Abstract
Anthracyclines cause severe irreversible cardiac toxicity. The study of changes in cardiac permeability with chemotherapy could enhance the understanding of mechanisms behind cardiac damage, and provide useful information to evaluate anthracycline cardiotoxicity. Thirty-six rats (12 Sprague-Dawley, 12 Wistar, 12 Fischer-344) were randomly assigned to control (n= 21) or doxorubicin (n = 15), and injected i.p. with a cumulative dose of 18 mg/kg doxorubicin in saline (vehicle) or vehicle over 12 days. Echocardiography was performed at baseline and on day 11. An isolated heart experiment was done on day 12 to obtain perfused heart pressure values, and to measure cardiac capillary permeability using a Texas Red/sodium fluorescein multiple indicator dilution method. Control animals had significantly lower average permeability-surface-area-products (0.035±0.013 cm3/s) than doxorubicin animals (0.066±0.023 cm3/s), PSP±SD, p<0.001. These permeability changes correlated with significant functional changes. There was a significant decline in cardiac function with a deleterious effect of chemotherapy on fractional shortening (p<0.001), left ventricular developed pressure (p<0.001), contractility (p<0.001), and relaxation (p=0.02). Based on our results, cardiac capillary permeability changes can be detected after in vivo chemotherapy treatment using our fluorescent multiple indicator dilution technique, and may provide valuable information in evaluating cardiotoxicity of novel drugs.
Keywords: Cardiotoxicity, Doxorubicin, Endothelial damage, Fluorescence
1. INTRODUCTION
Advances in early cancer diagnosis and therapy have improved the survival statistics of individuals with cancer in the last few decades. Current data reported by the American Cancer Society shows that the 5-year relative survival rate in cancer patients is 68%, or 19% higher than three decades prior.1 In children, mortality rates have dropped by more than 50% in the period between 1975 and 2010.1 These increased survival rates are bringing attention to the long-term effects of chemotherapy in children and adults, and to how chemotherapy affects organ systems.
Anthracyclines such as doxorubicin (DOX) are chemotherapy agents which are very effective in causing tumor regression or preventing tumor growth, but have serious systemic toxicity effects that constrain their use and acceptable dosages.2,3 Cardiac toxicity in particular is a major concern when using these agents. Damage caused by anthracyclines on the heart is chronic and irreversible, and can significantly impact functional ability and long-term quality of life in cancer survivors. For some patients, especially individuals with prior cardiac dysfunction and in children, chronic cardiac dysfunction after anthracycline treatment can be life-threatening.3 Anthracyclines damage myocardial tissue by producing reactive radical species which cause fibrosis, myocardial cell degeneration, cardiac dilatation, reduced contractile function, and interstitial edema, among other effects.4–7 Research efforts geared towards learning more about the mechanisms that mediate cardiac toxicity could assist in the design and screening process of new cancer drugs with reduced side effects. In turn, this could have a significant impact on the prognosis and quality of life of patients diagnosed with cancer.
Anthracyclines cause toxic side-effects in healthy cells through radical generation, oxidative stress, and damage of mitochondrial DNA by reactive species.8 Heart cells are especially affected because of their high mitochondrial content, which constitutes approximately 40% of the cell volume.4 The traditional focus of research in the field of anthracycline cardiotoxicity has been on contractile function and on the direct effect on cardiomyocytes. Wolf et al. reported that DOX also causes in vitro dysfunction in endothelial cells through the production of reactive oxygen and nitrogen species.9 Other authors have reported that DOX induces apoptosis in endothelial cells.10 Therefore, it seems that cardiotoxicity stems from a multifactorial combination of direct effects on cardiomyocytes along with indirect effects caused by damage to other cardiac structures such as the capillaries, with the resulting development of myocardial edema and dysfunction after chemotherapy. If this combination of factors is an accurate representation of the toxic environment created by DOX, administration of DOX should lead to in vivo damage in the cardiac endothelial layer, and consequently to significant changes in the permeability of the cardiac capillary endothelium. Changes in the capillary network would then compound on the direct effects of the drug on cardiac cells, and could have important implications in cardiotoxicity development and prevention. To our knowledge, there are no studies to this date on the effects of in vivo chemotherapy on cardiac capillary permeability other than those presented by our group in conference abstract form.11,12
Classical measurements of tissue permeability use radioactive indicators. Radioactive multiple indicator dilution protocols have previously been used to estimate coronary blood flow and capillary endothelial transport,13–15 and to study cardiac capillary permeability in hearts subjected to pathological stress conditions such as acidemia or ischemia/reperfusion.16,17 However, the application of these methods is limited by radiation exposure hazards. Our group has developed a fluorescent multiple indicator dilution method using Texas Red-conjugated Dextran (TR, MW 70,000 Da) as the reference dye, and sodium fluorescein (NaFL, MW 376 Da) as the diffusible dye to measure cardiac capillary permeability.11 These two dyes have been successfully used to label and track proteins such as albumin to measure capillary leakage,18 and dextrans have been used to study lung permeability after administration by infusion.19 Our group has shown that, based on their spectral features and size differential, NaFL and TR can be used in a fluorescent multiple indicator dilution method to measure cardiac capillary permeability with at least 10% reproducibility in repeat measurements.11 By comparing the output profiles of the two dyes versus time using a spectrofluorometer, we can estimate the permeability-surface-area-product (PSP) of the cardiac capillary network. The output curves of the dyes, the instantaneous extraction, and the PSP value are given by equations 1–3:
| (1) |
| (2) |
| (3) |
where h(t) is the fraction of dose per second emerging with the outflow, F is the flow of indicator containing fluid, Cout(t) represents the concentration-time curves for the indicator at the outflow, q0 is the dose of indicator injected, hD(t) is the normalized output response of the diffusible tracer, hR(t) is the normalized output response of the intravascular reference tracer, E(t) is the instantaneous extraction of the diffusible tracer, Emax is the maximum tracer extraction, and PSP is the permeability-surface-area product.15,20,21
The goal of the present work was to determine if DOX administration causes in vivo changes in cardiac capillary endothelium permeability along with contractile function changes, and whether permeability changes can be a variable of interest when determining the cardiac toxicity of a chemotherapy agent. We used a short-term rat model of cardiotoxicity and traditional measurements of cardiac function that are well established in the literature, such as echocardiography and pressure measurement in the isolated heart experiment, along with the TR/NaFL fluorescent multiple indicator dilution method for cardiac capillary permeability measurement developed by our group.
We hypothesized that the endothelial damage caused by DOX treatment would lead to increased cardiac capillary permeability in vivo consistent with other observed changes in cardiac function measured by echocardiography and pressure waveforms. Measurements of cardiac capillary permeability could thus be used as an additional data source when assessing the toxicity of novel cancer drugs. The study of changes in cardiac permeability could contribute to the understanding of the mechanisms behind cardiac damage caused by chemotherapy, and potentially provide an insight into new approaches to minimize cardiotoxicity of anthracyclines.
2. MATERIALS AND METHODS
2.1. Model of anthracycline cardiotoxicity
Our study used the rat short-term model of anthracycline cardiotoxicity that was originally proposed by Pouna et al, and subsequently followed by many other researchers.3,5,7,22–25 In this model, rats are treated over a period of twelve days with a cumulative dose of 18 mg/kg DOX, resulting in induction of detectable signs of cardiotoxicity. At the end of treatment (Pouna model day 12), an isolated heart experiment is performed to study contractility and cardiac function. For our purposes of studying cardiac capillary permeability along with heart function, the isolated heart procedure is an excellent tool because it allows control over heart rate, flow, and organ temperature, and the myocardium is perfused through the coronary capillaries.26 This ensures that permeability can be studied in an environment that mimics physiological perfusion. It also allows us to simultaneously obtain related functional measurements such as contractility under the same conditions for comparison. Another potential advantage of this method is the absence of hormonal and neural influences, so that experimental results are a direct reflection of the function of the myocardium.26
2.2. Study design
Twelve Sprague-Dawley rats, twelve Wistar rats, and twelve Fischer-344 rats (160–250 g) were purchased from Harlan (Indianapolis, IN), kept under standard housing conditions and fed ad libitum. All protocols followed the regulations of the Institutional Animal Care and Use Committee. Animals were randomly assigned to a control (n= 7 for each strain; total of 21 animals) or DOX group (n = 5 for each strain; total of 15 animals), and injected intraperitoneally with either 3 mg/kg DOX in 0.3 ml saline (vehicle) or vehicle on days 1, 3, 5, 7, 9, and 11. During the treatment period, animals were monitored every two days for general health, toxicity symptoms, and weight changes. A serial echocardiography study was performed in a total of 24 animals (n=12 for control, n=12 for DOX) at two time points. The first time point was before the start of the treatment and the second time point was on day 11, allowing for the investigation of cardiac functional changes during the experimental period. On day 12 for each animal (n=36), we performed an isolated heart experiment to obtain pressure values in the perfused heart, as well as cardiac capillary permeability values using our TR/NaFL fluorescent indicator dilution method.
2.3. Echocardiography
Rats were anesthetized using isoflurane administered through an inhalation cone with concurrent oxygen flow. Once anesthetized, the rat was placed in the left lateral decubitus position, the chest was shaved and then cleaned with isopropyl alcohol, and ultrasound gel was applied. A Hewlett-Packard Sonos 2500® echocardiography machine equipped with a pediatric 7.5 MHz transducer (Hewlett-Packard, Houston, TX) was used to obtain long-axis B-mode images from the left parasternal window, and M-mode images at the level of the papillary muscles. The following measurements were recorded: interventricular septum thickness in diastole and systole (IVSd and IVSs), left ventricular diameter in diastole and systole (LVDd and LVDs), and left ventricular posterior wall thickness in diastole and systole (LVPWd and LVPWs). The measurements were recorded in triplicate to eliminate the effect of beat to beat variability, and each variable was given as the average of the three measurements. Fractional shortening (FS) was calculated as given in equation 4 below.
| (4) |
2.4. Isolated heart setup for permeability and pressure measurements
We prepared a Langendorff isolated heart setup, in which the heart was surgically removed from the animal after an overdose of pentobarbital anesthesia (50 mg/kg, i.p.), and cannulated through the aorta in a perfusion setup (Harvard Apparatus, Holliston, MA). The heart was perfused with a Krebs-Henseleit buffer gassed with 95% O2 and 5% CO2 and warmed to 37°C. Pressures at the aortic inlet (AOP) and inside the left ventricle (LVP) were measured through transducers connected to a Biopac® MP-150 system (Biopac, Goleta, CA). Flow was adjusted to maintain aortic pressure between 50 mm Hg and 75 mm Hg (7–14 mL/min). The heart was paced at 300 bpm using a contact electrode connected to a Harvard Apparatus Stimulator P® system (Harvard Apparatus, Holliston, MA). After 30 minutes of stabilization to allow for anesthetic clearance and acclimation to the system, we performed permeability measurements in three replicates separated by 15 minutes. For each replicate, we injected 25 μL of the fluorescent dye mixture above the aortic cannula (12.5 μL each of 1.56 mg/mL TR and 1.56 μg/mL NaFL), and collected output samples for 45 seconds. Twenty samples were collected for each data set, distributed as follows: the first ten samples (immediately after injection) at 1 second per sample, the next five samples at 2 seconds per sample, and the last five samples at 5 seconds per sample. This sample collection strategy ensures that maximum temporal resolution is obtained near the expected peak when the concentrations are changing most rapidly. The fluorescent intensity of the samples was measured using a Fluorolog-3 spectrofluorometer (Jobin Yvon Horiba, Edison, NJ, USA) with characteristic excitation/emission wavelengths of 485/515 nm for NaFL, and 590/630 nm for TR. Pressures were also recorded in three replicates for each heart. Fluorescence and pressure data were processed using a custom Matlab algorithm developed in our lab.27 Calculated variables included (1) h(t) curves, extraction curves, and PSP values for the permeability data; and (2) +dP/dt (contractility), −dP/dt (relaxation), left ventricular developed pressure (LVDP), mean aortic pressure (MAP), time to peak (TTP), rate pressure product (RPP), and left ventricular end diastolic pressure (LVEDP) for hemodynamic data.
2.5. Statistical analysis
Two-way ANOVA was used to analyze permeability, pressure and echocardiography data. The factors under consideration were strain (Sprague-Dawley, Wistar, or Fischer-344) and treatment (control or DOX), and any interactions between factor levels. A Holm-Sidak method was used for post-hoc comparisons. A Mann-Whitney rank sum test was used to detect baseline differences between strains in fractional shortening values before treatment was initiated (echocardiography on day 1). For all statistical tests, significance was set at α = 0.05.
3. RESULTS
3.1. General toxicity of DOX treatment
Animals treated with DOX demonstrated general toxicity effects such as weight loss, hair loss, diarrhea, and porphyrin staining. These effects were absent in the control group. Average weight change was 33.0±6.3 g in the control group, and −21.3±5.9 g in the DOX group (p<0.001). None of the animals reached the threshold for termination of the experiment, > 20% weight loss compared to the baseline weight (defined as the body weight on day 1 of treatment), which was based on literature for humane endpoint determination.28–30
3.2. Changes in cardiac function with chemotherapy
3.2.1. Echocardiography
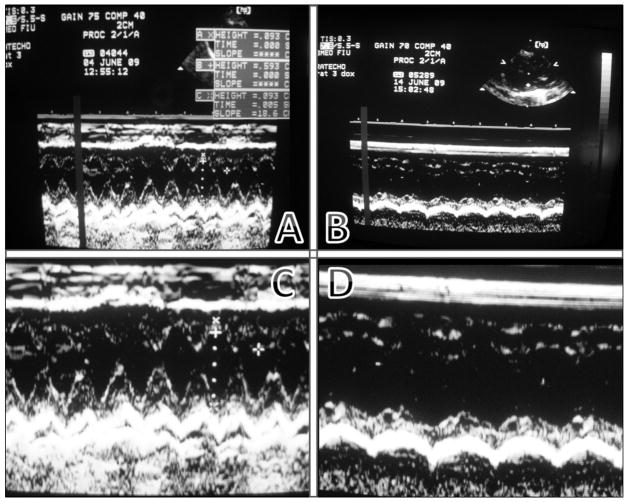
We completed echocardiography measurements at baseline and on day 11 on twenty-four rats. Fractional shortening (FS) measurements at baseline showed no significant differences between animals assigned to the control and DOX groups (FS control = 52.4%; FS DOX = 52.8%; p=0.21). However, by day 11, cardiac changes in the DOX animals were evident both qualitatively from the M-mode images as well as quantitatively from M-mode measurements. Figure 1 shows the marked contrast between M-mode images taken from the same animal on day 1 and images taken on day 11. The qualitative difference is remarkable.
Figure 1.
Top row: M-mode images for doxorubicin-treated rat baseline (A) vs. day 11 (B). Bottom row: Close up of both images (C = baseline, D = day 11). Notice the increase in left ventricular diameter, flattened contraction profile, and decreased fractional shortening (FS for this rat decreased by 22.8%).
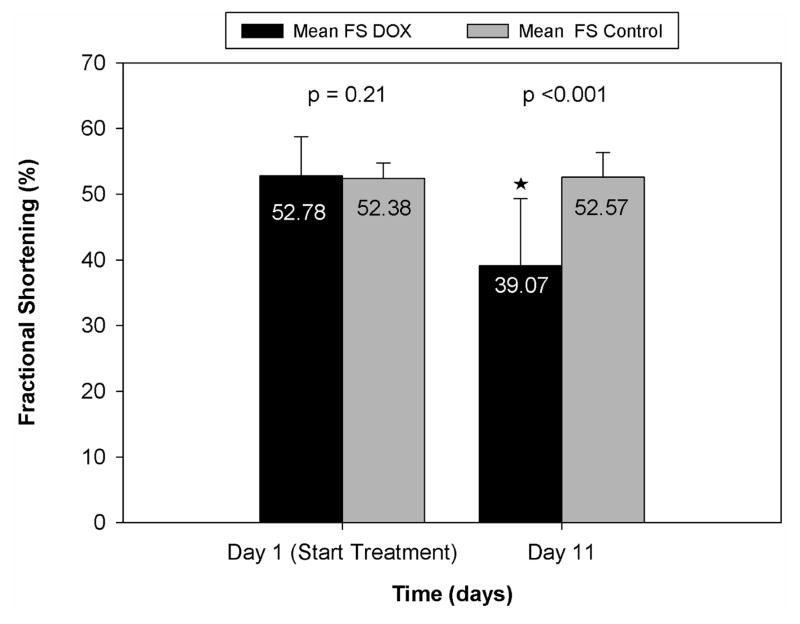
Quantitatively, we also found a significant difference in cardiac measurements between the two treatment groups. Fractional shortening averages for day 1 and day 11 for both groups are shown in Figure 2. There were no significant differences in FS values between control and DOX groups on day 1 (p =0.21). However, by day 11, the values for the groups were significantly different (FS control = 52.6%; FS doxorubicin = 39.1%, p <0.001). When looking at changes in FS over the experimental period, DOX-treated animals showed a significant (p<0.001) decrease between days 1 and 11, with an average FS change of −13.9%; whereas control animals did not undergo a significant change in fractional shortening (average change in FS = 0.20%). A two-way ANOVA showed no interaction between strain and treatment effects on FS changes (p=0.97).
Figure 2.
Comparative fractional shortening values (average±SD) for the control and DOX groups.
3.2.2. Pressure measurements
During the isolated heart experiment performed on day 12, we qualitatively observed that DOX-treated hearts were more likely to display visible pericardial effusion, and also displayed more difficulty adapting to pacing compared with control hearts, with occasional events of ventricular fibrillation that may indicate impairments in the electrical conduction system of the heart. Additionally, the wet/dry heart weight ratios for control hearts (2.76±0.41) and DOX hearts (3.05±0.39) were significantly different (p = 0.04), indicating the presence of edema in DOX-treated hearts. The average ± SD flow rate for control hearts was 7.9±1.4 mL/min, and for DOX-treated hearts it was 8.5±1.4 mL/min. Although the average flow for DOX hearts was slightly higher, there were no statistically significant differences in average flows used (p = 0.19).
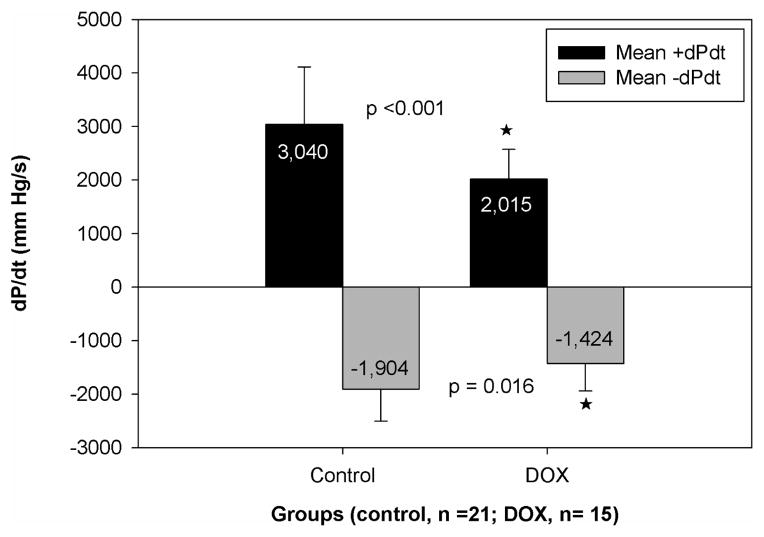
In terms of pressures, our results show a significant difference in +dP/dt (p<0.001) between the control group and the DOX group, as seen in Figure 3. This indicates that the contractility of the left ventricle is affected by chemotherapy treatment, resulting in lower +dP/dt values for the chemotherapy group (3040±1061 mm Hg/s for controls, 2015±557 mm Hg/s for DOX). There was a significant difference in +dP/dt based on animal strain (p<0.001), however, there was not a statistically significant interaction between strain and treatment (p = 0.07), so the treatment effects we observed were independent of strain.
Figure 3.
Comparison of left ventricular function assessed by dP/dt values for control and chemotherapy groups. Values are average±SD. A star denotes a significant difference in +dP/dt values (p<0.05)
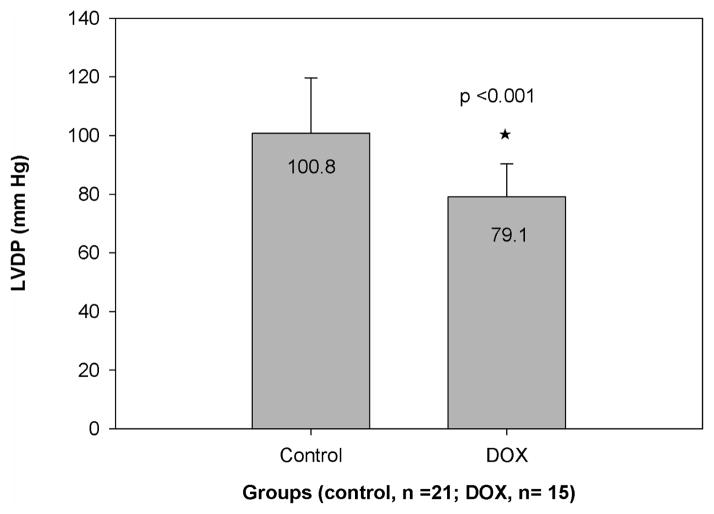
Although the effect was not as pronounced, the values of −dP/dt were also significantly decreased in the DOX group (−1903±600 mm Hg/s for control, −1424±516 mm Hg/s for DOX; p = 0.016). Therefore, the relaxation aspect of cardiac function was also significantly affected. There were no significant differences in −dP/dt based on strain (p=0.16), and no significant interaction between strain and treatment (p = 0.41). In addition, as shown in Figure 4, the DOX group had significantly lower (p<0.001) left ventricular developed pressure, which is consistent with the decrease in contractile function. There were no significant differences in LVDP based on strain (p =0.06), and no significant interaction between strain and treatment (p=0.14). There were no significant changes in mean aortic pressure, time to peak, left ventricular end diastolic pressure, or rate pressure product between control and treatment groups.
Figure 4.
Comparative LVDP values (average±SD) for the control and chemotherapy groups. The star denotes a significant difference between groups (p=0.0003)
3.3. Changes in cardiac capillary permeability with chemotherapy
Figure 5A shows a representative dye output curve for the fluorescent multiple indicator dilution experiment in a control Wistar rat. As expected, the TR output curve demonstrates a higher peak than NaFL, illustrating NaFL extraction from the capillaries. There is a clear separation between the two peaks within the first ten seconds, indicating extraction of the diffusible dye, followed by some backflow of diffusible dye into the system. Extraction of NaFL is further confirmed by the net extraction curve (Figure 5B), and the area under the curve shows full recovery of the reference tracer. Figures 6A and 6B show the same representative curves as Figures 5A and 5B, but for a Wistar rat treated with DOX. Figure 6 demonstrates increased NaFL extraction compared to Figure 5, with a larger separation between the NaFL and TR output curves.
Figure 5.
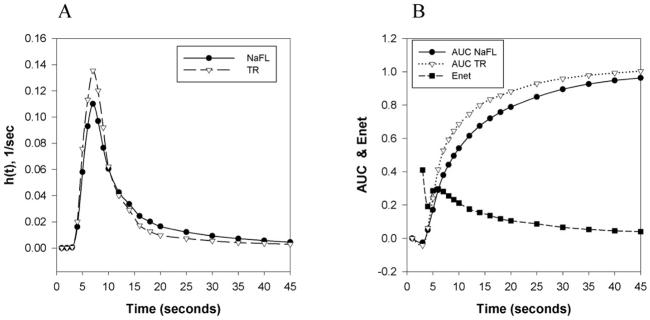
Multiple indicator fluorescent technique in isolated rat heart. A: Dye output curves versus time, h(t), for TR and NaFL dyes in a control Wistar rat. B: Sample net extraction (Enet) and areas under the curve (AUC) for TR and NaFL dyes in indicator dilution measurements in a control Wistar rat.
Figure 6.
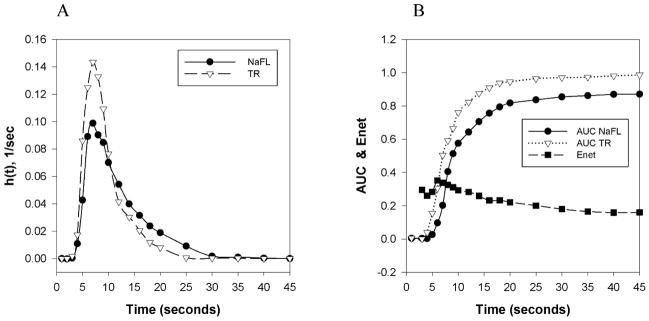
Multiple indicator fluorescent technique in isolated rat heart. A: Dye output curves versus time, h(t), for TR and NaFL dyes in a Wistar rat treated with DOX. B: Sample net extraction (Enet) and areas under the curve (AUC) for TR and NaFL dyes in indicator dilution measurements in a Wistar rat treated with DOX.
PSP values were calculated from the average of three replicates on each heart using the equations for the multiple indicator dilution method. All replicate measurements showed good reproducibility within 10% of each other.
The results show that control animals had a lower average PSP (0.035±0.013 cm3/s) than DOX animals (0.066±0.023 cm3/s), p<0.001. DOX treatment causes a significant increase in cardiac capillary permeability, with values that almost double those of the control group. These results support the hypothesis that cardiac capillary permeability changes can be detected after in vivo chemotherapy treatment using the NaFL/TR fluorescent indicator dilution method, and therefore are valuable to evaluate cardiotoxic effects.
An increase in permeability occurs after chemotherapy treatment consistently across strains. Average increases in permeability ranged from +0.02 cm3/s in Wistar rats to +0.04 cm3/s in Sprague Dawleys and Fischer-344 rats (Table 1). Although there are significant differences in PSP values between strains (p<0.001), a two-way ANOVA shows no significant interaction between strain and treatment effects (p=0.13). This indicates there is some strain-related variability in healthy cardiac capillary permeability, however, the deleterious effect of DOX on cardiac capillary permeability is consistent across strains and overcomes any differences in baseline permeability.
Table 1.
Permeability-surface-area product values in cm3/s.
| Strain | Control group (average±SD) | DOX group (average±SD) |
|---|---|---|
| Sprague-Dawley | 0.049±0.009 (†) | 0.084±0.026 (*) |
| Wistar | 0.035±0.008 (†) | 0.053±0.019 (*) |
| Fischer-344 | 0.021±0.003 (†) | 0.061±0.008 (*) |
significant difference from control group, p<0.05.
There were significant differences between strains, p<0.05, but no interaction between strain and treatment levels.
Overall percent dye recoveries for all experiments regardless of treatment group were 97.1±1.5% for TR, and 86.0±2.4% for NaFL. There were no significant differences in percent recovery of TR between the control and DOX groups, however, there was a significant difference in percent recovery of NaFL between the two groups (86.8±2.2% for control, 84.9±2.3% for DOX, p = 0.02. This may be related to tissue damage in the DOX group.
DISCUSSION
In this study, we used a rat short-term model of chemotherapy cardiotoxicity to examine changes in cardiac capillary permeability and cardiac function after DOX administration. Animals treated with DOX demonstrated general toxicity effects such as weight loss, hair loss, diarrhea, and porphyrin staining. This is consistent with reports from other authors who have used the Pouna protocol.24,25 The dosage administered during the treatment period is clinically relevant. Using normalization by body surface area as described by the FDA, a cumulative 18 mg/kg dose in rats corresponds to a 2.91 mg/kg or 107.67 mg/m2 dose in humans.31 The typical clinical dose of DOX is 75 mg/m2 administered intravenously every 3 weeks,32 and for most patients the cumulative dose is limited to 550 mg/m2 or less.10,33
Our results show that after the 12-day experimental period there are significant in vivo changes in cardiac capillary permeability that can be measured using a TR/NaFL fluorescent multiple indicator dilution method, and that these permeability changes coexist with significant functional changes as measured by echocardiography and pressure measurements in the isolated rat heart setup.
The dye output curves in the multiple indicator dilution method show full recovery of the reference tracer TR and extraction of the diffusible tracer NaFL, allowing for the determination of PSP values for the experiment. Back diffusion of NaFL after the peak is to be expected due to a reversal in the radial concentration gradient as the dye bolus starts progressing past a given region. After the initial output peak, the NaFL concentration gradient across the capillary wall reverses, driving the extracted dye back into the system and making it available during sample collection. This is represented in the h(t) graph after-peak region by a higher curve for NaFL when compared to TR, and by NaFL approaching an area under the curve (AUC) of 1. In our experiments, we do observe back diffusion of NaFL but it is not complete, and thus the AUC does not reach a value of 1. This may be due to the large volume of distribution, or because NaFL enters other compartments upon exiting the capillary bed. Lin et al report that NaFL can bind to positively charged extracellular matrix components such as collagen at physiological pH, as well as to proteins dissolved in the interstitium.34 Therefore, some of the NaFL that enters the tissue from the capillary bed may not be available to return to the capillary once the gradient reverses. We also have to consider that the collection time for a replicate is only 45 seconds, so there is a limited window of time for back diffusion to become part of a sample. As a result, any NaFL that may be available to diffuse back into the system after each 45-second collection period has ended would be discarded in the waste output between collections. The 15 minutes of continuous perfusion with fresh buffer in between replicates ensures that any possible back diffusion from the previous replicate does not affect the results. The AUC for TR approaches 1 in both control and experimental animals, indicating complete recovery of the reference tracer.
PSP values calculated from the dye output curves showed that cardiac capillary permeability was significantly increased in animals treated with DOX. There were significant differences in permeability based on treatment effect that were not strain-dependent. Thus, the effect of DOX on cardiac capillary permeability is reproducible across strains. Measurements in DOX animals showed higher variability as measured by their standard deviation, which is to be expected due to variations in individual response to chemotherapy. Changes in permeability do occur within a short period after chemotherapy exposure, as is illustrated by our 12-day study which models short-term exposures to DOX. This rapid response is consistent with reports by other authors that cardiac capillary permeability can change very fast in response to insults such as hypoxia or ischemia/reperfusion, even within the span of minutes or hours.35,36 There have been no reports of NaFL cardiac capillary permeability to which we can directly compare our results. However, there are some studies on the permeability of radioactively labeled solutes in perfused rat hearts. Using cyanocobalamin (MW 1355 Da), insulin (MW 5808 Da), and albumin (66463 Da), al Haboubi et al obtained permeability surface area product values in the order of 0.03 to 0.003 ml*s−1g−1 in the perfused rat heart, with the largest molecules having the smallest permeability values.37 Given that the average heart weight in control animals for our experiments was approximately 0.7 g, our average control PSP value for NaFL would be approximately 0.07 ml*s−1g−1. This seems consistent with the above results taking into consideration that differences in experimental flow rates can influence PSP values, that the MW of NaFL (375 Da) is one order of magnitude smaller than cyanocobalamin, and also that NaFL is a hydrophilic compound whereas cyanocobalamin is mostly hydrophobic, which would influence transport in physiological fluids.
We observed differences in PSP values based on strain. A reason for the observed difference could be variations in heart capillary surface areas across strains, or disparities in volume of distribution or pore sizes. The fact that treatment effects were independent of strain indicates that DOX damage to the endothelial wall affects all strains markedly and to an extent larger than any inherent differences between strains.
An important limitation in the interpretation of our PSP results comes from the inherent assumptions made in the indicator dilution method, and from the potential impact of DOX treatment on some of those assumptions. The model assumes that there is no net exchange of indicator between neighboring capillary-tissue units, so the organ can be viewed as consisting of parallel independent pathways. The existence of axial diffusion would violate this assumption. It is further assumed that exchange only takes place in the capillary-tissue units and that the input tubing, arteries, venues, arterioles and venules act only as dispersion vessels.38 The analysis we have presented also assumes homogeneity of flow, but we can probably anticipate that there will be perfusion heterogeneity, particularly in the presence of DOX-induced changes in the tissue. Heterogeneity of flow, both at the injection site and within the tissue, can affect effluent concentration for both the reference and the diffusible tracer. A possible approach to address this limitation would be to use a flow-limited indicator to account for transit time variability through the vessels,39 or to make an estimate of flow dispersion using a multi-capillary model or curve fitting.40
In the echocardiography and pressure measurement studies, we observed changes in cardiac function that were indicative of chemotherapy-induced cardiotoxicity and which were also strain-independent. This confirms that changes in cardiac capillary permeability occur alongside changes in contractile function of the heart. For the echocardiography studies, the literature and our results show that the technique can be used to monitor changes in rat heart function over time.41,42 Our measurements on day 1 and the qualitative changes we observed are comparable to other literature reports.33,43 The quantitative changes during the experimental period clearly indicate a significant strain-independent decline in cardiac function as measured by fractional shortening. Decreases in FS have also been reported by other authors, with the amount of decrease varying based on dosage, scheduling, and overall treatment duration.33,41,44 The individual decrease in fractional shortening was more pronounced in some of our animals than others, which is expected due to individual variability in the response to chemotherapy.
In hemodynamic measurements obtained using the isolated heart setup, there was also a significant decline in cardiac function with a deleterious effect of chemotherapy on left ventricular developed pressure, contractility (dP/dt), and relaxation (−dp/dt), and this effect was independent of strain, despite the fact there were some strain differences in +dP/dt. Literature reports differ regarding the significance of changes in these variables after chemotherapy depending on the treatment dose, treatment duration, and method used to collect pressure measurement. We have compared our results with groups who have used similar protocols, and have found that their overall values for +dP/dt and −dP/dt were comparable to our values and that all the groups find differences between control and doxorubicin treated rats for +dP/dt and −dP/dt after the 12-day treatment period, with a majority also finding differences in LVDP.23–25
Based on our results, we may conclude that +dP/dt changes, i.e., changes in contractility, are more pronounced than −dP/dt changes, which are indicative of relaxation ability, although both are affected in a statistically significant manner. In summary, we were able to detect significant changes in contractility and relaxation, as measured by LVDP and ±dP/dt, after doxorubicin treatment. These results are consistent with the expectation that the short-term DOX cardiotoxicity model would result in changes in cardiac function, and with our findings of significant changes in fractional shortening over the course of treatment as well as significant increases in permeability for DOX-treated animals.
4. CONCLUSION
Our TR/NaFL multiple indicator dilution technique can sensitively measure capillary permeability in the isolated rat heart, and can detect permeability changes in cardiac tissue due to chemotherapy. This technique may also be useful in diverse research applications where capillary permeability of an organ or tissue is a variable of interest, or to detect changes caused by disease or drug exposure.
The administration of DOX over the course of twelve days at clinically relevant dosages resulted in increased cardiac capillary permeability along with functional changes in cardiac contractile function. Significant permeability changes were detected regardless of rat strain. This confirms our hypothesis that cardiac capillary permeability is affected by DOX administration and permeability changes measured with our technique could be a useful parameter to include in cardiotoxicity studies for novel drug formulations. Current research aimed at developing new chemotherapy agents with reduced off-site toxicity would benefit from studying all possible variables that can influence cardiac toxicity and chemotherapy-related dysfunction.
Acknowledgments
Laboratory work was conducted using the facilities of the Biomedical Engineering Department at Florida International University. A.F.F. was supported by NIH/NIGMS R25 GM061347 during completion of a portion of this work. The same grant also provided partial experimental funding through the 2008 Biomedical Research Initiative Summer Research Award. D.A.C. was supported by the FIU BME Norman R Weldon Undergraduate Summer Research Internship during completion of a portion of this work.
NON-STANDARD ABBREVIATIONS AND ACRONYMS
- AOP
Aortic pressure (mm Hg)
- FS
Fractional shortening (%)
- IVSd
Interventricular septum thickness in diastole (mm)
- IVSs
Interventricular septum thickness in systole (mm)
- LVDd
Left ventricular diameter in diastole (mm)
- LVDP
Left ventricular developed pressure (mm Hg)
- LVDs
Left ventricular diameter in systole (mm)
- LVEDP
Left ventricular end diastolic pressure (mm Hg)
- LVP
Left ventricular pressure (mm Hg)
- LVPWd
Left ventricular posterior wall thickness in diastole (mm)
- LVPWs
Left ventricular posterior wall thickness in systole (mm)
- NaFL
Sodium fluorescein
- PSP
Permeability-surface-area-product (cm3s−1)
- TR
Texas Red-conjugated Dextran
- TTP
Time to peak (s)
References
- 1.American Cancer Society. Cancer Facts & Figures 2014. Atlanta: American Cancer Society; 2014. [Google Scholar]
- 2.Ferrans VJ. Overview of cardiac pathology in relation to anthracycline cardiotoxicity. Cancer Treat Rep. 1978;62(6):955–961. [PubMed] [Google Scholar]
- 3.Platel D, Bonoron-Adele S, Robert J. Role of daunorubicinol in daunorubicin-induced cardiotoxicity as evaluated with the model of isolated perfused rat heart. Pharmacol Toxicol. 2001;88(5):250–254. doi: 10.1034/j.1600-0773.2001.d01-112.x. [DOI] [PubMed] [Google Scholar]
- 4.Chen B, Peng X, Pentassuglia L, Lim CC, Sawyer DB. Molecular and cellular mechanisms of anthracycline cardiotoxicity. Cardiovasc Toxicol. 2007;7(2):114–121. doi: 10.1007/s12012-007-0005-5. [DOI] [PubMed] [Google Scholar]
- 5.Chen X, Chen Y, Bi Y, et al. Preventive cardioprotection of erythropoietin against doxorubicin-induced cardiomyopathy. Cardiovasc Drugs Ther. 2007;21(5):367–374. doi: 10.1007/s10557-007-6052-0. [DOI] [PubMed] [Google Scholar]
- 6.Minotti G, Menna P, Salvatorelli E, Cairo G, Gianni L. Anthracyclines: molecular advances and pharmacologic developments in antitumor activity and cardiotoxicity. Pharmacol Rev. 2004;56(2):185–229. doi: 10.1124/pr.56.2.6. [DOI] [PubMed] [Google Scholar]
- 7.Platel D, Pouna P, Bonoron-Adele S, Robert J. Preclinical evaluation of the cardiotoxicity of taxane-anthracycline combinations using the model of isolated perfused rat heart. Toxicol Appl Pharmacol. 2000;163(2):135–140. doi: 10.1006/taap.1999.8847. [DOI] [PubMed] [Google Scholar]
- 8.Menna P, Recalcati S, Cairo G, Minotti G. An introduction to the metabolic determinants of anthracycline cardiotoxicity. Cardiovasc Toxicol. 2007;7(2):80–85. doi: 10.1007/s12012-007-0011-7. [DOI] [PubMed] [Google Scholar]
- 9.Wolf MB, Baynes JW. The anti-cancer drug, doxorubicin, causes oxidant stress-induced endothelial dysfunction. Biochim Biophys Acta. 2006;1760(2):267–271. doi: 10.1016/j.bbagen.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 10.Takemura G, Fujiwara H. Doxorubicin-induced cardiomyopathy from the cardiotoxic mechanisms to management. Prog Cardiovasc Dis. 2007;49(5):330–352. doi: 10.1016/j.pcad.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 11.Fernandez-Fernandez A, McGoron AJ, Carvajal DA. Application of a fluorescent multiple indicator dilution method to study changes in cardiac capillary permeability with chemotherapy. Paper presented at: IFMBE Proceedings 25th Southern Biomedical Engineering Conference; 2009; Miami, FL. [Google Scholar]
- 12.Fernandez-Fernandez A, Carvajal DA, McGoron AJ. Measuring in vivo effects of chemotherapy treatment on cardiac capillary permeability. Paper presented at: IFMBE Proceedings 26th Southern Biomedical Engineering Conference; 2010; College Park, MD. [Google Scholar]
- 13.Bassingthwaighte JB, Sparks HV. Indicator dilution estimation of capillary endothelial transport. Annu Rev Physiol. 1986;48:321–334. doi: 10.1146/annurev.ph.48.030186.001541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bassingthwaighte JB, Strandell T, Donald DE. Estimation of coronary blood flow by washout of diffusible indicators. Circ Res. 1968;23(2):259–278. doi: 10.1161/01.res.23.2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zierler K. Indicator dilution methods for measuring blood flow, volume, and other properties of biological systems: a brief history and memoir. Ann Biomed Eng. 2000;28(8):836–848. doi: 10.1114/1.1308496. [DOI] [PubMed] [Google Scholar]
- 16.Meerdink DJ, Leppo JA. Myocardial transport of hexakis(2-methoxyisobutylisonitrile) and thallium before and after coronary reperfusion. Circ Res. 1990;66(6):1738–1746. doi: 10.1161/01.res.66.6.1738. [DOI] [PubMed] [Google Scholar]
- 17.McGoron AJ, Gerson MC, Biniakiewicz DS, Roszell NJ, Washburn LC, Millard RW. Extraction and retention of technetium-99m Q12, technetium-99m sestamibi, and thallium-201 in isolated rat heart during coronary acidemia. Eur J Nucl Med. 1997;24(12):1479–1486. doi: 10.1007/s002590050177. [DOI] [PubMed] [Google Scholar]
- 18.Assaly RA, Habib RH, Azizi M, Shapiro JI, Dignam JD. Use of multiple fluorophores for evaluating microvascular permeability in control rats and rats with sepsis. Clin Sci (Lond) 2008;114(2):123–130. doi: 10.1042/CS20070219. [DOI] [PubMed] [Google Scholar]
- 19.Sanders JR, Pou NA, Roselli RJ. Neutral and DEAE dextrans as tracers for assessing lung microvascular barrier permeability and integrity. J Appl Physiol. 2002;93(1):251–262. doi: 10.1152/japplphysiol.00635.2000. [DOI] [PubMed] [Google Scholar]
- 20.Meier P, Zierler KL. On the theory of the indicator-dilution method for measurement of blood flow and volume. J Appl Physiol. 1954;6(12):731–744. doi: 10.1152/jappl.1954.6.12.731. [DOI] [PubMed] [Google Scholar]
- 21.Crone C. The permeability of capillaries in various organs as determined by use of the ‘indicator diffusion’ method. Acta Physiol Scand. 1963;58:292–305. doi: 10.1111/j.1748-1716.1963.tb02652.x. [DOI] [PubMed] [Google Scholar]
- 22.Pouna P, Bonoron-Adele S, Gouverneur G, Tariosse L, Besse P, Robert J. Development of the model of rat isolated perfused heart for the evaluation of anthracycline cardiotoxicity and its circumvention. Br J Pharmacol. 1996;117(7):1593–1599. doi: 10.1111/j.1476-5381.1996.tb15326.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Robert J. Long-term and short-term models for studying anthracycline cardiotoxicity and protectors. Cardiovasc Toxicol. 2007;7(2):135–139. doi: 10.1007/s12012-007-0022-4. [DOI] [PubMed] [Google Scholar]
- 24.Plande J, Platel D, Tariosse L, Robert J. Experimental study of dexrazoxane-anthracycline combinations using the model of isolated perfused rat heart. Toxicol Lett. 2006;161(1):37–42. doi: 10.1016/j.toxlet.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 25.de Nigris F, Rienzo M, Schiano C, Fiorito C, Casamassimi A, Napoli C. Prominent cardioprotective effects of third generation beta blocker nebivolol against anthracycline-induced cardiotoxicity using the model of isolated perfused rat heart. Eur J Cancer. 2008;44(3):334–340. doi: 10.1016/j.ejca.2007.12.010. [DOI] [PubMed] [Google Scholar]
- 26.Skrzypiec-Spring M, Grotthus B, Szelag A, Schulz R. Isolated heart perfusion according to Langendorff---still viable in the new millennium. J Pharmacol Toxicol Methods. 2007;55(2):113–126. doi: 10.1016/j.vascn.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 27.Carvajal DA, Fernandez-Fernandez A, McGoron AJ. Development of Matlab algorithm to process pressure waveforms from isolated perfused heart experiments. Paper presented at: IFMBE Proceedings 25th Southern Biomedical Engineering Conference; 2009; Miami, FL. [Google Scholar]
- 28.Morton DB. A systematic approach for establishing humane endpoints. ILAR J. 2000;41(2):80–86. doi: 10.1093/ilar.41.2.80. [DOI] [PubMed] [Google Scholar]
- 29.Boston University. Tumor Policy for Mice and Rats. Section IX: Humane Endpoint Criteria. 2009 Available at: http://www.bu.edu/orccommittees/iacuc/policies-and-guidelines/tumor-policy-for-mice-and-rats/
- 30.University of Pennsylvania. IACUC Guideline: Humane Intervention and Endpoints for Laboratory Animal Species. 2011 Available at http://www.upenn.edu/regulatoryaffairs/Documents/iacuc/guidelines/iacucguideline-humaneendpoints-8%2023%2011.pdf.
- 31.Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. FASEB J. 2008;22(3):659–661. doi: 10.1096/fj.07-9574LSF. [DOI] [PubMed] [Google Scholar]
- 32.Wonders KY, Reigle BS. Trastuzumab and doxorubicin-related cardiotoxicity and the cardioprotective role of exercise. Integr Cancer Ther. 2009;8(1):17–21. doi: 10.1177/1534735408330717. [DOI] [PubMed] [Google Scholar]
- 33.Hayward R, Hydock DS. Doxorubicin cardiotoxicity in the rat: an in vivo characterization. J Am Assoc Lab Anim Sci. 2007;46(4):20–32. [PubMed] [Google Scholar]
- 34.Lin CW, Wang Y, Challa P, Epstein DL, Yuan F. Transscleral diffusion of ethacrynic acid and sodium fluorescein. Mol Vis. 2007;13:243–251. [PMC free article] [PubMed] [Google Scholar]
- 35.Svendsen J, Bjerrum P, Haunso S. Myocardial capillary permeability after regional ischemia and reperfusion in the in vivo canine heart. Effect of superoxide dismutase. Circulation Res. 1991;68:174–184. doi: 10.1161/01.res.68.1.174. [DOI] [PubMed] [Google Scholar]
- 36.Ward BJ, Donnelly JL. Hypoxia induced disruption of the cardiac endothelial glycocalyx: implications for capillary permeability. Cardiovasc Res. 1993;27(3):384–389. doi: 10.1093/cvr/27.3.384. [DOI] [PubMed] [Google Scholar]
- 37.Al-Haboubi HA, Ward BJ. Microvascular permeability of the isolated rat heart to various solutes in well-oxygenated and hypoxic conditions. Int J Microcirc Clin Exp. 1996;16(6):291–301. doi: 10.1159/000179188. [DOI] [PubMed] [Google Scholar]
- 38.Bassingthwaighte J, Goresky CA, Linehan JH. Modeling in the Analysis of the Processes of Uptake and Metabolism in the Whole Organ. In: Bassingthwaighte, et al., editors. Whole Organ Approaches to Cellular Metabolism. Springer; 1998. pp. 3–27. [Google Scholar]
- 39.Audi SH, Linehan JH, Krenz GS, Dawso CA. Accounting for the heterogeneity of capillary transit times in modeling multiple indicator dilution data. Ann Biomed Eng. 1998;26:914–930. doi: 10.1114/1.138. [DOI] [PubMed] [Google Scholar]
- 40.Bassingthwaighte JB, Levin M. Analysis of coronary outflow dilution curves for the estimation of cellular uptake rates in the presence of heterogeneous regional flows. Basic Res Cardiol. 1981;76(4):404–410. doi: 10.1007/BF01908332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schwarz ER, Pollick C, Dow J, Patterson M, Birnbaum Y, Kloner RA. A small animal model of non-ischemic cardiomyopathy and its evaluation by transthoracic echocardiography. Cardiovasc Res. 1998;39(1):216–223. doi: 10.1016/s0008-6363(98)00009-1. [DOI] [PubMed] [Google Scholar]
- 42.Schwarz ER, Pollick C, Meehan WP, Kloner RA. Evaluation of cardiac structures and function in small experimental animals: transthoracic, transesophageal, and intraventricular echocardiography to assess contractile function in rat heart. Basic Res Cardiol. 1998;93(6):477–486. doi: 10.1007/s003950050118. [DOI] [PubMed] [Google Scholar]
- 43.Watson LE, Sheth M, Denyer RF, Dostal DE. Baseline echocardiographic values for adult male rats. J Am Soc Echocardiogr. 2004;17(2):161–167. doi: 10.1016/j.echo.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 44.Teraoka K, Hirano M, Yamaguchi K, Yamashina A. Progressive cardiac dysfunction in adriamycin-induced cardiomyopathy rats. Eur J Heart Fail. 2000;2(4):373–378. doi: 10.1016/s1388-9842(00)00111-2. [DOI] [PubMed] [Google Scholar]