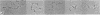Abstract
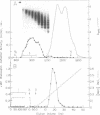
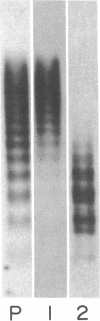
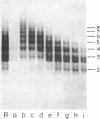
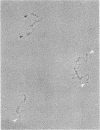
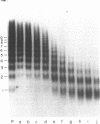
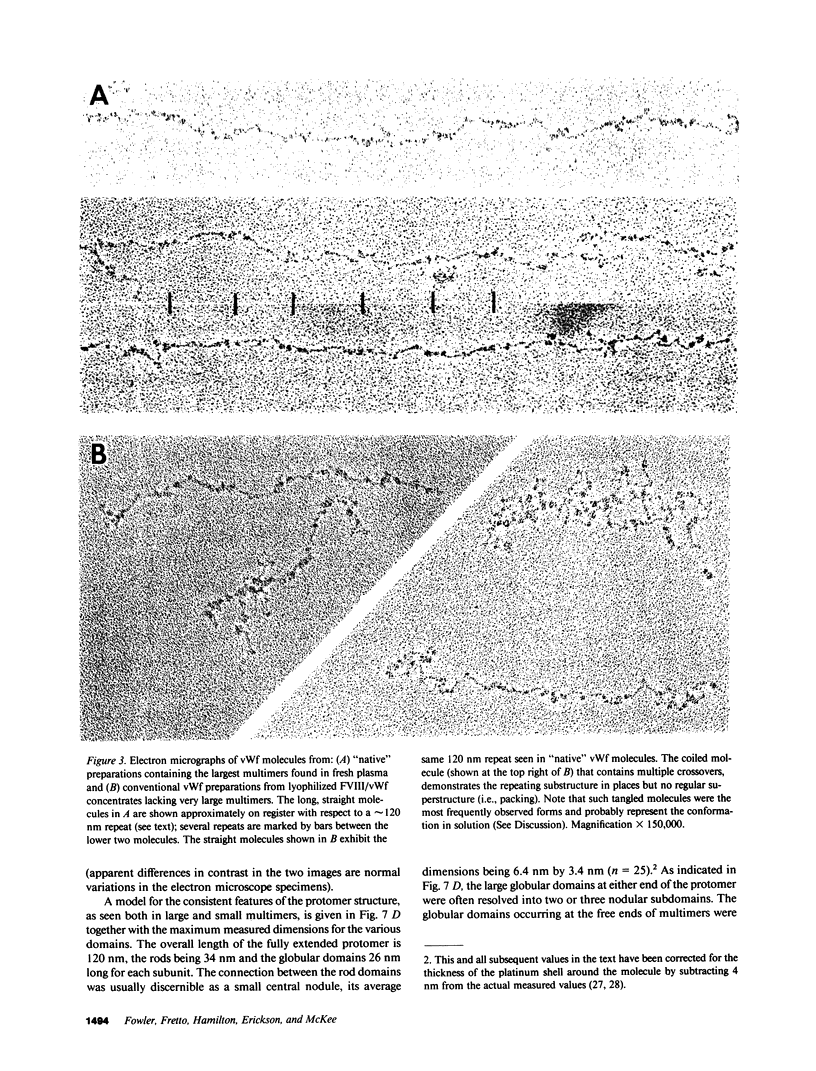
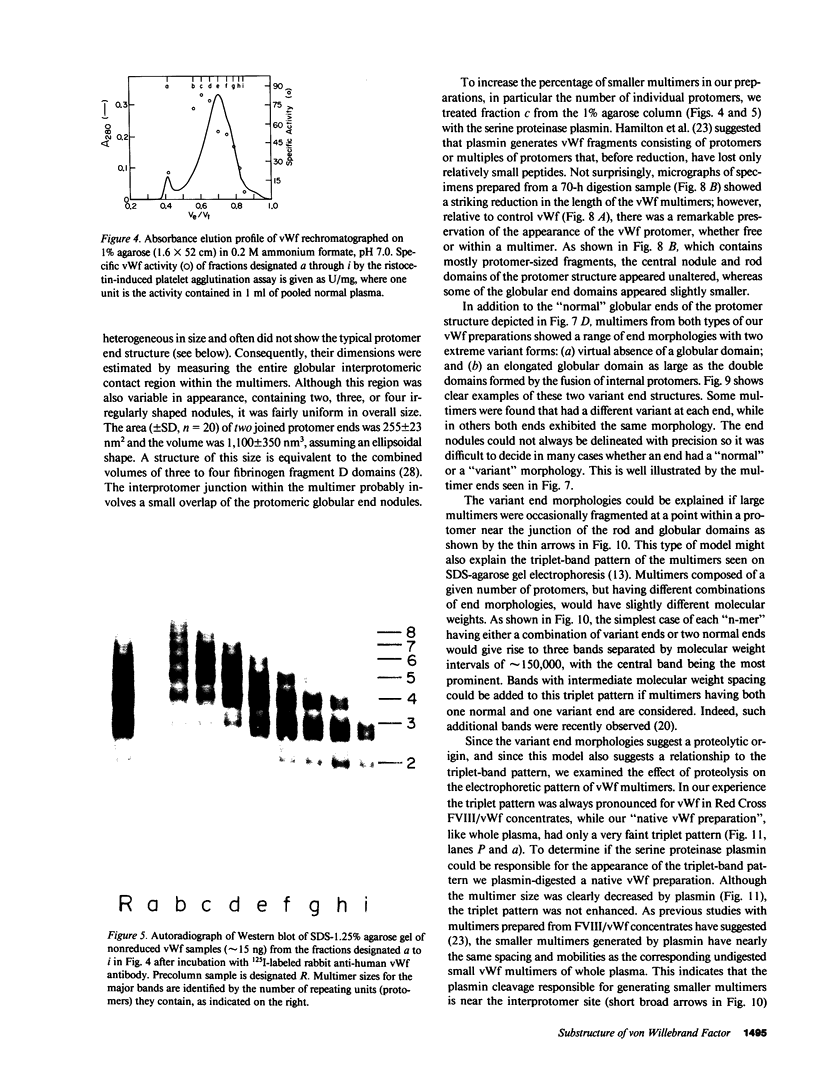
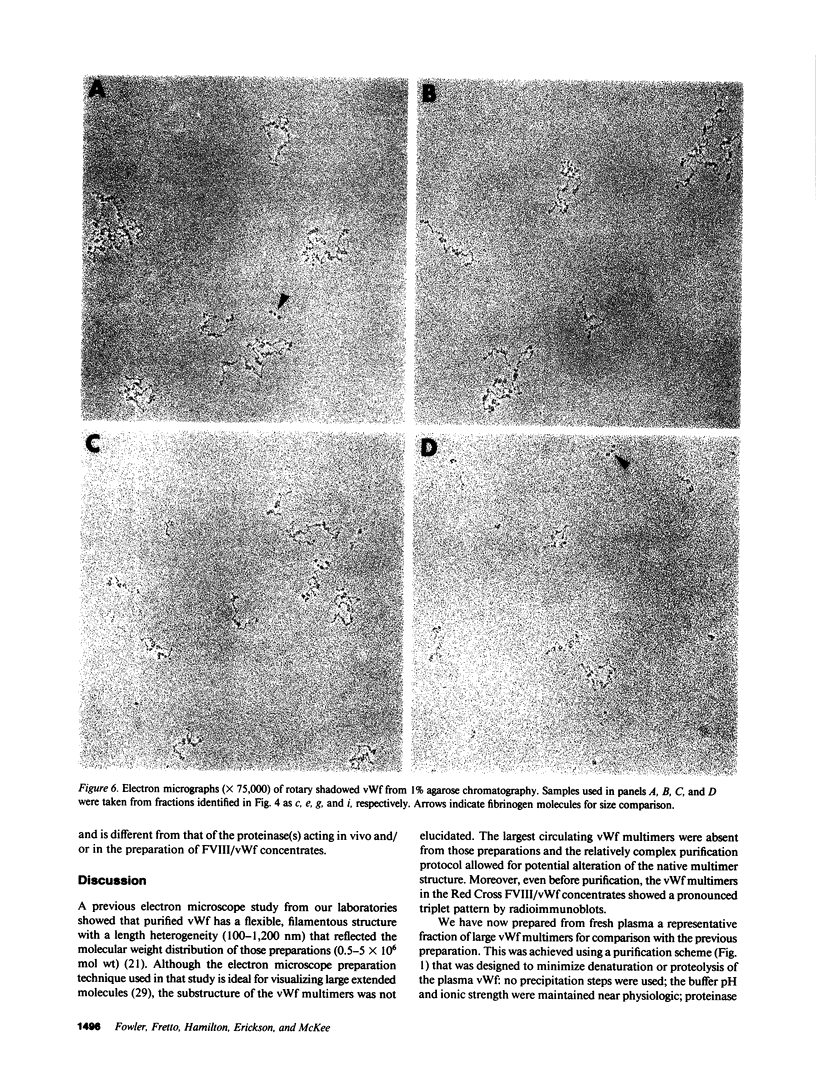
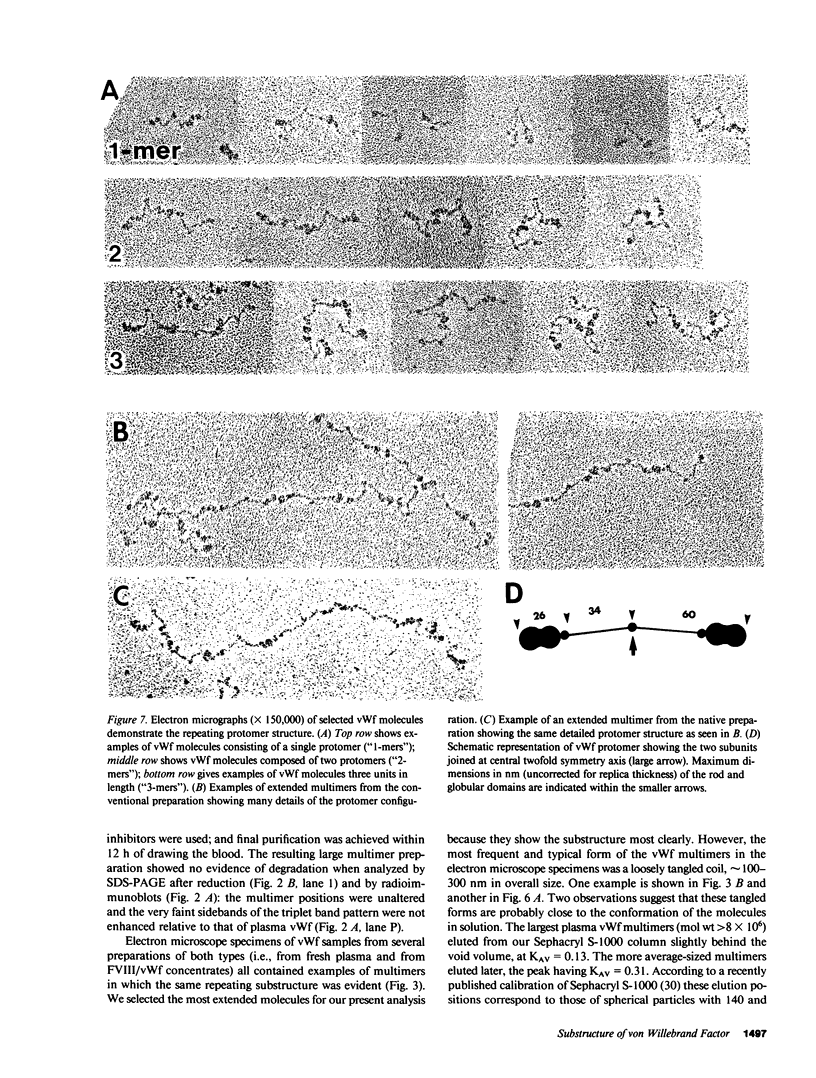
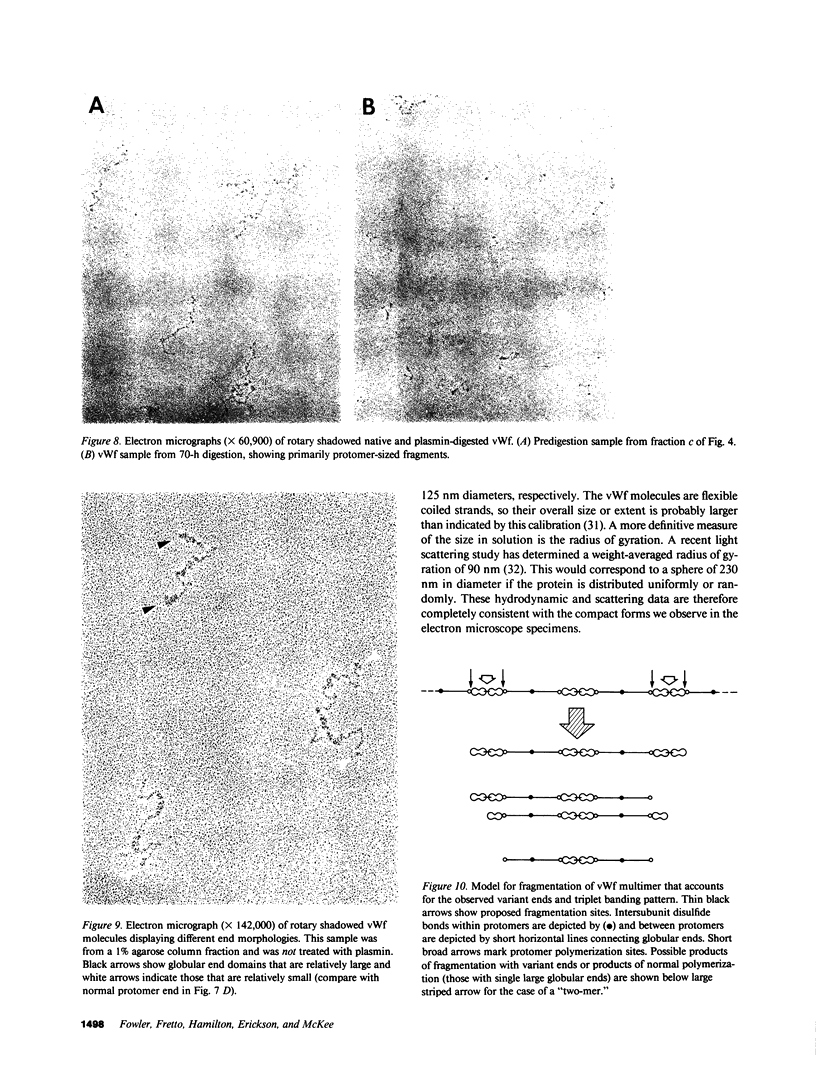
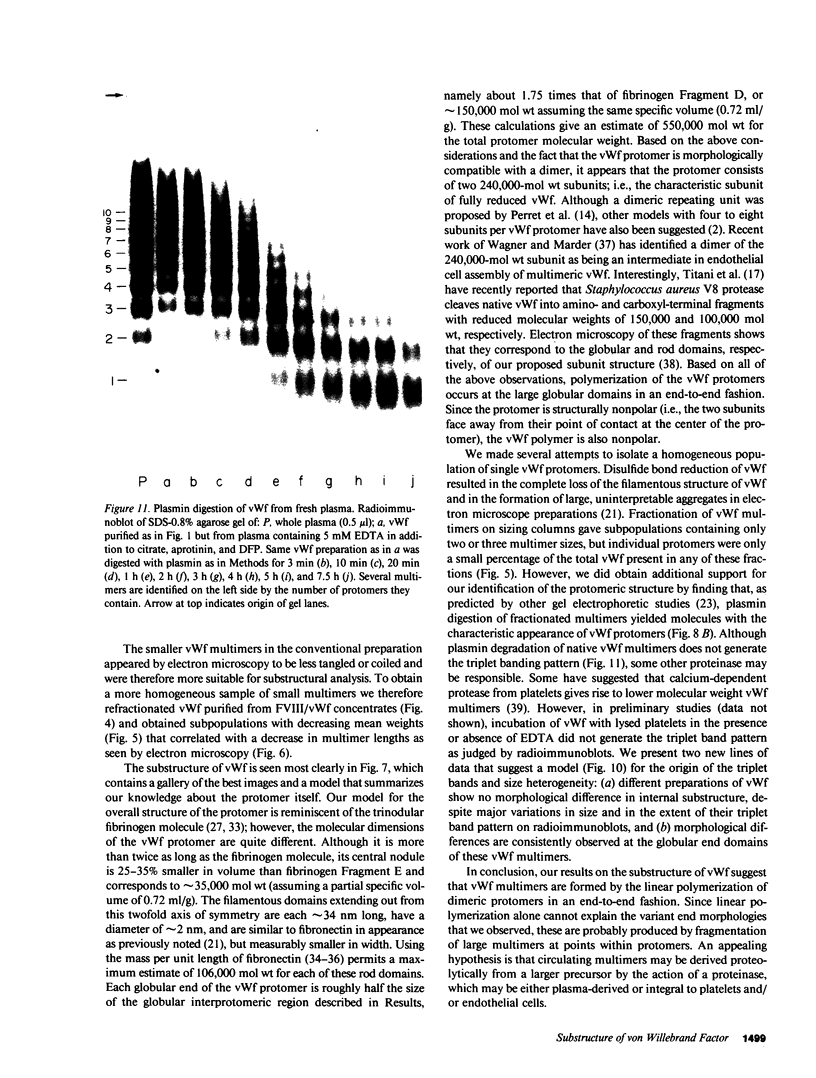
Using electron microscopy, we have visualized the substructure of human von Willebrand factor (vWf) purified by two different approaches. vWf multimers, which appear as flexible strands varying in length up to 2 micron, consist of dimeric units (protomers) polymerized linearly in an end-to-end fashion through disulfide bonds. Examination of small multimers (e.g., one-mers, two-mers, and three-mers) suggests that each protomer consists of two large globular end domains (22 X 6.5 nm) connected to a small central node (6.4 X 3.4 nm) by two flexible rod domains each approximately 34 nm long and approximately 2 nm in diameter. The protomer is 120 nm in length when fully extended. These same structural features are seen both in vWf molecules that were rapidly purified from fresh plasma by a new two-step procedure and in those purified from lyophilized intermediate-purity Factor VIII/vWf concentrates. The 240,000-mol wt subunit observed by gel electrophoresis upon complete reduction of vWf apparently contains both a rod domain and a globular domain and corresponds to one half of the protomer. Two subunits are disulfide-linked, probably near their carboxyl termini, to form the protomer; disulfide bonds in the amino-terminal globular ends link promoters to form vWf multimers. The vWf multimer strands have at least two morphologically distinct types of ends, which may result from proteolytic cleavage in the globular domains after formation of large linear polymers. In addition to releasing fragments that were similar in size and shape to the repeating protomeric unit, plasmic degradation of either preparation of vWf reduced the size of multimers, but had no detectable effect on the substructure of internal protomers.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Counts R. B., Paskell S. L., Elgee S. K. Disulfide bonds and the quaternary structure of factor VIII/von Willebrand factor. J Clin Invest. 1978 Sep;62(3):702–709. doi: 10.1172/JCI109178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel J., Odermatt E., Engel A., Madri J. A., Furthmayr H., Rohde H., Timpl R. Shapes, domain organizations and flexibility of laminin and fibronectin, two multifunctional proteins of the extracellular matrix. J Mol Biol. 1981 Jul 25;150(1):97–120. doi: 10.1016/0022-2836(81)90326-0. [DOI] [PubMed] [Google Scholar]
- Erickson H. P., Carrell N., McDonagh J. Fibronectin molecule visualized in electron microscopy: a long, thin, flexible strand. J Cell Biol. 1981 Dec;91(3 Pt 1):673–678. doi: 10.1083/jcb.91.3.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler W. E., Aebi U. Preparation of single molecules and supramolecular complexes for high-resolution metal shadowing. J Ultrastruct Res. 1983 Jun;83(3):319–334. doi: 10.1016/s0022-5320(83)90139-9. [DOI] [PubMed] [Google Scholar]
- Fowler W. E., Erickson H. P. Trinodular structure of fibrinogen. Confirmation by both shadowing and negative stain electron microscopy. J Mol Biol. 1979 Oct 25;134(2):241–249. doi: 10.1016/0022-2836(79)90034-2. [DOI] [PubMed] [Google Scholar]
- Fowler W. E., Fretto L. J., Erickson H. P., McKee P. A. Electron microsocpy of plasmic fragments of human fibrinogen as related to trinodular structure of the intact molecule. J Clin Invest. 1980 Jul;66(1):50–56. doi: 10.1172/JCI109834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gralnick H. R., Coller B. S., Sultan Y. Studies of the human factor VIII/von Willebrand factor protein. III. Qualitative defects in von Willebrand's disease. J Clin Invest. 1975 Oct;56(4):814–827. doi: 10.1172/JCI108160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HALL C. E., SLAYTER H. S. The fibrinogen molecule: its size, shape, and mode of polymerization. J Biophys Biochem Cytol. 1959 Jan 25;5(1):11–16. doi: 10.1083/jcb.5.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton K. K., Fretto L. J., Grierson D. S., McKee P. A. Effects of plasmin on von Willebrand factor multimers. Degradation in vitro and stimulation of release in vivo. J Clin Invest. 1985 Jul;76(1):261–270. doi: 10.1172/JCI111956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyer L. W., De los Santos R. P., Hoyer J. R. Antihemophilic factor antigen. Localization in endothelial cells by immunofluorescent microscopy. J Clin Invest. 1973 Nov;52(11):2737–2744. doi: 10.1172/JCI107469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyer L. W., Shainoff J. R. Factor VIII-related protein circulates in normal human plasma as high molecular weight multimers. Blood. 1980 Jun;55(6):1056–1059. [PubMed] [Google Scholar]
- Hoyer L. W. The factor VIII complex: structure and function. Blood. 1981 Jul;58(1):1–13. [PubMed] [Google Scholar]
- Jaffe E. A., Hoyer L. W., Nachman R. L. Synthesis of antihemophilic factor antigen by cultured human endothelial cells. J Clin Invest. 1973 Nov;52(11):2757–2764. doi: 10.1172/JCI107471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunicki T. J., Montgomery R. R., Schullek J. Cleavage of human von Willebrand factor by platelet calcium-activated protease. Blood. 1985 Feb;65(2):352–356. [PubMed] [Google Scholar]
- Laemmli U. K., Favre M. Maturation of the head of bacteriophage T4. I. DNA packaging events. J Mol Biol. 1973 Nov 15;80(4):575–599. doi: 10.1016/0022-2836(73)90198-8. [DOI] [PubMed] [Google Scholar]
- Legaz M. E., Schmer G., Counts R. B., Davie E. W. Isolation and characterization of human Factor VIII (antihemophilic factor). J Biol Chem. 1973 Jun 10;248(11):3946–3955. [PubMed] [Google Scholar]
- Lucas M. A., Fretto L. J., McKee P. A. The binding of human plasminogen to fibrin and fibrinogen. J Biol Chem. 1983 Apr 10;258(7):4249–4256. [PubMed] [Google Scholar]
- Mannucci P. M., Lombardi R., Pareti F. I., Solinas S., Mazzucconi M. G., Mariani G. A variant of von Willebrand's disease characterized by recessive inheritance and missing triplet structure of von Willebrand factor multimers. Blood. 1983 Nov;62(5):1000–1005. [PubMed] [Google Scholar]
- Martin S. E., Marder V. J., Francis C. W., Barlow G. H. Structural studies of the functional heterogeneity of von Willebrand protein polymers. Blood. 1981 Feb;57(2):313–323. [PubMed] [Google Scholar]
- McKee P. A. Observations on structure-function relationships of human antihemophilic/von Willebrand factor protein. Ann N Y Acad Sci. 1981;370:210–226. doi: 10.1111/j.1749-6632.1981.tb29734.x. [DOI] [PubMed] [Google Scholar]
- Merril C. R., Goldman D., Sedman S. A., Ebert M. H. Ultrasensitive stain for proteins in polyacrylamide gels shows regional variation in cerebrospinal fluid proteins. Science. 1981 Mar 27;211(4489):1437–1438. doi: 10.1126/science.6162199. [DOI] [PubMed] [Google Scholar]
- Meyer D., Obert B., Pietu G., Lavergne J. M., Zimmerman T. S. Multimeric structure of factor VIII/von Willebrand factor in von Willebrand's disease. J Lab Clin Med. 1980 Apr;95(4):590–602. [PubMed] [Google Scholar]
- Nozaki Y., Schechter N. M., Reynolds J. A., Tanford C. Use of gel chromatography for the determination of the Stokes radii of proteins in the presence and absence of detergents. A reexamination. Biochemistry. 1976 Aug 24;15(17):3884–3890. doi: 10.1021/bi00662a036. [DOI] [PubMed] [Google Scholar]
- Odermatt E., Engle J., Richter H., Hörmann H. Shape, conformation and stability of fibronectin fragments determined by electron microscopy, circular dichroism and ultracentrifugation. J Mol Biol. 1982 Jul 25;159(1):109–123. doi: 10.1016/0022-2836(82)90034-1. [DOI] [PubMed] [Google Scholar]
- Ohmori K., Fretto L. J., Harrison R. L., Switzer M. E., Erickson H. P., McKee P. A. Electron microscopy of human factor VIII/Von Willebrand glycoprotein: effect of reducing reagents on structure and function. J Cell Biol. 1982 Nov;95(2 Pt 1):632–640. doi: 10.1083/jcb.95.2.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perret B. A., Furlan M., Beck E. A. Studies on factor VIII-related protein. II. Estimation of molecular size differences between factor VIII oligomers. Biochim Biophys Acta. 1979 May 23;578(1):164–174. doi: 10.1016/0005-2795(79)90124-7. [DOI] [PubMed] [Google Scholar]
- Reynolds J. A., Nozaki Y., Tanford C. Gel-exclusion chromatography on S1000 Sephacryl: application to phospholipid vesicles. Anal Biochem. 1983 Apr 15;130(2):471–474. doi: 10.1016/0003-2697(83)90618-8. [DOI] [PubMed] [Google Scholar]
- Ruggeri Z. M., Zimmerman T. S. The complex multimeric composition of factor VIII/von Willebrand factor. Blood. 1981 Jun;57(6):1140–1143. [PubMed] [Google Scholar]
- Ruggeri Z. M., Zimmerman T. S. Variant von Willebrand's disease: characterization of two subtypes by analysis of multimeric composition of factor VIII/von Willebrand factor in plasma and platelets. J Clin Invest. 1980 Jun;65(6):1318–1325. doi: 10.1172/JCI109795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro G. A., Andersen J. C., Pizzo S. V., McKee P. A. The subunit structure of normal and hemophilic factor VIII. J Clin Invest. 1973 Sep;52(9):2198–2210. doi: 10.1172/JCI107405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sodetz J. M., Pizzo S. V., McKee P. A. Relationship of sialic acid to function and in vivo survival of human factor VIII/von Willebrand factor protein. J Biol Chem. 1977 Aug 10;252(15):5538–5546. [PubMed] [Google Scholar]
- Wagner D. D., Marder V. J. Biosynthesis of von Willebrand protein by human endothelial cells: processing steps and their intracellular localization. J Cell Biol. 1984 Dec;99(6):2123–2130. doi: 10.1083/jcb.99.6.2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman T. S., Roberts J., Edgington T. S. Factor-VIII-related antigen: multiple molecular forms in human plasma. Proc Natl Acad Sci U S A. 1975 Dec;72(12):5121–5125. doi: 10.1073/pnas.72.12.5121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman T. S., Ruggeri Z. M., Fulcher C. A. Factor VIII/von Willebrand factor. Prog Hematol. 1983;13:279–309. [PubMed] [Google Scholar]
- Zimmerman T. S., Ruggeri Z. M. Von Willebrand's disease. Prog Hemost Thromb. 1982;6:203–236. [PubMed] [Google Scholar]