Summary
HIV-associated nephropathy (HIVAN) is a common complication of HIV-1 infection in patients with African ancestry in general and with APOL1 gene risk variants in particular. Although collapsing glomerulopathy is considered a hallmark of HIVAN, significant numbers of glomeruli in patients with HIVAN also display other variants of focal segmental glomerulosclerosis (FSGS). We propose that collapsed glomeruli as well as glomeruli with other variants of FSGS are manifestations of HIVAN and their prevalence depends on associated host factors. We explored the role of the renin-angiotensin system (RAS) in the manifestation of any specific glomerular phenotype in HIVAN. To evaluate the role of the RAS we have used a genetically engineered mouse model of HIVAN (Tg26) with two and four copies of angiotensinogen (Agt) gene (Tg26/Agt2 and Tg26/Agt4). In Tg26/Agt2, 1 out of 6 glomeruli exhibited sclerosed phenotype, whereas 1 out of 25 glomeruli displayed collapsed phenotype; on the other hand, in Tg26/Agt4, 1 out of 3 glomeruli exhibited sclerotic phenotype and only 1 out of 7 glomeruli showed collapsed phenotype. To inhibit the effect of RAS, Tg26/Agt2 were administered captopril, aliskiren, aliskiren plus captopril or aliskiren plus telmisartan by miniosmotic pumps for 4 weeks. In all experimental groups there was a significant reduction in percentage of sclerosed glomeruli and only minimal reduction in collapsed glomeruli compared to normal saline receiving Tg26/Agt2. These findings suggest that the manifestation of the sclerosed phenotype in HIVAN is predominantly dependent on activation of the RAS.
Keywords: HIV-associated Nephropathy, Focal Glomerulosclerosis, Renin-angiotensin system, Angiotensinogen
Introduction
HIV-associated nephropathy, which is characterized by a collapsing variant of FSGS and micro cystic tubular dilatation (Pardo et., 1984; Rao et al., 1984; Cantor et al., 1991; Bourgoignie et al., 1991; Kopp et al., 1992; Sczech et., 2004; Berliner et al., 2008), is a manifestation of specific genetic, environmental, and host factors (Gharavi et al., 2004; Chan et al., 2009; Atta, 2010). Recently, APOL1 with polymorphism at G1 and G2 sites (risk allele) has been demonstrated to be a risk factor for non-diabetic glomerulosclerosis and for HIVAN patients with an African background (Freedman et al., 2010). Since Apol1 polymorphism contributes to both conventional FSGS and HIVAN, it is likely that some of glomeruli will develop sclerotic phenotype in addition to collapsing phenotype in HIVAN. This was further supported by epidemiologic studies showing that with the occurrence of HIVAN, the incidence of heroin nephropathy (predominantly sclerotic phenotype) has disappeared in the patients with African American ancestry (Fiedman et al., 1995). These studies suggest a possibility that in patients with HIV infection, the occurrence of sclerotic and collapsing lesions may be a part of the spectrum of the same entity - HIVAN.
The pathogenesis of glomerular lesions in HIVAN is characterized by the presence of proliferative and apoptosed cells (Atta, 2010). Under any stress, the fate (phenotype) of the cellular injury is dependent on the net outcome of forces promoting cell survival vs. cell death (Yadav et al., a, 2010). On that account, cells undergoing proliferation will be accompanied by some cells having apoptotic phenotype and cells undergoing the apoptotic process will be accompanied by some proliferating cells (Yadav et al., b, 2010). Nonetheless, the net outcome is defined by a net increase or decrease in the number of cells. Thus, theoretically, it is possible that during the time course of the disease there will be an increase or decrease in the severity of HIV-induced kidney cell injury depending on viral, genetic and host factors which will determine the emergence of a specific glomerular phenotype - sclerotic vs. collapsing. Since proliferating and apoptosed cells are the outcome of the loss of their native phenotype, it becomes difficult to determine the cell lineage. On that account, the exact lineage of proliferating cells in the Bowman’s space in collapsing variant of glomerulosclerosis still remains controversial (Barisoni et al., 2002; Dijkman et al., 2005; Dijkman et al., 2006). Several investigators suggested the lineage of glomerular proliferating cells to be of parietal cell origin rather than of visceral epithelial cells (Dijkman et al., 2005; Dijkman et al., 2006). However, we suggested that determination of the lineage of these cells depends on their location and the outcome of ongoing epithelial mesenchymal transition (Yadav et al., b, 2010).
The collapsing variant of glomerulosclerosis is characterized by segmental or global obliteration of the glomerular capillary lumina by wrinkling and collapse of the glomerular basement membrane associated with podocyte hypertrophy and hyperplasia (D’Agati., 2003). It has been shown in clinical studies that HIV and non-HIV patients with collapsing glomerulopathy develop higher rates of proteinuria and an accelerated decline in kidney function (Weiss et al., 1986; Valeri et al., 1996). Howie et al. (2001) suggested a method for measurement of chronic damage in renal biopsy specimens which proved to be prognostic in predicting renal survival in patients with chronic kidney diseases, including HIVAN (Howie et al., 2001; Post et al., 2008). Based on these studies, we separated HIVAN mice into two groups: severe disease and mild disease, depending on the percentage of glomeruli with global sclerosis and number of micro cystic tubular dilatations. We asked whether the presence of collapsed glomeruli in HIVAN is itself an independent marker of severity of the disease; in that scenario, the number of sclerotic glomeruli to one collapsed glomerulus has to increase with the severity of HIVAN. To test our hypothesis we evaluated the effects of RAS activation and down regulation on the prevalence of sclerosed vs. collapsed glomeruli in a transgenic mouse model of HIVAN. We also attempted to predict the probability of having an adequate biopsy specimen with sclerosed vs. collapsed glomeruli for the diagnosis of HIVAN.
Materials and methods
HIV transgenic mice (Tg26)
We have used Tg26 (with FVB/N background) mice. Breeding pairs to develop Tg26 colonies were kindly gifted by Prof. Paul E. Klotman M.D., President and CEO, Baylor College of Medicine, Houston, TX. Tg26 transgenic animals have the proviral transgene, pNL4-3: d1443, which encodes all the HIV-1 genes except gag and pol and therefore the mice are noninfectious (Lu et al., 2006). We are maintaining colonies of these mice in our animal facility. For genotyping, tail tips were clipped, DNA was isolated and PCR studies were carried out using following primers for Tg26.
HIV-F 5′ ACATGAGCAGTCAGTTCTGCCGCAGAC
HIV-R 3′ CAAGGACTCTGATGCGCAGGTGTG
The experimental protocol was approved by the Ethics Review Committee for Animal Experimentation of Long Island Jewish Medical Center.
Angiotensinogen (Agt) Transgenic Mice
We obtained Agtdup/Agtdup mice from Jackson Laboratories, Bar Harbor (ME). Since these mice were not on an FVB/N background, we have bred them with FVB/N for eight generations. Homozygous Agt mice (Agtdup/Agtdup) are viable and fertile. They show blood pressure levels ~16 mm Hg higher than their normal wild-type siblings (Agt+/Agt+). Heterozygous mice (Agt+/Agtdup) show blood pressure levels ~8 mm Hg higher than their normal wild-type siblings. This transgenic mouse model demonstrates the causality between genotype at the angiotensinogen locus and blood pressures. Blood pressures of one-copy (Agttm1Unc/+) through four-copy (Agtdup/Agtdup) animals show significant and almost linear increases of approximately 8 mm Hg per gene copy despite their normal compensatory mechanisms being intact.
To generate Tg26 mice with variable Agt copies, Tg26 mice were bred with Agtdup/Agtdup mice. Genotyping assays to distinguish between the different allele of the Agt gene (Agt+ and Agtdup) have been established at the Jackson Laboratory. The tail DNA of the progeny was analyzed by PCR. The Agtdup allele produced a 190-bp fragment when amplified with the D8Mit56 marker, whereas Agt+ gave a 160-bp fragment. The sequences of the two primers that were used are as follows: 5′-ACACTCAGAGACCATGAGTACACC-3′ SSLP primer D11Mit 258 and 5′-GAGTTCACTACCCACAAGTCTCC-3′ SSLP primer D11Mit258. Twelve week old Tg26 mice (8 in each group) with 2 and 4 copies of Agt were used for renal histology scoring.
To determine the effect of down regulation of the RAS on HIVAN, Tg26 mice (in groups of four) were administered either normal saline, angiotensin converting enzyme (ACE) inhibitor, Captopril (5 mg/Kg body weight/day); inhibitor of renin activity, Tekturna (Tekturna, 50mg/Kg body weight/day); a combination of Tekturna and Captopril; and a combination of Tekturna with angiotensin receptor (AT1) blocker, Telmisartan (300 μg/day, calculated for average weight of the mouse) via miniosmotic pumps for four weeks.
Renal Histology
At the end of the experimental periods, mice were sacrificed, mouse kidneys were isolated and prepared for histology and immunohistochemistry, and blood and urine samples were collected for biomarker assay. Renal cortical sections were stained with hematoxylin and eosin (H&E), and periodic acid-Schiff (PAS) stain. Slides were coded and examined under light microscopy by two investigators. Twenty random fields per section were examined and scored for glomerular lesions and micro cystic tubular dilatations. Glomerular lesions were classified as segmental glomerular sclerosis, SGS (including focal segmental glomerular sclerosis not otherwise specified, perihilar variant, tip variant), global glomerular sclerosis (GGS), and collapsing glomerulopathy (CG). Microcysts were considered tubules that are dilated at least 3-fold compared to normal tubules. The term sclerosis was used in aggregate for both segmental and global sclerosis since we believe that segmental sclerosis eventually leads to global sclerosis and these glomerular lesions are in the spectrum of the same disease process. Moreover, the same glomerulus at another level may show segmental or global sclerosis.
Statistical Analysis
For comparison of mean values between two groups, the unpaired t-test was used. To compare values between multiple groups, analysis of variance and a Bonferroni multiple range test was used to calculate a P value. Statistical significance was defined as P < 0.05.
Results
Tg26 animals develop kidney lesions typical of HIVAN and endogenous RAS activation results in glomerular sclerosis
Tg26 is the most frequently used mouse model of HIV-associated nephropathy. These animals develop histological changes typical of patients with HIVAN (Figure 1), which were shown in previous studies (Pardo et., 1984; Rao et al., 1984; Cantor et al., 1991; Bourgoignie et al., 1991; Kopp et al., 1992; Sczech et., 2004; Berliner et al., 2008). In the present study we show that the prevalence of different types of glomerular lesions (sclerotic vs. collapsing) depends on RAS status (Figure 2). Renal cortical sections of twelve week old Tg26 mice with four copies of the angiotensinogen (Agt) gene (Tg26/Agt4) showed a higher percentage of sclerosed glomeruli when compared to Tg26 mice with two copies of the Agt gene (Tg26/Agt2), whereas the difference in percent of collapsed glomeruli was not statistically significant (p>0.05).
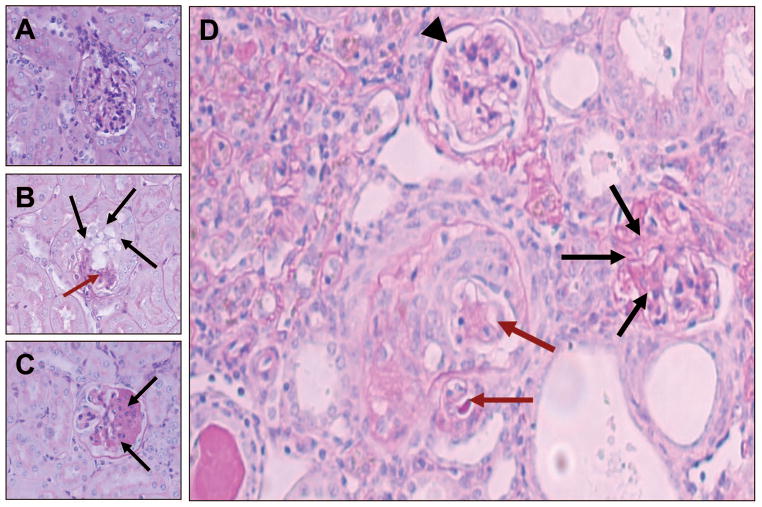
Figure 1. Representative microphotographs displaying glomerular phenotype.
A. A normal glomerulus in a control (FVB/N) mouse.
B. A collapsed glomerulus in Tg26 mouse. There is collapsed glomerular tuft (brown arrows), podocyte hypertrophy and hyperplasia (black arrows).
C. Segmental glomerulosclerosis in a Tg26 mouse. Segmental obliteration of glomerular tuft by increased matrix and hyaline material (black arrows). PAS; Mag X400
D. A renal cortical section from Tg26 mouse (Agt2) displaying presence of a hypercellularity in Bowman’s space with collapsed capillary loops (brown arrows), a glomeruls with sclerosed capillary tufts (black arrows) and a normal glomerulus (arrowhead). PAS; Mag X 1000
Figure 2.

Cumulative renal histology data of twelve week old Tg26/Agt2 and Tg26/Agt4 mice. Animals with four Agt copies displayed 38% of sclerosed glomeruli vs. 17% of sclerosed glomeruli in mice with two Agt copies. *<0.05 vs. respective Agt2; **P<0.05 vs. respective Agt2.
Tg26 mice with RAS inhibition show less glomerulosclerosis and improved kidney function
In order to down regulate RAS, we administered Captopril, Tekturna or combinations of Captopril plus Tekturna, and Tekturna plus Telmisartan to Tg26 mice for four weeks. The percentage of sclerosed glomeruli drastically decreased in treatment groups compared to Tg26 mice receiving normal saline. The percentage of collapsing glomeruli did not show a significant difference in animals receiving Captopril or Tekturna alone, but more aggressive RAS inhibition by the combinations of Captopril plus Tekturna and Tekturna plus Telmisartan resulted in a significant decrease in collapsing glomeruli as well (Figure 3).
Figure 3.

Cumulative data of renal histology in mice administered normal saline, captopril, tekturna, tekturan + captopril, and tekturna + telmisartan via miniosmotic pumps for four weeks. *P<0.05 vs. respective N/Saline groups.
Along with a decrease in percentage of sclerosed glomeruli, RAS inhibition also resulted in improved kidney function which was reflected in lower levels of blood urea nitrogen (BUN) compared to control group (Figure 4).
Figure 4.

Blood urea nitrogen data of Tg26 mice treated with normal saline, Captopril, Tekturna, Tekturna plus Captopril and Tekturna plus Telmisartan. *P<0.05 vs. Saline.
Tg26/Agt4 mice have larger glomeruli comparing to Tg26/Agt2 mice
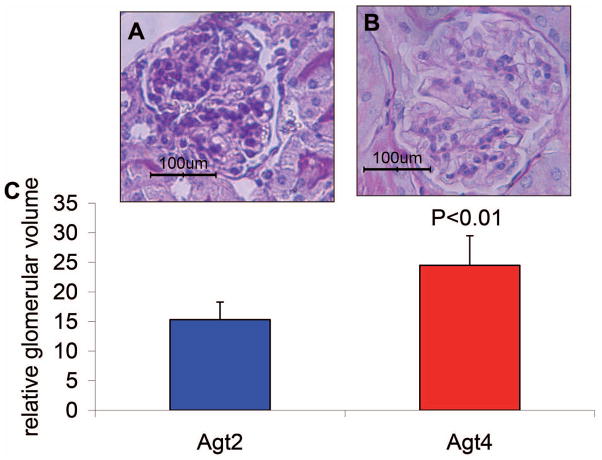
The size of glomeruli has been shown to be correlated with the progression of FSGS in many animal models and in humans (Barisoni et al., 2002; Dijkman et al., 2005; Dijkman et al., 2006). We explored the correlation of glomerular size with activation of RAS in Tg26 mice. Our data show that glomeruli of Tg26/Agt4 mice are 30% larger than those of Tg26/Agt2 mice (Figure 5).
Figure 5. Glomerular volume in Tg26Agt2 and Tg26Agt4 mice. A.
A representative unsclerosed glomerulus in Agt2 group.
B. A representative unsclerosed glomerulus in Agt4 group.
C. Cumulative data of glomerular volume in twelve weeks old Tg26/Agt2 and Tg26/Agt4 mice.
Discussion
The present study demonstrates that HIVAN is accompanied by both sclerotic and collapsing glomerular lesions in HIV transgenic mice; nonetheless, the number of sclerosed glomeruli is many times greater than the number of collapsed glomeruli. A higher number of Agt copies in HIVAN mice displayed a greater percentage of sclerosed glomeruli, while down regulation of the RAS was associated with reduced number of sclerosed glomeruli. These findings indicate that the number of sclerosed glomeruli have a higher correlation to the activation of RAS than to the number of collapsed glomeruli. The ratio of sclerosed to collapsed glomeruli was higher in HIVAN mice with two Agt copies when compared to HIVAN mice with four Agt copies. However, down regulation of RAS with ACE, ARBs and renin inhibitors attenuated the progression of sclerotic lesions more effectively when compared to that of collapsed glomerular lesions.
Since Tg26 mice with 2 Agt copies also displayed sclerosed glomeruli in addition to collapsed glomeruli, it was likely that sclerosis was a manifestation of a viral induced podocytopathy. Nonetheless, Tg26 mice with 2 Agt copies are also likely to have de novo activation of the renin angiotensin system. It has been reported that HIV has the potential to stimulate renin generation by podocytes (Salhan et al., 2012); this effect of HIV has been attributed to its down regulating effect on liganded vitamin D receptor. Secondly, HIVAN is a proteinuric disease leading to hypoproteinemia and hypovolemia, which would also enhance activation of the renin angiotensin system. Moreover, Ang II has been demonstrated to promote podocyte apoptosis in both in vitro and in vivo studies (Bird et al., 1989; Burns et al., 1999). On that account, both attenuation of production of Ang II by captopril as well as Ang II blockade have been reported to slow down the progression of renal lesions in Tg26 mice and HIVAN patients (Ding et al., 2002; Jia et al., 2008). To amplify the role of the renin angiotensin system we have used Tg26 mice with variable Agt copies.
The renin angiotensin system has been demonstrated to play an important role in the development and progression of renal lesions (Egido, 1996; Matsusaka et al., 1996). Modalities which either inhibit the generation of Ang II or block the effects of Ang II have been demonstrated to slow down the progression of HIVAN in both animal experimental models and humans (Bird et al., 1989; Burns et al., 1999; Kimmel et al., 2003). Additionally, inhibition of renin was also associated with retardation of the progression of HIVAN (Kumar et al., 2012). Moreover, infusion of Ang II in a mouse model of HIVAN accelerated the progression of HIVAN (Ideura et al., 2007). Furthermore, the activation of the RAS converted clinically occult HIVAN to overt HIVAN (Kumar et al., 2011). In the present study, too, the activation of the RAS in Tg26 mice was associated with more advanced renal lesions in the form of a higher number of sclerosed glomeruli. Interestingly, inhibition of the RAS predominantly reduced the number of sclerosed glomeruli.
In the present study, we observed a positive correlation between the number of Agt copies, the percentage of sclerosed glomeruli, and glomerular size. Tg26/Agt4 mice displayed glomeruli which were 30% larger than those of Tg26/Agt2 mice. Other investigators also reported an increase in glomerular volume in FSGS (Young et al., 2000; Hughson et al., 2002). It has been suggested that renal injury results in stimulation of compensatory growth stimuli in hyperperfusion of relatively preserved glomeruli, causing enlarged glomeruli. Since hyperperfusion of glomeruli is also the manifestation of the activation of the RAS, an increase in the number of larger glomeruli in mice with 4 Agt copies is consistent with our hypothesis. On the basis of this hypothesis, we speculate that enlarged glomeruli in mice with 4 Agt copies are destined to sclerose.
To make the diagnosis of HIVAN, one has to carry out a kidney biopsy (Atta, 2010). The average number of glomerular profiles required for a biopsy specimen to be considered adequate ranges from five to ten (Fuiano et al., 1996; Fogo, 2003; Hoy et al., 2006). Out of 10 profiles at least one has to be sclerosed in order for the diagnosis to be made, which makes 10% of the glomeruli. Because the number of sclerosed glomeruli is dependent on the severity of the disease we grouped mice according to severity of their glomerular disease: A) mice with less than 10% of sclerosed glomeruli and B) mice with ten or more percent of sclerosed glomeruli. Thirty three percent of mice belonged to group A and 67% to group B. In terms of biopsy specimens, 33% of mice would have no sclerosed glomeruli if the specimen contained a total of 10 or less glomerular profiles. In group B, 27% of glomeruli or one in every 3.7 were sclerosed and 7% of glomeruli or one in every 14 were collapsed (animals from group A were not considered because of negligible number of collapsed glomeruli in those mice, 0.6%). This means that the chances of having a sclerosed glomerulus in a biopsy sample are 3.7 times higher than having a collapsed one. In order to decide the diagnosis of collapsing glomerulopathy, there has to be at least one glomerulus showing collapsed glomerular capillaries along with podocyte hypertrophy and hyperplasia. These findings point out those that sclerosed glomeruli in the presence of microcystic dilatation of tubules may also be the manifestation of HIV-induced podocytopathy.
Based on our data and results, it appears that the number of sclerosed glomeruli correlate with the activation of the RAS in HIVAN. In terms of diagnosis of HIV-induced podocytopathy via biopsy specimen, there is more likelihood to find a sclerosed glomerulus than a collapsed one. On the other hand, during advanced HIV-induced podocytopathy there is more likelihood to have both collapsed and sclerosed glomeruli.
The presence of a single collapsed glomerulus qualifies for the diagnosis of HIVAN and the presence of sclerosed glomeruli does not alter its importance. However, data in the present study suggest that sclerosed glomeruli may also be part of a viral induced podocytopathy and more so in the presence of the activated renin angiotensin system. In that scenario the presence of FSGS needs to be considered to be part of a viral induced podocytopathy, more so if they are associated with microcystic dilatation of tubules.
Acknowledgments
This work was supported by grants RO1DK084910, RO1 DK083931 (PCS) from National Institutes of Health, Bethesda, MD.
Abbreviations
- RAS
Renin Angiotensin system
- Agt
Angiotensinogen
- HIVAN
HIV-associated nephropathy
References
- Atta MG. Diagnosis and natural history of HIV-associated nephropathy. Adv Chronic Kidney Dis. 2010;17:52–58. doi: 10.1053/j.ackd.2009.08.005. [DOI] [PubMed] [Google Scholar]
- Barisoni L, Kopp JB. Modulation of podocyte phenotype in collapsing glomerulopathies. Microsc Res Tech. 2002;57:254–262. doi: 10.1002/jemt.10084. [DOI] [PubMed] [Google Scholar]
- Berliner AR, Fine DM, Lucas GM, Rahman MH, Racusen LC, Scheel PJ, Atta MG. Observations on a cohort of HIV-infected patients undergoing native renal biopsy. Am J Nephrol. 2008;28:478–486. doi: 10.1159/000112851. [DOI] [PubMed] [Google Scholar]
- Bird JE, Durham SK, Giancarli MR, Gitliz PH, Pandya DG, Dambach DM, Mozes MM, Kopp B. Captopril prevents nephropathy in HIV-transgenic mice. J Am Soc Nephrol. 1989;9:1441–1447. doi: 10.1681/ASN.V981441. [DOI] [PubMed] [Google Scholar]
- Bourgoignie JJ, Pardo V. The nephropathology in human immunodeficiencyvirus (HIV-1) infection. Kidney Int. 1991;40 (suppl):S19–S23. [PubMed] [Google Scholar]
- Burns C, Paul SK, Toth IR, Siva L. Effect of angiotensin-converting enzyme inhibition in HIV-associated nephropathy. J Am Soc Nephrol. 1999;8:1140–1146. doi: 10.1681/ASN.V871140. [DOI] [PubMed] [Google Scholar]
- Cantor ES, Kimmel PL, Bosch JP. Effect of race on expression of acquired immunodeficiency syndrome-associated nephropathy. Arch Intern Med. 1991;151:125–128. [PubMed] [Google Scholar]
- Chan KT, Papeta N, Martino J, Zheng Z, Frankel RZ, Klotman PE, D’Agati VD, Lifton RP, Gharavi AG. Accelerated development of collapsing glomerulopathyin mice congenic for the HIVAN1 locus. Kidney Int. 2009;75:366–372. doi: 10.1038/ki.2008.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Agati V. Pathologic classification of focal segmental glomerulosclerosis. Semin Nephrol. 2003;23:117–134. doi: 10.1053/snep.2003.50012. [DOI] [PubMed] [Google Scholar]
- Dijkman H, Smeets B, van der Laak J, Steenbergen E, Wetzels J. The parietal epithelial cell is crucially involved in human idiopathic focal segmental glomerulosclerosis. Kidney Int. 2005;68:1562–1572. doi: 10.1111/j.1523-1755.2005.00568.x. [DOI] [PubMed] [Google Scholar]
- Dijkman HB, Weening JJ, Smeets B, Verrijp KC, van Kuppevelt TH, Assmann KK, Steenbergen EJ, Wetzels JF. Proliferating cells in HIV and pamidronate-associated collapsing focal segmental glomerulosclerosis are parietal epithelial cells. Kidney Int. 2006;70:338–344. doi: 10.1038/sj.ki.5001574. [DOI] [PubMed] [Google Scholar]
- Ding G, Kapasi AA, Franki N, Gibbons N, Kasinath BS, Singhal PC. Angiotensin II induces glomerular epithelial cell apoptosis. Am J Physiol. 2002;283:F173–F180. doi: 10.1152/ajprenal.00240.2001. [DOI] [PubMed] [Google Scholar]
- Egido J. Vasoactive hormones and renal sclerosis. Kidney Int. 1996;49:578–598. doi: 10.1038/ki.1996.82. [DOI] [PubMed] [Google Scholar]
- Fogo AB. Approach to renal biopsy. Am J Kidney Dis. 2003;42:826–836. [PubMed] [Google Scholar]
- Freedman BI, Kopp JB, Langefeld CD, Genovese G, Friedman DJ, Nelson GW, Winkler CA, Bowden DW, Pollak MR. The apolipoprotein L1 (APOL1) gene and nondiabetic nephropathy in African Americans. J Am Soc Nephrol. 2010;21:1422–1426. doi: 10.1681/ASN.2010070730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman EA, Tao TK. Disappearance of uremia due to heroin-associated nephropathy. Am J Kidney Dis. 1995;25:689–689. doi: 10.1016/0272-6386(95)90543-x. [DOI] [PubMed] [Google Scholar]
- Fuiano G, Comi N, Magri P, Sepe V, Balletta MM, Esposito C, Uccello F, Dal Canton A, Conte G. Serial morphometric analysis of sclerotic lesion ns in primary “focal” segmental glomerulosclerosis. Am Soc Nephrol. 1996;7:49–55. doi: 10.1681/ASN.V7149. [DOI] [PubMed] [Google Scholar]
- Gharavi AG, Ahmad T, Wong RD, Hooshyar R, Vaughn J, Oller S, Frankel RZ, Bruggeman LA, D’Agati VD, Klotman PE, Lifton RP. Mapping a locus for susceptibility to HIV-1-associated nephropathy to mouse chromosome 3. Proc Natl Acad Sci USA. 2004;101:2488–2489. doi: 10.1073/pnas.0308649100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howie AJ, Ferreira MAS, Adu D. Prognostic value of simple measurement of chronic damage in renal biopsy specimens. Nephrol Dial Transplant. 2001;16:1163–1169. doi: 10.1093/ndt/16.6.1163. [DOI] [PubMed] [Google Scholar]
- Hoy WE, Samuel T, Hughson MD, Nicol JL, Bertram JF. How many glomerular profiles must be measured to obtain reliable estimates of mean glomerular areas in human renal biopsies? J Am Soc Nephrol. 2006;17:556–563. doi: 10.1681/ASN.2005070772. [DOI] [PubMed] [Google Scholar]
- Hughson MD, Johnson K, Young RJ, Hoy WE, Bertram JF. Glomerular size and glomerulosclerosis: relationships to disease categories, glomerular solidification, and ischemic obsolescence. Am J Kidney Dis. 2002;39:679–688. doi: 10.1053/ajkd.2002.31980. [DOI] [PubMed] [Google Scholar]
- Ideura H, Hiromura K, Hiramatsu N, Shigehara T, Takeuchi S, Tomioka M, Sakairi T, Yamashita S, Maeshima A, Kaneko Y, Kuroiwa T, Kopp JB, Nojima Y. Angiotensin II provokes podocyte injury in murine model of HIV-associated nephropathy. Am J Physiol Renal Physiol. 2007;293:F1214–1221. doi: 10.1152/ajprenal.00162.2007. [DOI] [PubMed] [Google Scholar]
- Jia J, Ding G, Zhu J, Chen C, Liang W, Franki N, Singhal PC. Angiotensin II infusion induces nephrin expression changes and podocyte apoptosis. Am J Nephrol. 2008;28:500–507. doi: 10.1159/000113538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmel PL, Barisoni L, Kopp JB. Pathogenesis and treatment of HIV- associated renal diseases: lessons from clinical and animal studies, molecular pathologic correlations, and genetic investigations. Ann Intern Med. 2003;139:214–226. [PubMed] [Google Scholar]
- Kopp JB, Klotman ME, Adler SH, Bruggeman LA, Dickie P, Marinos NJ, Eckhaus M, Bryant JL, Notkins AL, Klotman PE. Progressive glomerulosclerosis and enhanced renal accumulation of basement membrane components in mice transgenic for human immunodeficiency virus type 1 genes. Proc Natl Acad Sci USA. 1992;89:1577–1581. doi: 10.1073/pnas.89.5.1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar D, Plagov A, Yadav I, Torri DD, Sayeneni S, Sagar A, Rai P, Adabala M, Lederman R, Chandel N, Ding G, Malhotra A, Singhal PC. Inhibition of Renin Activity Slows down the Progression of HIVAN. Am J Physiol Renal Physiol. 2012;303:F711–220. doi: 10.1152/ajprenal.00643.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar D, Salhan D, Magoon S, Torri DD, Sayeneni S, Sagar A, Bandhlish A, Malhotra A, Chander PN, Singhal PC. Adverse host factors exacerbate occult HIV-associated nephropathy. Am J Pathol. 2011;179:1681–92. doi: 10.1016/j.ajpath.2011.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu TC, He JC, Klotman P. Animal models of HIV- associated nephropathy. Curr Opin Nephrol Hypertens. 2006;15:233–237. doi: 10.1097/01.mnh.0000222688.69217.8e. [DOI] [PubMed] [Google Scholar]
- Matsusaka T, Hymes J, Ichikawa I. Angiotensin in progressive renal diseases: Theory and practice. J Am Soc Nephrol. 1996;7:2025–2043. doi: 10.1681/ASN.V7102025. [DOI] [PubMed] [Google Scholar]
- Möller CC, Pollak MR, Reiser The genetic basis of human glomerular disease. J Adv Chronic Kidney Dis. 2006;13:166–173. doi: 10.1053/j.ackd.2006.01.009. [DOI] [PubMed] [Google Scholar]
- Pardo V, Aldana M, Colton RM, Fischl MA, Jaffe D, Moskowitz L, Hensley GT, Bourgoignie JJ. Glomerular lesions in acquired immunodeficiency syndrome. Ann Intern Med. 1984;101:429–434. doi: 10.7326/0003-4819-101-4-429. [DOI] [PubMed] [Google Scholar]
- Post FA, Campbell LJ, Hamzah L, Collins L, Jones R, Siwani R, Johnson L, Fisher M, Holt SG, Bhagani S, Frankel AH, Wilkins E, Ainsworth JG, Larbalestier N, Macallan DC, Banerjee D, Baily G, Thuraisingham RC, Donohoe P, Hendry BM, Hilton RM, Edwards SG, Hangartner R, Howie AJ, Connolly JO, Easterbrook PJ. Predictors of renal outcome in HIV-associated nephropathy. Clin Infect Dis. 2008;46:1282–1289. doi: 10.1086/529385. [DOI] [PubMed] [Google Scholar]
- Rao TKS, Filppone EJ, Nicastri AD, Landesman SH, Frank E, Chen CK, Friedman EA. Associated focal segmental glomerulosclerosis in the acquired immunodeficiency syndrome. N Engl J Med. 1984;310:664–673. doi: 10.1056/NEJM198403153101101. [DOI] [PubMed] [Google Scholar]
- Salhan D, Husain M, Subrati A, Goyal R, Singh T, Rai P, Malhotra A, Singhal PC. HIV-induced kidney cell injury: role of ROS-induced downregulated vitamin D receptor. Am J Physiol Renal Physiol. 2012;303:F503–514. doi: 10.1152/ajprenal.00170.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szczech LA, Gupta SK, Habash R, Guasch A, Kalayjian R, Appel R, Fields TA, Svetkey LP, Flanagan KH, Klotman PE, Winston JA. The clinical epidemiology and course of the spectrum of renal diseases associated with HIV infection. Kidney Int. 2004;66:1145–1152. doi: 10.1111/j.1523-1755.2004.00865.x. [DOI] [PubMed] [Google Scholar]
- Valeri A, Barisoni L, Appel GB, Seigle R, D’Agati V. Idiopathic collapsing focal segmental glomerulosclerosis: a clinicopathologic study. Kidney Int. 1996;50:1734–1746. doi: 10.1038/ki.1996.493. [DOI] [PubMed] [Google Scholar]
- Weiss MA, Daquioag E, Margolin EG, Pollak VE. Nephrotic syndrome, progressive irreversible renal failure, and glomerular “collapse”: a new clinicopathologic entity? Am J Kidney Dis. 1986;7:20–28. doi: 10.1016/s0272-6386(86)80052-x. [DOI] [PubMed] [Google Scholar]
- Yadav A, Vallabu S, Arora S, Tandon P, Slahan D, Teichberg S, Singhal PC. ANG II promotes autophagy in podocytes. Am J Physiol Cell Physiol. 2010a;299:C488–C4896. doi: 10.1152/ajpcell.00424.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav A, Vallabu S, Kumar D, Ding G, Charney DN, Chander PN, Singhal PC. HIVAN phenotype: consequence of epithelial mesenchymal transdifferentiation. Am J Physiol Renal Physiol. 2010b;298:F734–F44. doi: 10.1152/ajprenal.00415.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young RJ, Hoy WE, Kincaid-Smith P, Seymour AE, Bertram JF. Glomerular size and glomerulosclerosis in Australian aborigines. Am J Kidney Dis. 2000;36:481–489. doi: 10.1053/ajkd.2000.9788. [DOI] [PubMed] [Google Scholar]