRCULATIONAHA.111.041806. [DOI] [PubMed] [Google Scholar]
- Winks JS, Hughes S, Filippov AK, Tatulian L, Abogadie FC, Brown DA, et al. Relationship between membrane phosphatidylinositol-4,5-bisphosphate and receptor-mediated inhibition of native neuronal M channels. J Neurosci. 2005;25:3400–3413. doi: 10.1523/JNEUROSCI.3231-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Q, Yu K, Perez-Cornejo P, Cui Y, Arreola J, Hartzell HC. Voltage- and calcium-dependent gating of TMEM16A/Ano1 chloride channels are physically coupled by the first intracellular loop. Proc Natl Acad Sci U S A. 2011;108:8891–8896. doi: 10.1073/pnas.1102147108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie W, Kaetzel MA, Bruzik KS, Dedman JR, Shears SB, Nelson DJ. Inositol 3,4,5,6-tetrakisphosphate inhibits the calmodulin-dependent protein kinase II-activated chloride conductance in T84 colonic epithelial cells. J Biol Chem. 1996;271:14092–14097. doi: 10.1074/jbc.271.24.14092. [DOI] [PubMed] [Google Scholar]
- Yamazaki J, Kitamura K. Cell-to-cell communication via nitric oxide modulation of oscillatory Cl(−) currents in rat intact cerebral arterioles. J Physiol. 2001;536:67–78. doi: 10.1111/j.1469-7793.2001.00067.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang YD, Cho H, Koo JY, Tak MH, Cho Y, Shim WS, et al. TMEM16A confers receptor-activated calcium-dependent chloride conductance. Nature. 2008;455:1210–1215. doi: 10.1038/nature07313. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Acute dispersal of rPASMC is also sensitive to PI(4,5)P2. (A) IClCa was attenuated with inclusion of 1 μM PI(4,5)P2 (Aii) compared to same-day controls (Ai), with mean I–V shown (Aii, n = 5). (B) Application of 3 mg·mL−1 α-cyclodextrin (orange) increased IClCa after 5 min application, with mean data shown at +70 mV (n = 5).


