Abstract
The purpose was to examine age differences and varying levels of step response inhibition on the performance of a voluntary lateral step initiation task. Seventy older adults (70 – 94 y) and twenty younger adults (21 – 58 y) performed visually-cued step initiation conditions based on direction and spatial location of arrows, ranging from a simple choice reaction time task to a perceptual inhibition task that included incongruous cues about which direction to step (e.g. a left pointing arrow appearing on the right side of a monitor). Evidence of postural adjustment errors and step latencies were recorded from vertical ground reaction forces exerted by the stepping leg. Compared with younger adults, older adults demonstrated greater variability in step behavior, generated more postural adjustment errors during conditions requiring inhibition, and had greater step initiation latencies that increased more than younger adults as the inhibition requirements of the condition became greater. Step task performance was related to clinical balance test performance more than executive function task performance.
Keywords: Balance, Aging, Gait, Posture, Executive Function, Inhibition, Biomechanics, Reaction Time
Introduction
Unintentional falls are associated with significant morbidity and mortality in individuals over the age of 65. Between 2006 and 2010, the hospitalization and fatality rate from unintentional falls doubled for each 5-year interval over the age of 65 (CDC 2012). Falls have been linked to multiple factors including diminished sensory function (vision, proprioception, and vestibular), poor sensorimotor processing, reduced strength and impaired cognition (Panel on Prevention of Falls in Older Persons and American Geriatrics Society and British Geriatrics Society 2011). Weight shifting during gait initiation, responses to perturbations, and stepping are complex tasks that require a number of these factors in everyday life. Improper weight shifting can result in loss of balance and falls, as has been documented in the long term care setting (Robinovitch et al. 2013). Thus, weight shifting can be used to gain insight into the control of balance and gait in older adults, and perhaps be a marker of changes in the postural control system.
Step initiation may provide a useful model for investigating the biomechanics of postural responses in older adults. Increased time to step may be a determinant of fall risk as the ability to respond quickly is a critical factor in arresting a fall (van den Bogert et al. 2002). In addition to typical age-related delays in voluntary step initiation times in healthy older adults compared with younger adults, (Luchies et al. 2002; Rogers et al. 2003a; Melzer and Oddsson 2004) older adults with balance impairments and higher fall risk have greater voluntary step initiation times compared with older adults without balance impairments (Medell and Alexander 2000; Lord and Fitzpatrick 2001; St. George et al. 2007) Longer step execution times are also associated with greater fall events in both single and dual task conditions (Melzer et al. 2010). Furthermore, whereas younger adults generally display consistent postural reactions across subjects, the behavior of older adults is more variable. For example, in response to destabilizing lateral surface perturbations that required a step to maintain equilibrium, older adults were more likely to generate multiple steps, use arm reactions, and have inter-limb collisions than younger adults (Maki et al. 2000; Mille et al. 2005; Mille et al. 2013).
Although posture and gait are seemingly effortless, automatic perceptual-motor processes, there are higher order processes (termed executive functions) that intervene in the presence of uncertainty or unexpected barriers. The influence of executive function processes on postural responses has been well-documented (Stelmach et al. 1989; Shumway-Cook et al. 1997; Brown et al. 1999; Redfern et al. 2001). Executive function processes also influence step initiation. For instance, the time to make a step increases when there is uncertainty about the direction of the step, or when a cognitive task is simultaneously performed (Patla et al. 1993; Lord and Fitzpatrick 2001; Brauer et al. 2002; Luchies et al. 2002; Rogers et al. 2003a; Melzer and Oddsson 2004; Melzer et al. 2007; St. George et al. 2007). In addition, several studies have detailed errors in the generation of initial postural responses when uncertainty about the appropriate postural response is created by presenting multiple stimulus-response options (Cohen et al. 2011; Sparto et al. 2013; Uemura et al. 2013a; Uemura et al. 2013b). However, the number of initial postural adjustment errors increase with age (Cohen et al. 2011). It has been suggested that deficits in inhibitory function (i.e. a component of executive function) underlie these errors (Cohen et al. 2011; Sparto et al. 2013). Because of the relationship between aging and inhibition, investigating their interaction in relation to a postural control task may help to inform us about factors that may challenge balance maintenance in older adults (Hasher and Zacks 1988; Hasher et al. 1991; Lustig et al. 2007). The study of response inhibition is also important because failing to suppress an inappropriate response may directly result in loss of balance, or indirectly by delaying the corrective response.
We have developed a voluntary step initiation task that requires stepping with increasing requirements for executive function, specifically inhibition (Sparto et al. 2013). In this task, subjects step laterally in response to a left or right pointing arrow that appears on the left or right side of a display monitor. One part is a simple reaction time (SRT) task that consists of one stimulus/response set (e.g. left arrow on left side of the monitor) with a response of stepping to the left. This simple test assesses age-related changes sensory and motor function. We also include a forced choice reaction time (CRT) task which consists of 2 stimulus/response options (i.e. left step response to a left arrow on the left side of the monitor, or, right step response to a right arrow on the right side of the monitor, randomly presented) that add decision processing requirements (Sternberg 2013). Lastly, we incorporate inhibitory functioning into the step initiation process by introducing conflict between two competing stimulus/response dimensions: 1) the direction that the arrow pointed (left or right), or 2) the location where the arrow appeared (left or right side of the monitor). Before a given block of trials, subjects are instructed to step laterally based upon the target characteristics. Thus, if the target stimulus is the arrow direction, and the competing distractor is the location of the arrow, which is incongruent with its direction (e.g. a left arrow, ←, appearing on the right side of the monitor), the subject is required to inhibit a response toward the location of the arrow. The three tasks form a continuum of requirements for executive function from basic sensory/motor function (SRT), the integration of this with a choice decision (CRT), to the inhibition of a strong sensory-motor association during choice (interference task). The test parallels a manual reaction time test of perceptual inhibition (Nassauer and Halperin 2003; Germain and Collette 2008; Jennings et al. 2011). In contrast with balance experiments that use a secondary dual task to divide attention, this study design integrates response inhibition function into the planning and execution of the postural task.
Consequently, the purpose of the research was to examine age differences and varying levels of executive function/inhibitory requirements on the performance of this voluntary lateral step initiation task. In contrast to our previous work investigating inhibition during step initiation,(Sparto et al. 2013) the sample size was increased from 40 to 70 older adults in order to better define variability in performance in older adults. In addition, the performance of younger adults was added so that we could make stronger inferences about age-related changes in inhibition. Finally, we compared the stepping performance with functional balance and neurocognitive assessment to determine the clinically-relevant correlates of step performance. The primary hypothesis was that an interaction between age and level of inhibition requirements of the step task would result in a larger increase in postural adjustment errors, onset of first postural response and foot liftoff times in older adults as the task required greater inhibitory control.
Methods
Subjects
After providing informed consent, two groups of community-dwelling, healthy adults participated in this study: an Older group (n=70, 41F, age 76 +/−5 y), and a Younger group (n=20, 10F, age 38 +/−11 y). Males and females did not differ in age within each group (p > 0.66). The subjects constituted a larger older adult sample and included a sample of younger adults compared with a previous report that introduced the experimental design (Sparto et al. 2013). After subjects provided informed consent, they underwent a comprehensive screening examination to exclude subjects with a history of neurological, cardiopulmonary, or orthopedic disease that would limit their mobility and balance function (e.g. stroke, Parkinson’s Disease, uncontrolled hypertension, significant peripheral neuropathy, significant vision loss, severe arthritis). In addition, subjects were excluded if they had impaired neurocognitive function as determined by a score more than 1.5 SDs below age-adjusted means in the Repeatable Battery for the Assessment of Neuropsychological Status (Pearson Education, San Antonio TX (Randolph et al. 1998). Approval for the study was granted by the Institutional Review Board in accordance with the ethical standards established in the 1964 Declaration of Helsinki.
Step Initiation Test Procedures
The full experimental design appears in a previous publication (Sparto et al. 2013). Briefly, subjects stood with one foot on each of two independent, side-by-side force plates (Bertec Corp. BP5050, 50 × 50 cm) and stepped laterally in response to visual stimuli that appeared on a monitor. The visual stimuli were generated by E-Prime 1.1 (Psychology Software Tools, Pittsburgh, PA). Non-slip, 10 cm diameter color-coded circles were placed on the force plates designating the starting and target step locations. The initial starting location was consistent for all subjects, with a 25 cm separation distance of the feet. The lateral distance of the target locations from the starting position was 25% of the subject’s leg length, defined as the distance from the floor to the greater trochanter. Subjects wore their own comfortable shoes. Prior to each trial, equal weight distribution on both feet was required, as measured by the force plates. Trials occurred randomly during a 4–6 s interval. Subjects were given additional verbal feedback to equalize their weight distribution as needed.
Two blocks of the simple reaction time (SRT) trials were performed, each with a single visual cue paired with a single step direction (Table 1). In one of the blocks, a left step was performed when a left pointing arrow appeared on the left side of the monitor (DL:LL, Direction Left: Location Left) in 22 of the 24 trials. In the other block, a right pointing arrow appeared on the right side of the monitor (DR:LR, Direction Right: Location Right). Each SRT block also included two catch trials in which no arrow was displayed. The color of the directional arrows was black and the size of the arrows was 4° horizontal and 2° vertical. The duration of each SRT block was 3 minutes. Data from both of these SRT blocks were combined.
Table 1.
Description of executive function task conditions, with mapping between stimulus and response.
| Condition | Stimuli per block | Display | Response |
|---|---|---|---|
| Simple Reaction Time (SRT) Blocks 1 and 2 |
1 | ||
| Block 1: Left arrow on left side |
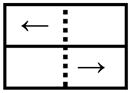
|
Step Left | |
| Block 2: Right arrow on right side | Step Right | ||
|
| |||
| Choice Reaction Time (CRT) Block 3 |
2 | ||
| Left arrow on left side |
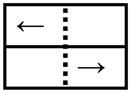
|
Step Left | |
| Right arrow on right side | Step Right | ||
|
| |||
| Perceptual Inhibition-Location (PIL) Blocks 4, 6 |
4 | ||
| CON: Left arrow on left side |
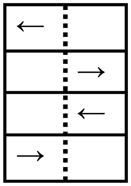
|
Step Left | |
| CON: Right arrow on right side | Step Right | ||
| INC: Left arrow on right side | Step Right | ||
| INC: Right arrow on left side | Step Left | ||
|
| |||
| Perceptual Inhibition-Direction (PID) Blocks 5, 7 |
4 | ||
| CON: Left arrow on left side |
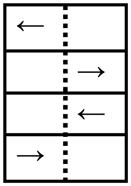
|
Step Left | |
| CON: Right arrow on right side | Step Right | ||
| INC: Left arrow on right side | Step Left | ||
| INC: Right arrow on left side | Step Right | ||
CON: Congruous Location and Direction of arrow. INC: Incongruous Location and Direction of arrow.
One block of the choice reaction time (CRT) task was performed and consisted of 2 possible visual cues (DL:LL and DR:LR) that appeared in random order. The duration of the CRT block was 4 minutes, allowing 32 steps (16 in each step direction) to be performed. Subjects were instructed to step laterally in the direction that the arrow was pointing.
Two blocks of the perceptual inhibition-location (PIL) task were performed, consisting of 4 possible visual cues presented in random order: DL:LL, DR:LR, DL:LR (Direction Left: Location Right), DR:LL (Direction Right: Location Left). Subjects were instructed to step laterally toward the side according to where the arrow was located (i.e. left or right side of the monitor), regardless of the arrow direction. Thus subjects had to inhibit a response based on the direction that the arrow was pointing. The duration of each block was 4 minutes, again allowing 32 steps to be performed in each block. Data from both PIL blocks were combined.
Two blocks of the perceptual inhibition-direction (PID) task were performed, consisting of the same four visual cues as the PIL task. However, the instructions were to step laterally in the direction where the arrow pointed, regardless of its location on the monitor. Thus subjects had to inhibit a response toward the location of the arrow. Data from both PID blocks were combined.
For the PIL and PID tasks, half of the stimuli were congruous (CON), i.e. the location of the arrow and the direction it was pointing conveyed the same meaning (e.g. DL:LL and DR:LR). The other half of the stimuli were incongruous (INC) because the location and direction provided conflicting cues for step direction (e.g. DL:LR and DR:LL). The number of CON and INC stimuli was equal because interference increases as the ratio of CON to INC trials nears 1:1 (Logan and Zbrodoff 1979; MacLeod 1991). Within each perceptual inhibition task, the CON and INC trials were processed separately as distinct conditions.
For the older adults, the order of blocks was fixed (SRT-left, SRT-right, CRT, PIL, PID, PIL, PID). In the younger adults, the perceptual inhibition blocks appeared after the SRT and CRT, but not in strict order since other blocks that were not relevant to this study were also performed.
Outcome measures
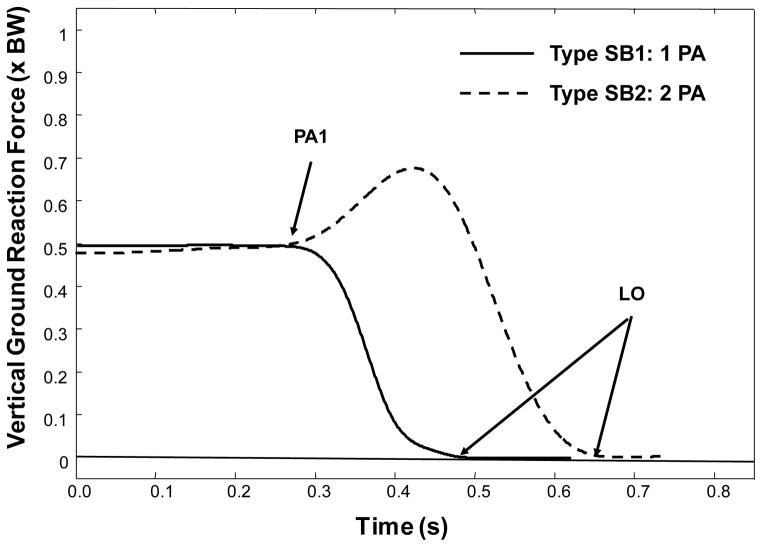
Ground reaction forces and moments were collected using the two force plates and a sampling frequency of 1000 Hz. Several critical events occur during the stepping process, and can be identified using the vertical ground reaction force data (VGRF) from the stepping leg (Figure 1) (Rogers et al. 2003a; Melzer and Oddsson 2004). For lateral steps, the VGRF time-history data assume one of two characteristic profiles.(Patla et al. 1993) Step Behavior 1 (SB1) has a single postural adjustment, where the stepping-leg VGRF unloads monotonically from 50% body weight to 0% body weight. The onset of the first VGRF deflection from 50% body weight marks the first postural adjustment (PA1); the point when the VGRF falls to 0% marks the liftoff time (LO). Detection of PA1 is accomplished by selecting the point when the derivative of the vertical ground reaction force exceeds a threshold of 2% body weight per second. Step Behavior 2 (SB2) has two postural adjustments; the VGRF initially increases, representing a loading of the stance leg, and then decreases by unloading the stepping-leg to permit step initiation. In SB2, following the initial PA1, the peak in the VGRF identifies the second postural adjustment (PA2). In some cases, additional loading and unloading deflections in the VGRF are observed before the subject eventually steps with the correct leg. As a result, each VGRF deflection point subsequent to PA1 is described as an additional postural adjustment (PA2, PA3, etc.).
Figure 1.
Classification of step behavior types based on vertical ground reaction force, relative to body weight, BW. Stimulus onset occurs at 0.0 s. The initial postural adjustment (PA1) and liftoff time (LO) are shown. Type SB1 is a step behavior characterized by a monotonic unloading from PA1 to LO. Type SB2 first loads the step limb before unloading.
PA1 measures reaction time, i.e. the instant when there is observable evidence of response to the stimulus. LO represents the functional outcome of the task; when the foot has completely lifted from the force platform, the subject has committed to taking a step in that direction. The median value of PA1 and LO were determined from all steps made in the correct direction, no matter how many postural adjustments were made, given the following restrictions. Left and right steps were combined, as side to side differences in PA1 were less than 10 ms. PA1 times quicker than 100 ms, which occurred in 0.1% of the trials, were considered to be false start errors and excluded. The median value was calculated only if there were 5 or more valid steps performed for that condition. If fewer than 5 valid steps were performed, the value was considered to be missing.
Based on previous work, each subject was classified according to his/her stepping behavior using the combined left and right SRT data (Sparto et al. 2014). Subjects who exhibited SB1 in greater than 64% of steps were designated to the SB1 group. Likewise, subjects who displayed SB2 in greater than 64% of steps were assigned to the SB2 group. Some subjects did not demonstrate a statistically significant preference for either SB1 or SB2; these subjects were categorized into step behavior group 3 (SB3). The 64% threshold criterion was based on the binomial test (α=0.05) to evaluate the likelihood of a subject primarily using SB1 versus SB2. For subjects assigned to the SB1 and SB2 groups, we computed the proportion of steps made with their preferred behavior (i.e. for a subject in SB1 group, percentage of steps made with SB1 steps), and proportion of steps demonstrating non-preferred behavior (i.e. steps with additional postural adjustments). The assumption in this article is that steps with additional postural adjustments constitute an error in stepping. It is considered to be an error because the subjects are changing their behavior or from the simple step condition, when they know which direction to step. For a subject who typically displays the SB1 direct unloading behavior, an error would be indicated by first loading the stepping leg and then unloading (i.e. 2 postural adjustments made). For a subject who normally displays the SB2 loading-unloading behavior, the subject would initially unload the stepping leg prior to executing the typical loading-unloading sequence, resulting in three postural adjustments.
Clinical Balance and Neurocognitive Assessment
In order to relate step test performance with commonly used balance and neurocognitive function measures, the older adult subjects performed the following tests: 4 m usual gait speed, Five Times Sit-To-Stand (5xSTS),(Csuka and McCarty 1985; Bohannon 1995) Four-Square Step Test (FSST),(Dite and Temple 2002) Dynamic Gait Index (DGI),(Shumway-Cook and Woollacott 1995), Digit Symbol Coding Test (DSCT) from the RBANS,(Randolph et al. 1998) the Stroop Color-Word Interference Test,(Delis et al. ; MacLeod 1991) and the Trail Making Test, Part B.(Delis et al.). The balance tests assessed walking speed, functional lower extremity strength (5xSTS), weight transfers and motor planning (FSST) and walking adaptability (DGI). The neurocognitive tests assessed different aspects of executive function, including processing speed (DSCT), selective attention/interference control (Stroop) and set shifting (Trail Making Test, Part B).
Statistical analyses
We used chi-square, Fisher’s exact and analysis of variance (ANOVA) tests to compare participant characteristics between older participant step groups. We fit a series of linear mixed models with each of the outcomes (percent preferred steps, PA1, LO) as the dependent variable; age-step behavior group (Age-SB), step task condition (Condition) and their interaction as fixed effects of interest; and an unstructured working correlation structure to account for multiple observations from same participant. For the PA1 and LO variables we used all valid steps, no matter how many postural adjustments were made. We constructed appropriate means contrasts to estimate magnitude and statistical significance of between-condition differences of each participant group, and between-group differences under each task condition. We used Pearson correlation coefficients to examine associations between step performance, and clinical balance and neurocognitive measures under each condition.
While examination of all step trials provides information about a subject’s average performance, analysis of the temporal measures based on a subject’s step behavior provides additional insight about the influence of inhibition on step initiation. We examined differences in performance during the steps in which subjects demonstrated their preferred step behavior, compared with steps in which they made additional postural adjustments (SB1: 1 PA v. greater than 1 PA; SB2: 2 PAs v. greater than 2 PAs). This analysis was limited to the PID-INC condition because it was the only condition in which there were enough trials in order to obtain stable estimates of PA1 and LO for both preferred and non-preferred step behaviors; and a similar linear mixed model was employed.
Results
Step behavior
When comparing older adults with younger adults, the preferred step behavior observed during the SRT condition was significantly associated with Age group (χ2 = 19.7, p < 0.001). All 20 younger adult subjects displayed the SB1 behavior, whereas only 31/70 (44%) of the older adults subjects clearly demonstrated the SB1 pattern. Twenty-six (37%) of the older subjects exhibited a preference for the SB2 pattern, and 13 (19%) did not have preferred step behavior during the SRT. Within the older subject group, the age of the SB1, SB2, and SB3 groups did not differ (F2,67 = 0.79, p = 0.46, Table 2). However, the older step behavior groups differed in the gender composition (χ2 = 6.4, p = 0.041). Whereas 32% of the females were classified in the SB1 group, 62% of the males had SB1 behavior.
Table 2.
Demographics, Clinical balance, Neurocognitive, and Manual Motor and Perceptual Inhibition Test Performance in the three older subject groups, classified by age-step behavior group (SB).
| Measure | Older-SB1 | Older-SB2 | Older-SB3 | p-value |
|---|---|---|---|---|
| N | 31 | 26 | 13 | - |
| Gender | 18 M, 13 F | 7 M, 19 F | 4 M, 9 F | 0.04 |
| Age1 | 75.5 (3.8) | 77.1 (6.0) | 76.3 (5.3) | 0.46 |
|
| ||||
| Clinical Balance | ||||
| Gait Speed1 | 1.30 (0.17) | 1.20 (0.21) | 1.18 (0.21) | 0.06 |
| Five Times Sit to Stand1 | 9.8 (1.7) | 12.2 (2.4) | 10.8 (2.3) | < 0.001 |
| Four-Square Step Test1 | 8.4 (1.3) | 10.1 (2.2) | 9.2 (1.4) | 0.002 |
| Dynamic Gait Index2 | 23 (20–24) | 23 (18–24) | 23 (21–24) | 0.57 |
|
| ||||
| Neurocognitive | ||||
| Digit Symbol Coding1 | 48 (9) | 44 (10) | 44 (10) | 0.25 |
| Stroop Color-Word Test2 | 55 (35 – 98) | 57 (40–105) | 59 (50 – 123) | 0.41 |
| Trail Making Test, Part B2 | 74 (41 – 207) | 77 (36 – 240) | 92 (54 – 134) | 0.09 |
Values represent Mean (SD), and p-value determined from one-way ANOVA.
Values represent Median (Range), and p-value determined from Kruskal-Wallis Test.
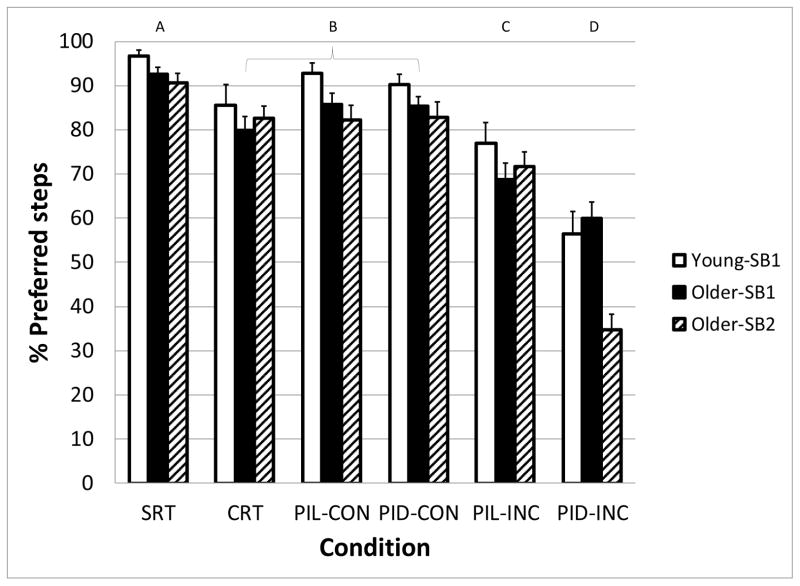
Stepping behavior changes were associated with the stimulus Condition and Age-SB group (Figure 2). There were significant main effects of Age-SB group (F2,74 = 3.8, p = 0.026), Condition (F5,74 = 58.7, p < 0.001) and a significant Age-SB*Condition interaction (F10,74 = 2.8, p = 0.005). The proportions of preferred steps in all groups were reduced under all conditions compared with the SRT baseline. The congruent PI conditions were similar to the CRT condition. The incongruous conditions significantly reduced the percent of preferred steps in all groups because of a concomitant increase in steps with additional postural adjustments. For example, the younger and older adult subjects who almost always used one postural adjustment during the SRT task now more frequently used 2 postural adjustments, while the older-SB2 group increasingly used more than two postural adjustments during the incongruous tasks. Of particular note is the reduction in percent of preferred step behaviors in the PI Direction – incongruous (PID-INC) tasks, with all means below 60 %. Also note that the SB2 group fell below 40 %. Although the Older-SB3 subjects did not have a preferred stepping behavior during the SRT, and thus we were not able to compute a proportion of preferred steps across conditions, it was evident that this group had a greater percentage of trials with 3 or more postural adjustments as the difficulty of the task increased, ranging from less than 1% in the SRT condition to 24% in the PID-INC condition (F5,12 = 8.9, p = 0.001).
Figure 2.
Percentage of step trials in which subject groups (Young-SB1, Older-SB1, and Older-SB2) displayed their preferred step behavior as a function of the task condition. Preferred step behavior was determined from the Simple Reaction Time task condition. Letters above graph indicate task conditions that are statistically different from the others. SRT: Simple Reaction Time, CRT: Choice Reaction Time, PIL: Perceptual Inhibition-Location, PID: Perceptual Inhibition-Direction, CON: Congruous trials, INC: Incongruous trials. Error bars represent standard error of the mean.
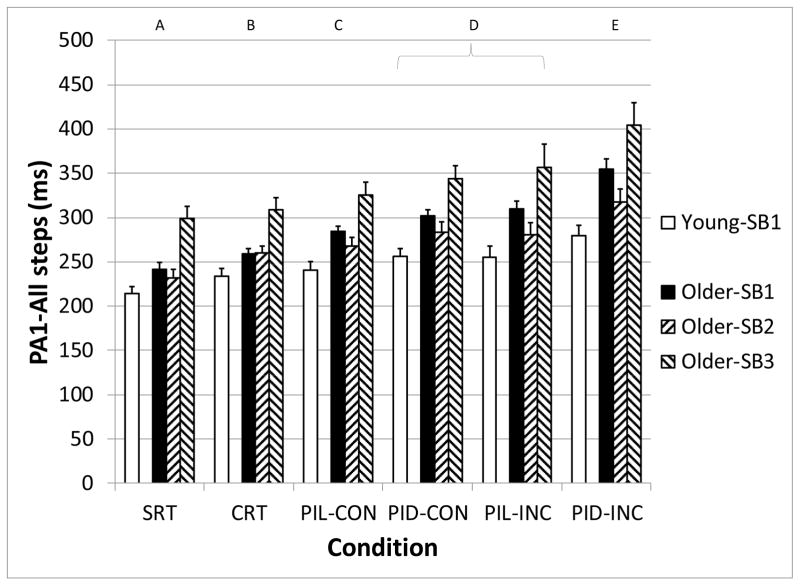
PA1 and LO – all steps
The initial postural reaction (PA1) was significantly affected by Age-SB group (F3,86 = 11.5, p < 0.001) and Condition (F5,86 = 61.3, p < 0.001), but not the Age-SB*Condition interaction (F15,86 = 1.7, p = 0.07). (Figure 3) PA1 increased as the executive function requirements grew. All conditions were significantly different, except for the PID-CON and PIL-INC conditions. Across all conditions, older adults had a mean delay in PA1 of 47 ms compared with younger adults. Post-hoc testing revealed group Older-SB3 to have greater PA1 compared with all of the subject groups, and group Older-SB1 to have greater PA1 compared with the Younger-SB1. However, Older-SB2 did not have significantly different PA1 compared with the younger adults. Although the difference in PA1 between the younger adults and older adult subgroups increased as the difficulty of the executive function task condition increased, this interaction effect did not reach the level of statistical significance.
Figure 3.
Median initial postural adjustment (PA1) for all steps, as a function of subject group (Young-SB1, Older-SB1, Older-SB2, and Older-SB3) and task condition. Letters above graph indicate task conditions that are statistically different from the others. SRT: Simple Reaction Time, CRT: Choice Reaction Time, PIL: Perceptual Inhibition-Location, PID: Perceptual Inhibition-Direction, CON: Congruous trials, INC: Incongruous trials. Error bars represent standard error of the mean.
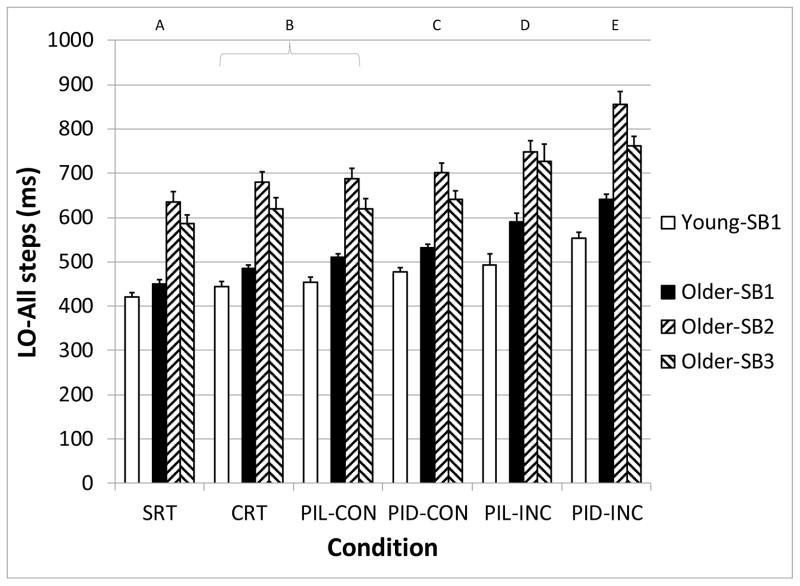
The main effects of Age-SB group (F3,86 = 45.5, p < 0.001) and Condition (F5,86 = 186.8, p < 0.001) on LO were significant (Figure 4). Post-hoc testing of the Condition effect demonstrated a progressive increase in LO from the SRT (513 ms) to the PID-INC (648 ms) condition, with significant pairwise differences between all conditions except for CRT and PIL-CON (Figure 4). The LO times of all older adults differed by approximately 150 ms across all conditions compared with younger adults. The LO times of the Older-SB2 and Older-SB3 groups were significantly greater than the Younger and Older-SB1 groups in all conditions. Additionally, the LO times of the Older-SB1 were greater than the Younger-SB1 only during the incongruous conditions of the perceptual inhibition tasks. A significant interaction between Age-SB*Condition also occurred (F15,86 = 3.4, p < 0.001), reflecting that the delay in LO in older adults compared with younger adults became progressively larger as the conditions became more difficult. The difference between Younger-SB1 and Older-SB1 increased from 29 ms in the SRT to 88 ms in the PID-INC conditions. The difference between Younger-SB1 and Older-SB3 ranged from 167 ms in the SRT to 233 ms during PIL-INC. Finally, Younger-SB1 and Older-SB2 differed in LO latency by 215 ms in the SRT and 302 ms in the PID-INC conditions.
Figure 4.
Median liftoff (LO) latency for all steps, as a function of subject group (Young-SB1, Older-SB1, Older-SB2, and Older-SB3) and task condition. Letters above graph indicate task conditions that are statistically different from the others. SRT: Simple Reaction Time, CRT: Choice Reaction Time, PIL: Perceptual Inhibition-Location, PID: Perceptual Inhibition-Direction, CON: Congruous trials, INC: Incongruous trials. Error bars represent standard error of the mean.
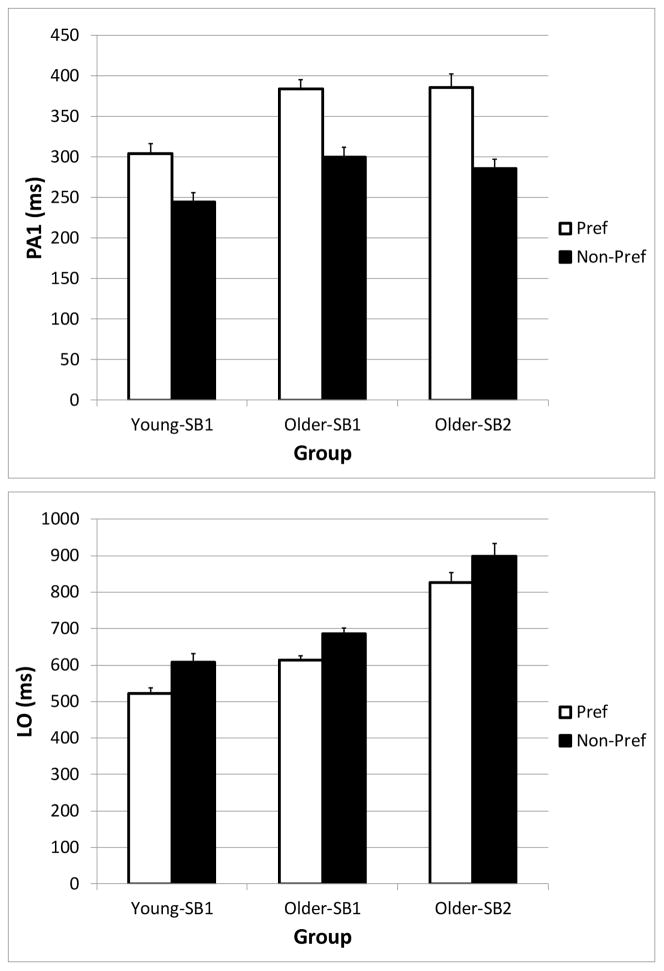
PA1 and LO – effect of step behavior
A pattern emerged during the PID-INC condition in which steps made with the non-preferred behavior occurred with an earlier PA1 latency, and a delayed LO compared with a subject’s preferred step behavior (Figure 5). The number of subjects who had to be excluded from the analysis because of not having sufficient data to compute a median value was 5/31 of the subjects in the Older-SB1 group, 4/26 of the subjects in the Older-SB2 group, and 1/20 of the subjects in the Younger-SB1 group. The linear mixed model showed significant Age-SB group (F2,74.8 = 9.9, p < 0.001) and Step Behavior main effects (F1,67.8 = 243.8, p < 0.001) on PA1. A significant interaction (F2,67.9 = 4.7, p = 0.013) indicated that the reduction in PA1 during the non-preferred steps was greater in the Older-SB2 group (−101 ms) compared with the Younger-SB1 group (−60 ms). The reduction for Older-SB1 was −86 ms. Because the non-preferred steps had additional postural adjustments, those steps resulted in greater LO times. While there were significant main effects of Age-SB group (F2,74.6 = 51.8, p < 0.001) and Step Behavior (F1,67.5 = 107.7, p < 0.001), the interaction was not significant (F2,67.6 = 0.5, p = 0.60), indicating that the increase in LO was similar across subjects groups (+72 to 86 ms). Although the data of Older-SB3 could not be used in the statistical analysis, we found an approximate 100 ms reduction in PA1 during the steps with multiple postural adjustments, compared with one PA.
Figure 5.
Differences in the initial postural adjustment (PA1, top) and liftoff (LO, bottom) for trials in which subject groups (Young-SB1, Older-SB1, and Older-SB2) made a correct initial postural adjustment (Preferred), and trials in which subjects made a postural adjustment error (Non-Preferred), during the Perceptual Inhibition-Direction incongruous condition. Error bars represent standard error of the mean.
Because the more difficult conditions contained more trials with postural adjustment errors that had an earlier PA1 latency, it must be noted that performance differences between older and younger adults as the difficulty of the task increased may have been muted somewhat when we performed the original analysis of all steps. Analysis of preferred step responses only (i.e. when the subjects made no errors) resulted in a significant interaction between Age-SB and Condition (F10,79.1 = 4.2, p < 0.001). The delays in PA1 between Younger-SB1 and Older-SB1 increased from 29 ms in the SRT to 81 ms in the PID-INC conditions. Likewise, the PA1 difference between Younger-SB1 and Older-SB2 ranged from 11 ms in the SRT to 79 ms in the PID-INC condition.
Association between step performance, clinical balance and neurocognitive measures
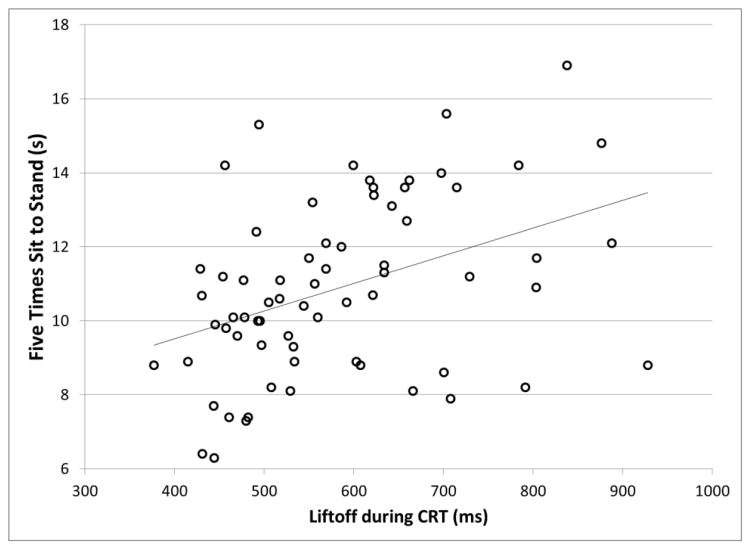
The step behavior group was associated to a greater extent with clinical balance measures than with the neurocognitive measures (Table 2). In particular, during the 5xSTS and the FSST, Older-SB2 subjects were significantly slower than Older-SB1 subjects. Older-SB3 subjects had values between the other groups. The clinical balance measures were significantly correlated with the LO times of all the conditions (0.28 < r < 0.44, p < 0.02), such that worse performance on the clinical balance measures was related to longer LO (Figure 6). Of the clinical balance measures, the 5xSTS test generally had the highest correlation with LO. The only significant relationship found between the clinical balance measures and PA1 was between gait speed and PA1-SRT (r = −0.25; p = 0.039). Neurocognitive measures were not associated with step performance for the most part. The DSCT results had a significant correlation with LO-SRT and LO-PID-INC times (r = 0.25, p = 0.035), indicating that worse performance on the DSCT was related to longer liftoff times. Tests of inhibition (Stroop) and set-shifting were not significantly associated with PA1 and LO.
Figure 6.
Association between liftoff latency during performance of the Choice Reaction Time (CRT) step task, and clinical balance performance measured by the Five Times Sit to Stand test. Pearson r = 0.40, p = 0.001.
Discussion
Main findings
The results demonstrated that, unlike Younger adults who consistently use a SB1 stepping behavior during simple reaction time step tasks, Older adults do not form a homogeneous group with regard to step behavior strategies during SRT tasks. Changes in step behavior (i.e. the proportion of preferred steps) occurred depending upon the demands of the stimulus-response condition. Incongruous stimulus-response conditions (directional arrows and screen locations being incongruous) increased the number of postural adjustments during steps, presumably due to an initial erroneous movement that is then corrected in subsequent adjustments. The Older-SB2 group was particularly affected by the incongruous arrow direction condition (PID-INC), which required inhibition of a response toward an irrelevant stimulus location, with postural adjustment errors during 60 % of the trials. In steps without postural adjustment errors, all three older adult groups experienced greater delays relative to the younger adults in the initial postural adjustment and foot liftoff as the executive function requirements of the task increased, in particular during the PID-INC condition, which had the greatest inhibition challenge. Step performance was more related with clinical balance test performance than standard tests of executive function.
Step behavior
The greater variability in stepping behaviors demonstrated by older adults (i.e. SB1, SB2, and SB3 subgroups in Older adults compared with SB1 group only in Younger adults) indicated that a simple comparison of older and younger adults may not be sufficient for understanding the complexity of balance control in older adults. Often, studies will create sub groupings of older adults based on presence of fall risk factors or clinical disease groupings (Maki et al. 1994; Brauer et al. 2002; St. George et al. 2007; Hass et al. 2008). However, in this study and a previous report,(Sparto et al. 2014) we found meaningful groupings based on a simple analysis of postural adjustments in healthy community dwelling adults.
Older adults clearly differed in step behavior strategies during the SRT condition. Some had one postural adjustment, similar to younger adults. Other older adults had two postural adjustments, which included a loading of the stepping leg prior to the step. A third smaller group was inconsistent in their stepping behavior. These behaviors did not appear to be directly related to chronological age, indicated by the relatively equal mean age across groups (approximately 76 years). On the other hand, there was a significant gender effect, resulting from a greater proportion of females demonstrating SB2 and SB3 responses compared with males. It is not likely that females had a fundamentally different step behavior than males, since in younger adults, all of the females had the SB1 behavior. However, in older adults, gender may reflect a proxy measure of some other variable that influenced step behavior. For example, differences in step behavior may reflect differences in lower extremity strength and power (Mille et al. 2005). Individuals with reduced strength and power may not be able to quickly abduct the stepping leg when stepping laterally, and thus may need to load the stepping leg to shift the center of mass toward the stance leg in a more stable position. In fact, a balance measure considered to represent functional lower extremity strength, the 5xSTS test,(Guralnik et al. 1994; Brown et al. 1995; Lord et al. 2002) was found to vary significantly amongst the groups (F2,67 = 9.8, p < 0.001). In particular, the time to complete the task was significantly worse in the SB2 group compared with the SB1 group (12.2 v. 9.8 s, p < 0.001). The SB3 group had intermediate times (10.8 s). Thus, reduced strength may be one factor contributing to the differences in step behavior. The Older-SB2 subjects also performed the FSST more slowly than the Older-SB1 group. The FSST involves elements of motor planning and quickly transferring weight, both of which play critical roles in determining step behavior. Furthermore, both the 5xSTS and FSST have been shown to identify individuals who have fallen multiple times,(Dite and Temple 2002; Tiedemann et al. 2008) and preliminary evidence suggests that step behavior type during the SRT condition may identify fallers as well (Sparto et al. 2014). Others have shown that increased step execution time is related to increased falls, and increased injurious falls (Melzer et al. 2009; Melzer et al. 2010).
A change in a subject’s step behavior during conditions that required increased decision processing and inhibition suggests that these cognitive functions are involved in stimulus-response stepping. Increasing the decision requirements (such as in the CRT and congruous tasks) caused a similar increase in number of postural adjustments in both younger and older adults, suggesting that the relatively simple process of a forced choice decision (having 2 possible outcomes) is preserved in older adults. When incongruous tasks were introduced, an additional increase in postural adjustments was also seen in both groups. In particular, the PID-INC condition produced 40–65% postural adjustment errors, which was a significantly greater amount compared with the PIL-INC condition. Not only was it more difficult for all subjects to inhibit responses toward irrelevant stimulus location cues (compared with irrelevant direction cues), Older-SB2 subjects had particular difficulty with this, as there was a marked difference in the error rates of Older-SB2 subjects (65%), compared with the Older-SB1 (40%) and Younger-SB1 subjects (44%).
The relative increase in postural adjustments between age and stepping groups for the tasks inducing interference (PIL and PID incongruous) seems best understood as the consequence of task demands for inhibitory executive function and aging decrements in this function. The examination of individual differences in executive function has established first that executive functions are only required when well-learned responses no longer meet task requirements and second that multiple executive functions exist and are only modestly—correlated even within those broadly labeled as inhibition (Miyake et al. 2000; Miyake and Friedman 2012). Relative to our design the successful execution of a CRT task was relatively well-learned and seemed likely not to have required intervention of an executive function. In contrast, the incongruous task presentations directly called for incorrect responses to be inhibited. Performance specifically on these tasks showed greater age/stepping group differentials; aligning with the literature showing aging deficits in inhibitory function (Hasher et al. 1991; Lustig et al. 2007). Thus, this stepping task may be a good measure of inhibitory functional changes that have influence on locomotion and postural control. Another benefit of this task is that despite making errors in postural adjustments, subjects completed the task of stepping in the correct direction without knowledge of the making the postural adjustments errors in most cases. As a result, assessment of inhibition could proceed with subjects using feedback to improve performance.
The increase in postural adjustments during the non-SRT trials are believed to be due to errors in response inhibition. Similar postural adjustment errors have been observed previously during forward stepping when subjects were unsure about which limb to step with during choice reaction time step tasks (Patla et al. 1993; Jacobs and Horak 2007; Cohen et al. 2011). The error rates for the Younger-SB1 and Older-SB1 groups are consistent with error rates produced in younger adults during performance of forward stepping, where the direction of forward step was cued by directional arrows embedded in congruous and incongruous flankers (Uemura et al. 2013a; Uemura et al. 2013b). Specifically, the error rate when incongruous flankers were displayed was approximately 30% greater than the error rate when congruous flankers were shown. What could explain the presence of additional postural adjustments? A likely possibility is that the subject made an error in the initial direction to step, which would result in one additional postural adjustment (i.e. a loading of the stepping leg for the SB1 group, and an initial unloading of the stepping leg for the SB2 group). More concretely, a relatively automatic, fast response to an incongruous spatial position when the arrow indicated the correct response would result in step initiation aimed in the wrong direction and hence requiring inhibition. The observation that older subjects made 2 or more additional postural adjustments more frequently during the incongruous conditions provides additional evidence of the complex interactions between executive function and postural control. The 13 Older-SB3 subjects also were susceptible to the incongruous conditions in that their frequency of steps with three or more postural adjustments, which undeniably indicated errors in step performance, increased. However, their mixture of step behaviors makes it difficult to specifically place their performance on a single continuum with SB1 and SB2.
PA1 and LO – all steps
The analysis of PA1 of all steps made in the correct direction, including steps made with an error in postural adjustments, revealed typical age-related delays between the younger adults and the full sample of older adults. The smallest difference between Younger and all Older subjects, at 34 ms, occurred during the SRT condition. This delay is consistent with several other studies that have measured age-related differences in step initiation, which were approximately 30 ms for comparable tasks.(Rogers et al. 2001; Tseng et al. 2009) The delay in older adults during SRT reflected age-related changes in sensorimotor speed and central processing, although the contributions of each could not be resolved. In addition, we found progressive delays in PA1 that were dependent on the task condition. These delays followed expected patterns based on the choice and inhibition requirements of the task. For instance, the CRT task, which had an additional stimulus/response option compared with the SRT task, generated PA1 delays of about 20 ms in both younger and older adults, respectively. The lack of an age effect on PA1 times between the SRT and CRT conditions supports the finding of no age-related change in error rate for these conditions, again suggesting that the executive function component that is engaged in this two forced choice paradigm is preserved in this group of older adults. The congruous trials of the perceptual inhibition location and direction tasks produced even greater PA1 compared with the CRT that can be explained by the presence of two additional visual stimuli during the tasks and the mixture of incongruous trials.
The increase in PA1 in the incongruous trials of the PIL and PID tasks, compared with the congruous trials of the very same tasks reflected the influence of inhibition on the generation of the step initiation responses. It was during the incongruous conditions that the age-related influence of inhibition on step responses was most evident. Whereas the delay in PA1 between older and younger adults was 34 ms during the SRT condition, the delay during the PID-INC condition increased to about 80 ms for the steps without postural adjustment errors, indicating substantially greater central nervous system processing time in selecting the correct response in older adults. Furthermore, we observed greater evidence of impairment in inhibition of older adults, specifically those in group Older-SB2, reflected by a faster response in PA1 during steps with postural adjustment errors, compared with other groups.
The PID-INC condition appeared to impose a greater load on inhibitory function than the PIL-INC condition, based on a reduction in the number of preferred steps and delay of the PA1 onset. In a similar manner to the way that one must inhibit a pre-potent reading response to name colors during the Stroop test, stepping in the direction of the spatial location of the arrow stimulus appears to take precedence over responding to the direction of the arrow. Thus inhibition of this more potent response toward the spatial location is more difficult in the PID-INC condition. This finding may be related to the Simon effect, which is the tendency to respond more quickly toward the spatial source of a stimulus.(Simon 1969)
When considering older adults as a whole group, the pattern of findings observed relative to the interaction between age and executive function on PA1 was also seen for LO; the significant difference in LO between younger adults and older adults that existed during the SRT became larger as the executive function requirements became larger. The explanation for this finding is simple. Because older adults generated more errors than younger adults as the executive function task difficulty increased, and because additional postural adjustments add to the LO, the result was an increasing rise in older adult LO compared with younger adults from the SRT to the PID-INC. However, examination of the LO values in the distinct older subject groups provides a stark contrast to the PA1 results. Although the Older-SB2 group had shorter PA1 compared with the Older-SB1 group (albeit not statistically significant), their LO times were significantly greater than the Older-SB1 group, averaging about 184 ms across all conditions. Thus, the step behavior of the Older-SB2 group dictated longer functional step outcomes. Meanwhile, even though group Older-SB3 had longer PA1 compared with both of the other older adult groups, their LO times were intermediate between the other older adults, because they had more steps with single postural adjustments compared with the Older-SB2 group.
As mentioned previously, increased error rates observed in the Older-SB2 group during the PID-INC conditions appear to have resulted from faster PA1 during these steps,(Uemura et al. 2013b) and suggests impairment in inhibitory function (Cohen et al. 2011). A negative outcome of this deficiency is that it delays the liftoff even more. Referring to Figure 5, a reduction in PA1 during the postural adjustment error trials of 100 ms resulted in an increase in liftoff of 72 ms.
The investigation of inhibition responses during postural tasks is clinically important. In a study by Schoene et al. (2014) that examined response inhibition in the context of stepping, subjects who produced more errors and delays in stepping had a greater risk of falls. (Schoene et al. 2014) Potential mechanisms for this relationship can be hypothesized. If older adults select the wrong postural responses, i.e., produce a PA error, or take longer to step, i.e., have a longer step time, the increased fall risk becomes evident. In the current study, a retrospective history of falls in the past year was not significantly related to the stepping parameters during the conditions requiring more inhibitory function even though the older adults who reported a fall in the previous year had a 73 ms delay in liftoff compared with those who did not. This lack of significance may be explained by the low rate of falls in our older population and thus inadequate power to detect differences.
Association between step performance, clinical balance and neurocognitive measures
Assessment of clinical balance and neurocognitive performance revealed that the step performance was more related to the clinical balance performance measures than isolated measures of executive function. It is possible that the contributions of the motor and balance control systems to step performance, which are also needed for high performance of the clinical balance measures, outweigh the cognitive/inhibition contributions to step performance. In particular, the 5xSTS test had the greatest correlations with the LO times, suggesting that lower extremity strength and power may be important determinants of the ability to step quickly as has been previously suggested.(Mille et al. 2005) Nonetheless, the contribution of inhibitory function to step performance was revealed by the variation in performance across the different conditions.
The lack of a relationship between step performance and neurocognitive performance is in contrast to previous large scale studies that have found relationships between impaired executive function and mobility measures such as reduced gait speed and greater fall risk (Rapport et al. 1998; Rosano et al. 2005; Atkinson et al. 2007; Persad et al. 2008; Anstey et al. 2009; Herman et al. 2010; Mirelman et al. 2012). One reason for the lack of correlation of the executive function measures and the step performance was that the subject groups, which differed in step performance, did not differ in the performance of the neurocognitive tests. Of the neurocognitive tests that were measured, the Stroop test would have been expected to have the greatest relationship with the step performance because it is a test of inhibition, but this relationship was not observed. In contrast, Cohen et al. (2011) found a significant relationship between a greater number of postural adjustment errors and worse Stroop interference, in part because of the significant difference in Stroop test performance between the young and older adults.(Cohen et al. 2011)
Conclusions
When inhibition requirements were included in the task, a significant number of responses included postural adjustment errors. This was particularly true when the location of the stimulus on the screen was incongruous and a response needed to be inhibited (PID-INC). The Older-SB1 group was most like the younger adults in terms of stepping behavior. In addition, when they made errors, the reduction in PA1 that occurred with erroneous postural adjustments was not significantly different than the reduction observed in younger adults. These findings indicate that the Older-SB1 group had relatively preserved inhibitory function, despite having typical age-related slowing of PA1 and LO.
The Older-SB2 group had a preferred method for lateral stepping that included an initial loading of the stepping leg before moving towards the intended step. This group was impacted by the PID-INC condition to a greater extent that the Younger or Older-SB1 group, resulting in a greater number of errors in the initial postural adjustment. This result may indicate more failures of inhibition of the location cue, highlighted by a greater error rate during the PID-INC condition, as well as greater discrepancy in PA1 between the preferred steps and steps with postural adjustment errors. This group did not have a timing delay in PA1 compared with the Younger-SB1, reinforcing the interpretation that the SB2 group changes were related to inhibitory functional limitations. Group SB3 did not have a clear step behavior. It is possible that they are in a period of transition from SB1 to SB2. Thus, aging does not appear to affect stepping responses uniformly. Older adults’ stepping behavior can be categorized into subgroups based upon stepping behaviors, and this categorization may be indicative of changes in cognitive function that interacts with motoric function. In the future, clinical tests may be developed that could identify subjects belonging to the SB2 group, who may benefit from step-based exercise programs.(Rogers et al. 2003b; Schoene et al. 2013)
Acknowledgments
The authors thank Susan Strelinski, James Cook, Rob Cavanaugh and Michelle Lin for their instrumental work in conducting the experiment. This research was supported by funding from the National Institutes of Health (R01 AG031118, P30 AG024827, P30 DC005205), including the Pittsburgh Claude D. Pepper Older Americans Independence Center (P30 AG024827), and the Eye and Ear Foundation.
Footnotes
Disclosure Statement
No conflicts of interest, financial or otherwise, are declared by the author(s).
References
- Anstey KJ, Wood J, Kerr G, Caldwell H, Lord SR. Different cognitive profiles for single compared with recurrent fallers without dementia. Neuropsychology. 2009;23:500–508. doi: 10.1037/a0015389. [DOI] [PubMed] [Google Scholar]
- Atkinson HH, Rosano C, Simonsick EM, et al. Cognitive function, gait speed decline, and comorbidities: the health, aging and body composition study. Journals of Gerontology Series A-Biological Sciences & Medical Sciences. 2007;62:844–850. doi: 10.1093/gerona/62.8.844. [DOI] [PubMed] [Google Scholar]
- Bohannon RW. Sit-to-stand test for measuring performance of lower extremity muscles. Percept Mot Skills. 1995;80:163–166. doi: 10.2466/pms.1995.80.1.163. [DOI] [PubMed] [Google Scholar]
- Brauer SG, Woollacott M, Shumway-Cook A. The influence of a concurrent cognitive task on the compensatory stepping response to a perturbation in balance-impaired and healthy elders. Gait & Posture. 2002;15:83–93. doi: 10.1016/s0966-6362(01)00163-1. [DOI] [PubMed] [Google Scholar]
- Brown LA, Shumway-Cook A, Woollacott MH. Attentional demands and postural recovery: the effects of aging. Journals of Gerontology Series A-Biological Sciences & Medical Sciences. 1999;54:M165–171. doi: 10.1093/gerona/54.4.m165. [DOI] [PubMed] [Google Scholar]
- Brown M, Sinacore DR, Host HH. The relationship of strength to function in the older adult. J Gerontol A Biol Sci Med Sci. 1995;50(Spec No):55–59. doi: 10.1093/gerona/50a.special_issue.55. [DOI] [PubMed] [Google Scholar]
- CDC. 2006 – 2010, United States Unintentional Fall Deaths and Rates per 100,000. 2012;2012 [Google Scholar]
- Cohen RG, Nutt JG, Horak FB. Errors in postural preparation lead to increased choice reaction times for step initiation in older adults. Journals of Gerontology Series A-Biological Sciences & Medical Sciences. 2011;66:705–713. doi: 10.1093/gerona/glr054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csuka M, McCarty DJ. Simple method for measurement of lower extremity muscle strength. Am J Med. 1985;78:77–81. doi: 10.1016/0002-9343(85)90465-6. [DOI] [PubMed] [Google Scholar]
- Delis DC, Kaplan E, Kramer JH. Delis-Kaplan Executive Function System (D-KEFS) The Psychological Corporation; [Google Scholar]
- Dite W, Temple VA. A clinical test of stepping and change of direction to identify multiple falling older adults. Archives of Physical Medicine & Rehabilitation. 2002;83:1566–1571. doi: 10.1053/apmr.2002.35469. [DOI] [PubMed] [Google Scholar]
- Germain S, Collette F. Dissociation of perceptual and motor inhibitory processes in young and elderly participants using the Simon task. Journal of the International Neuropsychological Society. 2008;14:1014–1021. doi: 10.1017/S135561770808123X. [DOI] [PubMed] [Google Scholar]
- Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. Journal of Gerontology. 1994;49:M85–94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- Hasher L, Stoltzfus ER, Zacks RT, Rypma B. Age and inhibition. J Exp Psychol Learn Mem Cogn. 1991;17:163–169. doi: 10.1037//0278-7393.17.1.163. [DOI] [PubMed] [Google Scholar]
- Hasher L, Zacks R. Working memory, comprehension, and aging: A review and new view. In: Bower GK, editor. The psychology of learning and motivation. Academic Press; San Diego, CA: 1988. pp. 193–225. [Google Scholar]
- Hass CJ, Waddell DE, Wolf SL, Juncos JL, Gregor RJ. Gait initiation in older adults with postural instability. Clinical Biomechanics. 2008;23:743–753. doi: 10.1016/j.clinbiomech.2008.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman T, Mirelman A, Giladi N, Schweiger A, Hausdorff JM. Executive control deficits as a prodrome to falls in healthy older adults: a prospective study linking thinking, walking, and falling. Journals of Gerontology Series A-Biological Sciences & Medical Sciences. 2010;65:1086–1092. doi: 10.1093/gerona/glq077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs JV, Horak FB. External postural perturbations induce multiple anticipatory postural adjustments when subjects cannot pre-select their stepping foot. Experimental Brain Research. 2007;179:29–42. doi: 10.1007/s00221-006-0763-5. [DOI] [PubMed] [Google Scholar]
- Jennings JR, Mendelson DN, Redfern MS, Nebes RD. Detecting age differences in resistance to perceptual and motor interference. Experimental Aging Research. 2011;37:179–197. doi: 10.1080/0361073X.2011.554512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan GD, Zbrodoff NJ. When it helps to be misleed: Facilitative effects of increasing the frequency of conflicting stimuli in a Stroop-like task. Memory & Cognition. 1979;7:166–174. [Google Scholar]
- Lord SR, Fitzpatrick RC. Choice stepping reaction time: a composite measure of falls risk in older people. Journals of Gerontology Series A-Biological Sciences & Medical Sciences. 2001;56:M627–632. doi: 10.1093/gerona/56.10.m627. [DOI] [PubMed] [Google Scholar]
- Lord SR, Murray SM, Chapman K, Munro B, Tiedemann A. Sit-to-stand performance depends on sensation, speed, balance, and psychological status in addition to strength in older people. Journals of Gerontology Series A-Biological Sciences & Medical Sciences. 2002;57:M539–543. doi: 10.1093/gerona/57.8.m539. [DOI] [PubMed] [Google Scholar]
- Luchies CW, Schiffman J, Richards LG, Thompson MR, Bazuin D, DeYoung AJ. Effects of age, step direction, and reaction condition on the ability to step quickly. Journals of Gerontology Series A-Biological Sciences & Medical Sciences. 2002;57:M246–249. doi: 10.1093/gerona/57.4.m246. [DOI] [PubMed] [Google Scholar]
- Lustig C, Hasher L, Zacks R. Inhibitory deficit theory: recent developments in a “new view”. In: Gorfein DS, McLeod CM, editors. Inhibition in Cognition. American Psychological Association; Washington, DC: 2007. pp. 145–162. [Google Scholar]
- MacLeod CM. Half a century of research on the Stroop effect: an integrative review. Psychological Bulletin. 1991;109:163–203. doi: 10.1037/0033-2909.109.2.163. [DOI] [PubMed] [Google Scholar]
- Maki BE, Edmondstone MA, McIlroy WE. Age-related differences in laterally directed compensatory stepping behavior. Journals of Gerontology Series A-Biological Sciences & Medical Sciences. 2000;55:M270–277. doi: 10.1093/gerona/55.5.m270. [DOI] [PubMed] [Google Scholar]
- Maki BE, Holliday PJ, Topper AK. A prospective study of postural balance and risk of falling in an ambulatory and independent elderly population. Journal of Gerontology. 1994;49:M72–84. doi: 10.1093/geronj/49.2.m72. [DOI] [PubMed] [Google Scholar]
- Medell JL, Alexander NB. A clinical measure of maximal and rapid stepping in older women.[see comment] Journals of Gerontology Series A-Biological Sciences & Medical Sciences. 2000;55:M429–433. doi: 10.1093/gerona/55.8.m429. [DOI] [PubMed] [Google Scholar]
- Melzer I, Kurz I, Shahar D, Levi M, Oddsson L. Application of the voluntary step execution test to identify elderly fallers. Age Ageing. 2007;36:532–537. doi: 10.1093/ageing/afm068. [DOI] [PubMed] [Google Scholar]
- Melzer I, Kurz I, Shahar D, Oddsson LI. Predicting injury from falls in older adults: comparison of voluntary step reaction times in injured and noninjured fallers--a prospective study. J Am Geriatr Soc. 2009;57:743–745. doi: 10.1111/j.1532-5415.2009.02198.x. [DOI] [PubMed] [Google Scholar]
- Melzer I, Kurz I, Shahar D, Oddsson LI. Do voluntary step reactions in dual task conditions have an added value over single task for fall prediction? A prospective study. Aging Clin Exp Res. 2010;22:360–366. doi: 10.1007/BF03324940. [DOI] [PubMed] [Google Scholar]
- Melzer I, Oddsson LI. The effect of a cognitive task on voluntary step execution in healthy elderly and young individuals. Journal of the American Geriatrics Society. 2004;52:1255–1262. doi: 10.1111/j.1532-5415.2004.52353.x. [DOI] [PubMed] [Google Scholar]
- Mille ML, Johnson-Hilliard M, Martinez KM, Zhang Y, Edwards BJ, Rogers MW. One step, two steps, three steps more... Directional vulnerability to falls in community-dwelling older people. J Gerontol A Biol Sci Med Sci. 2013;68:1540–1548. doi: 10.1093/gerona/glt062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mille ML, Johnson ME, Martinez KM, Rogers MW. Age-dependent differences in lateral balance recovery through protective stepping. Clinical Biomechanics. 2005;20:607–616. doi: 10.1016/j.clinbiomech.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Mirelman A, Herman T, Brozgol M, et al. Executive function and falls in older adults: new findings from a five-year prospective study link fall risk to cognition. PLoS One. 2012;7:e40297. doi: 10.1371/journal.pone.0040297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake A, Friedman NP. The Nature and Organization of Individual Differences in Executive Functions: Four General Conclusions. Curr Dir Psychol Sci. 2012;21:8–14. doi: 10.1177/0963721411429458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A, Wager TD. The unity and diversity of executive functions and their contributions to complex “Frontal Lobe” tasks: a latent variable analysis. Cogn Psychol. 2000;41:49–100. doi: 10.1006/cogp.1999.0734. [DOI] [PubMed] [Google Scholar]
- Nassauer KW, Halperin JM. Dissociation of perceptual and motor inhibition processes through the use of novel computerized conflict tasks. Journal of the International Neuropsychological Society. 2003;9:25–30. doi: 10.1017/s1355617703910034. [DOI] [PubMed] [Google Scholar]
- Panel on Prevention of Falls in Older Persons, American Geriatrics Society and British Geriatrics Society. Summary of the Updated American Geriatrics Society/British Geriatrics Society clinical practice guideline for prevention of falls in older persons. Journal of the American Geriatrics Society. 2011;59:148–157. doi: 10.1111/j.1532-5415.2010.03234.x. [DOI] [PubMed] [Google Scholar]
- Patla AE, Frank JS, Winter DA, Rietdyk S, Prentice S, Prasad S. Age-related changes in balance control system: initiation of stepping. Clinical Biomechanics. 1993;8:179–184. doi: 10.1016/0268-0033(93)90012-7. [DOI] [PubMed] [Google Scholar]
- Persad CC, Jones JL, Ashton-Miller JA, Alexander NB, Giordani B. Executive function and gait in older adults with cognitive impairment. Journals of Gerontology Series A-Biological Sciences & Medical Sciences. 2008;63:1350–1355. doi: 10.1093/gerona/63.12.1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randolph C, Tierney MC, Mohr E, Chase TN. The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS): preliminary clinical validity. Journal of Clinical & Experimental Neuropsychology. 1998;20:310–319. doi: 10.1076/jcen.20.3.310.823. [DOI] [PubMed] [Google Scholar]
- Rapport LJ, Hanks RA, Millis SR, Deshpande SA. Executive functioning and predictors of falls in the rehabilitation setting. Archives of Physical Medicine & Rehabilitation. 1998;79:629–633. doi: 10.1016/s0003-9993(98)90035-1. [DOI] [PubMed] [Google Scholar]
- Redfern MS, Jennings JR, Martin C, Furman JM. Attention influences sensory integration for postural control in older adults. Gait & Posture. 2001;14:211–216. doi: 10.1016/s0966-6362(01)00144-8. [DOI] [PubMed] [Google Scholar]
- Robinovitch SN, Feldman F, Yang Y, et al. Video capture of the circumstances of falls in elderly people residing in long-term care: an observational study. The Lancet. 2013;381:47–54. doi: 10.1016/S0140-6736(12)61263-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers MW, Hedman LD, Johnson ME, Martinez KM, Mille ML. Triggering of protective stepping for the control of human balance: age and contextual dependence. Cognitive Brain Research. 2003a;16:192–198. doi: 10.1016/s0926-6410(02)00273-2. [DOI] [PubMed] [Google Scholar]
- Rogers MW, Johnson ME, Martinez KM, Mille ML, Hedman LD. Step training improves the speed of voluntary step initiation in aging. Journals of Gerontology Series A-Biological Sciences & Medical Sciences. 2003b;58:46–51. doi: 10.1093/gerona/58.1.m46. [DOI] [PubMed] [Google Scholar]
- Rogers MW, Kukulka CG, Brunt D, Cain TD, Hanke TA. The influence of stimulus cue on the initiation of stepping in young and older adults. Archives of Physical Medicine & Rehabilitation. 2001;82:619–624. doi: 10.1053/apmr.2001.20833. [DOI] [PubMed] [Google Scholar]
- Rosano C, Simonsick EM, Harris TB, et al. Association between physical and cognitive function in healthy elderly: the health, aging and body composition study. Neuroepidemiology. 2005;24:8–14. doi: 10.1159/000081043. [DOI] [PubMed] [Google Scholar]
- Schoene D, Lord SR, Delbaere K, Severino C, Davies TA, Smith ST. A randomized controlled pilot study of home-based step training in older people using videogame technology. PLoS One. 2013;8:e57734. doi: 10.1371/journal.pone.0057734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoene D, Smith ST, Davies TA, Delbaere K, Lord SR. A Stroop Stepping Test (SST) using low-cost computer game technology discriminates between older fallers and non-fallers. Age Ageing. 2014;43:285–289. doi: 10.1093/ageing/aft157. [DOI] [PubMed] [Google Scholar]
- Shumway-Cook A, Woollacott A. Motor Control Theory and Practical Applications. Williams and Wilkins; Baltimore: 1995. [Google Scholar]
- Shumway-Cook A, Woollacott M, Kerns KA, Baldwin M. The effects of two types of cognitive tasks on postural stability in older adults with and without a history of falls. Journals of Gerontology: Series A-Biological Sciences & Medical Sciences. 1997;52:M232–240. doi: 10.1093/gerona/52a.4.m232. [DOI] [PubMed] [Google Scholar]
- Simon JR. Reactions toward the source of stimulation. Journal of Experimental Psychology. 1969;81:174–176. doi: 10.1037/h0027448. [DOI] [PubMed] [Google Scholar]
- Sparto PJ, Fuhrman SI, Redfern MS, Jennings JR, Perera S, Nebes RD, Furman JM. Postural adjustment errors reveal deficits in inhibition during lateral step initiation in older adults. Journal of Neurophysiology. 2013;109:415–428. doi: 10.1152/jn.00682.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparto PJ, Jennings JR, Furman JM, Redfern MS. Lateral step initiation behavior in older adults. Gait Posture. 2014;39:799–803. doi: 10.1016/j.gaitpost.2013.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St George RJ, Fitzpatrick RC, Rogers MW, Lord SR. Choice stepping response and transfer times: effects of age, fall risk, and secondary tasks. Journals of Gerontology: Series A-Biological Sciences & Medical Sciences. 2007;62:537–542. doi: 10.1093/gerona/62.5.537. [DOI] [PubMed] [Google Scholar]
- Stelmach GE, Teasdale N, Di Fabio RP, Phillips J. Age related decline in postural control mechanisms. International Journal of Aging & Human Development. 1989;29:205–223. doi: 10.2190/KKP0-W3Q5-6RDN-RXYT. [DOI] [PubMed] [Google Scholar]
- Sternberg S. The meaning of additive reaction-time effects: some misconceptions. Front Psychol. 2013;4:744. doi: 10.3389/fpsyg.2013.00744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiedemann A, Shimada H, Sherrington C, Murray S, Lord S. The comparative ability of eight functional mobility tests for predicting falls in community-dwelling older people. Age Ageing. 2008;37:430–435. doi: 10.1093/ageing/afn100. [DOI] [PubMed] [Google Scholar]
- Tseng SC, Stanhope SJ, Morton SM. Impaired reactive stepping adjustments in older adults. J Gerontol A Biol Sci Med Sci. 2009;64:807–815. doi: 10.1093/gerona/glp027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uemura K, Oya T, Uchiyama Y. Hum Mov Sci. 2013a. Effects of speed and accuracy strategy on choice step execution in response to the flanker interference task. [DOI] [PubMed] [Google Scholar]
- Uemura K, Oya T, Uchiyama Y. Effects of visual interference on initial motor program errors and execution times in the choice step reaction. Gait Posture. 2013b;38:68–72. doi: 10.1016/j.gaitpost.2012.10.016. [DOI] [PubMed] [Google Scholar]
- van den Bogert AJ, Pavol MJ, Grabiner MD. Response time is more important than walking speed for the ability of older adults to avoid a fall after a trip. Journal of Biomechanics. 2002;35:199–205. doi: 10.1016/s0021-9290(01)00198-1. [DOI] [PubMed] [Google Scholar]