Abstract
Background
Despite the increasing prevalence of heart failure (HF) with preserved ejection fraction (HFpEF) in humans, there remains no therapeutic options for HFpEF. Adiponectin (APN), an adipocyte-derived cytokine exerts cardioprotective actions and its deficiency is implicated in the development of hypertension and HF with reduced ejection fraction. Similarly APN deficiency in HFpEF exacerbates left ventricular hypertrophy (LVH), diastolic dysfunction and HF. However, the therapeutic effects of APN in HFpEF remain unknown. We sought to test the hypothesis that chronic APN overexpression protects against the progression of HF in a murine model of HFpEF.
Methods and Results
APN transgenic (APNTG) and wild-type (WT) mice underwent uninephrectomy, a continuous saline or d-aldosterone infusion and given 1.0% sodium chloride drinking water for 4-weeks. Aldosterone-infused WT mice developed HFpEF with hypertension, LVH and diastolic dysfunction. Aldosterone infusion increased myocardial oxidative stress and decreased sarcoplasmic reticulum Ca2+-ATPase (SERCA2a) protein expression in HFpEF. Although total phospholamban (PLN) protein expression was unchanged, there was decreased expression of PKA-dependent PLN phosphorylation at Ser16 and CaMKII-dependent PLN phosphorylation at Thr17. APN overexpression in aldosterone-infused mice ameliorated LVH, diastolic dysfunction, lung congestion and myocardial oxidative stress without affecting blood pressure (BP) and LVEF. This improvement in diastolic function parameters in aldosterone-infused APNTG mice was accompanied by preserved protein expression of PKA-dependent phosphorylation of PLN at Ser16. APN replacement prevented the progression of aldosterone-induced HFpEF, independent of BP, by improving diastolic dysfunction and modulating cardiac hypertrophy.
Conclusions
These findings suggest that APN may have therapeutic effects in patients with HFpEF.
Keywords: adiponectin, heart failure with preserved ejection fraction, left ventricular hypertrophy, diastolic dysfunction, oxidative stress, calcium handling proteins
Heart failure with preserved ejection fraction (HFpEF), also known as “diastolic heart failure”, is a clinical syndrome characterized by signs and symptoms of heart failure (HF) with preservation of left ventricular (LV) ejection fraction (LVEF)1. HFpEF accounts for up to 50% of all patients presenting with HF2, yet there remains no therapies for HFpEF. In addition to associated comorbidities, there are likely many divergent pathophysiological mechanisms similar to HF with reduced EF (HFrEF). HFpEF is highly prevalent in obese individuals3, 4 and hypertension remains the major cause of HFpEF2, 5. Emerging evidence also indicates that factors secreted by adipocytes play a role in hypertension-related diseases6–8.
Adiponectin (APN), an adipocyte-derived cytokine, is abundant in human plasma9 with low APN levels seen in diseases such as hypertension, coronary artery disease, obesity and insulin resistance10–12. As such in experimental models, APN deficiency exacerbates the development of obesity-related hypertension13, adverse cardiac remodeling14, 15 in ischemia-reperfusion injury16 and myocardial infarction17. Recently, we showed that lack of APN in a murine model of diastolic HF, increased the propensity to develop diastolic HF and diastolic dysfunction18. Although, hypoadiponectinemia in aldosterone-induced HFpEF exacerbates hypertension, LVH, diastolic dysfunction, and HF18, the pathophysiological role and therapeutic effects of APN repletion in HFpEF are unknown. We thus sought to test the hypothesis that chronic APN overexpression protects against the progression of HFpEF and sought to investigate the proposed mechanism.
Methods
An expanded Materials and Methods section is available in the Online Data Supplement. The Institutional Animal Care and Use Committee at Boston University School of Medicine approved all study procedures related to the handling and surgery of the mice. APN transgenic (APNTG) and wild-type (WT) mice in a C57BL/6J background were generated as previously described19.
Experimental Model
Twelve-week old APNTG mice and WT littermates were anesthetized with pentobarbital sodium (50 mg/kg, intraperitoneally). They underwent uninephrectomy and intraperitoneal implantation of osmotic mini-pumps (Durect Corp., Cupertino, CA) that delivered a continuous infusion of either saline or 0.15 µg/hr d-aldosterone (Sigma-Aldrich Co., St. Louis, MO) for 4 weeks. See online supplement for mice groups.
Physiological Measurements
Heart rate and blood pressure (BP) were measured weekly using a noninvasive tail-cuff BP analyzer, BP-2000 Blood Pressure Analysis System (Visitech Systems, Inc., Apex, NC)18, 20.
Echocardiography
Transthoracic echocardiography was performed at the end of 4 weeks using the Vevo 770 High-Resolution In Vivo Micro-Imaging System and a Real-Time Micro Visualization 707B Scanhead (VisualSonic Inc., Toronto, Ontario, Canada)18, 20. For details of LV structure, function and Doppler measurements see online supplement.
Mice were sacrificed 4 weeks after saline or aldosterone infusion and biomarker, organ weight, tissue analysis and cardiomyocyte sizes were determined. Myocardial oxidative stress by 3-nitrotyrosine staining and immunoblotting of calcium handling proteins and signaling pathway were also measured. qRT-PCR for atrial natriuretic peptide (ANP) mRNA expression was also determined. See online supplement for all details.
Statistical Analysis
Data are expressed as mean± standard error of the mean (SEM). For comparisons of multiple groups, Kruskal–Wallis 1-way ANOVA was performed with a post hoc Dunn test for multiple comparisons. Paired data were evaluated by Mann–Whitney test. P<0.05 values were considered significant. All statistical analyses were performed using Graph Pad Prism (GraphPad Software, Inc., La Jolla, CA).
Results
General characteristics
There were no deaths in the mice during the 4-week period. Characteristics of WT and APNTG mice 4-weeks after saline or aldosterone infusion are summarized in Table 1. Body weights (BW) were comparable between WT and APNTG mice regardless of saline or aldosterone infusion.
Table 1.
Characteristics of WT/APNTG Mice 4 Weeks after Saline/Aldosterone Infusion
| Groups | WT-saline n = 7 |
APNTG-saline n = 7 |
WT-aldo n = 15 |
APNTG-aldo n = 15 |
|---|---|---|---|---|
| General | ||||
| Body Weight (g) | 21.9±1.2 | 22.5±2.4 | 22.5±0.7 | 24.2±1.0 |
| Heart Rate (beats/min) | 676±13 | 679±18 | 654±13 | 675±12 |
| Systolic Blood Pressure (mm Hg) | 105±2 | 100±2 | 127±3†† | 125±4‡‡ |
| Heart weight/Body Weight (mg/g) | 4.35±0.78 | 4.42±0.21 | 5.69±0.15†† | 5.14±0.07‡‡, * |
| Wet-Lung Weight/Dry-Lung Weight | 3.97±0.06 | 3.99±0.11 | 4.48±0.05†† | 4.25±0.06‡, * |
| Fibrosis area (%) | 1.0±0.1 | 0.9±0.2 | 6.7±0.7†† | 4.3±0.8‡, * |
| Blood Chemical Analysis | ||||
| Serum aldosterone levels (pg/mL) | 626.0±66.4 | 810.7±85.9 | 6542.6±324.2†† | 5317.4±551.7‡‡ |
| Serum adiponectin levels (µg/mL) | 14.5±1.3 | 27.0±1.0†† | 14.0±0.9 | 29.2±0.3‡‡, * |
Data are expressed as mean ± SEM.
Abbreviations: WT; wild-type, APNTG; adiponectin transgenic, aldo; aldosterone
P < 0.01 vs. WT-saline,
P < 0.05 vs. APNTG-saline,
P < 0.01 vs. APNTG-saline,
P < 0.05 vs. WT-aldo,
P < 0.01 vs. WT-aldo.
WT-saline, ‡P < 0.05 vs. APNTG-saline, ‡‡P < 0.01 vs. APNTG-saline, *P < 0.05 vs. WT-aldo, **P < 0.01 vs. WT-aldo.
Hemodynamic parameters
Heart rates (HR) were comparable between WT and APNTG mice regardless of saline or aldosterone infusion (Table 1). Systolic BP (SBP) was measured weekly (Supplemental Figure 1) and was significantly increased by 4-weeks of aldosterone infusion in WT-aldosterone (127±3 vs. 105±2 mmHg; P<0.01) and APNTG-aldosterone mice (125±4 vs. 100±2 mmHg; P<0.01) compared with their respective saline-infused controls. There was no difference in SBP between the aldosterone-infused WT and APNTG mice.
Serum aldosterone levels
Serum aldosterone levels in WT-aldosterone (6,542.6±324.2 vs. 626.0±66.4 pg/mL; P<0.01) and APNTG-aldosterone mice (5,317.4±551.7 vs. 810.7±85.9 pg/mL; P<0.01) were significantly elevated compared with respective saline-infused mice. There was no difference in serum aldosterone levels between WT and APNTG mice regardless of saline or aldosterone infusion (Table 1).
Serum adiponectin levels
Serum APN levels in APNTG-saline mice (27.0±1.0 µg/mL) were ~1.9 times higher than in WT-saline mice (14.5±1.3 µg/mL; P<0.01). Serum APN levels in APNTG-aldosterone mice (29.2±0.3 µg/mL) were significantly elevated vs. APNTG-saline mice (27.0±1.0µg/mL; P<0.05). There was no difference in APN levels between WT-saline and WT-aldosterone mice (Table 1).
LV structure and systolic function
Echocardiographic parameters for LV structure and systolic function are summarized in Table 2. Aldosterone infusion significantly increased total wall thickness (TWT) and LV mass in WT-aldosterone (1.04±0.02 mm and 140.4±6.5 mg) and APNTG-aldosterone mice (0.93±0.02 mm and 110.9±12.7 mg). Consistent with these findings, the heart weight (HW) to BW ratio (HW/BW) was also significantly increased in the WT-aldosterone (5.69±0.15) and APNTG-aldosterone mice (5.14±0.07; Table 1). There were no significant differences in cardiac hypertrophy between WT-saline and APNTG-saline mice (Table 1 and 2). However, cardiac hypertrophy in APNTG-aldosterone mice was significantly less vs. WT-aldosterone mice (P<0.01 for TWT; P<0.05 for LV mass and P<0.05 for HW/BW). There was no difference in LV chamber size and LVEF between the WT and APNTG mice regardless of saline or aldosterone infusion (Table 2).
Table 2.
Echocardiographic Parameters of WT/APNTG Mice 4 Weeks after Saline/Aldosterone Infusion
| Group | WT-saline n = 7 |
APNTG-saline n = 7 |
WT-aldo n = 15 |
APNTG-aldo n = 15 |
|---|---|---|---|---|
| LV Structure and Systolic Function | ||||
| TWT (mm) | 0.75±0.01 | 0.73±0.01 | 1.04±0.02†† | 0.93±0.02‡‡, ** |
| LVEDD (mm) | 3.42±0.06 | 3.44±0.08 | 3.49±0.08 | 3.34±0.23 |
| LVESD (mm) | 1.95±0.06 | 2.04±0.12 | 2.03±0.07 | 1.95±0.09 |
| LVEF (%) | 75.12±1.59 | 73.83±1.65 | 73.96±0.93 | 75.69±1.62 |
| LV mass (mg) | 83.40±2.26 | 81.38±3.72 | 140.4±6.52 †† | 110.9±12.69‡* |
| Diastolic Function | ||||
| Peak E (mm/s) | 654.83±13.79 | 677.60±14.50 | 1008.73±27.19†† | 873.75±20.96‡‡, ** |
| Peak A (mm/s) | 454.04±9.37 | 449.61±15.34 | 490.50±40.89 | 591.24±34.94‡ |
| E/A | 1.45±0.04 | 1.51±0.04 | 2.17±0.17†† | 1.50±0.09** |
| DT (ms) | 22.08±1.00 | 21.88±0.63 | 15.56±1.00†† | 20.42±0.77** |
| IVRT (ms) | 19.58±0.77 | 20.63±1.88 | 19.72±0.77 | 20.00±1.12 |
| eˈ (mm/s) | 27.26±1.25 | 28.26±1.56 | 17.81±0.33†† | 20.55±0.69‡‡, ** |
| E/eˈ | 24.17±0.72 | 24.14±1.02 | 56.84±1.96†† | 42.67±1.28‡‡, ** |
Data are expressed as mean± SEM.
Abbreviations: WT; wild-type, APNTG; adiponectin transgenic, aldo; aldosterone, LV; left ventricular, LVEDD; LV end-diastolic diameter, LVESD; LV end-systolic diameter, TWT; total wall thickness, LVEF; LV ejection fraction, E; early, A; late, DT; early filling deceleration time, IVRT; isovolumic relaxation time, eˈ; peak early diastolic myocardial velocity.
P < 0.01 vs. WT-saline,
P < 0.05 vs. APNTG-saline,
P < 0.01 vs. APNTG-saline,
P < 0.05 vs. WT-aldo,
P < 0.01 vs. WT-aldo.
Diastolic function
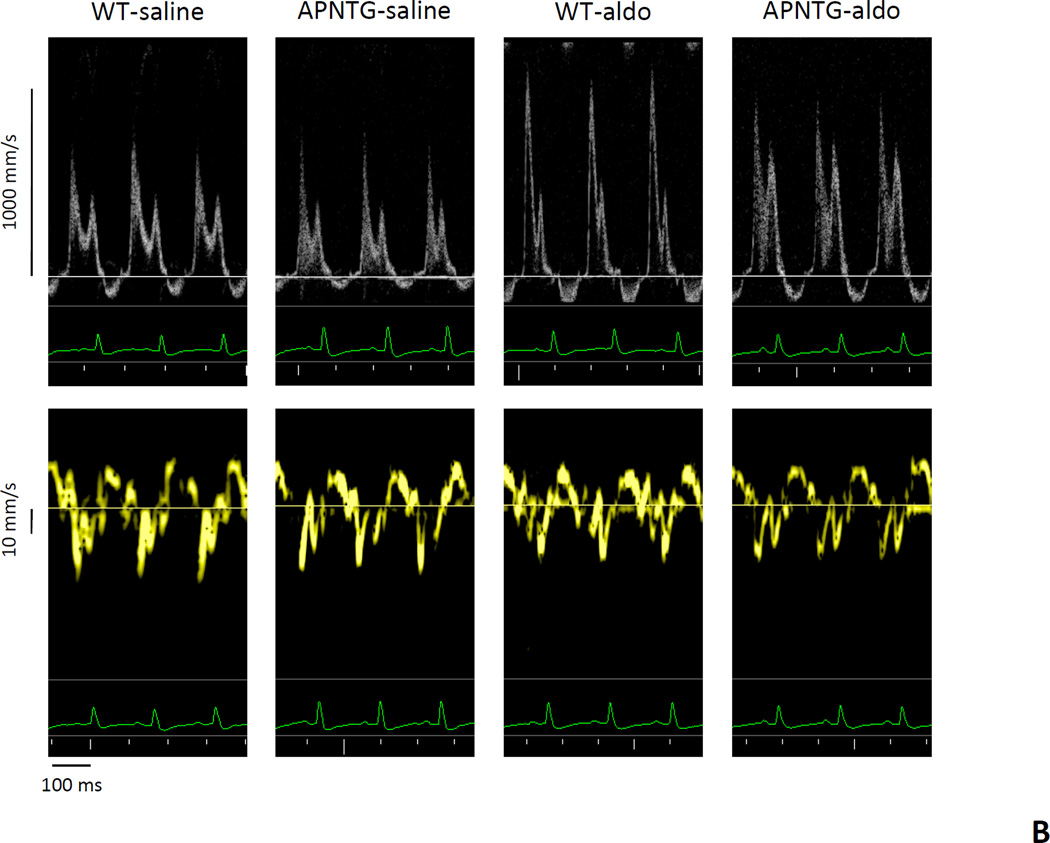
Echocardiographic parameters (mitral and tissue Doppler) for LV diastolic function in the mice are summarized in Table 2; Figure 1A–B. Mitral Doppler: Aldosterone infusion significantly increased peak E velocity in the WT-aldosterone vs. WT-saline mice and in APNTG-aldosterone vs. APNTG-saline mice (Table 2; Figure 1B). The peak E velocity in APNTG-aldosterone mice was however, significantly lower than in WT-aldosterone mice (P<0.01). Aldosterone infusion significantly increased peak A velocity in APNTGaldosterone vs. APNTG-saline mice, but not in WT-aldosterone vs. WT-saline mice (P=NS). The resultant ratio of peak E velocity to peak A velocity (E/A) was significantly higher in WT-aldosterone (2.17±0.17) vs. WT-saline mice (1.45±0.04; P<0.01). This increase in E/A ratio in WT-aldosterone mice, indicating impaired LV compliance, was attenuated in APNTG-aldosterone mice (1.50±0.09; P<0.01). Deceleration time (DT) was significantly shortened in WT-aldosterone vs. WT-saline mice (P<0.01). The shortened of DT in WT-aldosterone mice, indicates abnormal LV relaxation and was attenuated in APNTG-aldosterone mice (P<0.01). There was no difference in isovolumic relaxation time (IVRT) between WT and APNTG mice regardless of saline or aldosterone infusion. Tissue Doppler: (Table 2) Aldosterone infusion significantly decreased peak e’ velocity in WT-aldosterone vs. WT-saline mice (P<0.01) and APNTG-aldosterone mice vs. APNTG-saline mice (P<0.01). However, the peak e’ velocity in APNTG-aldosterone mice was significantly higher than in WT-aldosterone mice (P<0.01). The resultant ratio of peak E velocity to peak e’ velocity (E/e’) was significantly higher in WT-aldosterone mice compared with WT-saline mice (P<0.01). The increase in E/e’ in WT-aldosterone mice, indicates elevated diastolic filling pressure and was significantly attenuated in APNTG-aldosterone mice (P<0.01).
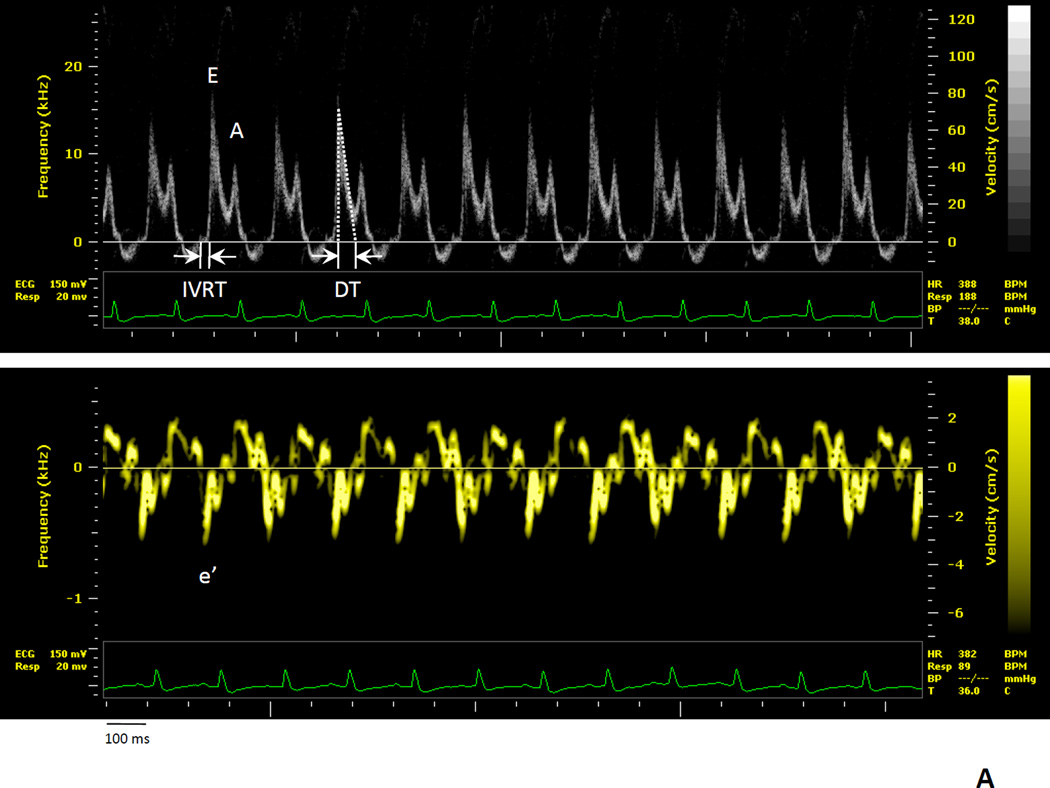
Figure 1.
Representative pulse wave and tissue Doppler images in WT and APNTG mice 4 weeks after saline or aldosterone infusion. A) WT-saline; B) WT-saline, APNTG-saline, WT-aldosterone, and APNTG-aldosterone. IVRT indicates isovolumetric relaxation time, E; peak early transmitral flow velocity, A; peak late transmitral flow velocity, DT; early filling deceleration time, e’; peak early diastolic myocardial velocity.
APNTG-aldosterone mice showed a reduction in the ratio of peak E velocity to peak A velocity compared with WT-aldosterone mice. However relative to the saline-infused mice this ratio of peak E velocity to peak A velocity was elevated. The reduction in E/A ratio in APNTG-aldosterone mice is due to a decrease in peak E velocity demonstrating an improvement in early transmitral flow (E wave) in the restrictive filling pattern. Despite a reduction in E/A ratio in APNTG mice the E/e’ remained elevated suggesting elevated filling pressures or restrictive filling.
Lung congestion
Aldosterone infusion significantly increased wet/dry lung weight, an indicator of pulmonary congestion, in WT-aldosterone vs. WT-saline mice (P<0.01) and APNTG-aldosterone vs. APNTG-saline mice (P<0.05; Table 1). There was no difference in wet/dry lung weight, between saline-infused WT and APNTG mice. However, the wet/dry lung weight in APNTG-aldosterone mice was significantly lower compared with WT-aldosterone mice indicating less pulmonary congestion (P < 0.05).
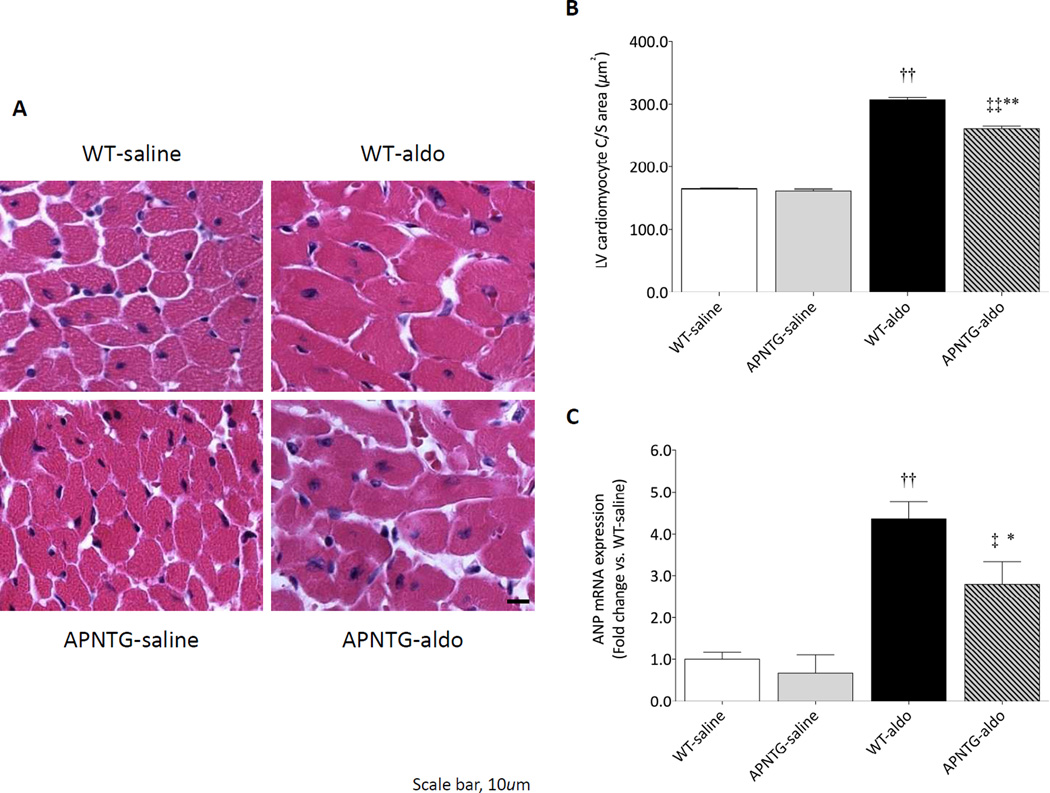
LV cardiomyocyte hypertrophy
Aldosterone infusion significantly increased LV cardiomyocyte C/S area in the WT-aldosterone and APNTG-aldosterone mice compared with respective saline-infused mice (P<0.01 for both; Figure 2A–B). Consistent with these findings, ANP mRNA expression, a molecular marker of cardiomyocyte hypertrophy, was increased in the LV of WT-aldosterone vs. WT-saline mice (P<0.01) and APNTG-aldosterone vs. APNTG-saline mice (P<0.05; Figure 2C). There was no difference in the LV cardiomyocyte C/S area and ANP mRNA expression in saline-infused WT and APNTG mice. However, both LV cardiomyocyte C/S area and ANP mRNA expression in APNTG-aldosterone mice were significantly decreased vs. WT-aldosterone mice (P<0.01 and P<0.05, respectively; Figure 2).
Figure 2.
LV cardiomyocyte hypertrophy in WT and APNTG mice 4 weeks after saline or aldosterone infusion. A) Representative H&E staining. Scale bar is 10µm; B) LV cardiomyocyte C/S area; C) ANP mRNA expression (n=4–9 per group). ††P<0.01 vs. WT-saline, ‡P<0.05 vs. APNTG-saline, ‡‡P<0.01 vs. APNTG-saline, *P<0.05 vs. WT-aldosterone, **P<0.01 vs. WT-aldosterone.
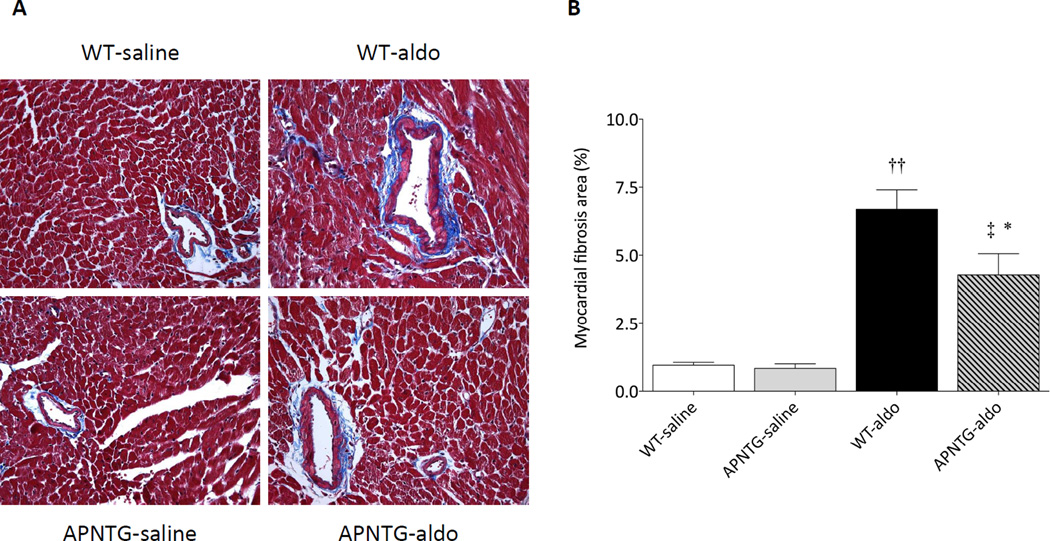
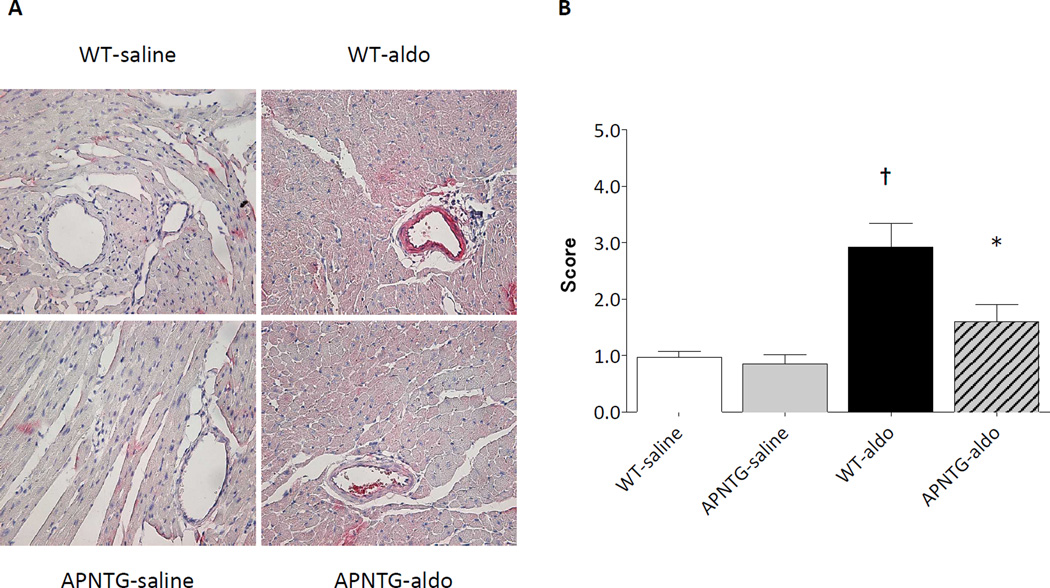
Myocardial fibrosis
Aldosterone infusion significantly increased the area of myocardial fibrosis in WT-aldosterone vs. WT-saline mice (P<0.01) and APNTG-aldosterone vs. APNTG-saline mice (P<0.05; Figure 3). Myocardial fibrosis in APNTG-aldosterone mice was significantly less than the WT-aldosterone mice (P<0.05).
Figure 3.
Myocardial fibrosis in WT and APNTG mice 4 weeks after saline or aldosterone infusion. A) Representative Masson trichrome staining. Original magnification x400; B) Myocardial fibrosis area (n=4–9/group); ††P<0.01 vs. WT-saline, ‡P<0.05 vs. APNTG-saline, *P<0.05 vs. WT-aldosterone.
Myocardial oxidative stress
Myocardial oxidative stress, assessed by 3-nitrotyrosine staining, was markedly increased in WT-aldosterone mice vs. WT-saline mice. The increase in nitrotyrosine staining in WT-aldosterone mice was attenuated in APNTG-aldosterone mice (Figure 4A–B). There was a 54% reduction in nitrotyrosine staining in APNTG-aldosterone mice (P<0.05 vs. WT-aldosterone mice).
Figure 4.
Myocardial oxidative stress in WT and APNTG mice 4 weeks after saline or aldosterone infusion. A) Representative 3-nitrotyrosine staining. Original magnification ×400. B) Semiquantitative analysis of nitrotyrosine staining of C/S of murine myocardium. Nitrotyrosine staining was scored using an arbitrary grade from 1 to 4. (n=4–9/group). †P<0.01 vs. WT-saline, *P<0.05 vs. WT-aldosterone.
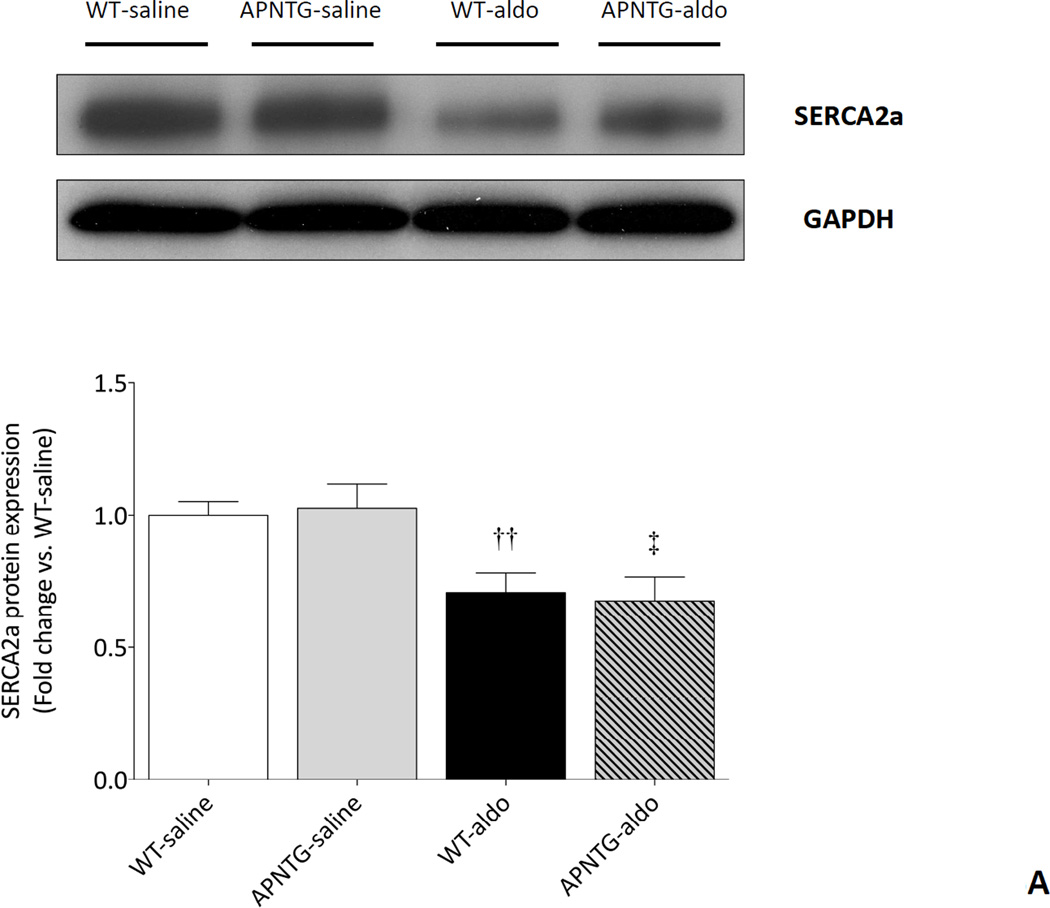
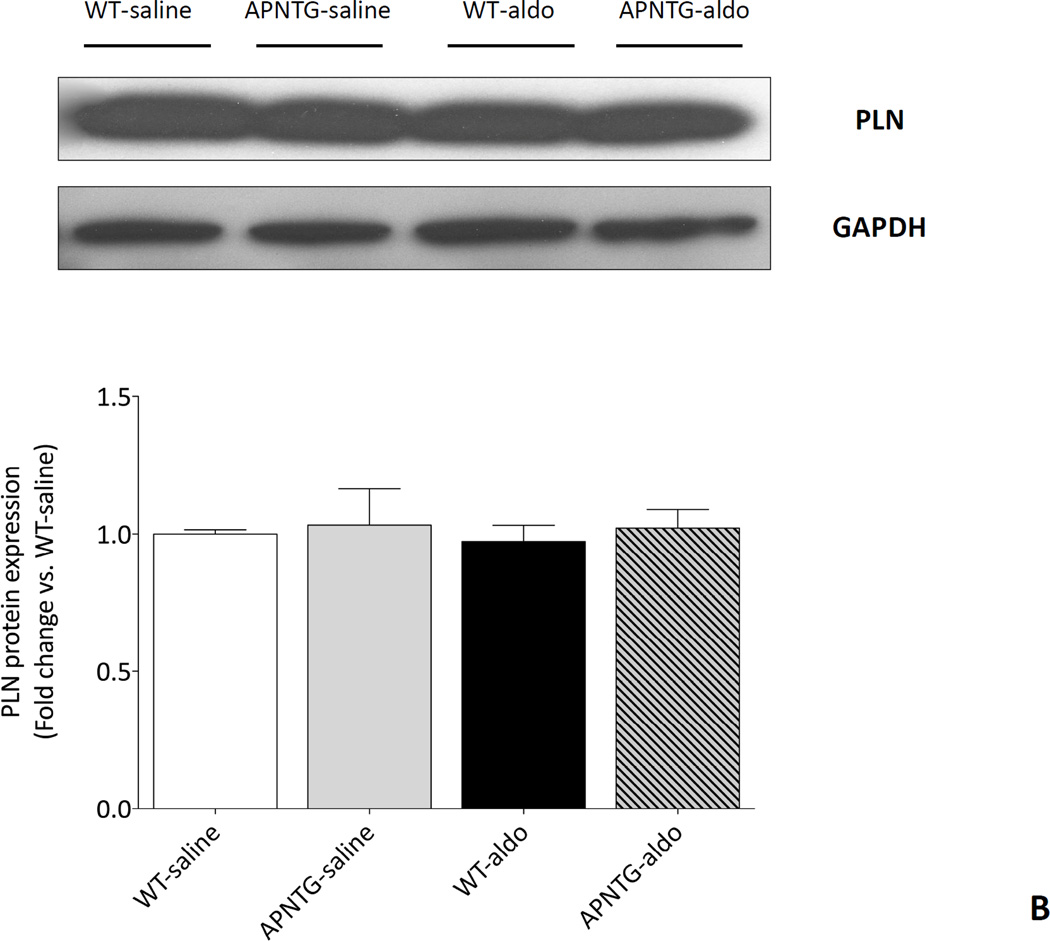
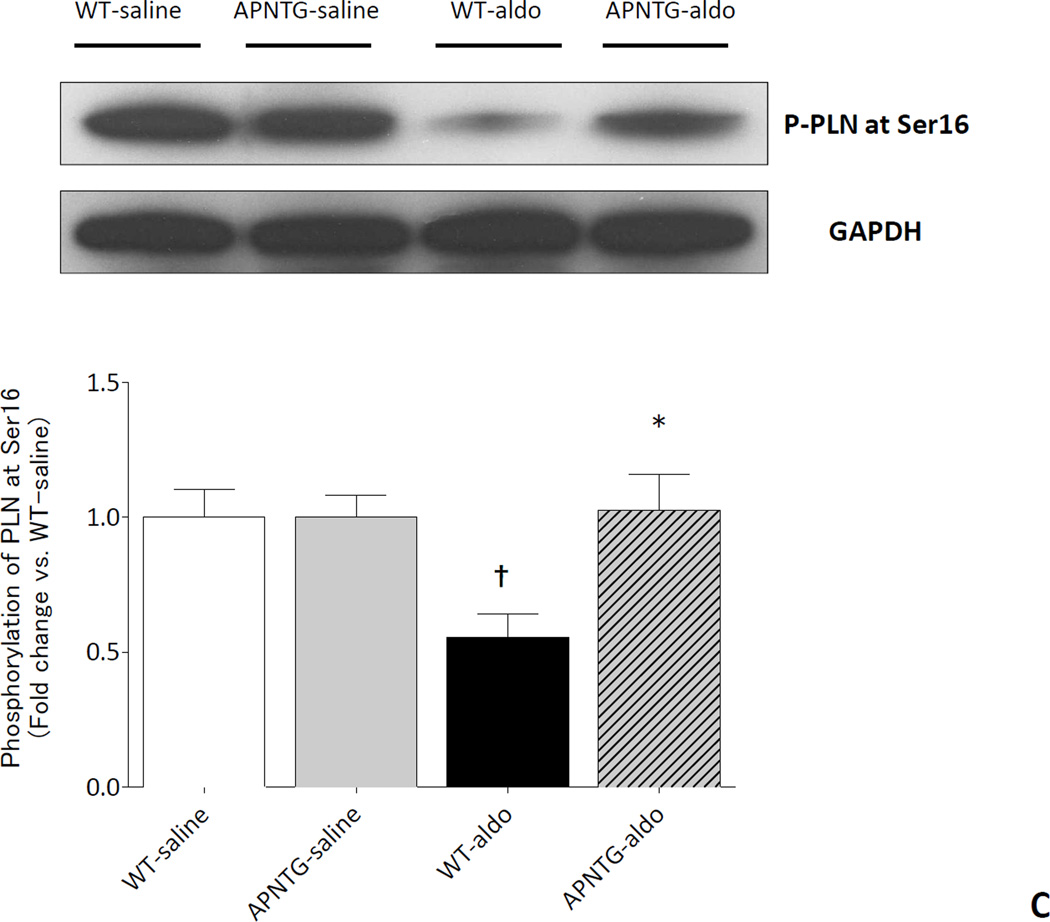
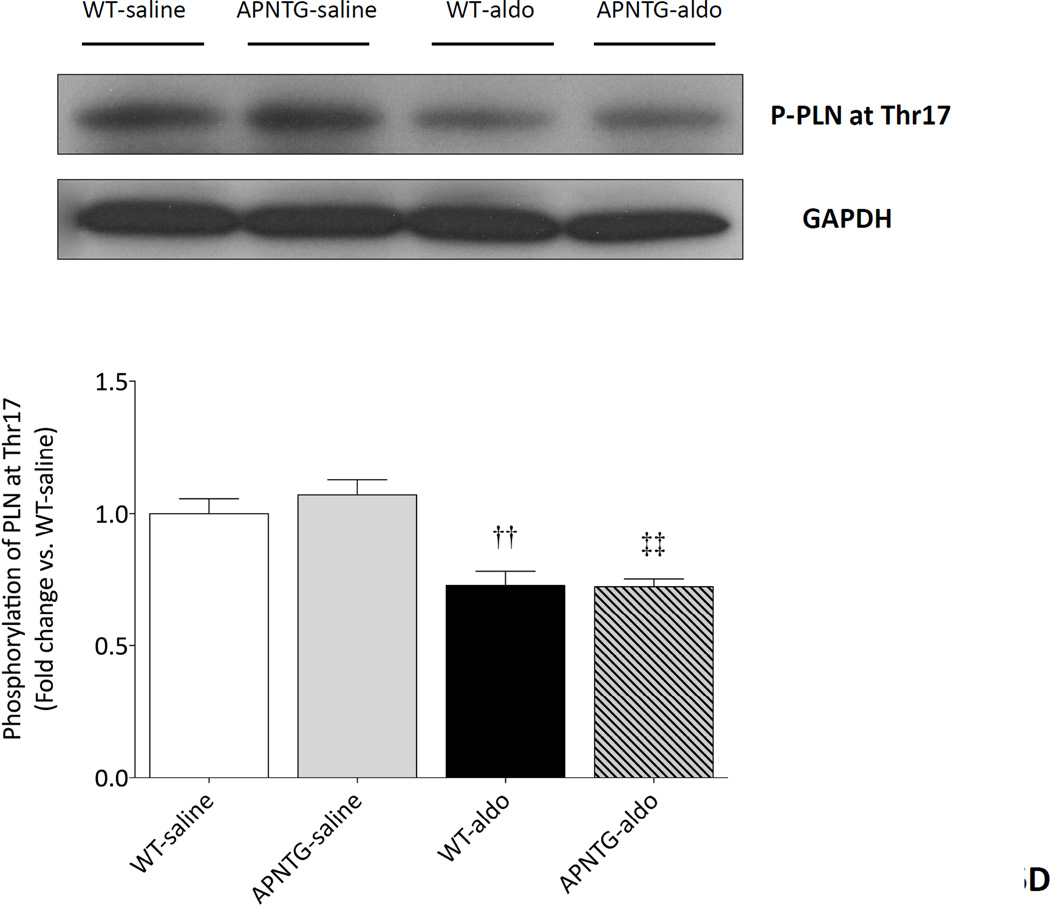
Calcium handling proteins: SERCA2a and PLN
Four weeks of aldosterone infusion significantly decreased SERCA2a protein expression in WT-aldosterone vs. WT-saline mice (0.71-fold; P<0.01) and APNTG-aldosterone vs. APNTG-saline mice (0.66-fold; P<0.05). The decrease in SERCA2a protein expression was comparable between WT-aldosterone and APNTG-aldosterone mice (Figure 5A). There was no difference in PLN protein expression between WT and APNTG mice regardless of saline or aldosterone infusion (Figure 5B). Four weeks of aldosterone infusion significantly decreased phosphorylation of PLN at Ser16 in WT-aldosterone vs. WT-saline mice (0.55-fold; P<0.01; Figure 5C). However, this decrease in PLN phosphorylation at Ser16 in WT-aldosterone mice was significantly attenuated in APNTG-aldosterone mice (P<0.05; Figure 5C). Four weeks of aldosterone infusion significantly decreased phosphorylation of PLN at Thr17 in WT-aldosterone vs. WT-saline mice (0.73-fold; P<0.01) and APNTG-aldosterone vs. APNTG-saline mice (0.68-fold; P<0.01; Figure 5D). PLN phosphorylation of at Thr17 was similar between WT-aldosterone and APNTG-aldosterone mice (Figure 5D).
Figure 5.
Expression of calcium handling regulatory proteins in WT and APNTG mice 4 weeks after saline or aldosterone infusion. A) (Upper) Representative blots of SERCA2a and GAPDH. (Lower) Quantitative analysis of SERCA2a protein expression; B) (Upper) Representative blots of PLN and GAPDH. (Lower) Quantitative analysis of PLN protein expression; C) (Upper) Representative blots of PLN phosphorylation at Ser16 and GAPDH. (Lower) Quantitative analysis of PLN phosphorylation at Ser16; D) (Upper) Representative blots of PLN phosphorylation at Thr17 and GAPDH. (Lower) Quantitative analysis of PLN phosphorylation at Thr17. ††P<0.01 vs. WT-saline, ‡P<0.05 vs. APNTG-saline, ‡‡P<0.01 vs. APNTG-saline, *P<0.05 vs. WT-aldosterone; (n=3–8/group).
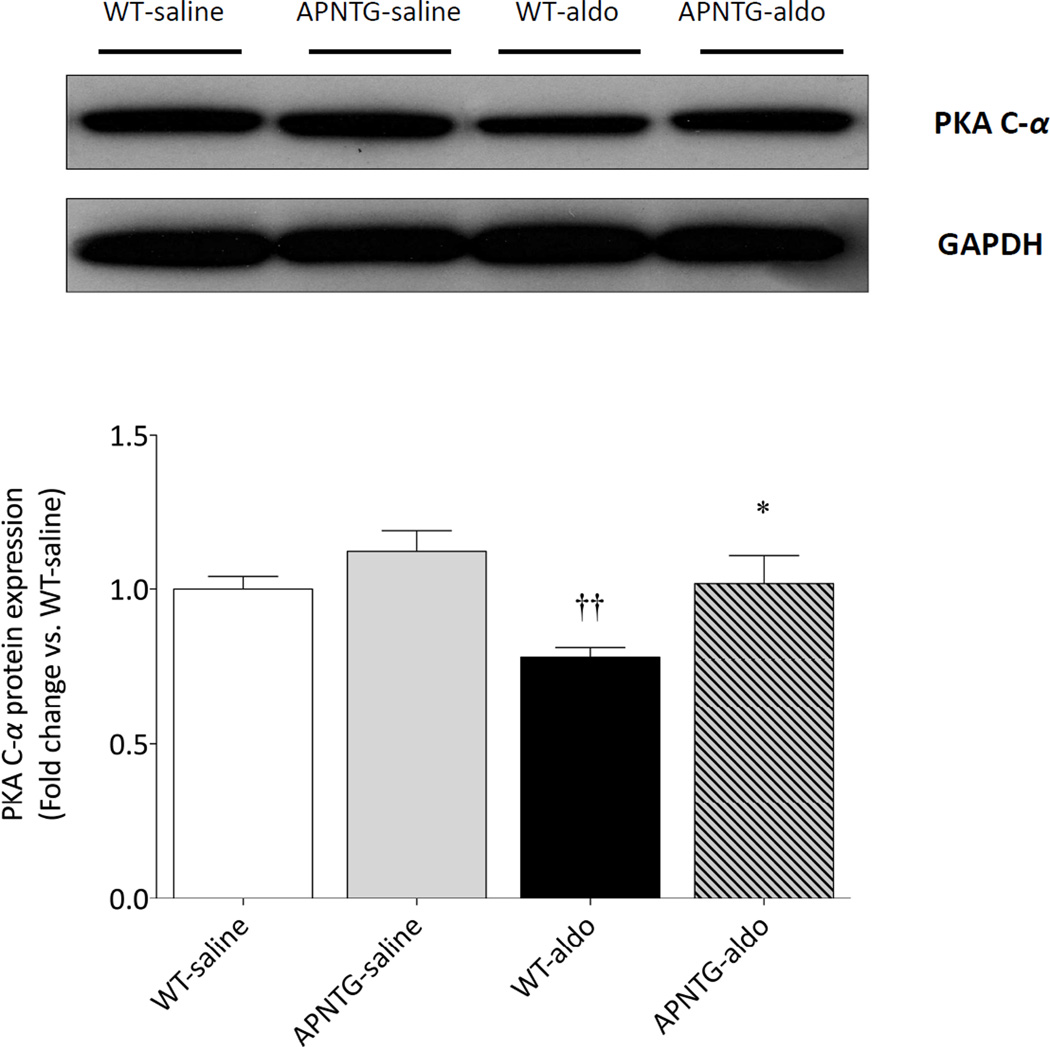
PKA expression
Four weeks of aldosterone infusion significantly decreased PKA C-α protein expression in WT-aldosterone vs. WT-saline mice (0.78-fold; P<0.01). This decrease of PKA C-α protein expression in WT-aldosterone mice was however significantly attenuated in APNTG-aldosterone mice (P<0.05; Figure 6).
Figure 6.
PKA C-α protein expression in WT and APNTG mice 4 weeks after saline or aldosterone infusion. (Upper) Representative blots of PKA C-α and GAPDH. (Lower) Quantitative analysis of PKA C-α expression (n=3–8/group); ††P<0.01 vs. WT-saline, *P<0.05 vs. WT-aldosterone.
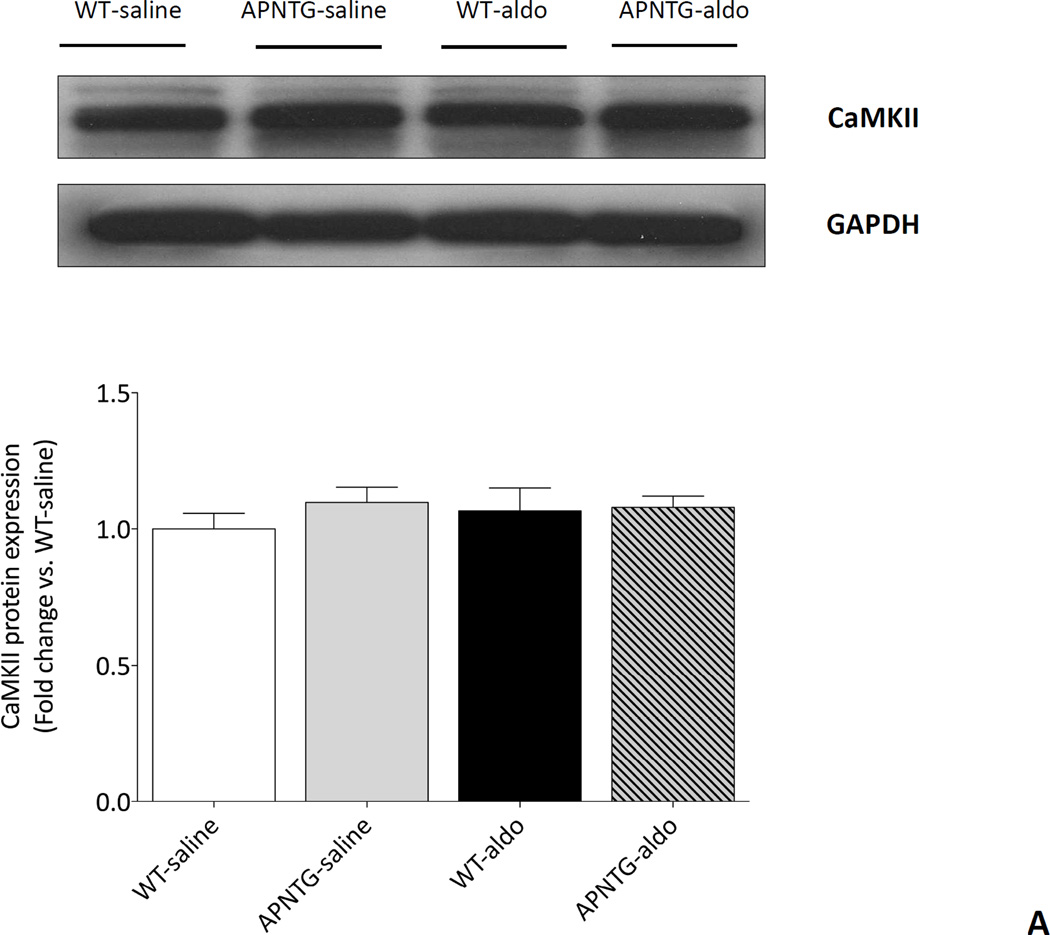
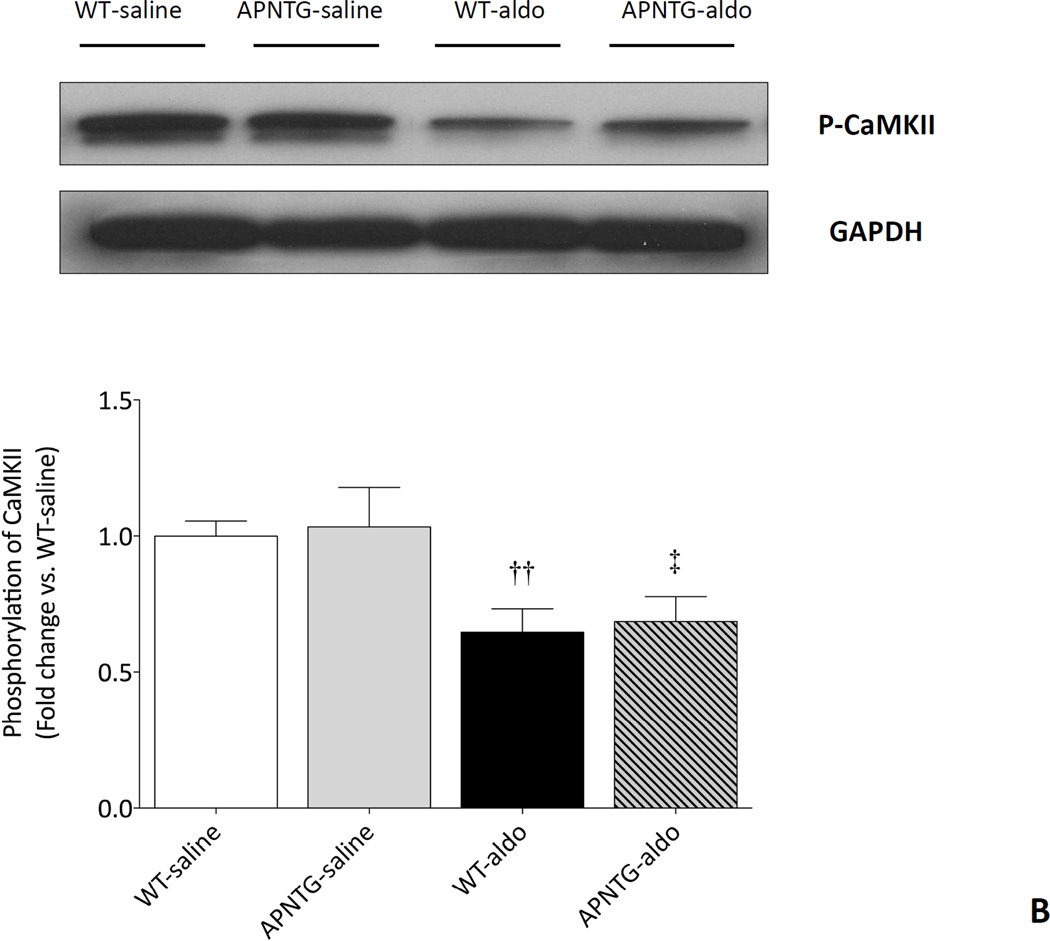
CaMKII expression
There was no difference in CaMKII protein expression between WT and APNTG mice regardless of saline or aldosterone infusion (Figure 7A). Four weeks of aldosterone infusion significantly decreased phosphorylation of CaMKII in WT-aldosterone vs. WT-saline mice (0.65-fold; P<0.01) and APNTG-aldosterone vs. APNTG-saline mice (0.66-fold; P<0.05; Figure 7B). There was no difference in phosphorylation of CaMKII between WT-aldosterone and APNTG-aldosterone mice.
Figure 7.
CaMKII protein expression and phosphorylation of CaMKII in WT and APNTG mice 4 weeks after saline or aldosterone infusion. A) (Upper) Representative blots of CaMKII and GAPDH. (Lower) Quantitative analysis of CaMKII protein expression; B) (Upper) Representative blots of phosphorylation of CaMKII and GAPDH. (Lower) Quantitative analysis of phosphorylation of CaMKII (n=3–8/group); ††P<0.01 vs. WT-saline, ‡P<0.05 vs. APNTG-saline.
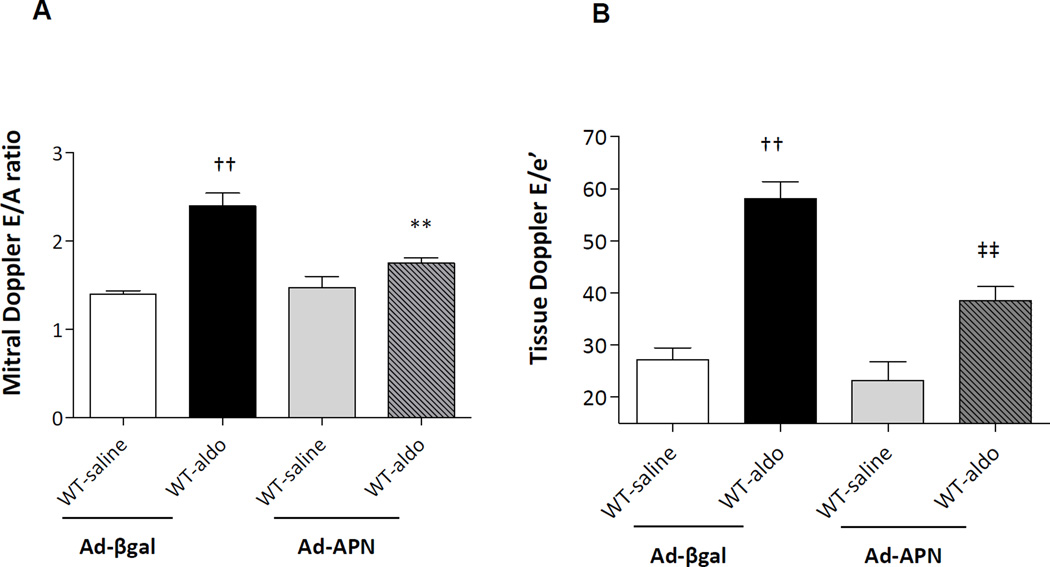
APN supplementation (Figure 8A–B)
Figure 8.
Ad-APN supplementation modulates diastolic dysfunction in aldosterone-infused WT. A) Ad-APN attenuated the E/A ratio by 37% in WT-aldosterone mice treated with Ad-APN vs. WT-aldosterone mice treated with Ad-βgal (**P<0.01). B) Ad-APN decreased E/e’ by 34% in WT-aldosterone mice vs. WT-aldosterone mice treated with Ad-βgal (††P<0.01).
To examine whether Ad-APN supplementation ameliorated aldosterone-induced diastolic dysfunction in vivo, both WT-saline and WT-aldosterone mice were treated with either adenovirus-APN (Ad-APN) or adenovirus-β-galactosidase (Ad-βgal). Ad-APN or Ad-βgal were injected into the jugular vein of mice 14 days after surgery. This dose of Ad-APN raises APN levels in the physiological range and contains the 3 isoforms14, 21 that are present in mice with the hexamer form being dominant. Neither Ad-APN nor Ad-βgal had an effect on SBP in WT-saline and WT-aldosterone mice (data not shown).
Ad-APN attenuated the aldosterone-induced changes in diastolic dysfunction: E/A ratio WT-aldosterone treated with Ad-APN (2.4) vs. WT-aldosterone mice treated with Ad-βgal (1.75; P<0.01). Similarly, E/e’ decreased 34% vs. WT-aldosterone mice treated with Ad-βgal (P<0.01).
Discussion
In this study, aldosterone-infused WT mice developed hypertension, LVH, diastolic dysfunction, and increased lung congestion while maintaining a preserved LVEF, thus resulting in HFpEF. Aldosterone infusion also increased myocardial oxidative stress, decreased SERCA2a protein expression, decreased PKA-dependent PLN phosphorylation at Ser16, and decreased CaMKII-dependent phosphorylation of PLN at Thr17. Chronic hyperadiponectinemia ameliorated LVH, diastolic dysfunction, and lung congestion without effects on BP or LVEF in HFpEF mice. Chronic APN overexpression also decreased myocardial nitrotyrosine staining, a measure of oxidative stress. The improvement of diastolic dysfunction parameters was associated with preserved PKA-dependent PLN phosphorylation at Ser16. In addition, APN supplementation with Ad-APN improved measures of diastolic dysfunction in HFpEF mice infused with aldosterone.
We previously showed that APN deficiency in aldosterone-induced HFpEF mice exacerbated hypertension and LVH18. Although it has been reported that hypoadiponectinemia is a risk factor for hypertension11, the therapeutic effect of APN on hypertension is largely unknown. Ohashi, et al. reported that adenovirus-mediated overexpression of APN ameliorated obesity-related hypertension in mice13. However, in our study, transgenic mice with chronic APN overexpression and Ad-APN supplementation of WT mice did not affect BP. This difference may be due to pathophysiological differences of targeted experimental models and less likely due to the difference of APN levels in each experimental model. APN levels in our APNTG mice were about 2-fold higher than those in WT mice19, and although we did not measure APN levels in the APN supplementation experiments, it was likely similar to adenovirus-mediated APN levels in Ohashi’s study where APN levels were about 6-fold higher than those at baseline. Further studies are needed to determine the therapeutic effect of APN in hypertension, but the importance of our study is that chronic APN overexpression ameliorates the progression of LVH, independent of alterations in BP. Aldosterone-induced LVH, which is composed of LV cardiomyocyte hypertrophy and myocardial fibrosis20, 22 is accompanied by oxidative stress via mineralocorticoid receptor activation23–25. Thus, suppression of oxidative stress might be an important therapeutic target in HFpEF26, 27. Several studies have recently shown that APN exerts its cardioprotective effect by inhibiting oxidative stress28–30. Consistent with these findings, we showed that APN overexpression diminished aldosterone-induced myocardial 3-nitrotyrosine production, a marker of oxidative stress. APN likely mitigates aldosterone-induced adverse cardiac remodeling partly by suppressing oxidative stress29, 30. In a high fat experimental model, APN prevented platelet aggregation by attenuating oxidative and nitrosative stress31 and modulated ROS metabolite levels and increased antioxidant levels in an ischemia/reperfusion porcine model32. Mitochondrial targeted antioxidant peptide, SS-31 may prove to be a therapeutic option in HFpEF patients as it targets mitochondrial ROS, and modulates LVH, fibrosis, and LV diastolic dysfunction in an experimental model33.
Both cardiomyocyte hypertrophy and myocardial fibrosis contribute to impaired active relaxation and increased passive stiffness of the LV, and subsequently leads to diastolic dysfunction and clinical HF34, 35. In our study, hyperadiponectinemia decreased cardiac hypertrophy and improved some measures of diastolic dysfunction, independent of BP. Thus, chronic hyperadiponectinemia may improve diastolic dysfunction and HF by ameliorating LVH18. In human studies, it has been reported that low plasma APN is associated with diastolic dysfunction36.
Diastolic intracellular calcium handling is a major determinant of LV relaxation35, 37. Dephosphorylated PLN is an inhibitor of SERCA2a, but PKA-catalyzed (or CaMKII), phosphorylation of PLN results in the dissociation of PLN from SERCA2a, thus activating this Ca2+ pump and augmenting SERCA2a activity. In our study, chronic aldosterone infusion decreased SERCA2a protein expression and PLN phosphorylation at both Ser16 and Thr17, but did not alter PLN protein expression. In addition, PKA C-α, an active catalytic subunit of PKA, and protein expression and phosphorylation of CaMKII, an indicator of CaMKII activity, were both decreased by chronic aldosterone infusion. Accumulating evidence indicates that β-adrenergic receptor stimulation regulates PKA and CaMKII activity38–40. Additionally, it has also been reported that Ser16 phosphorylation is mainly affected by PKA activity whereas Thr17 phosphorylation is affected by CaMKII activity38, 39, 41. Although β-adrenergic signaling affected by chronic aldosterone infusion remains to be clarified in this study, decreased PKA and CaMKII activity suggest that β-adrenergic signaling is down-regulated by chronic aldosterone infusion. Collectively, our data indicates that chronic aldosterone infusion decreased PKA-dependent phosphorylation of PLN at Ser16 and CaMKII-dependent phosphorylation of PLN at Thr17, presumably followed by β-adrenergic signaling downregulation, and subsequently suppressed SERCA2a protein expression. We did not measure intracellular Ca2+ concentrations. However, evidence indicates that diastolic intracellular calcium handling is mainly regulated by SERCA2a and its modulator PLN35, 37, 41. Thus, changes in SERCA2a protein expression and/or phosphorylation of PLN in our study, may be associated with abnormal intracellular diastolic calcium handling and subsequent diastolic dysfunction, as demonstrated by impaired LV compliance (increased E/A), abnormal LV relaxation (shortened DT), and elevated diastolic filling pressure (increased E/e’). Nonetheless, in our study hyperadiponectinemia and chronic APN overexpression ameliorated these measures of diastolic dysfunction in HFpEF indicating that the improvement in these measures might be due to alterations in calcium handling protein signaling.
At cellular and molecular levels, APN induces Ca2+ influx via AdipoR1 and subsequently activates CaMKK, AMPK and SIRT1 in skeletal muscle42. However in the myocardium, SERCA2a protein expression in chronic aldosterone infusion was not affected by chronic APN overexpression. Likewise, CaMKII activity and CaMKII-dependent phosphorylation of PLN at Thr17 were both decreased and to comparable levels. Yet chronic APN overexpression ameliorated the decrease of PLN phosphorylation at Ser16, followed by preserved PKA activity. SERCA2a function is determined not only by SERCA2a protein expression43, but also by the phosphorylation status of PLN44. In our study, chronic APN overexpression did not affect the SERCA2a protein expression, but improved diastolic dysfunction. These findings may be partly explained by improvement of SERCA2a function through phosphorylation of PLN. In addition, several studies have shown that APN is associated with cyclic adenosine monophosphate (cAMP)-dependent PKA signaling45, 46. Thus, our finding suggests that chronic APN overexpression preserves phosphorylation of PLN at Ser16 through PKA activation, and improves SERCA2a dysfunction.
Finally, the potential beneficial effects of APN may extend to the downregulation of inflammatory cytokines or the upregulation of anti-inflammatory cytokines which may impact cardiac hypertrophy, the extracellular matrix, diastolic dysfunction and HFpEF. Pro-inflammatory cytokines, such as TNF-α which is pro-hypertrophic47, are increased in diastolic dysfunction and HFpEF18 (Supplemental Figure 2). We recently showed that IFN-γ, a pro-inflammatory cytokine attenuated cardiac hypertrophy and is a regulator of cardiac hypertrophy in HFpEF thus disputing the notion that inflammatory cytokines mediate only adverse effects48.
In conclusion, chronic APN overexpression and supplementation prevented the progression of aldosterone-induced HFpEF, independent of blood pressure. The beneficial effect of APN was associated with reduced myocardial oxidative stress and modulation of intracellular calcium handling regulatory proteins. Our findings indicate that APN and its signaling pathway may be a therapeutic target for patients with HFpEF.
Supplementary Material
Acknowledgments
Sources of Funding
This work was supported by the National Institutes of Health (HL117153 to F.S.) and research grant for study abroad from The Japanese Circulation Society (to K.T.).
Footnotes
Disclosures
None.
References
- 1.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL. 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;128:e240–e327. doi: 10.1161/CIR.0b013e31829e8776. [DOI] [PubMed] [Google Scholar]
- 2.Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355:251–259. doi: 10.1056/NEJMoa052256. [DOI] [PubMed] [Google Scholar]
- 3.Goodfriend TL, Calhoun DA. Resistant hypertension, obesity, sleep apnea, and aldosterone: theory and therapy. Hypertension. 2004;43:518–524. doi: 10.1161/01.HYP.0000116223.97436.e5. [DOI] [PubMed] [Google Scholar]
- 4.Calhoun DA, Jones D, Textor S, Goff DC, Murphy TP, Toto RD, White A, Cushman WC, White W, Sica D, Ferdinand K, Giles TD, Falkner B, Carey RM. Resistant Hypertension: Diagnosis, Evaluation, and Treatment: A Scientific Statement From the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Circulation. 2008;117:e510–e526. doi: 10.1161/CIRCULATIONAHA.108.189141. [DOI] [PubMed] [Google Scholar]
- 5.Bhatia RS, Tu JV, Lee DS, Austin PC, Fang J, Haouzi A, Gong Y, Liu PP. Outcome of Heart Failure with Preserved Ejection Fraction in a Population-Based Study. N Engl J Med. 2006;355:260–269. doi: 10.1056/NEJMoa051530. [DOI] [PubMed] [Google Scholar]
- 6.Engeli S, Bohnke J, Gorzelniak K, Janke J, Schling P, Bader M, Luft FC, Sharma AM. Weight Loss and the Renin-Angiotensin-Aldosterone System. Hypertension. 2005;45:356–362. doi: 10.1161/01.HYP.0000154361.47683.d3. [DOI] [PubMed] [Google Scholar]
- 7.Ehrhart-Bornstein M, Lamounier-Zepter V, Schraven A, Langenbach J, Willenberg HS, Barthel A, Hauner H, McCann SM, Scherbaum WA, Bornstein SR. Human adipocytes secrete mineralocorticoid-releasing factors. Proc Natl Acad Sci U S A. 2003;100:14211–14216. doi: 10.1073/pnas.2336140100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vleugels K, Schinner S, Krause D, Morawietz H, Bornstein SR, Ehrhart-Bornstein M, Krug AW. ERK1/2 MAPKs and Wnt signaling pathways are independently involved in adipocytokine-mediated aldosterone secretion. Exp Clin Endocrinol Diabetes. 2011;119:644–648. doi: 10.1055/s-0031-1284367. [DOI] [PubMed] [Google Scholar]
- 9.Arita Y, Kihara S, Ouchi N, Takahashi M, Maeda K, Miyagawa J, Hotta K, Shimomura I, Nakamura T, Miyaoka K, Kuriyama H, Nishida M, Yamashita S, Okubo K, Matsubara K, Muraguchi M, Ohmoto Y, Funahashi T, Matsuzawa Y. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun. 1999;257:79–83. doi: 10.1006/bbrc.1999.0255. [DOI] [PubMed] [Google Scholar]
- 10.Hotta K, Funahashi T, Arita Y, Takahashi M, Matsuda M, Okamoto Y, Iwahashi H, Kuriyama H, Ouchi N, Maeda K, Nishida M, Kihara S, Sakai N, Nakajima T, Hasegawa K, Muraguchi M, Ohmoto Y, Nakamura T, Yamashita S, Hanafusa T, Matsuzawa Y. Plasma concentrations of a novel, adipose-specific protein, adiponectin, in type 2 diabetic patients. Arterioscler Thromb Vasc Biol. 2000;20:1595–1599. doi: 10.1161/01.atv.20.6.1595. [DOI] [PubMed] [Google Scholar]
- 11.Iwashima Y, Katsuya T, Ishikawa K, Ouchi N, Ohishi M, Sugimoto K, Fu Y, Motone M, Yamamoto K, Matsuo A, Ohashi K, Kihara S, Funahashi T, Rakugi H, Matsuzawa Y, Ogihara T. Hypoadiponectinemia is an independent risk factor for hypertension. Hypertension. 2004;43:1318–1323. doi: 10.1161/01.HYP.0000129281.03801.4b. [DOI] [PubMed] [Google Scholar]
- 12.Kumada M, Kihara S, Sumitsuji S, Kawamoto T, Matsumoto S, Ouchi N, Arita Y, Okamoto Y, Shimomura I, Hiraoka H, Nakamura T, Funahashi T, Matsuzawa Y. Association of hypoadiponectinemia with coronary artery disease in men. Arterioscler Thromb Vasc Biol. 2003;23:85–89. doi: 10.1161/01.atv.0000048856.22331.50. [DOI] [PubMed] [Google Scholar]
- 13.Ohashi K, Kihara S, Ouchi N, Kumada M, Fujita K, Hiuge A, Hibuse T, Ryo M, Nishizawa H, Maeda N, Maeda K, Shibata R, Walsh K, Funahashi T, Shimomura I. Adiponectin replenishment ameliorates obesity-related hypertension. Hypertension. 2006;47:1108–1116. doi: 10.1161/01.HYP.0000222368.43759.a1. [DOI] [PubMed] [Google Scholar]
- 14.Shibata R, Ouchi N, Ito M, Kihara S, Shiojima I, Pimentel DR, Kumada M, Sato K, Schiekofer S, Ohashi K, Funahashi T, Colucci WS, Walsh K. Adiponectin-mediated modulation of hypertrophic signals in the heart. Nat Med. 2004;10:1384–1389. doi: 10.1038/nm1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liao Y, Takashima S, Maeda N, Ouchi N, Komamura K, Shimomura I, Hori M, Matsuzawa Y, Funahashi T, Kitakaze M. Exacerbation of heart failure in adiponectin-deficient mice due to impaired regulation of AMPK and glucose metabolism. Cardiovasc Res. 2005;67:705–713. doi: 10.1016/j.cardiores.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 16.Shibata R, Sato K, Pimentel DR, Takemura Y, Kihara S, Ohashi K, Funahashi T, Ouchi N, Walsh K. Adiponectin protects against myocardial ischemia-reperfusion injury through. Nat Med. 2005;11:1096–1103. doi: 10.1038/nm1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shibata R, Izumiya Y, Sato K, Papanicolaou K, Kihara S, Colucci WS, Sam F, Ouchi N, Walsh K. Adiponectin protects against the development of systolic dysfunction following myocardial infarction. J Mol Cell Cardiol. 2007;42:1065–1074. doi: 10.1016/j.yjmcc.2007.03.808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sam F, Duhaney TA, Sato K, Wilson RM, Ohashi K, Sono-Romanelli S, Higuchi A, De Silva DS, Qin F, Walsh K, Ouchi N. Adiponectin deficiency, diastolic dysfunction, and diastolic heart failure. Endocrinology. 2010;151:322–331. doi: 10.1210/en.2009-0806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Combs TP, Pajvani UB, Berg AH, Lin Y, Jelicks LA, Laplante M, Nawrocki AR, Rajala MW, Parlow A, Cheeseboro L, Ding YY, Russell RG, Lindemann D, Hartley A, Baker GRC, Obici S, Deshaies Y, Ludgate M, Rossetti L, Scherer PE. A Transgenic Mouse with a Deletion in the Collagenous Domain of Adiponectin Displays Elevated Circulating Adiponectin and Improved Insulin Sensitivity. Endocrinology. 2004;145:367–383. doi: 10.1210/en.2003-1068. [DOI] [PubMed] [Google Scholar]
- 20.Wilson RM, De Silva DS, Sato K, Izumiya Y, Sam F. Effects of fixed-dose isosorbide dinitrate/hydralazine on diastolic function and exercise capacity in hypertension-induced diastolic heart failure. Hypertension. 2009;54:583–590. doi: 10.1161/HYPERTENSIONAHA.109.134932. [DOI] [PubMed] [Google Scholar]
- 21.Shibata R, Sato K, Kumada M, Izumiya Y, Sonoda M, Kihara S, Ouchi N, Walsh K. Adiponectin accumulates in myocardial tissue that has been damaged by ischemia-reperfusion injury via leakage from the vascular compartment. Cardiovascular Research. 2007;74:471–479. doi: 10.1016/j.cardiores.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 22.Qin W, Rudolph AE, Bond BR, Rocha R, Blomme EA, Goellner JJ, Funder JW, McMahon EG. Transgenic model of aldosterone-driven cardiac hypertrophy and heart failure. Circ Res. 2003;93:69–76. doi: 10.1161/01.RES.0000080521.15238.E5. [DOI] [PubMed] [Google Scholar]
- 23.Rude MK, Duhaney TA, Kuster GM, Judge S, Heo J, Colucci WS, Siwik DA, Sam F. Aldosterone stimulates matrix metalloproteinases and reactive oxygen species in adult rat ventricular cardiomyocytes. Hypertension. 2005;46:555–561. doi: 10.1161/01.HYP.0000176236.55322.18. [DOI] [PubMed] [Google Scholar]
- 24.Kuster GM, Kotlyar E, Rude MK, Siwik DA, Liao R, Colucci WS, Sam F. Mineralocorticoid receptor inhibition ameliorates the transition to myocardial failure and decreases oxidative stress and inflammation in mice with chronic pressure overload. Circulation. 2005;111:420–427. doi: 10.1161/01.CIR.0000153800.09920.40. [DOI] [PubMed] [Google Scholar]
- 25.Stas S, Whaley-Connell A, Habibi J, Appesh L, Hayden MR, Karuparthi PR, Qazi M, Morris EM, Cooper SA, Link CD, Stump C, Hay M, Ferrario C, Sowers JR. Mineralocorticoid receptor blockade attenuates chronic overexpression of the renin-angiotensin-aldosterone system stimulation of reduced nicotinamide adenine dinucleotide phosphate oxidase and cardiac remodeling. Endocrinology. 2007;148:3773–3780. doi: 10.1210/en.2006-1691. [DOI] [PubMed] [Google Scholar]
- 26.Hummel SL, Seymour EM, Brook RD, Kolias TJ, Sheth SS, Rosenblum HR, Wells JM, Weder AB. Low-sodium dietary approaches to stop hypertension diet reduces blood pressure, arterial stiffness, and oxidative stress in hypertensive heart failure with preserved ejection fraction. Hypertension. 2012;60:1200–1206. doi: 10.1161/HYPERTENSIONAHA.112.202705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mohammed SF, Ohtani T, Korinek J, Lam CSP, Larsen K, Simari RD, Valencik ML, Burnett JC, Redfield MM. Mineralocorticoid Accelerates Transition to Heart Failure With Preserved Ejection Fraction Via "Nongenomic Effects". Circulation. 2010;122:370–378. doi: 10.1161/CIRCULATIONAHA.109.915215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tao L, Gao E, Jiao X, Yuan Y, Li S, Christopher TA, Lopez BL, Koch W, Chan L, Goldstein BJ, Ma XL. Adiponectin Cardioprotection After Myocardial Ischemia/Reperfusion Involves the Reduction of Oxidative/Nitrative Stress. Circulation. 2007;115:1408–1416. doi: 10.1161/CIRCULATIONAHA.106.666941. [DOI] [PubMed] [Google Scholar]
- 29.Essick EE, Ouchi N, Wilson RM, Ohashi K, Ghobrial J, Shibata R, Pimentel DR, Sam F. Adiponectin mediates cardioprotection in oxidative stress-induced cardiac myocyte remodeling. Am J Physiol Heart Circ Physiol. 2011;301:H984–H993. doi: 10.1152/ajpheart.00428.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Essick EE, Wilson RM, Pimentel DR, Shimano M, Baid S, Ouchi N, Sam F. Adiponectin Modulates Oxidative Stress-Induced Autophagy in Cardiomyocytes. PLoS ONE. 2013;8:e68697. doi: 10.1371/journal.pone.0068697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang WQ, Zhang HF, Gao GX, Bai QX, Li R, Wang XM. Adiponectin inhibits hyperlipidemia-induced platelet aggregation via attenuating oxidative/nitrative stress. Physiol Res. 2011;60:347–354. doi: 10.33549/physiolres.932044. [DOI] [PubMed] [Google Scholar]
- 32.Kondo K, Shibata R, Unno K, Shimano M, Ishii M, Kito T, Shintani S, Walsh K, Ouchi N, Murohara T. Impact of a single intracoronary administration of adiponectin on myocardial ischemia/reperfusion injury in a pig model. Circ Cardiovasc Interv. 2010;3:166–173. doi: 10.1161/CIRCINTERVENTIONS.109.872044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maack C, Bohm M. Targeting mitochondrial oxidative stress in heart failure throttling the afterburner. J Am Coll Cardiol. 2011;58:83–86. doi: 10.1016/j.jacc.2011.01.032. [DOI] [PubMed] [Google Scholar]
- 34.Zile MR, Baicu CF, Gaasch WH. Diastolic Heart Failure -- Abnormalities in Active Relaxation and Passive Stiffness of the Left Ventricle. N Engl J Med. 2004;350:1953–1959. doi: 10.1056/NEJMoa032566. [DOI] [PubMed] [Google Scholar]
- 35.Kass DA, Bronzwaer JG, Paulus WJ. What mechanisms underlie diastolic dysfunction in heart failure? Circ Res. 2004;94:1533–1542. doi: 10.1161/01.RES.0000129254.25507.d6. [DOI] [PubMed] [Google Scholar]
- 36.Negi SI, Jeong EM, Shukrullah I, Raicu M, Dudley SC., Jr Association of low plasma adiponectin with early diastolic dysfunction. Congest Heart Fail. 2012;18:187–191. doi: 10.1111/j.1751-7133.2011.00276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zile MR, Gaasch WH. Abnormal calcium homeostasis: one mechanism in diastolic heart failure. J Am Coll Cardiol. 2011;58:155–157. doi: 10.1016/j.jacc.2010.10.068. [DOI] [PubMed] [Google Scholar]
- 38.Kuschel M, Karczewski P, Hempel P, Schlegel WP, Krause EG, Bartel S. Ser16 prevails over Thr17 phospholamban phosphorylation in the beta-adrenergic regulation of cardiac relaxation. Am J Physiol. 1999;276:H1625–H1633. doi: 10.1152/ajpheart.1999.276.5.H1625. [DOI] [PubMed] [Google Scholar]
- 39.Grimm M, Brown JH. Beta-adrenergic receptor signaling in the heart: role of CaMKII. J Mol Cell Cardiol. 2010;48:322–330. doi: 10.1016/j.yjmcc.2009.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.He BJ, Anderson ME. Aldosterone and cardiovascular disease: the heart of the matter. Trends Endocrinol Metab. 2013;24:21–30. doi: 10.1016/j.tem.2012.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yano M, Ikeda Y, Matsuzaki M. Altered intracellular Ca2+ handling in heart failure. J Clin Invest. 2005;115:556–564. doi: 10.1172/JCI24159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Iwabu M, Yamauchi T, Okada-Iwabu M, Sato K, Nakagawa T, Funata M, Yamaguchi M, Namiki S, Nakayama R, Tabata M, Ogata H, Kubota N, Takamoto I, Hayashi YK, Yamauchi N, Waki H, Fukayama M, Nishino I, Tokuyama K, Ueki K, Oike Y, Ishii S, Hirose K, Shimizu T, Touhara K, Kadowaki T. Adiponectin and AdipoR1 regulate PGC-1alpha and mitochondria by Ca2+ and AMPK/SIRT1. Nature. 2010;464:1313–1319. doi: 10.1038/nature08991. [DOI] [PubMed] [Google Scholar]
- 43.Meyer M, Dillmann WH. Sarcoplasmic reticulum Ca(2+)-ATPase overexpression by adenovirus mediated gene transfer and in transgenic mice. Cardiovasc Res. 1998;37:360–366. doi: 10.1016/s0008-6363(97)00270-8. [DOI] [PubMed] [Google Scholar]
- 44.Nef HM, Mollmann H, Skwara W, Bolck B, Schwinger RH, Hamm C, Kostin S, Schaper J, Elsasser A. Reduced sarcoplasmic reticulum Ca2+ -ATPase activity and dephosphorylated phospholamban contribute to contractile dysfunction in human hibernating myocardium. Mol Cell Biochem. 2006;282:53–63. doi: 10.1007/s11010-006-1171-7. [DOI] [PubMed] [Google Scholar]
- 45.Ouchi N, Kihara S, Arita Y, Okamoto Y, Maeda K, Kuriyama H, Hotta K, Nishida M, Takahashi M, Muraguchi M, Ohmoto Y, Nakamura T, Yamashita S, Funahashi T, Matsuzawa Y. Adiponectin, an adipocyte-derived plasma protein, inhibits endothelial NF-kappaB signaling through a cAMP-dependent pathway. Circulation. 2000;102:1296–1301. doi: 10.1161/01.cir.102.11.1296. [DOI] [PubMed] [Google Scholar]
- 46.Xu SQ, Mahadev K, Wu X, Fuchsel L, Donnelly S, Scalia RG, Goldstein BJ. Adiponectin protects against angiotensin II or tumor necrosis factor α-induced endothelial cell monolayer hyperpermeability: role of cAMP/PKA signaling. Arterioscler Thromb Vasc Biol. 2008;28:899–905. doi: 10.1161/ATVBAHA.108.163634. [DOI] [PubMed] [Google Scholar]
- 47.Sun M, Chen M, Dawood F, Zurawska U, Li JY, Parker T, Kassiri Z, Kirshenbaum LA, Arnold M, Khokha R, Liu PP. Tumor necrosis factor-α mediates cardiac remodeling and ventricular dysfunction after pressure overload state. Circulation. 2007;115:1398–1407. doi: 10.1161/CIRCULATIONAHA.106.643585. [DOI] [PubMed] [Google Scholar]
- 48.Garcia AG, Wilson RM, Heo J, Murthy NR, Baid S, Ouchi N, Sam F. Interferon-gamma Ablation Exacerbates Myocardial Hypertrophy in Diastolic Heart Failure. Am J Physiol Heart Circ Physiol. 2012;303:H587–H596. doi: 10.1152/ajpheart.00298.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.