Abstract
Objective
To assess the frequency of obstructive sleep apnea among women with and without hypertensive disorders of pregnancy.
Design
Cohort study.
Setting
Obstetric clinics at an academic medical center.
Population
Pregnant women with hypertensive disorders (chronic hypertension, gestational hypertension, or pre-eclampsia) and normotensive women.
Methods
Women completed a questionnaire about habitual snoring and underwent overnight ambulatory polysomnography.
Main Outcome Measures
The presence and severity of obstructive sleep apnea.
Results
Obstructive sleep apnoea was found among 21 of 51 women with hypertensive disorders (41%), but in only three of 16 women who were normotensive (19%, chi-square test, P = 0.005). Non-snoring hypertensive women typically had mild obstructive sleep apnea but >25% of snoring hypertensive women had moderate-to-severe obstructive sleep apnea. Among the hypertensive women, the mean apnea/hypopnea index was substantially higher in snorers than non-snorers (19.9±34.1 vs. 3.4±3.1, p=0.013) and the oxyhemoglobin saturation nadir was significantly lower (86.4±6.6 vs. 90.2±3.5, p=0.021). Among hypertensive women, after stratification by obesity the pooled relative risk for obstructive sleep apnea in snoring women with hypertension compared to non-snoring hypertension was 2.0 [95%CI 1.4–2.8].
Conclusions
Pregnant women with hypertension are at high risk for unrecognised obstructive sleep apnea. While longitudinal and intervention studies are urgently needed, it would seem pertinent given the known relationship between obstructive sleep apnea and hypertension in the general population, that hypertensive pregnant women who snore should be tested for obstructive sleep apnea, a condition believed to cause or promote hypertension.
Keywords: obstructive sleep apnea, snoring, hypertension, pregnancy
Introduction
Hypertensive disorders affect approximately 10% of pregnancies and increase the risk for adverse outcomes.1 In addition, they substantially increase healthcare costs.2 Of particular concern is preeclampsia, characterised by new-onset hypertension and proteinuria after 20 weeks’ gestation.3 Prompt recognition, evaluation, and management are required to prevent end-organ damage. From a public health perspective it is alarming that the incidence of pre-eclampsia has increased by almost one third in the past decade and is responsible for over 60,000 maternal deaths each year.4 Moreover, women with pre-eclampsia are at increased risk of cardiovascular disease later in life.5, 6
In the non-pregnant population, a key contributor to hypertensive disease is obstructive sleep apnea,7 a disorder characterised by nocturnal airway collapse with disruption of normal ventilation, hypoxemia, and sleep fragmentation. The prevalence of obstructive sleep apnea in women 30–39 years of age is approximately 6.5%, with moderate or severe obstructive sleep apnea affecting 1–5%.8–10 The prevalence of obstructive sleep apnea also increases with increasing body mass index.11 Nonetheless, it remains highly under-diagnosed: more than 90% of women with obstructive sleep apnea do not know they have it.12 Treatment of obstructive sleep apnea, using the gold-standard positive airway pressure, reduces cardiovascular morbidity and mortality, with improvement in daytime and nocturnal blood pressure.13, 14
Accumulating evidence shows that habitual snoring, the hallmark symptom of obstructive sleep apnea, increases in frequency during pregnancy15–18 and affects up to one-third of women by the third trimester.17, 19 Although the prevalence of objectively documented obstructive sleep apnea in pregnancy remains unknown, a recent study found that 15% of obese pregnant women have obstructive sleep apnea in the first trimester.20 Importantly, most studies that have queried pregnant women about snoring or performed overnight polysomnography (sleep study) demonstrate an association with gestational hypertension and pre-eclampsia.15, 17, 19–24 In the largest prospective study to date, we have recently shown that snoring, specifically new-onset snoring during pregnancy, is independently associated with gestational hypertension and pre-eclampsia even after accounting for other contributing factors.17 Moreover, a recent polysomnographic study of women with and without gestational hypertension found a higher frequency of obstructive sleep apnea in the former (53% vs. 12%, p<0.001).24
As treatment for obstructive sleep apnea is readily available and can reduce blood pressure,13 confirmation of its frequency during pregnancy and identification of ways to screen for it should be high priority. The goal of this study was to investigate the frequency of unrecognised obstructive sleep apnea and symptoms that may help to identify it among hypertensive pregnant women as compared to healthy, normotensive pregnant women.
Methods
These data represent the initial analyses of a longitudinal treatment intervention trial in hypertensive pregnancies. Hypertensive pregnant women were recruited from high-risk prenatal clinics or inpatient units within the University of Michigan between March 2009 and July 2013. Women were eligible if they were ≥14 years old and had a clinical diagnosis of chronic hypertension, gestational hypertension, or pre-eclampsia as obtained from obstetric notes in the medical record and verified by medical coding using the International Classification of Diseases, Ninth Revision (ICD-9).25 Women were eligible at any gestational age. For comparison, normotensive women, without comorbidities, were recruited from prenatal clinics in a parallel study using identical methods. Written informed consent was obtained from all subjects. Approval was obtained by the University of Michigan Institutional Review Board (IRBMed).
At enrollment, women completed a questionnaire about sleep and symptoms of obstructive sleep apnea. Participants were asked a) whether they snored (“How often do you snore?” response options: “Almost every night”, “3–4 times per week”, 1–2 times per week”, “1–2 times per month”, or “Never or almost never”). Habitual snoring was considered present if women reported snoring “3–4 times per week” or “Almost every night”; b) whether they stopped breathing or gasped for air (“During this pregnancy have you stopped breathing or gasped for air?” response options: “Almost every night”, “3–4 times per week”, 1–2 times per week”, “1–2 times per month”, or “Never or almost never”); and c) the gestational timing of snoring onset to identify women who had chronic symptoms prior to pregnancy (“If you have snored during this pregnancy, when did the snoring begin?” response options: “Already snored before pregnancy”, “Snoring started in the 1st trimester”, “Snoring started in the 2nd trimester” or “Snoring started in the 3rd trimester”).
In addition, all women underwent polysomnography either at home or in the hospital (for women who were inpatients) using a portable device (Medipalm, Braebon, Ontario, Canada or Embletta Gold, Embla, Bromfield, CO). A trained technologist visited all subjects, initiated the monitoring, and returned on the following morning to retrieve the equipment and download the data. The following channels were recorded: 6-channel electroencephalogram (EEG), submental electromyogram, electro-oculogram, electrocardiogram, nasal and oral airflow (thermistor, nasal pressure transducer), chest and abdominal respiratory movement using respiratory inductance plethysmography, oxygen saturation (SpO2), snoring microphone, and body position sensor. All of the sleep studies were manually scored by a single board-certified sleep technician blinded to study group (maternal hypertensive status) and were reviewed by a board-certified sleep physician (AVS) who was also masked to study group. Scoring followed American Academy of Sleep Medicine recommendations.26 Sleep duration, based upon standard EEG scoring, was available for n=50 subjects (75%). Subjects also had a parallel Watch-PAT study, (Peripheral Arterial Tonometry; a wrist-worn finger plethysmograph) at the same time as the ambulatory polysomnography. The Watch-PAT is a well-validated measure of sleep27–29 that utilises an inbuilt actigraph to measure sleep duration.30 Thus sleep duration was calculated from the Watch-PAT for those in whom EEG was unavailable, generally because of technical failures that are common when full polysomnography is attempted outside a laboratory setting.31, 32 We have previously demonstrated the validity of Watch-PAT in pregnancy.33 An apnea was defined by a drop in peak thermistor excursion by >90% of the pre-event baseline where at least 90% of duration met amplitude reduction criteria for apnea, with ≥10 seconds’ duration. An obstructive apnea was defined as an apnea with continued respiratory effort. Hypopneas were scored if the nasal pressure signal excursion dropped by >50% of baseline for ≥10 seconds with ≥3% desaturation or an arousal. The apnea hypopnea index (AHI) was calculated as the number of apneas and hypopneas per hour of total sleep time. The presence of mild obstructive sleep apnea was defined as an AHI ≥5 and <15; moderate obstructive sleep apnea as an AHI ≥15 and <30, and severe obstructive sleep apnea as an AHI ≥30.26
Medical records were accessed to ascertain key variables including maternal age, gravidity, parity, gestational age at time of study, height, and weight. As body mass index (BMI) is strongly associated with obstructive sleep apnea, pre-pregnancy or early first trimester BMI was categorised according to Institute of Medicine recommendations.34 Subjects were classified as underweight (BMI<18.5kg/m2); normal weight (BMI 18.5–24.9kg/m2); overweight (BMI 25.0–29.9kg/m2); or obese (BMI ≥30.0kg/m2). In addition, clinical diagnoses, and medication status were recorded. In reporting this study, guidelines from Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) group35 were followed.
Statistical analysis
Data were double entered into a database and checked for outliers and normality of the distribution. Analyses were performed using SPSS version 20 (IBM SPSS Statistics, Armonk, NY). Means and standard deviation (SD) were provided for normally distributed data and medians and interquartile ranges (IQR) were provided for non-normally distributed data. For normally distributed data, between-group comparisons of continuous variables were conducted with t-tests (hypertension vs. no hypertension) and ANOVA (chronic hypertension vs. gestational hypertension vs. pre-eclampsia) where appropriate. Dichotomised variables were compared with Chi Square tests. Non-normally distributed data were analysed using non-parametric statistics. A p-value <0.05 was considered statistically significant. Pooled relative risks were calculated using the Mantel Haenszel method and confidence intervals were calculated using the Greenland-Robin variance formula.36
Results
A total of 181 hypertensive pregnant women and 70 healthy normotensive women were invited to participate; 53 hypertensive women (29%) and 18 normotensive women (26%) agreed. After signing the consent form but prior to the overnight study, two withdrew (n=1 hypertensive and n=1 normotensive) and one hypertensive woman delivered her infant. The number of hypertensive women included in the final analysis was therefore 51 and the number of normotensive women was 16. There were no demographic differences nor differences in the frequency of snoring between those who declined participation and those who participated (data not shown). Overall, among the hypertensive women, at the time of the overnight study 59% had chronic hypertension (cHTN), 23% had gestational hypertension (GHTN), and 18% had pre-eclampsia (Pre-E). Demographic data are shown in Table 1.
Table 1.
Demographic data of the total sample
| Normotensive controls (n=16) | Chronic Hypertension (n=30) | Gestational Hypertension (n=12) | Pre- eclampsia (n=9) | |
|---|---|---|---|---|
| Mean age (years) | 28.1 (9.2) | 33.3 (4.0)* | 31.9 (6.8) | 30.4 (7.1) |
| Mean pre- pregnancy BMI (kg/m2) | 23.7 (4.8) | 41.0 (10.4)a,e,f | 34.1 (11.2)b | 31.1 (9.6) |
| Obese pre-pregnancy (%) | 2 (13%) | 27 (90%)a,g | 8 (67%)a | 5 (56%)c |
| BMI at study entry (kg/m2) | 28.1 (4.7) | 43.6 (9.3)a,e,g | 37.2 (11.3)b | 36.0 (8.2)c |
| Race (%) | ||||
| Caucasian | 9 (56%) | 15 (50%) | 9 (75%) | 5 (56%) |
| African American | 3 (19%) | 15 (50%) | 3 (25%) | 3 (33%) |
| Asian | 3 (19%) | 0 (0%)c | 0 (0%)c | 0 (0%)c |
| Biracial | 1 (6%) | 0 (0%) | 0 (0%) | 1 (11%) |
| Gestational age (weeks) | 33.8 (3.8) | 24.6 (8.1)a,d,g | 33.0 (2.9) | 30.1 (4.2) |
| Gravidity | 2.5 (1.9) | 3.4 (2.4) | 2.5 (1.3) | 4.6 (3.9)c |
| Parity | 0.8 (1.1) | 1.5 (1.7) | 0.5 (0.7)g | 1.9 (1.6) |
| First pregnancy (%) | 6 (38%) | 8 (27%) | 4 (33%) | 3 (33%) |
| Diabetes Mellitus (%) | 0 (0%) | 5 (17%) | 0 (0%) | 1 (11%) |
| Gestational diabetes (%) | 0 (0%) | 2 (7%) | 1 (8%) | 0 (0%) |
| Previous history of GHTN/Pre-E | 0 (0%) | 8 (27%)c | 1 (8%) | 1 (11%) |
| Smoker (%) | 2 (12%) | 9 (30%)e | 0 (0%) | 1 (11%) |
p<0.001 compared to controls;
p<0.01 compared to controls;
p<0.05 compared to controls
p<0.001 compared to GHTN;
p<0.05 compared to GHTN
p=0.01 compared to pre-eclampsia;
p<0.05 compared to pre-eclampsia
Mean and standard deviations are shown in the table; all continuous data were normally-distributed.
BMI=Body Mass Index (kg/m2); GHTN=gestational hypertension; Pre-E=pre-eclampsia
In total, hypertensive women in comparison to normotensive women were significantly more likely to report snoring: n=31 (61%) vs. n=3 (19%), p=0.008. After stratification by obesity, the pooled relative risk for snoring in hypertensive women was 3.4 [95%CI 2.7–4.3].
Within the hypertensive group, snoring at least 3 nights/week was reported by n=19 (63%) of the women with chronic hypertension, n=7 (58%) of the women with GHTN, and n=5 (56%) of the women with Pre-E. Notably, the majority of women with cHTN (as well as normotensive women who snored) reported habitual snoring before pregnancy, whereas those with GHTN and Pre-E who snored were more likely to report onset during pregnancy rather than before pregnancy (Table 2). Few women reported that they stopped breathing at night (“witnessed apnea”); with the exception of GHTN where no woman endorsed a positive answer, only 1 woman in each of the other groups answered positively (3% of cHTN, 11% of Pre-E, and 5% of normotensive).
Table 2.
Sleep variables
| Normotensive controls (n=16) | Chronic Hypertension (n=30) | Gestational Hypertension (n=12) | Pre- eclampsia (n=9) | p- value | |
|---|---|---|---|---|---|
| Chronic snoring (n; %) | 3 (19%) | 16 (53%) | 1 (8%) | 2 (22%) | |
| Pregnancy- onset snoring (n; %) | 0 (0%) | 3 (10%) | 6 (50%) | 3 (33%) | |
| TST (mins) | 378.5 (60.3) | 325.0 (134.0) | 362.0 (87.0) | 342.0 (227.5) | |
| AHI | 2.5 (3.3) | 4.0 (23.5) | 2.0 (5.0) | 4.0 (7.0) | |
| OAI | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | |
| CAI | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.1) | 0.0 (0.0) | |
| HI | 2.5 (3.3) | 3.5 (10.3) | 1.0 (4.5) | 4.0 (6.0) | |
| AHI ≥5 (n; %) | 3 (19%) | 13 (43%) | 3 (25%) | 5 (56%) | |
| AHI ≥15 (n; %) | 1 (6%) | 5 (17%) | 2 (17%) | 1 (11%) | |
| AHI ≥30 (n; %) | 0 (0%) | 4 (13%) | 1 (8%) | 1 (11%) | |
| Mean SpO2 (%)(n; %) | 96.0 (1.3) | 96.0 (2.0) | 97.0 (2.0) | 95.0 (3.0) | 0.023 |
| SpO2 nadir (%)(n; %) | 92.0 (2.3) | 89.0 (5.5) | 90.0 (10.0) | 90.0 (4.0) | 0.035 |
| SpO2 nadir ≤80% (n; %) | 0 (0%) | 3 (10%) | 1 (8%) | 0 (0%) |
Continuous data shown as median and IQR as non-normal distribution. Dichotomous data shown as raw numbers and percentage.
IQR=Interquartile Range; TST=Total Sleep Time; AHI=Apnea/Hypopnea Index; OAI=Obstructive Apnea Index; CAI=Central Apnea Index; HI=Hypopnea Index; SpO2=oxygen saturation
Results from the evaluation of obstructive sleep apnea are shown in Table 2. Women with pre-E as well as those with cHTN had the highest number of respiratory events compared to other women but this did not reach statistical significance. The only statistically significant differences between groups were found with mean SpO2 and SpO2 nadir. A sub analysis of only women with EEG-based sleep duration measures did not change any of the findings and so all analyses included the total sample.
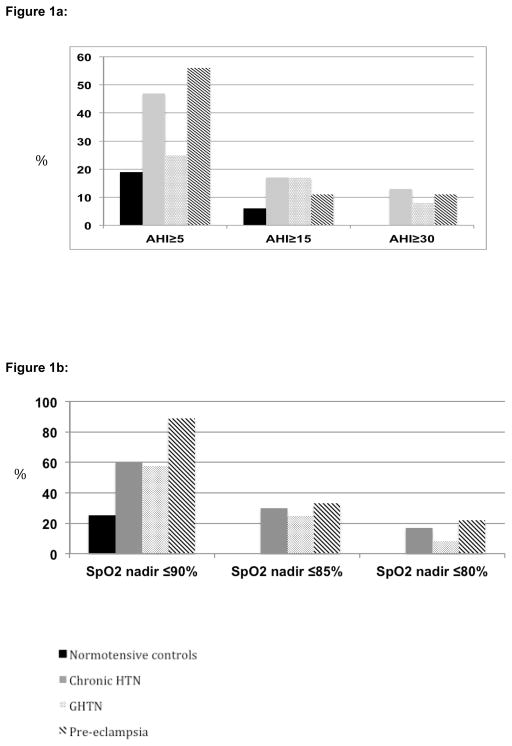
Figure 1a illustrates the proportion of women in each threshold of apnea severity. Approximately half of all women with cHTN and pre-E were found to have unrecognised obstructive sleep apnea. One quarter of those with GHTN had obstructive sleep apnea compared to one in five normotensive women. Of note, approximately 10% of hypertensive women had severe obstructive sleep apnea (AHI ≥30). Figure 1b illustrates the proportion of women falling at or below thresholds of minimum nocturnal oxygen saturation for each group. The majority of hypertensive women had dips in oxygen saturation below 90%; however almost one in four women with pre-E had nocturnal saturation nadirs below 80%.
Figure 1.
Figure 1a: Proportion of women with each threshold of AHI by hypertensive status.
AHI=Apnea/hypopnea index; HTN=hypertension; GHTN=gestational hypertension
Figure 1b: Proportion of women falling at or below thresholds of minimum nocturnal oxygen saturation by hypertensive status
SpO2=oxygen saturation; HTN=hypertension; GHTN=gestational hypertension
Comparisons between hypertensive groups
Analyses were then conducted between snoring and non-snoring women with hypertension. The mean AHI was significantly higher in hypertensive snoring women compared to hypertensive non-snoring women (19.9±34.1 vs. 3.4±3.1, p=0.013). Mean SpO2 was not different between these groups (95.3±1.8 vs. 95.7±1.9, p=0.40); however SpO2 nadir was significantly lower in women with hypertension and snoring compared to those with hypertension and no snoring (86.4±6.6 vs. 90.2±3.5, p=0.021). Notably, hypertensive women who reported habitual snoring were significantly more likely than non-snoring hypertensive women to have undiagnosed obstructive sleep apnea (AHI ≥5; 53% vs. 24%, p=0.03); Table 3. After stratification by obesity the pooled relative risk for obstructive sleep apnea in snoring women with hypertension compared to non-snoring hypertension was 2.0 [95%CI 1.4–2.8]. Furthermore, approximately one in four hypertensive snoring women had moderate-to-severe obstructive sleep apnea and only the hypertensive, snoring women had oxygen desaturations ≤80%.
Table 3.
Comparison of hypertensive women with and without snoring
| Hypertension and snoring (n=30) | Hypertension without snoring (n=21) | |
|---|---|---|
| AHI ≥5 | 16 (53%)* | 5 (24%) |
| AHI ≥15 | 8 (27%)** | 0% |
| AHI ≥30 | 6 (20%)* | 0% |
| SpO2 nadir ≤80% | 6 (20%)* | 0% |
p=0.05;
p=0.01
AHI=Apnea/hypopnea index; SpO2= oxygen saturation
Discussion
Main Findings
This study demonstrates that a substantial proportion of hypertensive pregnant women have obstructive sleep apnea and that snoring may be an excellent marker in clinical practice for this condition. Although non-snoring hypertensive women frequently had mild obstructive sleep apnea, women who self-identified as snorers had moderate-to-severe obstructive sleep apnea with clinically significant oxygen desaturation. This suggests that pregnant women with snoring in the setting of hypertension strongly merit evaluation for underlying obstructive sleep apnea.
Interestingly, in women with hypertension, the timing of snoring onset appeared related to hypertension type. Women who reported chronic snoring were most likely to have chronic hypertension, whereas those who reported pregnancy-onset snoring were more likely to have gestational hypertension. This finding strengthens similar observations from our prospective study, in which we demonstrated that pregnancy-onset snoring independently predicted gestational hypertension.17 In contrast, the timing of snoring onset was variable for women with pre-eclampsia.
Strengths and limitations
A major strength of this study is the objective measure of obstructive sleep apnea via polysomnography in a relatively large sample of women. We chose ambulatory rather than laboratory-based polysomnography because of the inherent difficulties of laboratory-based assessment in pregnancy, particularly high-risk women. While laboratory-based monitoring is the “gold-standard”, it is not often an option for pregnant women and frequently encounters barriers in clinical practice. Indeed, previous polysomnographic studies in pregnancy have smaller sample sizes than the current study,24, 37–42 limiting interpretation when results are non-significant. Use of ambulatory polysomnography allowed women to spend the night in their home, minimised potential anxiety and discomfort and still provided consistent apnea measures.
In the context of research, home monitoring is widely used, e.g., in NIH-funded multi-centre epidemiological studies such as the Sleep Heart Health Study43 and the Study of Women’s Health Across the Nation.44 The number of respiratory events obtained from ambulatory monitors is strongly correlated to those obtained from laboratory-based polysomnography43, 45–47 whether or not full polysomnography or cardiopulmonary monitoring is used. In a meta-analysis48 home studies were found to provide similar diagnostic information to laboratory polysomnography but may underestimate obstructive sleep apnea severity. If so, then our findings may have underestimated obstructive sleep apnea severity.
An additional strength was the inclusion of women with chronic hypertension, gestational hypertension, and pre-eclampsia. This allowed assessment for the first time of differential patterns of association with obstructive sleep apnea. A limitation is that, although we have demonstrated a clear association between hypertension during pregnancy and obstructive sleep apnea, we were unable to determine causality. We suggest that future studies include a prospective cohort of women that are recruited in early pregnancy and followed through the postnatal period to determine the temporal relationship between hypertension and obstructive sleep apnea in this population. In addition, large treatment intervention studies in pregnancy using positive airway pressure therapy are also required. Small pilot studies of positive airway pressure in hypertensive pregnancies are encouraging and suggest that maternal blood pressure can be reduced by treatment of obstructive sleep apnea;49, 39 however no large, randomised studies exist.
As discussed below, a wealth of information including randomised clinical trials demonstrates a casual pathway between obstructive sleep apnea and hypertension in non-pregnant adults,50 and similar pathways are likely in pregnancy.51, 52 However, caution may be warranted in pregnancy due to the occurrence of other physiological changes as hypertension may promote obstructive sleep apnea.53 Secondly, only approximately one third of women who were invited to take part actually participated. It is possible that those who agreed were more likely to have sleep problems; however sleep data collected on those who declined participation demonstrated that this was not the case. This suggests that our data are unlikely to have significant bias in this regard.
Interpretation (finding in light of other evidence)
The causal association between snoring/obstructive sleep apnea has been reported in multiple epidemiological studies of non-pregnant adults.54–56 It is well established that obstructive sleep apnea affects autonomic function57–59 and subsequently modifies cardiovascular regulation.60 The cyclic deoxygenation-reoxygenation during repeated apneic events, as well as sleep fragmentation from arousals, contributes to alterations in the autonomic nervous system. In addition, chronic intermittent hypoxia and exaggerated negative intrathoracic pressure swings can provoke systemic inflammation, oxidative stress, endothelial dysfunction, atherosclerosis, and hypertension.61, 62 Positive airway pressure, the first-line treatment for obstructive sleep apnea, has been shown in meta-analyses of randomized controlled trials in the non-pregnant population to reduce both daytime and nocturnal blood pressure.13, 14
Data from pregnant women suggest that snoring/obstructive sleep apnea is associated with gestational hypertension and pre-eclampsia15, 17, 19–22 as well as fetal growth restriction, operative delivery, prematurity, and admission to the neonatal intensive care unit.15, 20, 63–65 We previously demonstrated a clear independent association with pregnancy-onset snoring, and maternal hypertension.17 Moreover, a repeat polysomnographic study of women 1–2 years following gestational hypertension (as well as healthy controls) has shown that hypertensive women experienced a decrease in the respiratory disturbance index while the controls did not.66 The authors suggested that the physiologic effects of pregnancy may have a pathologic role in the development of obstructive sleep apnea during pregnancy in women with gestational hypertension.
The mechanisms of sleep disruption that affect cardiovascular morbidity in non-pregnant individuals are remarkably similar to the biological pathways for pre-eclampsia. Although the pathogenic process for pre-eclampsia likely originates in the placenta, the pathways include endothelial dysfunction, oxidative stress, and inflammation.51 These shared mechanistic pathways have been discussed.52 Nonetheless, although the timing of snoring onset appears to be associated with the timing of the hypertension, this could also reflect an association in the opposite direction; i.e., snoring could be a manifestation of hypertension or associated oedema. While this is less likely, the cross-sectional nature of our study precludes a definitive answer.
Our findings are consistent with those of Reid et al24 who studied women with gestational hypertension/pre-eclampsia and healthy pregnant controls with laboratory-based polysomnography; this study also found that approximately half of hypertensive women had undiagnosed obstructive sleep apnea. No women with chronic hypertension were included.
In the present study, although half of hypertensive women had unrecognised obstructive sleep apnea, those who reported snoring were at particular risk. These women were found exclusively to have moderate-to-severe obstructive sleep apnea with significantly lower oxyhemoglobin nadirs, while non-snoring hypertensive women had only mild obstructive sleep apnea. This suggests that self-report of habitual snoring in hypertensive pregnancies may be a useful clinical marker for moderate-to-severe obstructive sleep apnea that can be easily ascertained in the clinic. Nonetheless mild obstructive sleep apnea may not be benign. In non-pregnant populations mild obstructive sleep apnea is associated with autonomic alterations67 and cardiac structural changes.68, 69 Data from pregnant women show that airflow limitation in the absence of obstructive sleep apnea is frequent in pre-eclampsia70 and is reversible with positive airway pressure treatment, with simultaneous blood pressure reduction.49
A previous study of 24-hour ambulatory blood pressure in 186 hypertensive pregnant women has shown that over half of women also have nocturnal hypertension,71 and that this was most prevalent in pre-eclampsia (79%) compared to gestational or essential hypertension (45%). Of note, marked augmentation of the hemodynamic response to obstructive apneas is observed in pre-eclampsia.49 In pre-eclampsia, sleep is associated with adverse hemodynamic changes, which can be minimised by positive airway pressure therapy.40 This suggests that continuous fluctuations of blood pressure due to nocturnal obstructive events may have relevance to altered blood pressure control and this has clinical implications for monitoring and treatment of hypertensive pregnant women.
Conclusion
Approximately half of pregnant women with hypertension may have unrecognised obstructive sleep apnea. Women presenting with hypertension during pregnancy who also report snoring are at particularly high risk for moderate-to-severe obstructive sleep apnea with clinically significant oxyhemoglobin desaturation. While further studies are urgently required, including longitudinal as well as treatment intervention studies, our findings support the need for the obstetric healthcare provider to consider a sleep evaluation in hypertensive pregnancies, especially when snoring is present.
Acknowledgments
The authors would like to thank the pregnant women who participated and the medical, nursing, and clinic staff who supported this study. We are indebted to Lori A. Kempf, BA, CCRP and Mary Groll-Brown, BAS, PSGT, research coordinators for their dedication to subject recruitment. Thanks also to Robert Hagbloom, RPSGT, who performed blind-scoring for all of the PSG studies. We extend our thanks to Braebon for loaning the MediPalm device.
Funding:
This project was supported by the Gene and Tubie Gilmore Fund for Sleep Research and the National Heart, Lung, and Blood Institute (NHLBI) K23 HL095739. Dr. O’Brien was also supported by NHLBI R21 HL089918 and in part by R21 HL087819.
Footnotes
ClinicalTrials.gov Identifier: NCT01029691
The other authors have indicated no financial conflicts of interest.
Contribution to Authorship:
LMO and RDC conceived the study, LMO, RDC, TRBJ, ASB, and MCC designed the study with input from RA, CG, and CES. LMO, ASB, MCC, and RA contributed to data acquisition; AVS contributed to the analysis of PSGs and provided all PSG interpretations. LMO analyzed and interpreted the final data, and drafted the manuscript, with all authors providing critical appraisal and final approval of the submitted version.
Details of Ethics Approval:
This study was approved by the University of Michigan Institutional Review Board (IRBMed), HUM#00022596. Initial approval was obtained on 19th May 2009.
Disclosures:
Dr. O’Brien has received equipment support from Philips Respironics Inc. and is an advisory board member for the non-profit Star Legacy Foundation. Dr. Chames participates in a study that receives equipment from Cura Surgical. Dr. Chervin receives educational grants from Philips Respironics Inc., Fisher Paykel Inc., receives honoraria as section editor for UpToDate, receives fees for technology licensed by Zansors Inc., is an advisory board member for the non-profit Sweet Dreamzzz Inc., is named in patents owned by the University of Michigan for signal analysis diagnostic algorithms and hardware relevant to the assessment and treatment of sleep disorders, and serves on the Board of Directors of the American Academy of Sleep Medicine, American Board of Sleep Medicine, American Sleep Medicine Foundation, and the International Pediatric Sleep Association. Dr. Armitage received equipment support from Braebon.
References
- 1.Duley L. The global impact of pre-eclampsia and eclampsia. Semin Perinatol. 2009;33(3):130–7. doi: 10.1053/j.semperi.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 2.Liu A, Wen SW, Bottomley J, Walker MC, Smith G. Utilization of health care services of pregnant women complicated by preeclampsia in Ontario. Hypertens Pregnancy. 2009;28(1):76–84. doi: 10.1080/10641950802366252. [DOI] [PubMed] [Google Scholar]
- 3.Report of the National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy. American journal of obstetrics and gynecology. 2000;183(1):S1–S22. [PubMed] [Google Scholar]
- 4.World Health Report 2005: Make every mother and child count. Geneva: WHO; 2005. [DOI] [PubMed] [Google Scholar]
- 5.Fraser A, Nelson SM, Macdonald-Wallis C, Cherry L, Butler E, Sattar N, Lawlor DA. Associations of pregnancy complications with calculated cardiovascular disease risk and cardiovascular risk factors in middle age: the Avon Longitudinal Study of Parents and Children. Circulation. 2012;125(11):1367–80. doi: 10.1161/CIRCULATIONAHA.111.044784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith GN, Pudwell J, Walker M, Wen SW. Ten-year, thirty-year, and lifetime cardiovascular disease risk estimates following a pregnancy complicated by preeclampsia. Journal of obstetrics and gynaecology Canada: JOGC = Journal d’obstetrique et gynecologie du Canada: JOGC. 2012;34(9):830–5. doi: 10.1016/S1701-2163(16)35381-6. [DOI] [PubMed] [Google Scholar]
- 7.Somers VK, White DP, Amin R, Abraham WT, Costa F, Culebras A, Daniels S, Floras JS, Hunt CE, Olson LJ, Pickering TG, Russell R, Woo M, Young T. Sleep apnea and cardiovascular disease: an American Heart Association/american College Of Cardiology Foundation Scientific Statement from the American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology, Stroke Council, and Council On Cardiovascular Nursing. In collaboration with the National Heart, Lung, and Blood Institute National Center on Sleep Disorders Research (National Institutes of Health) Circulation. 2008;118(10):1080–111. doi: 10.1161/CIRCULATIONAHA.107.189375. [DOI] [PubMed] [Google Scholar]
- 8.Young T. Analytic epidemiology studies of sleep disordered breathing--what explains the gender difference in sleep disordered breathing? Sleep. 1993;16(8 Suppl):S1–2. doi: 10.1093/sleep/16.suppl_8.s1. [DOI] [PubMed] [Google Scholar]
- 9.Redline S, Kump K, Tishler PV, Browner I, Ferrette V. Gender differences in sleep disordered breathing in a community-based sample. Am J Respir Crit Care Med. 1994;149(3 Pt 1):722–6. doi: 10.1164/ajrccm.149.3.8118642. [DOI] [PubMed] [Google Scholar]
- 10.Bixler EO, Vgontzas AN, Lin HM, Ten Have T, Rein J, Vela-Bueno A, Kales A. Prevalence of sleep-disordered breathing in women: effects of gender. Am J Respir Crit Care Med. 2001;163(3 Pt 1):608–13. doi: 10.1164/ajrccm.163.3.9911064. [DOI] [PubMed] [Google Scholar]
- 11.Svensson M, Lindberg E, Naessen T, Janson C. Risk factors associated with snoring in women with special emphasis on body mass index: a population-based study. Chest. 2006;129(4):933–41. doi: 10.1378/chest.129.4.933. [DOI] [PubMed] [Google Scholar]
- 12.Young T, Evans L, Finn L, Palta M. Estimation of the clinically diagnosed proportion of sleep apnea syndrome in middle-aged men and women. Sleep. 1997;20(9):705–6. doi: 10.1093/sleep/20.9.705. [DOI] [PubMed] [Google Scholar]
- 13.Bazzano LA, Khan Z, Reynolds K, He J. Effect of nocturnal nasal continuous positive airway pressure on blood pressure in obstructive sleep apnea. Hypertension. 2007;50(2):417–23. doi: 10.1161/HYPERTENSIONAHA.106.085175. [DOI] [PubMed] [Google Scholar]
- 14.Haentjens P, Van Meerhaeghe A, Moscariello A, De Weerdt S, Poppe K, Dupont A, Velkeniers B. The impact of continuous positive airway pressure on blood pressure in patients with obstructive sleep apnea syndrome: evidence from a meta-analysis of placebo-controlled randomized trials. Arch Intern Med. 2007;167(8):757–64. doi: 10.1001/archinte.167.8.757. [DOI] [PubMed] [Google Scholar]
- 15.Franklin KA, Holmgren PA, Jonsson F, Poromaa N, Stenlund H, Svanborg E. Snoring, pregnancy-induced hypertension, and growth retardation of the fetus. Chest. 2000;117(1):137–41. doi: 10.1378/chest.117.1.137. [DOI] [PubMed] [Google Scholar]
- 16.Pien GW, Fife D, Pack AI, Nkwuo JE, Schwab RJ. Changes in symptoms of sleep-disordered breathing during pregnancy. Sleep. 2005;28(10):1299–305. doi: 10.1093/sleep/28.10.1299. [DOI] [PubMed] [Google Scholar]
- 17.O’Brien LM, Bullough AS, Owusu JT, Tremblay KA, Brincat CA, Kalbfleisch JD, Chervin RD. Pregnancy-Onset Habitual Snoring, Gestational Hypertension, and Pre-eclampsia: Prospective Cohort Study. Am J Obstet Gynecol. 2012;207(6):487, e1–9. doi: 10.1016/j.ajog.2012.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Facco FL, Kramer J, Ho KH, Zee PC, Grobman WA. Sleep disturbances in pregnancy. Obstet Gynecol. 2010;115(1):77–83. doi: 10.1097/AOG.0b013e3181c4f8ec. [DOI] [PubMed] [Google Scholar]
- 19.Bourjeily G, Raker CA, Chalhoub M, Miller MA. Pregnancy and fetal outcomes of symptoms of sleep-disordered breathing. Eur Respir J. 2010;36(4):849–55. doi: 10.1183/09031936.00021810. [DOI] [PubMed] [Google Scholar]
- 20.Louis J, Auckley D, Miladinovic B, Shepherd A, Mencin P, Kumar D, Mercer B, Redline S. Perinatal outcomes associated with obstructive sleep apnea in obese pregnant women. Obstetrics and gynecology. 2012;120(5):1085–92. doi: 10.1097/AOG.0b013e31826eb9d8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ursavas A, Karadag M, Nalci N, Ercan I, Gozu RO. Self-reported snoring, maternal obesity and neck circumference as risk factors for pregnancy-induced hypertension and preeclampsia. Respiration. 2008;76(1):33–9. doi: 10.1159/000107735. [DOI] [PubMed] [Google Scholar]
- 22.Champagne K, Schwartzman K, Opatrny L, Barriga P, Morin L, Mallozzi A, Benjamin A, Kimoff RJ. Obstructive sleep apnoea and its association with gestational hypertension. The European respiratory journal: official journal of the European Society for Clinical Respiratory Physiology. 2009;33(3):559–65. doi: 10.1183/09031936.00122607. [DOI] [PubMed] [Google Scholar]
- 23.Izci B, Martin SE, Dundas KC, Liston WA, Calder AA, Douglas NJ. Sleep complaints: snoring and daytime sleepiness in pregnant and pre-eclamptic women. Sleep medicine. 2005;6(2):163–9. doi: 10.1016/j.sleep.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 24.Reid J, Skomro R, Cotton D, Ward H, Olatunbosun F, Gjevre J, Guilleminault C. Pregnant women with gestational hypertension may have a high frequency of sleep disordered breathing. Sleep. 2011;34(8):1033–8. doi: 10.5665/SLEEP.1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Organization WH. Family of International Classifications. [Google Scholar]
- 26.Iber CA-IS, Chesson A, Quan S for the American Academy of Sleep Medicine. The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications. American Academy of Sleep Medicine; Westchester, IL: 2007. [Google Scholar]
- 27.Bar A, Pillar G, Dvir I, Sheffy J, Schnall RP, Lavie P. Evaluation of a portable device based on peripheral arterial tone for unattended home sleep studies. Chest. 2003;123(3):695–703. doi: 10.1378/chest.123.3.695. [DOI] [PubMed] [Google Scholar]
- 28.Pittman SD, Ayas NT, MacDonald MM, Malhotra A, Fogel RB, White DP. Using a wrist-worn device based on peripheral arterial tonometry to diagnose obstructive sleep apnea: in-laboratory and ambulatory validation. Sleep. 2004;27(5):923–33. doi: 10.1093/sleep/27.5.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Choi JH, Kim EJ, Kim YS, Choi J, Kim TH, Kwon SY, Lee HM, Lee SH, Shin C. Validation study of portable device for the diagnosis of obstructive sleep apnea according to the new AASM scoring criteria: Watch-PAT 100. Acta Otolaryngol. 2010;130(7):838–43. doi: 10.3109/00016480903431139. [DOI] [PubMed] [Google Scholar]
- 30.Hedner J, Pillar G, Pittman SD, Zou D, Grote L, White DP. A novel adaptive wrist actigraphy algorithm for sleep-wake assessment in sleep apnea patients. Sleep. 2004;27(8):1560–6. doi: 10.1093/sleep/27.8.1560. [DOI] [PubMed] [Google Scholar]
- 31.Portier F, Portmann A, Czernichow P, Vascaut L, Devin E, Benhamou D, Cuvelier A, Muir JF. Evaluation of home versus laboratory polysomnography in the diagnosis of sleep apnea syndrome. Am J Respir Crit Care Med. 2000;162(3 Pt 1):814–8. doi: 10.1164/ajrccm.162.3.9908002. [DOI] [PubMed] [Google Scholar]
- 32.Dingli K, Coleman EL, Vennelle M, Finch SP, Wraith PK, Mackay TW, Douglas NJ. Evaluation of a portable device for diagnosing the sleep apnoea/hypopnoea syndrome. The European respiratory journal. 2003;21(2):253–9. doi: 10.1183/09031936.03.00298103. [DOI] [PubMed] [Google Scholar]
- 33.O’Brien LM, Bullough AS, Shelgikar AV, Chames MC, Armitage R, Chervin RD. Validation of Watch-PAT-200 Against Polysomnography During Pregnancy. J Clin Sleep Med. 2012;8(3):287–94. doi: 10.5664/jcsm.1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Council NR. In: Weight Gain During Pregnancy: Reexamining the Guidelines. Rasmussen KM, Yaktine AL, editors. Washington (DC): The National Academies Press; 2009. [PubMed] [Google Scholar]
- 35.von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370(9596):1453–7. doi: 10.1016/S0140-6736(07)61602-X. [DOI] [PubMed] [Google Scholar]
- 36.Greenland S, Robins JM. Estimation of a common effect parameter from sparse follow-up data. Biometrics. 1985;41(1):55–68. [PubMed] [Google Scholar]
- 37.Guilleminault C, Kreutzer M, Chang JL. Pregnancy, sleep disordered breathing and treatment with nasal continuous positive airway pressure. Sleep Med. 2004;5(1):43–51. doi: 10.1016/j.sleep.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 38.Guilleminault C, Palombini L, Poyares D, Takaoka S, Huynh NT, El-Sayed Y. Pre-eclampsia and nasal CPAP: part 1. Early intervention with nasal CPAP in pregnant women with risk-factors for pre-eclampsia: preliminary findings. Sleep medicine. 2007;9(1):9–14. doi: 10.1016/j.sleep.2007.04.020. [DOI] [PubMed] [Google Scholar]
- 39.Poyares D, Guilleminault C, Hachul H, Fujita L, Takaoka S, Tufik S, Sass N. Pre-eclampsia and nasal CPAP: part 2. Hypertension during pregnancy, chronic snoring, and early nasal CPAP intervention. Sleep medicine. 2007;9(1):15–21. doi: 10.1016/j.sleep.2007.04.019. [DOI] [PubMed] [Google Scholar]
- 40.Blyton DM, Sullivan CE, Edwards N. Reduced nocturnal cardiac output associated with preeclampsia is minimized with the use of nocturnal nasal CPAP. Sleep. 2004;27(1):79–84. doi: 10.1093/sleep/27.1.79. [DOI] [PubMed] [Google Scholar]
- 41.Edwards N, Blyton CM, Kesby GJ, Wilcox I, Sullivan CE. Pre-eclampsia is associated with marked alterations in sleep architecture. Sleep. 2000;23(5):619–25. [PubMed] [Google Scholar]
- 42.Edwards N, Blyton DM, Kirjavainen TT, Sullivan CE. Hemodynamic responses to obstructive respiratory events during sleep are augmented in women with preeclampsia. Am J Hypertens. 2001;14(11 Pt 1):1090–5. doi: 10.1016/s0895-7061(01)02190-2. [DOI] [PubMed] [Google Scholar]
- 43.Iber C, Redline S, Kaplan Gilpin AM, Quan SF, Zhang L, Gottlieb DJ, Rapoport D, Resnick HE, Sanders M, Smith P. Polysomnography performed in the unattended home versus the attended laboratory setting--Sleep Heart Health Study methodology. Sleep. 2004;27(3):536–40. doi: 10.1093/sleep/27.3.536. [DOI] [PubMed] [Google Scholar]
- 44.Zheng H, Sowers M, Buysse DJ, Consens F, Kravitz HM, Matthews KA, Owens JF, Gold EB, Hall M. Sources of variability in epidemiological studies of sleep using repeated nights of in-home polysomnography: SWAN Sleep Study. J Clin Sleep Med. 2012;8(1):87–96. doi: 10.5664/jcsm.1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Redline S, Tosteson T, Boucher MA, Millman RP. Measurement of sleep-related breathing disturbances in epidemiologic studies. Assessment of the validity and reproducibility of a portable monitoring device. Chest. 1991;100(5):1281–6. doi: 10.1378/chest.100.5.1281. [DOI] [PubMed] [Google Scholar]
- 46.Reichert JA, Bloch DA, Cundiff E, Votteri BA. Comparison of the NovaSom QSG, a new sleep apnea home-diagnostic system, and polysomnography. Sleep medicine. 2003;4(3):213–8. doi: 10.1016/s1389-9457(02)00234-4. [DOI] [PubMed] [Google Scholar]
- 47.Erman MK, Stewart D, Einhorn D, Gordon N, Casal E. Validation of the ApneaLink for the screening of sleep apnea: a novel and simple single-channel recording device. J Clin Sleep Med. 2007;3(4):387–92. [PMC free article] [PubMed] [Google Scholar]
- 48.Ghegan MD, Angelos PC, Stonebraker AC, Gillespie MB. Laboratory versus portable sleep studies: a meta-analysis. Laryngoscope. 2006;116(6):859–64. doi: 10.1097/01.mlg.0000214866.32050.2e. [DOI] [PubMed] [Google Scholar]
- 49.Edwards N, Blyton DM, Kirjavainen T, Kesby GJ, Sullivan CE. Nasal continuous positive airway pressure reduces sleep-induced blood pressure increments in preeclampsia. Am J Respir Crit Care Med. 2000;162(1):252–7. doi: 10.1164/ajrccm.162.1.9905006. [DOI] [PubMed] [Google Scholar]
- 50.Lavie L. Oxidative stress--a unifying paradigm in obstructive sleep apnea and comorbidities. Prog Cardiovasc Dis. 2009;51(4):303–12. doi: 10.1016/j.pcad.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 51.Powe CE, Levine RJ, Karumanchi SA. Preeclampsia, a disease of the maternal endothelium: the role of antiangiogenic factors and implications for later cardiovascular disease. Circulation. 2011;123(24):2856–69. doi: 10.1161/CIRCULATIONAHA.109.853127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Izci-Balserak B, Pien GW. Sleep-disordered breathing and pregnancy: potential mechanisms and evidence for maternal and fetal morbidity. Curr Opin Pulm Med. 2010;16(6):574–82. doi: 10.1097/MCP.0b013e32833f0d55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pratt-Ubunama MN, Nishizaka MK, Boedefeld RL, Cofield SS, Harding SM, Calhoun DA. Plasma aldosterone is related to severity of obstructive sleep apnea in subjects with resistant hypertension. Chest. 2007;131(2):453–9. doi: 10.1378/chest.06-1442. [DOI] [PubMed] [Google Scholar]
- 54.Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342(19):1378–84. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- 55.Young T, Finn L, Peppard PE, Szklo-Coxe M, Austin D, Nieto FJ, Stubbs R, Hla KM. Sleep disordered breathing and mortality: eighteen-year follow-up of the Wisconsin sleep cohort. Sleep. 2008;31(8):1071–8. [PMC free article] [PubMed] [Google Scholar]
- 56.Marin JM, Agusti A, Villar I, Forner M, Nieto D, Carrizo SJ, Barbe F, Vicente E, Wei Y, Nieto FJ, Jelic S. Association between treated and untreated obstructive sleep apnea and risk of hypertension. Jama. 2012;307(20):2169–76. doi: 10.1001/jama.2012.3418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Somers VK, Dyken ME, Mark AL, Abboud FM. Sympathetic-nerve activity during sleep in normal subjects. N Engl J Med. 1993;328(5):303–7. doi: 10.1056/NEJM199302043280502. [DOI] [PubMed] [Google Scholar]
- 58.Somers VK, Dyken ME, Clary MP, Abboud FM. Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest. 1995;96(4):1897–904. doi: 10.1172/JCI118235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Khoo MC, Kim TS, Berry RB. Spectral indices of cardiac autonomic function in obstructive sleep apnea. Sleep. 1999;22(4):443–51. doi: 10.1093/sleep/22.4.443. [DOI] [PubMed] [Google Scholar]
- 60.Murali NS, Svatikova A, Somers VK. Cardiovascular physiology and sleep. Front Biosci. 2003;8:s636–52. doi: 10.2741/1105. [DOI] [PubMed] [Google Scholar]
- 61.Lavie L. Intermittent hypoxia: the culprit of oxidative stress, vascular inflammation and dyslipidemia in obstructive sleep apnea. Expert Rev Respir Med. 2008;2(1):75–84. doi: 10.1586/17476348.2.1.75. [DOI] [PubMed] [Google Scholar]
- 62.Bradley TD, Floras JS. Obstructive sleep apnoea and its cardiovascular consequences. Lancet. 2009;373(9657):82–93. doi: 10.1016/S0140-6736(08)61622-0. [DOI] [PubMed] [Google Scholar]
- 63.Micheli K, Komninos I, Bagkeris E, Roumeliotaki T, Koutis A, Kogevinas M, Chatzi L. Sleep patterns in late pregnancy and risk of preterm birth and fetal growth restriction. Epidemiology. 2011;22(5):738–44. doi: 10.1097/EDE.0b013e31822546fd. [DOI] [PubMed] [Google Scholar]
- 64.O’Brien L, Bullough AS, Owusu JT, Tremblay KA, Brincat CA, Chames MC, Kalbfleisch JD, Chervin RD. Habitual Snoring During Pregnancy and Delivery Outcomes: Prospective Cohort Study. Sleep. 2013;36(11):1625–32. doi: 10.5665/sleep.3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fung AM, Wilson DL, Lappas M, Howard M, Barnes M, O’Donoghue F, Tong S, Esdale H, Fleming G, Walker SP. Effects of maternal obstructive sleep apnoea on fetal growth: a prospective cohort study. PLoS One. 2013;8(7):e68057. doi: 10.1371/journal.pone.0068057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Reid J, Glew RA, Skomro R, Fenton M, Cotton D, Olatunbosun F, Gjevre J, Guilleminault C. Sleep disordered breathing and gestational hypertension: postpartum follow-up study. Sleep. 2013;36(5):717–21B. doi: 10.5665/sleep.2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Narkiewicz K, Montano N, Cogliati C, van de Borne PJ, Dyken ME, Somers VK. Altered cardiovascular variability in obstructive sleep apnea. Circulation. 1998;98(11):1071–7. doi: 10.1161/01.cir.98.11.1071. [DOI] [PubMed] [Google Scholar]
- 68.Sukhija R, Aronow WS, Sandhu R, Kakar P, Maguire GP, Ahn C, Lehrman SG. Prevalence of left ventricular hypertrophy in persons with and without obstructive sleep apnea. Cardiol Rev. 2006;14(4):170–2. doi: 10.1097/01.crd.0000184455.52778.00. [DOI] [PubMed] [Google Scholar]
- 69.Altekin RE, Karakas MS, Yanikoglu A, Ozel D, Ozbudak O, Demir I, Deger N. Determination of right ventricular dysfunction using the speckle tracking echocardiography method in patients with obstructive sleep apnea. Cardiol J. 2012;19(2):130–9. doi: 10.5603/cj.2012.0024. [DOI] [PubMed] [Google Scholar]
- 70.Connolly G, Razak AR, Hayanga A, Russell A, McKenna P, McNicholas WT. Inspiratory flow limitation during sleep in pre-eclampsia: comparison with normal pregnant and nonpregnant women. The European respiratory journal. 2001;18(4):672–6. doi: 10.1183/09031936.01.00053501. [DOI] [PubMed] [Google Scholar]
- 71.Brown MA, Bowyer L, McHugh L, Davis GK, Mangos GJ, Jones M. Twenty-four-hour automated blood pressure monitoring as a predictor of preeclampsia. American journal of obstetrics and gynecology. 2001;185(3):618–22. doi: 10.1067/mob.2001.117664. [DOI] [PubMed] [Google Scholar]