Abstract
Background
Conveying the complex trade-offs of continuous-flow left ventricular assist devices (CF LVAD) is challenging, and made more difficult by absence of an evidence summary for the full range of possible outcomes. We aimed to summarize the current evidence on outcomes of CF LVAD.
Methods and Results
PubMed and Cochrane Library were searched from January 2007–December 2013, supplemented with manual review. Three reviewers independently assessed each study for saliency regarding patient-centered outcomes. Data were summarized in tabular form. Overall study characteristics encouraged inclusion of all indications (destination therapy and bridge to transplant) and prevented meta-analysis. The electronic search identified 465 abstracts, of which 50 met inclusion criteria; manual review added 2 articles in press. The articles included 10 industry-funded trials and registries, 10 multi-center reports, and the remainder single-center observational experiences. Estimated actuarial survival after CF LVAD ranged from 56–87% at 1-year, 43–84% at 2-years, and 47% at 4-years. Improvements in functional class and quality of life were reported, but missing data complicated interpretation. Adverse events were experienced by the majority of patients, but estimates for bleeding, stroke, infection, right heart failure, arrhythmias, and rehospitalizations varied greatly.
Conclusions
The totality of data for CF LVADs show consistent improvements in survival and quality of life counterbalanced by a range of common complications. While this summary should provide a practical resource for health care provider-led discussions with patients, it highlights the critical need for high-quality patient-centered data collected with standard definitions.
Keywords: heart-assist device, heart failure, left ventricular assist device, health outcomes
Left ventricular assist devices (LVADs) are becoming an increasingly viable treatment option for patients with end-stage heart failure. Newer generation continuous-flow (CF) LVADs have taken the place of the first generation pulsatile-flow (PF) LVADs due to their smaller size and greater durability. Ideal informed consent and shared decision making for LVADs should be grounded in a thorough review of expected risks and benefits.1 This process should compare and contrast LVAD therapy to alternative approaches, and include not only estimates of survival but also major adverse events, health-related quality of life (HRQoL), symptom burden, functional limitations, and obligations for caregivers.2
Although a variety of trial and registry data are available to complement individual clinician experience and patient testimonials, there is currently no comprehensive systematic review of CF LVADs that attempts to summarize and organize available information into a practical format. Existing guidelines and standard consent forms do not provide such summary data with any degree of detail.3 This absence of accurate and easily accessible information from which to anchor risk-benefit communication leads to a potentially non-standardized and variable informed consent and decision-making process around LVADs that may be incomplete, confusing, or biased.4–5
Therefore, we aimed to summarize the current evidence on risks and benefits of CF LVADs. Our objectives were to: 1) capture contemporary clinical data regarding outcomes for patients offered CF LVADs; 2) describe the nature and quality of this evidence; 3) organize the data in a way that conveys the full range of expected outcomes for CF LVADs, with direct comparisons to outcomes without implantation; and 4) identify critical gaps in the scientific data that should be a priority of future research. Our primary goal was to provide a practical document that could guide a more standardized informed consent process and future development of decision aids for CF LVADs.
Methods
Search Strategy
Our methods directly adhered to the guidelines set forth in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement.6 We searched PubMed (MEDLINE) and the Cochrane Library full text databases for English-language studies concerning human subjects published between January 1, 2007 and December 13, 2013 related to CF LVAD outcomes. The year 2007 was chosen, as this was the time when data on contemporary CF LVADs started to become available. The search algorithm, developed and then replicated for accuracy by four members of the study team (CKM, KHM, JST, LAA), included a combination of Medical Subject Headings and free-text terms related to LVAD, CF, and associated outcomes such as survival, HRQoL, bleeding, stroke, or infection (see Supplemental Material: Figure 1). The original intent was to summarize data for LVAD used as destination therapy (DT); however, due to the complexities around indication reporting and because many aspects of LVAD therapy are applicable across indications, studies including both bridge to transplant (BTT) and DT were included. Where possible, care was taken to clearly identify and separate BTT-only and DT-only data within the review. We included all forms of research, such as meta-analyses, trials, retrospective and prospective studies and expert opinion papers without restriction by journal. The reference lists of selected studies were manually reviewed to identify any relevant studies that were potentially missed in the database search.
Study Selection
Eligible articles were presented in English and provided primary data about outcomes of CF LVADs. Two study team members independently reviewed all titles and abstracts for initial selection for inclusion (AVA, LAA). When the title and abstract provided insufficient information to determine study relevance, a full text copy of the article was retrieved and reviewed. For final selection, full text copies of all initially selected articles were examined for study eligibility.
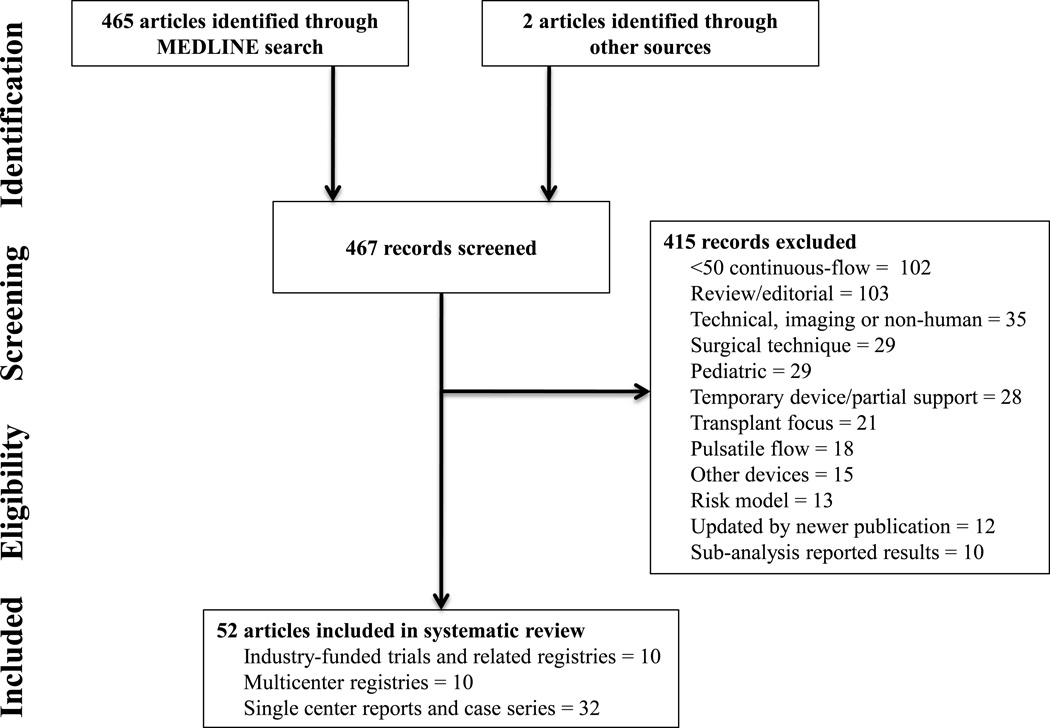
The following exclusion criteria were applied: studies that included less than 50 patients with CF devices, reviews or editorials on CF LVADs, studies that were non-human or focused on imagine with CF LVADs, studies that focused on surgical techniques, studies restricted to pediatric populations, studies that reported outcomes on temporary devices or partial support, studies with a transplant focus, studies that did not separate data based on CF versus PF device, studies reporting on other devices such as intra-aortic balloon pumps, studies reporting data on risk modeling and CF LVADs, studies that were updated by newer publications, and sub-analyses of previously reported results. Additionally, for serial publications updating longitudinal registry findings, we included only the most recent publication that addressed a specific outcome. We also excluded papers focused on risk modeling for which the overall outcome frequency for the same population was published elsewhere. Exceptions were made for evidence sources that were frequently referenced by more contemporary publications. See Figure 1 for a summary of evidence search and selection. For each article excluded, a single most obvious exclusion criterion was chosen, even though many articles met multiple exclusion criteria.
Figure 1.
Search Flow Diagram
Data Extraction and Quality Assessment
From each study, two independent reviewers (KHM, JST) extracted total patients, total CF patients, type of device (CF, PF), specific model of device (e.g. Thoratec HeartMate II (HMII), HeartWare HVAD), duration of follow-up or defined time at risk, population characteristics (BTT, DT), and outcome measures (survival, functional status, HRQoL, bleeding, neurological events, infection, device malfunction, right heart failure, arrhythmia, aortic insufficiency, renal failure, and rehospitalizaion). These outcomes were chosen by a group of clinicians, patients, and families as most important to CF LVAD therapy. Definitions for each outcome are included in Supplemental Material: Figure 2. A third reviewer (CKM) independently reviewed and confirmed all data abstracted. Disagreements were resolved through discussion by the entire study team. We aimed to include all relevant studies that reported outcomes for CF devices that met our stated inclusion and exclusion criteria. Assessment of quality was based on study design. The studies were categorized in the order of rigor: 1) industry-funded trials and related prospective registries, which included all controlled trials related to CF devices; 2) multicenter registries; and 3) single center reports and case series.
Data Synthesis
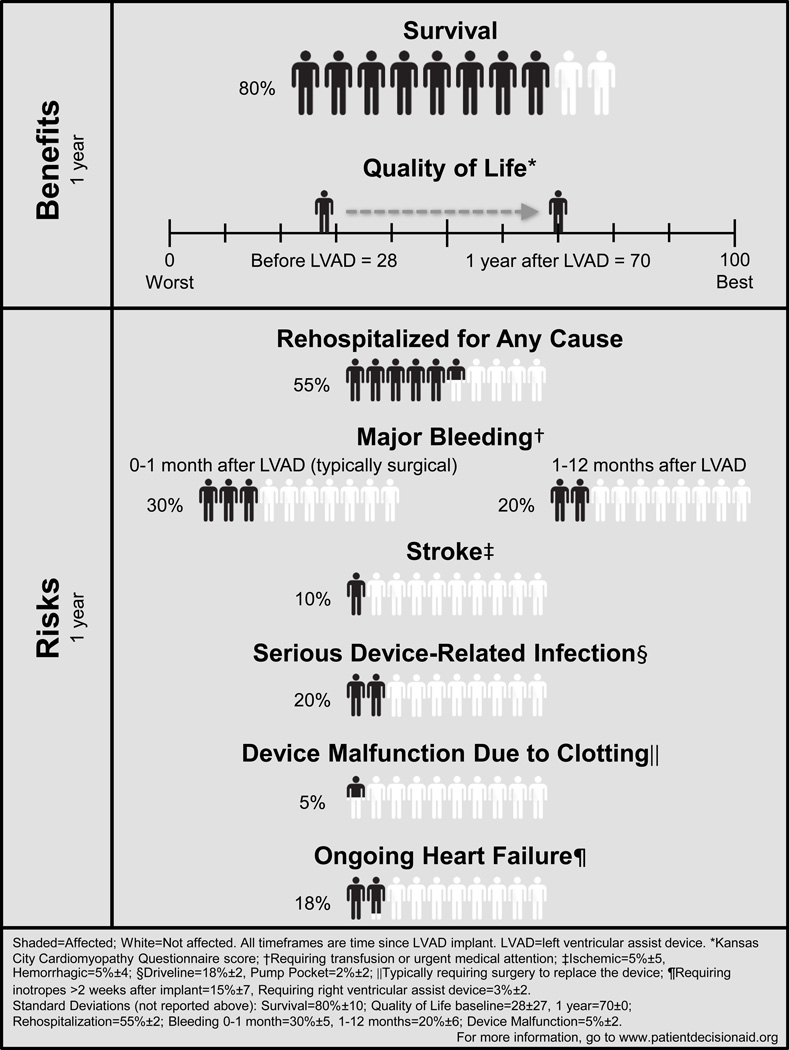
Data was summarized in tabular form. The resulting tables were organized according to the prospectively identified key patient-centered outcome domains of interest: survival, HRQoL and functional status, and adverse events. Where necessary, we manually calculated cumulative numbers when data was only reported separately for subgroups. When relevant data was only presented in graphic form, quantitative estimates were extracted and reported. Narrative syntheses of data were added to supplement the tables, incorporate nuanced information about data generation and quality, and provide a holistic summary. In order to create a clinically useful synopsis that may help facilitate communication with patients and families, we performed a crude analytic summary of the existing data. After reviewing the literature for all reported outcomes, we solicited feedback from physicians, physician assistants, nurse practitioners, nurses, a social worker, patients, and their families about which outcomes should be included in a summary figure. We consolidated hospitalization, bleeding, stroke, and infections, where appropriate, for ease of understanding. Event rates at 1-year were chosen as many studies did not report events past 1-year. Final outcomes are reported in Figure 2. We first collated the data from all studies for each outcome and subsequently removed redundant data. We excluded studies that did not report percentage data. Second, we applied the weighted average method based on total study sample and percentage reported. The weighted average method provides a summary treatment effect that more heavily emphasizes data reported from a registry with a large number of patients than a small single center study. It assumes fixed effects of CF LVAD across studies. The resultant weighted average included all trial, registry, and single-center studies and is reported as an estimated mean for all data. Finally, we applied the weighted average method to calculate the time period reported for each outcome. We were unable to calculate the summary effect sizes due to a variety of barriers, including the clinically diverse nature of the studies and lack of reporting standards.
Figure 2.
Simplified One-Year Outcomes Using Weighted Averages for Left Ventricular Assist Device (Combined Bridge-to-Transplant and Destination Therapy)
Results
The electronic search identified 465 titles and abstracts. An additional 2 studies related to pump thrombosis that were currently in press7–8 and not identified in the search were added. After inclusion and exclusion criteria were applied, a total of 52 full-text articles were included and reviewed (see Supplemental Material: Table 1).
The majority of studies were non-randomized, observational, small power, or single center. The studies included 10 industry-funded trials, 10 multi-center reports (many were different analyses of the same ongoing registry), and the remainder single-center observational experiences. A number of the multi-center registry publications that highlighted particular outcomes of interest were sub-analyses or post-hoc analyses of existing databases. Similarly, ongoing registries tended to be used for serial publications regarding individual outcomes.
Based on the data extracted, we identified 10 relevant patient-centered outcomes for which data was reported: survival, HRQoL, functional status, bleeding, neurological events, infection, device malfunction, right heart failure, arrhythmias, and rehospitalizations. All single-center studies and those with less than 100 CF LVAD patients are summarized in Supplemental Material: Tables 2–4. A summary of simplified one-year outcomes using weighted averages, including all data is provided in Figure 2. A pictograph was developed according to accepted patient communication methods9 and feedback from clinicians, nurses and patients was provided on content and readability.
Survival
(6 Industry Funded Trials and Related Registries10–14; 6 Multicenter Registries7,15–19; 9 Single Center Reports or Case Series. See Supplemental Material for all single-center studies and those with less than 100 CF LVAD patients.)
Survival is the outcome of dominant importance to the majority of patients.20 Extending life is a primary goal of LVADs, and therefore communicating statistics around mortality is critical. Although survival is an objective measure, characterizing long-term survival after LVAD implantation is complicated by a number of factors, including finite study time periods, patient loss to follow-up, and censoring of patients at the time of transplantation. Even in the DT population, a significant minority of patients will later become transplant eligible (approximately 10% in DT trial populations).12 Therefore, estimated actuarial survival is most often reported.
Most studies reported survival rates at times post-implantation ranging from 1 to 24 months, with less data beyond 2 years (Table 1). The pivotal HMII DT Trial showed estimated actuarial survival at 2 years of 58%.12 The Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) Fifth Annual Report showed 1, 12, 24, 36, and 48-month survival at 95%, 80%, 70%, 59% and 47%, respectively.19 These values represent some of the most recent data as well as some of the highest rates of survival for CF LVAD patients. The ADVANCE: HVAD BTT Trial Continued Access Protocol (CAP) also illustrated improved survival 6 months and 1 year after device implantation compared with trials performed in earlier years.14 However, to what extent progressive improvements in survival represent true improvements in the use of the device versus patient selection into less sick populations is unclear.
Table 1.
Estimated Actuarial Survival of CF LVAD - All Trial and Registry Data with Greater Than 100 CF Patients
| Study | First Author |
Year | Total CF |
BTT | DT | 1 Mo |
6 Mo |
12 Mo |
24 Mo |
36 Mo |
48 Mo |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Industry-Funded Trials and Related Registries | |||||||||||
| HMII BTT Trial10 | Miller | 2007 | 133 | 133 | 0 | 89% | 75% | 68% | - | - | - |
| HMII BTT Trial Registry11 | Pagani | 2009 | 281 | 281 | 0 | 92% | 82% | 73% | - | - | - |
| HMII DT Trial12 | Slaughter | 2009 | 133 | 0 | 133 | - | - | 68% | 58% | - | - |
| ADVANCE: HVAD BTT Trial13 | Aaronson | 2012 | 140 | 140 | 0 | 99% | 94% | 86% | - | - | - |
| ADVANCE: HVAD BTT Trial CAP14 | Slaughter | 2013 | 332 | 332 | 0 | 97% | 91% | - | 84% | - | - |
| Mutlicenter Registries | |||||||||||
| INCOR Analysis15 | Schmid | 2008 | 216 | - | - | - | - | 56% | 43% | - | - |
| 64 European Institutions16* | Lahpor | 2010 | 411 | 300 | 86 | - | 74% | 72% | - | - | - |
| U of Minnesota, Pittsburgh, & Columbia17 | Boyle | 2011 | 101 | 86 | 15 | 77% | - | - | - | - | - |
| John INTERMACS18 | John | 2011 | 1496 | 1496 | 0 | - | 89% | 85% | - | - | - |
| INTERMACS 201319 | Kirklin | 2013 | 5436 | 3742 | 1694 | 95% | - | 80% | 70% | 59% | 47% |
| INTERMACS Thrombosis7 | Kirklin | 2014 | 6910 | - | - | 95% | 87% | 80% | 69% | 58% | 46% |
See Supplemental Material for all single-center studies or those with less than 100 continuous flow patients
Study includes alternative indications for implant (e.g. bridge to decision, bridge to candidacy)
BTT=bridge to transplant; CAP=continued access protocol; CF=continuous-flow; DT=destination therapy; HMII= HeartMate II; HVAD=HeartWare ventricular assist device; INCOR=INCOR left ventricular assist device; INTERMACS=Interagency Registry for Mechanically Assisted Circulatory Support; mo=month; LVAD=left ventricular assist device
HRQoL and Functional Status
(6 Industry Funded Trials and Related Registries10–14,21; 1 Multicenter Registry19)
One of the main goals of LVAD implantation is to improve the HRQoL and functional capacity for patients with symptomatic heart failure. Improvements in New York Heart Association functional class are common after LVAD implantation. Approximately 80% of patients improved from New York Heart Association class IIIB or IV at baseline to New York Heart Association class I or II symptoms after LVAD in the HMII BTT and DT trials.10,12 In addition, instruments commonly used to assess HRQoL in heart failure patients that are often prospectively collected in LVAD studies include heart failure disease-specific measures such as the Minnesota Living with Heart Failure Questionnaire22, the Kansas City Cardiomyopathy Questionnaire23, as well as general measures such as the European Quality of Life-5 Dimensions.24 In addition to subscales of HRQoL questionnaires, functional status is most commonly assessed through 6-minute walk distance. Among patients who survive with LVADs, HRQoL measures improve markedly from baseline (Table 2). The collective HMII studies demonstrated significant improvements in Minnesota Living with Heart Failure Questionnaire and Kansas City Cardiomyopathy Questionnaire scores, as well as 6-minute walk distance from before surgery to all time points assessed after device implantation.10–12,21 Additionally, the most recent INTERMACS Annual Report demonstrated overall improvements in the European Quality of Life-5 Dimensions visual analog scale, and fewer patients identified themselves as having “extreme problems” with self-care and usual activities.19
Table 2.
HRQoL and Functional Status of CF Devices
| KCCQ - Scores range from 0 to 100. A higher score illustrates a better health status. | |||||||||||||
| Study | First Author | Year | # CF | Baseline* | # CF | 3 Mo* | # CF | 6 Mo* | # CF | 12 Mo* | # CF | 24 Mo* | |
| HMII BTT Trial10 | Miller | 2007 | 113 | 33±19 | 77 | 57±20 | - | - | - | - | - | - | |
| HMII BTT Trial Registry11 | Pagani | 2009 | 90 | 36±21 | - | - | 90 | 63±23 | - | - | - | - | |
| HMII DT Trial12 | Slaughter | 2009 | 115 | 27±16 | 89 | 63±19 | - | - | 76 | 66±20 | 47 | 70±19 | |
| HMII BTT DT Trial Registry21 | Rogers | 2010 | |||||||||||
| BTT | 226 | 26(m) | 167 | 58(m) | 119 | 60(m) | - | - | - | - | |||
| DT | 318 | 24(m) | 262 | 68(m) | 240 | 72(m) | 203 | 70(m) | 97 | 74(m) | |||
| ADVANCE: HVAD BTT Trial13 | Aaronson | 2012 | 128 | 35±19 | - | - | 70 | 67±21 | - | - | - | - | |
| ADVANCE: HVAD BTT Trial CAP14 | Slaughter | 2013 | 169 | 37±22 | - | - | 169 | 68±19 | - | - | - | - | |
| MLHFQ - Scores range from 0 to 105. A lower score illustrates a better quality of life. | |||||||||||||
| Study | First Author | Year | # CF | Baseline | # CF | 3 Mo | # CF | 6 Mo | # CF | 12 Mo | # CF | 24 Mo | |
| HMII BTT Trial10 | Miller | 2007 | 114 | 73±25 | 77 | 45±25 | - | - | - | - | - | - | |
| HMII BTT Trial Registry11 | Pagani | 2009 | 92 | 69±23 | - | - | 92 | 41±25 | - | - | - | - | |
| HMII DT Trial12 | Slaughter | 2009 | 116 | 75±18 | 89 | 37±22 | 76 | 34±22 | 44 | 30±22 | |||
| HMII BTT DT Trial Registry21 | Rogers | 2010 | |||||||||||
| BTT | 224 | 75(m) | 164 | 42(m) | 115 | 38(m) | - | - | - | - | |||
| DT | 323 | 75(m) | 258 | 34(m) | 234 | 32(m) | 197 | 32(m) | 90 | 34(m) | |||
| EQ-5D VAS - Scores ranges from 0 to 100, 0 representing the worst possible health state and 100 representing the best. | |||||||||||||
| Study | First Author | Year | # CF | Baseline | # CF | 3 Mo | # CF | 6 Mo | # CF | 12 Mo | # CF | 24 Mo | |
| ADVANCE: HVAD BTT Trial13 | Aaronson | 2012 | 130 | 40±24 | - | - | 72 | 70±20 | - | - | - | - | |
| ADVANCE: HVAD BTT Trial CAP14 | Slaughter | 2013 | 178 | 44±25 | - | - | 178 | 72±19 | - | - | - | - | |
| INTERMACS 201319 | Kirklin | 2013 | 852 | 41(m) | 528 | 69(m) | 466 | 73(m) | 281 | 72(m) | - | - | |
| 6MWD - Evaluates an individual’s functional exercise capacity by measuring the distance, in meters, he or she can walk in six minutes. | |||||||||||||
| Study | First Author | Year | # CF | Baseline | # CF | 3 Mo | # CF | 6 Mo | # CF | 12 Mo | # CF | 24 Mo | |
| HMII BTT Trial10 | Miller | 2007 | 25 | 42±97 | 56 | 292±212 | - | - | - | - | - | - | |
| HMII BTT Trial Registry11 | Pagani | 2009 | 14 | 201±140 | - | - | 109 | 347±179 | - | - | - | - | |
| HMII DT Trial12 | Slaughter | 2009 | 50 | 182±140 | 77 | 319±191 | 61 | 318±164 | 36 | 372±191 | |||
| HMII BTT DT Trial Registry21 | Rogers | 2010 | |||||||||||
| BTT | 38 | 214±215 | - | - | 97 | 372±199 | - | - | - | - | |||
| DT | 129 | 204±150 | - | - | 199 | 350±198 | - | - | 75 | 360±210 | |||
Time was reported variably among studies: mean/SD (x ± y), mean (x(m))
#=number; 6MWD=6 minute walk distance; BTT=bridge to transplant; CAP=continued access protocol; CF=continuous-flow; DT=destination therapy; EQ-5D=European Quality of Life-5 Dimensions; HRQoL=health-related quality of life; HMII=HeartMate II; HVAD= HeartWare ventricular assist device; INTERMACS=Interagency Registry for Mechanically Assisted Circulatory Support; KCCQ=Kansas City Cardiomyopathy Questionnaire; MLHFQ=Minnesota Living with Heart Failure Questionnaire; mo=month; VAS=Visual Analogue Scale.
It should be noted that HRQoL and functional status data has typically censored patients at the time of death, which can progressively enrich for a “healthier” population. Additionally, missing data for HRQoL and functional status measures are more common than for an outcome like survival, with 13–38% of patients unable to complete health status or functional assessments at a given time point.10,12 However this failure to complete health status assessments can itself be informative, with worse outcomes commonly seen in these patients.10,12 Finally, the HRQoL measures developed in patients with chronic heart failure may not perform as intended when applied to the LVAD population, given that many heart failure-related symptoms are traded for other unique symptoms and burdens.25
Common Adverse Events
Although LVADs offer patients the potential for improved survival, HRQoL, and functional status, there are also several risks associated with LVAD therapy. Some of the most commonly studied and reported major adverse events following LVAD implantation are bleeding, neurological events, and infection. Definitions for all adverse events are included in Supplemental Material: Figure 2.
Bleeding
(7 Industry Funded Trials and Related Registries10–14; 3 Multicenter Registries16,18–19; 14 Single Center Reports or Case Series. See Supplemental Material for all single-center studies and those with less than 100 CF LVAD patients.) Bleeding is the most commonly recorded adverse event of CF LVADS11–12,19, with the majority of patients in all published cohorts experiencing some type of bleeding. When possible, bleeding rates are reported by <30 days (early) and >30 days (late) in order to help differentiate post-operative bleeding from non-surgical bleeding. A recent study of 139 HMII patients showed the greatest risk of bleeding was within the first 2 weeks post-operatively and early bleeding was associated with decreased survival.26 Later gastrointestinal bleeding is reported with rates as high as 13% in the ADVANCE: HVAD BTT Trial CAP.14 A single-center study of 86 HMII patients reported gastrointestinal bleeding as a frequent source of morbidity for patients but not a factor that significantly impacts survival.27
Neurological Events
(7 Industry Funded Trials and Related Registries10–14; 4 Multicenter Registries15–16,18,19; 9 Single Center Reports or Case Series. See Supplemental Material for all single-center studies and those with less than 100 CF LVAD patients.) Neurological events, including ischemic stroke, hemorrhagic stroke, and transient ischemic attack, are relatively common and often severe complications following LVAD placement. In the INTERMACS Annual Report, there was a 3% risk of stroke at 1 month, 5% at 3 months, 7% at 6 months, 11% at 12 months, 17% at 24 months, and 19% at 36 months post-implant.19 Similarly, the HMII DT Trial showed rates of ischemic and hemorrhagic stroke as high as 8% and 11%, respectively, in the first 2 years after LVAD placement, with hemorrhagic stroke being the leading cause of death among patients with a CF LVAD.12 The reported rates of stroke from other reports is quite variable and likely reflects differences in study follow-up time and the patient population studied; however, the overall annual risk of stroke in CF LVAD patients appears to be substantial.
Infection
(7 Industry Funded Trials and Related Registries10–14; 4 Multicenter Registries16,18–19,28; 9 Single Center Reports or Case Series. See Supplemental Material for all single-center studies and those with less than 100 CF LVAD patients.) Patients are at increased risk of bacterial infections following LVAD implantation, occurring at the driveline, pump pocket, or systemically. Driveline infections in the International HVAD Trial are reported at 18% at 1-year29, while the HMII BTT trial and trial registry report rates as high as 14% at 6 months.10–11 Developing any type of infection is associated with decreased survival and quality of life;28 in one cohort, 2-year cumulative survival rate was 67% for patients with infections and 81% in those without.30
A summary of these outcomes, including all trial and registry data with greater than 100 CF LVAD patients, is provided in Table 3.
Table 3.
Common Adverse Events of CF LVAD - All Trial and Registry Data with Greater Than 100 CF Patients*
| Bleeding | Neurological Event | Infection | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study | First Author | Year | Total CF | BTT | DT | Defined Time at Risk‖ |
Early ≤30 days | Late > 30 days | GI | Ischemic | Hemorrhagic | TIA | Other | Local | Driveline | Sepsis | Other | |
| Industry-Funded Trials and Related Registries | ||||||||||||||||||
| HMII BTT Trial10† | Miller | 2007 | 133 | 133 | 0 | 6 mo | 75% | 8% | - | 6% | 2% | 4% | 6% | 28% | 14% | 0% | 20% | - |
| HMII BTT Trial Registry11† | Pagani | 2009 | 281 | 281 | 0 | 6 mo | 69% | 23% | - | 5% | 3% | 2% | 5% | 30% | 14% | 2% | 17% | - |
| HMII DT Trial12† | Slaughter | 2009 | 133 | 0 | 133 | 24 mo | 111% | - | 8% | 11% | - | 22% | 49% | - | - | 36% | 35% | |
| ADVANCE: HVAD BTT Trial13‡ | Aaronson | 2012 | 140 | 140 | 0 | 6 mo | 26% | 11% | 11% | 7% | 6% | 4% | - | - | 12% | - | 11% | - |
| ADVANCE: HVAD BTT Trial CAP14‡ | Slaughter | 2013 | 332 | 332 | 0 | 3-36 mo | 15% | 14% | 13% | 8% | 8% | 5% | - | - | 17% | - | 17% | - |
| Mutlicenter Registries | ||||||||||||||||||
| INCOR Analysis15 | Schmid | 2008 | 216 | - | - | 48 mo | - | - | - | 18% | 8% | - | - | - | - | - | - | - |
| 64 European Institutions16§ | Lahpor | 2010 | 184 | - | - | 8±7 mo | 52% | - | 4% | 2% | 4% | - | 20% | 22% | 5% | 30% | - | |
| John INTERMACS18‡ | John | 2011 | 1496 | 1496 | 0 | 9±7 mo | 36% | 10% | 4% | 2% | - | 4% | - | 13% | 2% | 3% | 26% | |
| Goldstein INTERMACS28ठ| Goldstein | 2012 | 2006 | 830 | 291 | 12 mo | - | - | - | - | - | - | - | 23% | 19% | - | 20% | - |
| INTERMACS 201319‡ | Kirklin | 2013 | 5358 | - | - | 12 mo | 9.5/100 pt-mo | 1.8/100 pt- mo |
- | - | 8.0/100 pt-mo | |||||||
Adverse events definitions in Supplemental Material: Figure 2.
See Supplemental Material for all single-center studies or those with less than 100 continuous flow patients
Study uses HeartMate II definitions for adverse events.
Study uses INTERMACS definitions for adverse events.
Study includes alternative indications for implant (i.e. bridge to decision, bridge to candidacy).
Time at risk was variable among studies: truncated time point (x mo), mean/SD (x ± mo), range (x–y mo).
BTT=bridge to transplant; CAP=continued access protocol; CF=continuous-flow; DT=destination therapy; GI=gastrointestinal; HMII= HeartMate II; HVAD=HeartWare ventricular assist device; INCOR=INCOR left ventricular assist device; INTERMACS=Interagency Registry for Mechanically Assisted Circulatory Support; mo=month; TIA=transient ischemic attack
Other Adverse Events
Although bleeding, neurological events, and infection are dominant in adverse events reporting, there are additional complications with CF LVAD therapy that can be equally devastating or have an impact on HRQoL (Table 4).
Table 4.
Other Adverse Events of CF LVAD - All Trial and Registry Data with Greater Than 100 CF Patients*
| Device Malfunction |
Right Heart Failure |
Arrhythmia | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study | First Author | Year | Total CF Pts | BTT | DT | Defined Time at Risk‖ | Thrombosis Requiring Exchange |
Other Requiring Exchange |
Inotropic Support | RVAD | VA | Other | Rehospitalization |
| Industry-Funded Trials and Related Registries | |||||||||||||
| HMII BTT Trial10† | Miller | 2007 | 133 | 133 | 0 | 6 mo | 2% | 2% | 13% | 4% | 24% | - | 41% |
| HMII BTT Trial Registry11† | Pagani | 2009 | 281 | 281 | 0 | 18 mo | 2% | 3% | 13% | 6% | 20% | - | 68% |
| HMII DT Trial12† | Slaughter | 2009 | 133 | 0 | 133 | 24 mo | 2% | 8% | 20% | 4% | 56% | 94% | |
| HMII BTT Trial Registry-RVF33† | Kormos | 2010 | 484 | 484 | 0 | 12 mo | - | - | 14% | 6% | - | - | - |
| ADVANCE: HVAD BTT Trial13‡ | Aaronson | 2012 | 140 | 140 | 0 | 6 mo | 2% | 5% | 16% | 3% | 21% | 20% | - |
| ADVANCE: HVAD BTT Trial CAP14‡ | Slaughter | 2013 | 332 | 332 | 0 | 3–36 mo | 4% | 5% | 25% | 3% | 21% | 21% | - |
| Multicenter Registries | |||||||||||||
| 64 European Institutions16§ | Lahpor | 2010 | 184 | - | - | 8±7 mo | 2% | - | 20% | 32% | - | - | |
| John INTERMACS18‡ | John | 2011 | 1496 | 1496 | 0 | 9±7 mo | 10% | 12% | 1% | 28% | - | 50% | |
| Holman INTERMACS31‡ | Holman | 2013 | 2816 | - | - | 36 mo | 0.9% | 0.8% | - | - | - | - | - |
| INTERMACS 201319‡ | Kirklin | 2013 | 5358 | - | - | 12 mo | 1.6/100 pt- mo |
1.8/100 pt- mo |
4.7/100 pt- mo |
- | |||
| INTERMACS Thrombosis7‡ | Kirklin | 2014 | 6910 | - | - | 12 mo | 5% | - | - | - | - | - | - |
| Najjar ADVANCE BTT & CAP8‡ | Najjar | 2014 | 382 | 382 | - | 12 mo | 5% | - | - | - | - | - | - |
| Cleveland Clinic, Barnes-Jewish, & Duke32 |
Starling | 2014 | 837 | - | - | 8-11 mo | 4% | - | - | - | - | - | - |
Adverse events definitions in Supplemental Material: Figure 2.
See Supplemental Material for all single-center studies or those with less than 100 continuous flow patients
Study uses HeartMate II definitions for adverse events.
Study uses INTERMACS definitions for adverse events.
Study includes alternative indications for implant (i.e. bridge to decision, bridge to candidacy)
Time at risk was variable among studies: truncated time point (x mo), mean/SD (x ± y mo), range (x–y mo), mean (x mo(m))
BTT=bridge to transplant; CAP=continued access protocol; CF=continuous-flow; DT=destination therapy; HMII= HeartMate II; HVAD=HeartWare ventricular assist device; INTERMACS=Interagency Registry for Mechanically Assisted Circulatory Support; mo=month; pt=patient; RVAD=right ventricular assist device; RVF=right ventricular failure; VA=ventricular arrhythmia; yr=year.
Device Malfunction
(7 Industry Funded Trials and Related Registries10–14; 7 Multicenter Registries7–8,12,16,18,31–32; 4 Single Center Reports or Case Series. See Supplemental Material for all single-center studies and those with less than 100 CF LVAD patients.) Device malfunction is a serious adverse event of LVADs, as treatment usually requires reoperation and its attendant risks. There are several causes of device malfunction, including thrombus formation with hemolysis, mechanical failure of the impeller, and driveline lead fractures with electrical failure. In earlier reports, the highest rate of thrombosis requiring pump exchange was in the International HVAD Trial at 8% at 2 years.29 However, a recent study from three high volume centers, including 837 HMII patients, shows an increase in the rate of confirmed pump thrombosis at 3 months post-implant from 2.2% before March 2011 to 8.4% by January 2013.32 Analysis of the multicenter INTERMACS registry also confirmed a temporal increase in the rates of HMII thrombosis.7 Temporal changes in thrombosis rates were not seen in a recent analysis of the HeartWare HVAD.8 These recent studies illustrate the dynamic potential for rates of adverse events over time, possibly reflecting changes in device technology, patient selection, surgical technique, and post-implantation management.
Right Heart Failure
(8 Industry Funded Trials and Related Registries10–14,33; 3 Multicenter Registries16,18–19; 7 Single Center Reports or Case Series. See Supplemental Material for all single-center studies and those with less than 100 CF LVAD patients.) Right heart failure following LVAD implantation contributes to increased post-operative morbidity and mortality. Outcomes of LVAD patients are dependent on right heart function due to the necessity of adequate flow through the pulmonary circuit to the left heart. In the HMII DT Trial, 20% of patients received extended inotropic therapy for persistent right heart failure and 4% required placement of a right ventricular assist device.12 In the ADVANCE: HVAD BTT Trial CAP, 25% of patients became dependent on inotropic therapy and 3% required a right ventricular assist device.14 The HMII BTT Trial-Registry and an analysis of 484 patients enrolled in the HMII BTT Trial reported right heart failure post-LVAD implantation is associated with a marked reduction in rates of survival and longer hospital length of stay.10,33
Cardiac Arrhythmias
(6 Industry Funded Trials and Related Registries10–14; 3 Multicenter Registries16,18–19; 5 Single Center Reports or Case Series. See Supplemental Material for all single-center studies and those with less than 100 CF LVAD patients.) Cardiac arrhythmias, both ventricular and supraventricular, can develop after LVAD implantation. A study of 184 HMII devices showed an incidence of ventricular arrhythmias up to 32% post-LVAD.16 Studies have reported conflicting associations of ventricular arrhythmias with survival. A study of 61 patients reported patients with post-LVAD ventricular arrhythmias had a significantly increased risk of mortality.34 In contrast, another cohort of 61 patients showed post-LVAD ventricular arrhythmias had no association with survival but did have greater morbidity, as patients with post-LVAD ventricular arrhythmias had greater rehospitalizaion rates.35 In addition, patients with post-LVAD ventricular arrhythmias had higher rates of appropriate (31%) and inappropriate (15%) defibrillator shocks.35
Rehospitalizations
(4 Industry Funded Trials and Related Registries10–12; 1 Multicenter Registries18; 7 Single Center Reports or Case Series. See Supplemental Material for all single-center studies and those with less than 100 CF LVAD patients.) Recurrent hospital admissions are a common occurrence in LVAD patients. One trial reported readmission rates as high as 94%12, and another reports up to 1.2 admissions per patient year.29 One study of 71 CF LVAD patients found that patients are most often readmitted within 6 months of discharge, with gastrointestinal bleeding as the most common cause.36
Additional Adverse Events
Renal function, neurocognitive function, and aortic insufficiency are additional patient-centered outcomes identified in our electronic search. There are limited studies that report this data; therefore, the outcomes were not included in the tables. Remaining patient-centered outcomes included the following: 1) one study of 107 HMII patients found that 15% required some form of renal replacement therapy post-LVAD implantation;37 2) one study of 96 HMII patients showed no change or improvement in neurocognitive testing post-LVAD implantation;38 and 3) one study of 58 HMII patients showed that aortic insufficiency of the native valve progresses with the duration of LVAD support.39
A summary of these outcomes, including all trial and registry data with greater than 100 CF LVAD patients, is provided in Table 4.
Discussion
Permanent mechanical circulatory support in the form of a CF LVAD offers the potential to fundamentally change the clinical course of severe heart failure. Existing evidence shows that, for populations of carefully selected patients, LVADs improve survival, HRQoL, and functional status. Because outcomes in these domains are so dismal without an LVAD, the absolute benefit is marked, with number needed to treat that dwarfs most existing medical therapies. However, despite these dramatic improvements in survival and heart failure symptoms, LVAD therapy remains associated with a significant residual risk of mortality after implantation and the potential for major adverse events in a significant percentage of patients. Thus, for eligible patients, whether to pursue these therapies should involve a careful assessment of the totality of expected risks and benefits over time and how these may relate to a patient’s values, goals, and preferences.
Given the complex tradeoffs of LVAD therapy, necessary components for shared decision making around CF LVAD must include optimal patient selection, extensive informed consent, and adequate time to review expected risks, benefits, and burdens. To ensure this, LVAD programs are required by the Joint Commission to have a process around education, decision making, and informed consent. While the ethical mandate surrounding informed consent is clear,2 achieving true informed consent in this setting is challenging.1 Additionally, providing accurate information to patients in an understandable format to facilitate shared-decision making is complicated.
There have been other reviews regarding LVAD outcomes40; however, there has been no major attempt at systematically reviewing this data according to accepted scientific standards in a patient-focused manner. Several studies have found that graphical presentation of risks and benefits enhances understanding of statistics for patients and families of varying numeracy and literacy skills.41 The pictograph developed from this systematic review (Figure 2) provides patients, their families, and clinicians an estimate of the full range of CF LVAD outcomes based on weighted averages of all trial, registry, and single-center data published to date. Research has shown that patients differ in their decision-making processes, with some patients viewing survival and quality of life as paramount while others weigh risks and burdens more heavily.20, 42 In response to this, the pictograph provides both benefits and risks; therefore, patients can weigh those outcomes they feel are most important to them. This pictograph could be easily incorporated into existing educational materials and informed consent documents. While the pictograph does not provide tailored risk estimates and does not show measures of uncertainty for individual patients, we feel it is a significant improvement over existing educational materials.43
The importance of considering tradeoffs for LVAD therapy is particularly salient to the subgroup of patients with a DT indication, who should expect to live the remainder of their life dependent on an LVAD and for whom the reasons making them transplant ineligible will usually persist (advanced age, comorbidity, and frailty). As such, the initial goal of this systematic review was to summarize the risks and benefits of DT LVAD therapy. However, the overall paucity of studies reporting outcomes confined to patients implanted with a DT indication—and the intermixing of DT and BTT patients in some reporting—led to the inclusion of data from BTT populations as well. This allowed for a wider range of evidence; however, the data for BTT patients is not ideal data for informed decision making around DT LVAD. The two indications yield different populations and different outcomes for a variety of reasons, including the temporary nature of BTT and the younger age and lower comorbidity of the BTT-eligible population. This limitation is not peculiar to the systematic review, but rather to the state of LVAD research and reporting. Where reported, we have recognized the DT versus BTT indication to help refine interpretation.
As only one device is currently approved in the United States for the DT indication, and only one large high-quality trial (HMII DT Trial) was conducted to describe outcomes in this setting, clinicians and patient educational materials for DT often default to the event rates reported from this study.12 This approach is reasonable given possible threats to scientific validity from lower quality observational data. However, the HMII DT Trial has a number of limitations that encourage supplementation from other sources. Although a randomized controlled trial, the study compared CF DT LVAD to the older PF LVAD, not to a control group of optimal medical therapy patients (OMT). Additionally, this comparison was un-blinded. The trial included only 133 patients in the HMII CF device arm, such that estimates of rare events are potentially unstable. Further, the randomized controlled trial setting, with highly selected patients treated at a limited number of participating centers, may not be particularly generalizable to the usual care setting. Recent reports of higher than expected pump thrombosis rates illustrate these potential concerns.7,32 Therefore, systematically reviewing all of the published literature for individual outcomes and then summarizing those estimates in tabular form hopefully offers a more complete picture of the expected range of risks and benefits that clinicians can convey to patients considering LVAD.
In addition to summarizing existing data, the conduct of this systemic review also clarifies deficiencies in the sum of existing data. Data regarding survival for DT eligible patients who decline LVAD and continue with OMT are relatively sparse and dated. All studies showed a higher survival rate at every increment of time for patients who received an LVAD as compared to patients who were treated with OMT.44–46 Further, the COSI trial showed a dismal 6% survival at one year for patients with end-stage heart failure managed with continuous inotropic support.46 With ranges in 2-year survival of 60–70% for CF DT LVAD12 and 6–8% for OMT44, the number needed to treat to save one life over a 2-year span is less than 2 patients. Although survival is significantly improved with LVAD implantation, the absolute rates of death remain relatively high with less than half of patients still alive 4 years after implantation.19 With improvements in technology, surgical technique, peri-operative care, and patient selection,47 summary data suggest that for the near term, CF DT LVAD patients should expect actuarial survival rates in the 2-years after implantation on the order of 65–75%. BTT survival rates are better, with 2-year survival reported as high as 79–84%.14,29 In a perfect world, studies would randomly compare DT LVADs to OMT. While randomizing patients to this comparison is essentially unfeasible in most contexts, obtaining additional data on outcomes among OMT populations is possible. The recently launched Medical Arm of INTERMACS (MEDAMACS)48 and Randomized Evaluation of VAD InterVEntion before Inotropic Therapy (REVIVE-IT)49 studies should provide additional data regarding the outcomes of OMT in a group of non-inotrope dependent advanced heart failure patients. Additionally, due to variation in outcomes by indication, future studies should aim to separate data collection and outcomes reporting based on BTT versus DT implantation status. Patients who decline DT LVAD may also provide important comparator information.
Furthermore, lack of rigorous standard methodology decreases the utility of study information. The non-uniform reporting of event rates limits the ability to pool data across studies. While events per patient time may be statistically sound, it complicates interpretation for patients with lower numeracy. Similarly, there is no standardized or patient-centered method for accounting for loss to follow up (e.g. death during longitudinal measures of quality of life) or alternative end points (e.g. transplantation when calculating survival in DT). Additionally, with standardized reporting, summarizing data for longitudinal outcomes reporting would be less problematic. Finally, due to the rapid evolution of mechanical circulatory support, flexible and efficient systems must be developed to help patients and their health care providers stay updated on contemporary rates of outcomes. Ultimately, not only is there a desperate need for additional high-quality data, but this data must be collected using standard event definitions, such as the set used by the INTERMACS registry, and reported in units that are mostly clinically meaningful.
Limitations
In addition to the above issues with mixing of BTT and DT, data quality, and non-standardized reporting, there are several additional limitations that deserve recognition. First, the vast amount of data was difficult to present in a complete and simultaneously understandable format, such that some detail was sacrificed for ease of interpretation, both in terms of excluding smaller studies and in simplifying the studies that were included. Second, we included all data on outcomes of CF LVADs without a rigorous exclusion process based on the quality of the study; therefore, interpretation of the data should be guided by the categorized rigor of the study. In depth review of individual studies may ultimately be required by individual health care providers to better answer nuanced clinical questions.
Conclusion
The totality of data for CF LVADs show consistent improvements in survival and quality of life counterbalanced by a range of common complications. While this summary of outcomes data for CF LVAD should help frame consent, education, and decision making around durable mechanical circulatory support, future action is required to remedy the lack of high quality data for CF LVAD, particularly in the DT setting. Additional randomized controlled trials with larger patient populations are needed to further examine survival, HRQoL and frequency of complications. Supplementary trials that report outcomes for a timeline that mirrors the lifespan of DT patients are also necessary. Further, registries for patients on OMT who decline LVAD therapy are critical. An exponential expansion in LVAD use without such information is likely to diminish the overall value that patients and society can derive from this remarkable therapy.
Supplementary Material
Acknowledgements
The authors would like to thank Molly Magid for providing comments and revisions on the manuscript.
Sources of Funding
Dr. Ambardekar is supported by a Scientist Development Grant from the American Heart Association and by the Boettcher Foundation’s Webb-Waring Biomedical Research Program.
Dr. Matlock is supported by a career development award from the National Institutes on Aging (K23AG040696). Dr. Matlock is also supported by the University of Colorado Department of Medicine Early Career Scholars Program.
Dr. Allen is supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under Award Number K23HL105896. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Dr. Allen is also supported by the University of Colorado Department of Medicine Early Career Scholars Program.
Footnotes
Disclosures
None.
References
- 1.Allen LA, Stevenson LW, Grady KL, Goldstein NE, Matlock DD, Arnold RM, Cook NR, Felker GM, Francis GS, Hauptman PJ, Havranek EP, Krumholz HM, Mancini D, Riegel B, Spertus JA. Decision making in advanced heart failure: a scientific statement from the American Heart Association. Circulation. 2012;125:1928–1952. doi: 10.1161/CIR.0b013e31824f2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beauchamp TL, Childress JF. Principles of Biomedical Ethics. 6th edition. Oxford: Oxford University Press; 2008. [Google Scholar]
- 3.Feldman D, Pamboukian SV, Teuteberg JJ, Birks E, Lietz K, Moore SA, Morgan JA, Arabia F, Bauman ME, Buchholz HW, Deng M, Dickstein ML, El-Banayosa A, Elliot T, Goldstein DJ, Grady KL, Jones K, Hryniewicz K, John R, Kaan A, Kusne S, Loebe M, Massicotte MP, Moazami N, Mohacsi P, Mooney M, Nelson T, Pagani F, Perry W, Potapov EV, Eduardo Rame J, Russell SD, Sorensen EN, Sun B, Strueber M, Mangi AA, Petty MG, Rogers J. The 2013 International Society for Heart and Lung Transplantation Guidelines for mechanical circulatory support: executive summary. J Heart Lung Transplant. 2013;32:157–187. doi: 10.1016/j.healun.2012.09.013. [DOI] [PubMed] [Google Scholar]
- 4.Peters E, Västfjäll D, Slovic P, Mertz CK, Mazzocco K, Dickert S. Numeracy and decision making. Psychological Science. 2006;17:408–414. doi: 10.1111/j.1467-9280.2006.01720.x. [DOI] [PubMed] [Google Scholar]
- 5.Matlock DD, Nowels CT, Masoudi FA, Sauer WH, Bekelman DB, Main DS, Kutner JS. Patient and cardiologist perceptions of decision making for implantable cardioverter-defibrillators: a qualitative study. Pacing Clin Electrophysiol. 2001;34:1634–1644. doi: 10.1111/j.1540-8159.2011.03237.x. [DOI] [PubMed] [Google Scholar]
- 6.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kirklin JK, Naftel DC, Kormos RL, Pagani FD, Myers SL, Stevenson LW, Acker MA, Goldstein DL, Silvestry SC, Milano CA, Timothy Baldwin J, Pinney S, Eduardo Rame J, Miller MA. Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) analysis of pump thrombosis in the Heartmate II left ventricular assist device. J Heart Lung Transplant. 2014;33:12–22. doi: 10.1016/j.healun.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 8.Najjar SS, Slaugther MS, Pagani FD, Starling RC, McGee EC, Eckman P, Tatooles AJ, Moazami N, Kormos RL, Hathaway DR, Najarian KB, Bhat G, Aaronson KD, Boyce SW. An analysis of pump thrombus events in patients in the HeartWare ADVANCE bridge to transplant and continued access protocol trial. J Heart Lung Transplant. 2014;33:23–34. doi: 10.1016/j.healun.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 9.Fagerlin A, Zikmund-Fisher BJ, Ubel PA. Helping patients decide: ten steps to better risk communication. J Natl Cancer Inst. 2011;103:1436–1443. doi: 10.1093/jnci/djr318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miller LW, Pagani FD, Russell SD, John R, Boyle AJ, Aaronson KD, Conte JV, Naka Y, Mancini D, Delgado RM, MacGillvray TE, Farrar DJ, Frazier OH. Use of a continuous-flow device in patients awaiting heart transplantation. N Engl J Med. 2007;357:885–896. doi: 10.1056/NEJMoa067758. [DOI] [PubMed] [Google Scholar]
- 11.Pagani FD, Miller LW, Russell SD, Aaronson KD, John R, Boyle AJ, Conte JV, Bogaev RC, MacGillvray TE, Naka Y, Mancini D, Massey HT, Chen L, Klodell CT, Aranda JM, Moazami N, Ewald GA, Farrar DJ, Frazier OH. Extended mechanical circulatory support with a continuous-flow rotary left ventricular assist device. J Am Coll Cardiol. 2009;54:312–321. doi: 10.1016/j.jacc.2009.03.055. [DOI] [PubMed] [Google Scholar]
- 12.Slaughter MS, Rogers JG, Milano CA, Russell SD, Conte JV, Feldman D, Sun B, Tatooles AJ, Delgado RM, 3rd, Long JW, Wozniak TC, Ghumman W, Farrar DJ, Frazier OH. Advanced heart failure treated with continuous-flow left ventricular assist device. N Engl J Med. 2009;361:2241–2251. doi: 10.1056/NEJMoa0909938. [DOI] [PubMed] [Google Scholar]
- 13.Aaronson KD, Slaughter MS, Miller LW, McGee EC, Cotts WG, Acker MA, Jessup ML, Gregoric ID, Loyalka P, Frazier OH, Jeevanandam V, Anderson AS, Kormos RL, Teuteberg JJ, Levy WC, Naftel DC, Bittman RM, Pagani FD, Hathaway DR, Boyce SW. Use of an intrapericardial, continuous-flow, centrifugal pump in patients awaiting heart transplant. Circulation. 2012;125:3191–3200. doi: 10.1161/CIRCULATIONAHA.111.058412. [DOI] [PubMed] [Google Scholar]
- 14.Slaughter MS, Pagani FD, McGee EC, Birks EJ, Cotts WG, Gregoric I, Howard Frazier O, Icenogle T, Najjar SS, Boyce SW, Acker MA, John R, Hathaway DR, Najarian KB, Aaronson KD. HeartWare ventricular assist system for bridge to transplant: combined results of the bridge to transplant and continued access protocol trial. J Heart Lung Transplant. 2013;32:675–683. doi: 10.1016/j.healun.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 15.Schmid C, Jurmann M, Birnbaum D, Colombo T, Falk V, Feltrin G, Garatti A, Genoni M, Gerosa G, Gottel P, Gummert J, Halfmann R, Hammel D, Hennig E, Kaufmann F, Lanfranconi M, Meyns B, Mohr F, Muller J, Nikolov D, Rucinskas K, Scheld HH, Schmid FX, Schneider M, Sirvydis V, Tandler R, Vitali E, Vlasselaers D, Weyland M, Wilhelm M, Hetzer R. Influence of inflow cannula length in axial-flow pumps on neurologic adverse event rate: results from a multi-center analysis. J Heart Lung Transplant. 2008;27:253–260. doi: 10.1016/j.healun.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 16.Lahpor J, Khaghani A, Hetzer R, Pavie A, Friedrich I, Sander K, Strueber M. European results with a continuous-flow ventricular assist device for advanced heart-failure patients. Eur J Cardiothorac Surg. 2010;37:357–361. doi: 10.1016/j.ejcts.2009.05.043. [DOI] [PubMed] [Google Scholar]
- 17.Boyle AJ, Ascheim DD, Russo MJ, Kormos RL, John R, Naka Y, Gelijns AC, Hong KN, Teuteberg JJ. Clinical outcomes for continuous-flow left ventricular assist device patients stratified by pre-operative INTERMACS classification. J Heart Lung Transplant. 2011;30:402–407. doi: 10.1016/j.healun.2010.10.016. [DOI] [PubMed] [Google Scholar]
- 18.John R, Naka Y, Smedira NG, Starling R, Jorde U, Eckman P, Farrar DJ, Pagani FD. Continuous flow left ventricular assist device outcomes in commercial use compared with the prior clinical trial. Ann Thorac Surg. 2011;92:1406–1413. doi: 10.1016/j.athoracsur.2011.05.080. [DOI] [PubMed] [Google Scholar]
- 19.Kirklin JK, Naftel DC, Kormos RL, Stevenson LW, Pagani FD, Miller MA, Timothy Baldwin J, Young JB. Fifth INTERMACS annual report: risk factor analysis from more than 6,000 mechanical circulatory support patients. J Heart Lung Transplant. 2013;32:141–156. doi: 10.1016/j.healun.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 20.Lewis EF, Johnson PA, Johnson W, Collins C, Griffin L, Stevenson LW. Preferences for quality of life or survival expressed by patients with heart failure. J Heart Lung Transplant. 2001;20:1016–1024. doi: 10.1016/s1053-2498(01)00298-4. [DOI] [PubMed] [Google Scholar]
- 21.Rogers JG, Aaronson KD, Boyle AJ, Russell SD, Milano CA, Pagani FD, Edwards BS, Park S, John R, Conte JV, Farrar DJ, Slaughter MS. Continuous flow left ventricular assist device improves functional capacity and quality of life of advanced heart failure patients. J Am Coll Cardiol. 2010;55:1826–1834. doi: 10.1016/j.jacc.2009.12.052. [DOI] [PubMed] [Google Scholar]
- 22.Rector TS, Kubo SH, Cohn JN. Patients’ self-assessment of their congestive heart failure. Part 2: content, reliability and validity of a new measure, The Minnesota Living with Heart Failure Questionnaire. Heart Failure. 1987:198–209. [Google Scholar]
- 23.Green CP, Porter CB, Bresnahan DR, Spertus JA. Development and evaluation of the Kansas City Cardiomyopathy Questionnaire: a new health status measure for heart failure. J Am Coll Cardiol. 2000;35:1245–1255. doi: 10.1016/s0735-1097(00)00531-3. [DOI] [PubMed] [Google Scholar]
- 24.European Quality of Life-5 Dimensions. [Accessed December 2013]; Available at http://www.euroqual.org. [Google Scholar]
- 25.Grady KL, Meyer PM, Mattea A, Dressler D, Ormaza S, White-Williams C, Chillcott S, Kaan A, Loo A, Todd B, Klemme A, Piccione W, Costanzo MR. Change in quality of life from before to after discharge following left ventricular assist device implantation. J Heart Lung Transplant. 2003;22:322–333. doi: 10.1016/s1053-2498(02)00668-x. [DOI] [PubMed] [Google Scholar]
- 26.Bunte MC, Blackstone EH, Thuita L, Fowler J, Joseph L, Ozaki A, Starling RC, Smedira NG, Mountis MM. Major bleeding during HeartMate II support. J Am Coll Cardiol. 2013;62:2188–2196. doi: 10.1016/j.jacc.2013.05.089. [DOI] [PubMed] [Google Scholar]
- 27.Morgan JA, Paone G, Nemeh HW, Henry SE, Patel R, Vavra J, Williams CT, Lanfear DE, Tita C, Brewer RJ. Gastrointestinal bleeding with the HeartMate II left ventricular assist device. J Heart Lung Transplant. 2012;31:715–718. doi: 10.1016/j.healun.2012.02.015. [DOI] [PubMed] [Google Scholar]
- 28.Goldstein DJ, Naftel D, Holman W, Bellumkonda L, Pamboukian SV, Pagani FD, Kirklin J. Continuous-flow devices and percutaneous site infections: clinical outcomes. J Heart Lung Transplant. 2012;31:1151–1157. doi: 10.1016/j.healun.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 29.Strueber M, O’Driscoll G, Jansz P, Khaghani A, Levy WC, Wieselthaler GM. Multicenter evaluation of an intrapericardial left ventricular assist system. J Am Coll Cardiol. 2011;57:1375–1382. doi: 10.1016/j.jacc.2010.10.040. [DOI] [PubMed] [Google Scholar]
- 30.Topkara VK, Kondareddy S, Malik F, Wang IW, Mann DL, Ewald GA, Moazami N. Infectious complications in patients with left ventricular assist device: etiology and outcomes in the continuous-flow era. Ann Thorac Surg. 2010;90:1270–1277. doi: 10.1016/j.athoracsur.2010.04.093. [DOI] [PubMed] [Google Scholar]
- 31.Holman WL, Naftel DC, Eckert CE, Kormos RL, Goldstein DJ, Kirklin JK. J Thorac Cardiovasc Surg. 2013;146:437–441. doi: 10.1016/j.jtcvs.2013.02.018. [DOI] [PubMed] [Google Scholar]
- 32.Starling RC, Moazami N, Silvestry SC, Ewald G, Rogers JG, Milano CA, Rame JE, Acker MA, Blackstone EH, Ehrlinger J, Thuita L, Mountis MM, Soltesz, Lytle BW, Smedira NG. Unexpected abrupt increase in left ventricular assist device thrombosis. N Engl J Med. 2014;370:33–40. doi: 10.1056/NEJMoa1313385. [DOI] [PubMed] [Google Scholar]
- 33.Kormos RL, Teuteberg JJ, Pagani FD, Russell SD, John R, Miller LW, Massey T, Milano CA, Moazami N, Sundareswaran KS, Farrar DJ. Right ventricular failure in patients with the HeartMate II continuous-flow left ventricular assist device: incidence, risk factors, and effect on outcomes. J Thorac Cardiovasc Surg. 2010;139:1316–1324. doi: 10.1016/j.jtcvs.2009.11.020. [DOI] [PubMed] [Google Scholar]
- 34.Brenyo A, Rao M, Koneru S, Hallinan W, Shah S, Massey HT, Chen L, Polonsky B, McNitt S, Huang DT, Goldenberg I, Aktas M. Risk of mortality for ventricular arrhythmia in ambulatory LVAD patients. J Cardiovasc Electrophysiol. 2012;23:515–520. doi: 10.1111/j.1540-8167.2011.02223.x. [DOI] [PubMed] [Google Scholar]
- 35.Raasch H, Jensen BC, Chang PP, Mounsey JP, Gehi AK, Chung EH, Sheridan BC, Bowen A, Katz JN. Epidemiology, management, and outcomes of sustained ventricular arrhythmias after continous-flow left ventricular assist device implanation. Am Heart J. 2012;164:373–378. doi: 10.1016/j.ahj.2012.06.018. [DOI] [PubMed] [Google Scholar]
- 36.Forest SJ, Bello R, Friedmann P, Casazza D, Nucci C, Shin JJ, D’Alessandro, Stevens G, Goldstein DJ. Readmissions after ventricular assist device: Etiologies, patterns, and days out of the hospital. Ann Thorac Surg. 2013;95:1276–1281. doi: 10.1016/j.athoracsur.2012.12.039. [DOI] [PubMed] [Google Scholar]
- 37.Demirozu ZT, Etheridge WB, Radovancevic R, Frazier OH. Results of HeartMate II left ventricular assist device implantation on renal function in patients requiring post-implant renal replacement therapy. J Heart Lung Transplant. 2011;30:182–187. doi: 10.1016/j.healun.2010.08.019. [DOI] [PubMed] [Google Scholar]
- 38.Petrucci RJ, Rogers JG, Blue L. Neurocognitive function in destination therapy patients receiving continuous-flow vs pulsatile-flow ventricular assist device support. J Heart Lung Transplant. 2012;31:27–36. doi: 10.1016/j.healun.2011.10.012. [DOI] [PubMed] [Google Scholar]
- 39.Cowger J, Pagani FD, Haft JW, Romano MA, Aaronson KD, Kolias TJ. The development of aortic insufficiency in left ventricular assist device-support patients. Circ Heart Fail. 2010;3:668–674. doi: 10.1161/CIRCHEARTFAILURE.109.917765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Boothroyd LJ, Lambert L, Ducharme A, Guertin J, Sas G, Charbonneau E, Carrier M, Morin JE, Bogaty P. Challenge of informing patient decision making: what can we tell patients considering long-term mechanical circulatory support about outcomes, daily life, and end-of-life issues? Circ Cardiovasc Outcomes. 2014;7:179-7. doi: 10.1161/CIRCOUTCOMES.113.000243. [DOI] [PubMed] [Google Scholar]
- 41.Sudore RL, Schillinger D. Interventions to improve care for patients with limited health literacy. J Clin Outcomes Manag. 2009;16:20–29. [PMC free article] [PubMed] [Google Scholar]
- 42.McIlvennan CK, Allen LA, Nowels CT, Cleveland JC, Brieke A, Matlock DD. Decision making for destination therapy left ventricular assist devices: “There was no choice” versus “I thought about it an awful lot”. Circ Cardiovasc Outcomes. 2014;7:374–380. doi: 10.1161/CIRCOUTCOMES.113.000729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Iacovetto M, Matlock DD, McIlvennan CK, Thompson JS, Bradley WJ, Allen LA. Existing tools for patients considering a left ventricular assist device: a cross-sectional review of internet, print and multimedia materials. Circ Cardiovasc Outcomes. doi: 10.1161/CIRCOUTCOMES.114.000892. [in press] [DOI] [PubMed] [Google Scholar]
- 44.Rose EA, Gelijns AC, Moskowitz AJ, Heitjan DF, Stevenson LW, Dembitsky W, Long JW, Ascheim DD, Tierney AR, Levitan RG, Watson JT, Meier P, Ronan NS, Shapiro PA, Lazar RM, Miller LW, Gupta L, Frazier OH, Desvigne-Nickens P, Oz MC, Poirier VL. Long-term use of a left ventricular assist device for end-stage heart failure. N Engl J Med. 2001;345:1435–1443. doi: 10.1056/NEJMoa012175. [DOI] [PubMed] [Google Scholar]
- 45.Rogers JG, Butler J, Lansman SL, Gass A, Portner PM, Pasque MK, Pierson RN., 3rd Chronic mechanical circulatory support for inotrope-dependent heart failure patients who are not transplant candidates: results of the INTrEPID trial. J Am Coll Cardiol. 2007;50:741–747. doi: 10.1016/j.jacc.2007.03.063. [DOI] [PubMed] [Google Scholar]
- 46.Hershberger RE, Nauman D, Walker TL, Dutton D, Burgess D. Care processes and clinical outcomes of continuous outpatient support with inotropes (COSI) in patients with refractory endstage heart failure. J Card Fail. 2003;9:180–187. doi: 10.1054/jcaf.2003.24. [DOI] [PubMed] [Google Scholar]
- 47.Miller LW, Guglin M. Patient selection for ventricular assist devices: a moving target. J Am Coll Cardiol. 2013;61:1209–1221. doi: 10.1016/j.jacc.2012.08.1029. [DOI] [PubMed] [Google Scholar]
- 48.National Heart, Lung, and Blood Institute. ClinicalTrials.gov [Internet] Bethedsa (MD): National Library of Medicine (US); Medical arm of the Interagency Registry for Mechanically Assisted Circulatory Support (MedaMACS) 2013 – [cited 2014 Apr 20] Available from: https://clinicaltrials.gov/ct2/show/NCT01932294. [Google Scholar]
- 49.National Heart, Lung, and Blood Institute, University of Michigan. ClinicalTrials.gov [Internet] Bethesda (MD): National Library of Medicine (US); The evaluation of VAD intervention before inotrope therapy (REVIVE-IT) 2013 – [cited 2013 Dec 10]. Available from: http://clinicaltrials.gov/show/NCT01369407 NLM Identifier: NCT01369407. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.