Abstract
Background
Statin use in nursing home (NH) residents with advanced dementia neither prolongs life nor promotes comfort.
Objectives
To describepatterns of,and factors associated with,statin use anddiscontinuation in NH residents progressing to advanced dementiaand followed for at least 90 days.
Design, Setting, Participants, Measurements
We constructed a retrospective inception cohort of NH residents with dementia from 5 states using a dataset linking 2007-2008 Minimum Data Set (MDS) to Medicare denominator and Part D files. Residents were observed from baseline (i.e. date of progression to very severe cognitive impairment with eating problems) and followedfor at least 90 days to statin discontinuation or death. Logistic regression identifiedbaseline factors associated with statin use. Cox proportional hazard regression identified factors associated with time to statin discontinuation.
Results
Of 10,212cohort residents,16.6% (n=1,699) used statins. Greater odds of statin use were associated withhaving diabetes (adjusted odds ratio [AOR] 1.24, 95% confidence interval [CI] 1.09-1.40), stroke (AOR 1.31, 95% CI 1.16-1.48), and hypertension (AOR 1.35, 95% CI 1.18-1.54); hospice enrollment was associated with lower odds(AOR = .75, 95% CI .64-.89).In follow-up, only 37.2% (n=632) discontinued statins. The median time to discontinuationwas36 days (interquartile range: 12 days-110 days). Shorter time to discontinuation was associated withhospitalization in prior 30 days (adjusted hazard ratio [AHR] 1.67, 95% CI 1.40-1.99) and greater total number of daily medications(AHR 1.02, 95% CI 1.01-1.04).When statins were discontinued, 15.0% (n=95) of residents stopped only statins while 47.5% (n=300) stopped at least one other medication.
Conclusion
Most NH residents who use statins at the time of progression to advanced dementia continue use in follow-up.
Keywords: pharmaceuticals, dementia, statins
INTRODUCTION
Forpatients with life-limiting illness, the Institute of Medicine (IOM) recommends clinical care that minimizes unnecessary or non-beneficial interventions.1Instead, end-of-life care should emphasize therapies that optimize quality of life.For patients with a life-limiting illness such as advanced dementia, an expert consensus panel suggests that certain medications such as statins may be inappropriate when the goal of care is comfort.2The rationale is that advanced dementia is characterized by very high mortality rates, high levels of functional disability, and eating dysfunction.3,4While statins are commonly prescribed for the primary and secondary prevention of cardiovascular events in at-risk patients, their ability to promote benefits such as prolonged survival and comfort is unclear in the last weeks to months of life.5-7
Few studies examinepatterns of chronic disease medication use8-10or statin use as death approaches.5-7,11Our prior study among nursing home (NH) residents with advanced dementia showed that 12% used a lipid-lowering drug, and that one-third discontinued that drug in the last weeks of life.10Up to 50% of patients with other types of advanced illness used statins in the last six months of life,7 with no distinction in discontinuation rates by whether the statin was prescribed for primary or secondary prevention.6Unfortunately,these prior studies were based on small samples drawn from specialized populations of managed care patients6, veterans7, or a limited geographic area.10
Therefore, we assembled an inception cohort of NHresidents with dementia who recently advanced from moderate or severe to very severe cognitive impairment with eating problems from 5 large U.S. states in order to describe: 1) the prevalenceand factors associated with statin use at time of decline to very severe cognitive impairment with eating problems; 2) incidence of statin discontinuation and time to discontinuation following decline; 3) the change in the number of other medications prescribed concurrent with the statin discontinuation; and 4) factors associated with statin discontinuation.
METHODS
Data Source
We used Minimum Data Set 2.0 data from January 1, 2007 to December 31, 2008 to assemblea cohort ofNH residents with dementia from all licensed facilities (N=3,371) in five states (Minnesota, Massachusetts, Pennsylvania, California and Florida).The MDS is a standardized, clinically-based instrument used in US NHs to assess resident condition for clinical and reimbursement purposes. It collects information on the demographic, functional, medical, psychological, and cognitive status of residents.12-13The Centers for Medicare and Medicaid Services (CMS) require that each certified facility conduct an MDS assessment of all residents on admission, quarterly thereafter, and with significant changes in clinical status. Welinked files to Medicare Part D data to measure medication use.
Study Population
To create the inception cohort, we initially identified all residents with dementia using Section I of the MDS 2.0, includingthose with dementia of the Alzheimer's type and dementia other than Alzheimer's type (n=367,410).We excluded residents who did not have an associated Medicare Part D file (n=80,768). We used the Cognitive Performance Scale (CPS) score to classify dementia severity. CPS scores correspond to levels of impairment: 1 (borderline intact); 2 (mild impairment); 3 (moderate impairment); 4 (moderately severe impairment); 5 (severe impairment); and 6 (very severe impairment with eating problems).14We defined advanced dementia as CPS 6, whichcorresponds to a mean Mini-Mental Status Examination score of 0.4.14We excluded residents with prevalent advanced dementia (CPS 6) on their first MDS assessment (n=27,327), andincluded residents with observed progression to CPS 6 on a subsequent MDS assessment (n=11,786). The baseline date for cohort entry was defined as the first MDS assessment with a CPS = 6. We excluded residents who did not have at least 90 days of observation following the baseline date (on or before October 1, 2008) or a recorded date of death (n=1,574), which resulted in n=10,212 comprising the inception cohort.
Study Variables
Statin Exposure
Statins were identified by the generic name in the Part D data and included: atorvastatin, fluvastatin, lovastatin, pravastatin, rosuvastatin, simvastatin.Part D data reports date of service including the date on which a prescription is filled and the days’ supply; we defined statin exposure periods based on these two parameters.
In order to be classified as a statin user at baseline, patients were required to have a statin exposure period overlapping with thebaseline date. Statin discontinuation was defined as the presence of a gap of at least 30 days after the last day for which pills were available to the patient (based on days’ supply of the most recently filled prescription). For those who discontinued statins, the date of discontinuation was defined as the date on which the last prescription was filled plus the days’ supply corresponding to the last prescription. For example, if January 1, 2007 were the date of the last prescription and this prescription provided 30 days’ supply, then (in the absence of another prescription in the following 30 days) the date of discontinuation would be January 31, 2007. If, however, there were a prescription filled on February 14, 2007 (or on any other day within the 30 days following January 31st) then the patient was defined as continuing their statin.
Overall Medication Use
We identified overall medication useat two times: the baselinedate and statin discontinuation date. We defined overall medication useon the baseline date to be equal to the count of unique drug names with an exposure period that overlapped with the baseline date. We also calculated the change in overall medication use at the time of statin discontinuationbased on the difference between the number of medications on day 15 after thestatin stop date and the medication burden 15 days prior to the statin stop date. The overall medication use measure includes statins and other chronic disease medications administered on a daily basis. As needed medications were not included in this measure because they are typically dispensed once at the beginning of the nursing home stay, and infrequently thereafter unless used on a regular basis.
Resident Characteristics
Resident characteristics that were potentially associated with medication use were ascertained from the residents’ full MDS assessments at theirbaseline date.These characteristics10,15,16included: sociodemographics (age; sex; race/ethnicity; marital status); whether Medicaid was the primary payor; comorbidities (diabetes mellitus, heart failure, hypertension, peripheral vascular disease, stroke, cardiac dysrhythmia, renal failure, cancer, depression); nutritional problems (≥25% of meals uneaten), and presence of a feeding tube; behavioral problems by the Behavioral Index (0-2, higher scores indicate more severe behaviors)17,18; functional status based on the sum of the activities of daily living (ADL) scores (0-28, higher scores indicate greater functional impairment); advance directives (living will, donot-resuscitate [DNR] order, do-not-hospitalize [DNH] order, medication restriction [i.e. resident or responsible party does not wish the resident to receive life-sustaining medications such as antibiotics or chemotherapy], and feeding restrictions [i.e. resident or responsible party does not wish the resident to be fed by artificial means if unable to be nourished orally]);enrollment in hospice; and hospitalization in the prior 90 days; and geographic location of the nursing home (California, Florida, Massachusetts, Minnesota, and Pennsylvania).
Data Analysis
We used descriptive statistics to describe resident characteristics, the proportion of residents using a statin at time of progression to advanced dementia and the number of non-statin medications stopped at the same time as the statin discontinuation. In an analysis including all residents in the cohort, we used logistic regression to compare residents using statins at baseline compared to those not using statins at baseline. In an analysis restricted to residents using statins at baseline, we used Cox proportional hazard models to identify the factors associated time to statin discontinuation. We followed each resident from the baseline date to death, censoring at the end of the observation period (December 31, 2008), or the last date a prescription was filled. For both the logistic regression and Cox models, unadjusted analyses were followed by adjusted analyses including factors associated with outcomes at the p<.05 level in unadjusted analysis. All analyses were performed using STATA 10.1 (StataCorp, College Station, TX). The institutional review board of the University of Massachusetts Medical School approved this study.
RESULTS
Thefinal cohort of 10,212 NH residentswith advanced dementia were 73.9% female and75.4% white. Overall, 68.4% had a DNR order,16.9% were enrolled in hospice, and the average number of medications used was 7 (standard deviation [SD]=4.8). (Table 1) Almost seventeen percent(16.6%; n=1,699)were prescribed statins at the time of decline to advanced dementia (i.e. time of entry into the cohort), and the average number of medications used by these residents was 10.3 (SD 4.8). In adjusted analysis, having a history ofdiabetes (adjusted odds ratio [AOR] 1.24, 95% confidence interval [CI], 1.09, 1.40), stroke (AOR 1.31, 95% CI, 1.16, 1.48), or hypertension (AOR 1.35, 95% CI, 1.18, 1.54) increased the odds of statin use at baseline, while being older (AOR .97, 95% CI .96, .98) and being enrolled in hospice (AOR .75 [95% CI, .64, .89]) decreased the odds of statin use at baseline. (Table 1)
Table 1.
Characteristics of Residents with Advanced Dementia Using Statins at Baseline
| Characteristic | Overall N=10,212 | Residents using statins N=1,699 | Adjusted Odds Ratio (95% CI) | |
|---|---|---|---|---|
| n (%)a | na | % | ||
| Mean Age, years (SD) | 85.0 (SD 7.3) | 83.1 (7.0) | n/a | .97 (.96, .98) |
| Sex | ||||
| Male | 2,663 (26.1) | 529 | 19.9 | Referent |
| Female | 7,549 (73.9) | 1,170 | 15.5 | .93 (.81, 1.06) |
| Race/ethnicity | ||||
| White, not Hispanic | 7,701 (75.4) | 1,221 | 15.9 | Referent |
| Asian/Pacific Islander | 424 (4.2) | 95 | 22.4 | 1.04 (.79, 1.37) |
| Black, not Hispanic | 1,088 (10.7) | 205 | 18.4 | 1.03 (.84, 1.21) |
| Hispanic | 970 (9.5) | 169 | 17.4 | .91 (.74, 1.11) |
| Other | 29 (.3) | <10 | 31.0 | 2.04 (.89, 4.68) |
| Marital Status | ||||
| Not currently married | 7,567 (74.1) | 1,189 | 15.7 | Referent |
| Currently Married | 2,645 (25.9) | 510 | 19.3 | 1.11 (.98, 1.27) |
| Medicaid | ||||
| No | 3,462 (33.9) | 563 | 16.3 | -- |
| Yes | 6,750 (66.1) | 1,136 | 16.8 | -- |
| State Residence | ||||
| California | 3,278 (32.1) | 578 | 17.6 | Referent |
| Florida | 2,686 (26.3) | 449 | 16.7 | .91 (.77, 1.07) |
| Massachusetts | 1,334 (13.1) | 67 | 17.1 | 1.17 (.97, 1.42) |
| Minnesota | 572 (5.6) | 377 | 11.7 | .77 (.57, 1.03) |
| Pennsylvania | 2,342 (22.9) | 2,342 | 16.1 | .93 (.79, 1.10) |
| Comorbid Conditions | ||||
| Diabetes Mellitus | 3,022 (29.6) | 714 | 23.6 | 1.24 (1.09, 1.40) |
| Heart Failure | 2,127 (20.8) | 397 | 18.7 | .89 (.78, 1.02) |
| Stroke/TIA | 2,489 (24.4) | 542 | 21.8 | 1.31 (1.16, 1.48) |
| Hypertension | 6,980 (68.4) | 1,318 | 18.9 | 1.35 (1.18, 1.54) |
| Peripheral Vascular Disease | 1,475 (14.4) | 254 | 17.2 | -- |
| Cardiac Dysrhythmia | 1,668 (16.3) | 298 | 17.9 | -- |
| Renal Failure | 1,126 (11.0) | 215 | 19.1 | .92 (.77, 1.09) |
| Cancer | 768 (7.5) | 139 | 18.1 | |
| Mental Health Diagnoses | ||||
| Depression | 4,928 (48.3) | 892 | 18.1 | 1.05 (.94, 1.18) |
| Anxiety | 2,153 (21.1) | 348 | 16.2 | -- |
| Bipolar | 249 (2.4) | 48 | 19.3 | -- |
| Schizophrenia | 291 (2.9) | 57 | 19.6 | -- |
| Behavioral Index Score | ||||
| 0 | 7,856 (76.9) | 1,304 | 16.6 | -- |
| 1-2 | 2,356 (23.1) | 395 | 16.8 | -- |
| Activities of Daily Living (ADL) score | ||||
| < 28 | 4,437 (43.4) | 760 | 17.1 | -- |
| 28 | 5,775 (56.6) | 939 | 16.3 | -- |
| Feeding tube | 2379 (23.3) | 389 | 16.4 | -- |
| Advance Directive | ||||
| Living Will | 2,013 (19.7) | 343 | 17.0 | -- |
| DNR | 6,983 (68.4) | 1,089 | 15.6 | 1.02 (.90, 1.17) |
| DNH | 1,266 (12.4) | 168 | 13.3 | .87 (.72, 1.07) |
| Feeding restrictionb | 2,126 (20.8) | 310 | 14.6 | 1.05 (.87, 1.24) |
| Medication restrictionc | 733 (7.2) | 90 | 12.3 | .81 (.63, 1.07) |
| Hospice (vs not) | 1,721 (16.9) | 239 | 13.9 | .75 (.64, .89) |
| Mean medication use, # (SD) | 7.07 (SD 4.8) | 10.3 (SD 4.8) | n/a | 1.16 (1.15, 1.18) |
| Hospitalized <30 days prior to baseline (vs none) | 3,994 (39.1) | 748 | 44.0 | 1.03 (.92, 1.17) |
“--” not significant at p<.05 level in unadjusted analysis.Bold indicates significant at p<.05 level. Adjusted for age, sex, race/ethnicity, marital status, state of residence, diabetes mellitus, heart failure, stroke/TIA, hypertension, renal failure, depression, DNR, DNH, feeding restriction, medication restriction, hospice enrollment, medication use (#), hospitalization < 30 days of baseline
Unless otherwise noted
Feeding Restrictions – The resident or responsible party (family or legal guardian) does not wish the resident to be fed by artificial means (e.g. tube, intervention nutrition) if unable to be nourished by oral means.
Medication Restrictions- The resident or responsible party (family or legal guardian) does not wish the resident to receive life-sustaining medications (e.g., antibiotics, chemotherapy).
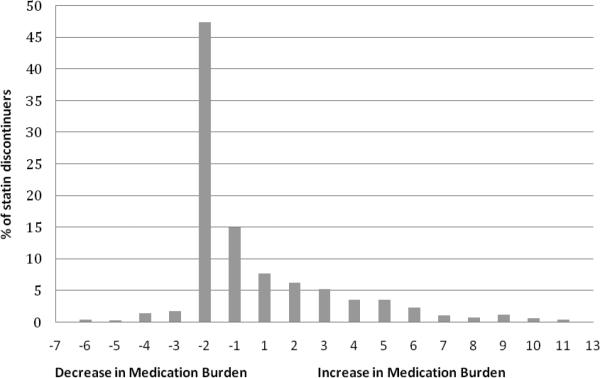
Among the statin userswith advanced dementia at baseline (N=1,699), 37.2% (n=632) discontinued statin usein the follow-up period. Mean follow-up time was 185 days, median follow-up time was 163 days. A total of n=924 (54.4%) statin users died in follow up. Of decedents, n=568 (61.5%) were using statins at death. When statins were discontinued, 15.0% (n=95) of residentsstopped only statins while other daily medications were continued (i.e. had a net decrease of one in their overall medication burden). (Figure 1)Another 47.5% (n=300)of statin users had statins stopped along with at least one other medication, resulting in a net decrease of at least two in their overall medication burden.In contrast, the net medication burden increased in 34.6% (n=211) of residents at the time of statin discontinuation due to the addition of other medications. Among residents with advanced dementia on statins at baseline who discontinued these drugs, the median time to discontinuation was 36 days after baseline (interquartile range: 12 days, 110 days).Factors associated with increased hazard of (i.e. shorter time to) discontinuation after adjustment for factors significant at the p<.05 level in unadjusted analysisincluded residence in a nursing home in Florida (relative to California) (adjusted hazard ratio [AHR] 1.30, 95% CI 1.06, 1.60), being hospitalized in the 30 days prior to decline to advanced dementia (AHR1.67, 95% CI 1.40, 1.99), having a greater medication burden at baseline (AHR 1.02, 95% CI 1.01, 1.04), and having a diagnosis of cancer (AHR 1.52, 95% CI 1.15, 1,98).(Table 2) At the end of the study period, 67% of those using statins at baseline remained on these drugs, representing 10% of the overall inception cohort with advanced dementia.
Figure 1.
Change in net medication burden at time of statin discontinuation, among residents discontinuing statins after diagnosis of advanced dementia (n=632)*
Table 2.
Unadjusted and Adjusted Cox Models for Time to Statin Discontinuation after Decline to Advanced Dementia (n=1671, including 28 statin discontinuations on entry)
| Characteristic | Unadjusted Hazard Ratio (95% CI) | Adjusted Hazard Ratio (95% CI) |
|---|---|---|
| Mean Age, years | 1.00 (.99, 1.01) | -- |
| Female sex (vs male) | .88 (.74, 1.04) | -- |
| Race/Ethnicity (vs White) | ||
| Asian/Pacific Islander | 1.03 (.47, 3.35) | .97 (.67, 1.41) |
| Black, not Hispanic | .98 (.77, 1.26) | .90 (.70, 1.16) |
| Hispanic | 1.28 (1.00, 1.65) | 1.13 (.87, 1.46) |
| American Indian/Alaskan | 1.25 (.47, 3.35) | 1.47 (.54, 3.98) |
| Currently Married (vs not currently married) | 1.12 (.94, 1.32) | -- |
| Medicaid (vs not) | .71 (.60, .83) | .80 (.68, .95) |
| State Residence (vs California) | ||
| Florida | 1.29 (1.05, 1.57) | 1.30 (1.06, 1.60) |
| Massachusetts | 1.01 (.78, 1.30) | 1.21 (.93, 1.59) |
| Minnesota | 1.07 (.68, 1.67) | 1.20 (.76, 1.90) |
| Pennsylvania | .84 (.67, 1.05) | .95 (.75, 1.21) |
| Comorbid Conditions | ||
| Diabetes Mellitus | .98 (.83, 1.15) | -- |
| Heart Failure | 1.18 (.98, 1.43) | -- |
| Stroke/TIA | 1.16 (.98, 1.37) | -- |
| Hypertension | 1.11 (.91, 1.35) | -- |
| Peripheral Vascular Disease | 1.04 (.84, 1.30) | -- |
| Cardiac Dysrhythmia | 1.08 (.87, 1.33) | -- |
| Renal Failure | 1.29 (1.02, 1.64) | 1.14 (.89, 1.45) |
| Cancer | 1.53 (1.17, 1.99) | 1.52 (1.16, 1.98) |
| Mental Health Diagnoses | ||
| Depression | 1.02 (.87, 1.19) | -- |
| Anxiety | 1.12 (.93, 1.36) | -- |
| Bipolar | 1.00 (.62, 1.62) | -- |
| Schizophrenia | .72 (.45, 1.17) | -- |
| Behavioral Index Score (vs 0) | ||
| 1-2 | .92 (.86, 1.11) | -- |
| Activities of Daily Living score (vs< 28) 28 | 1.14 (.97, 1.34) | -- |
| Feeding tube | .81 (.67, .97) | .96 (.78, 1.18) |
| Advance Directive | ||
| Living Will | .99 (.81, 1.21) | -- |
| DNR | .85 (.72, 1.00) | .91 (.77, 1.08) |
| DNH | 1.08 (.81, 1.44) | -- |
| Feeding restrictiona | .87 (.70, 1.09) | -- |
| Medication restrictionb | .99 (.69, 1.44) | -- |
| Hospice (vs not enrolled in hospice) | 1.12 (.86, 1.46) | |
| Mean medication useat baseline, # | 1.03 (1.01, 1.05) | 1.02 (1.01, 1.04) |
| Hospitalization<30 days prior to baseline (vs none) | 1.83 (1.56, 2.14) | 1.67 (1.40, 1.99) |
“--” not significant at p<.05 level in unadjusted analysis.Bold indicates significant at p<.05 level.
Feeding Restrictions – The resident or responsible party (family or legal guardian) does not wish the resident to be fed by artificial means (e.g. tube, intervention nutrition) if unable to be nourished by oral means.
Medication Restrictions- The resident or responsible party (family or legal guardian) does not wish the resident to receive life-sustaining medications (e.g., antibiotics, chemotherapy).
DISCUSSION
We found that about 1 in 6 NH residents with advanced dementia, defined as very severe impairment with eating problems, are prescribed a statin at the time of declineto advanced dementia. Further, two-thirdsof these residents continue on statins after reachingadvanced stage disease.
Because swallowing and eating difficulties make medication administration burdensome and difficult for both the resident and the NH staff,26 statin discontinuation is an intervention arguably consistent with the goal of maintaining patient comfort.2Further, there is little evidence to suggest that statins confer benefit that outweighs this burden among persons with weeks to months of remaining life expectancy. We know of no prior estimate of statin use and discontinuation in relation to the clinical challenge dysphagia or anorexia in this population, except our prior study showing that lipid-lowering medicationswere discontinued in about 30% of residents with advanced dementia.10
While one study previously reported that chronic medication use for comorbid medical conditions decreased over time among patients with life-limiting illness,8 another study suggests that the presence of a recognizable, life-limiting condition does not increase statin discontinuation.5, 27 Because the use of symptom management medications at the end-of-life increases the total number of drugs used near death,8careful balancing of chronic disease versus acute symptom management medications should be considered to optimize quality of life.1,2,19
We found that most discontinuations occurred within 30 days of decline to advanced dementia, and that many of these discontinuations were preceded by an acute hospitalization. Only one other study describes the timing of statin discontinuation in relation to the diagnosis of an advanced illness, and that study reported that patients diagnosed with lung cancer discontinued statins an average of 244 days after the time of cancer diagnosis.11 Time to discontinuation relative to development of metastatic disease or death was not described.
Our study also found that recent hospitalization is the factor most strongly associated with statin discontinuation. Reasons for this are unclear. Prior studies indicate that hospitalizations in populations with advanced illness have limited clinical benefit,20involve aggressive interventions21,22and result in distress for family members.23,24 The physical and medical setbacks associated with such hospitalizationsmay trigger end-of-life discussions, and such goals of care discussions have been shown to be associated with less aggressive medical care near death.25 However, we are unable to distinguish whether the relationship between statin discontinuation and recent hospitalization was due to intentional discontinuationto meet comfort as the goal of care, was a marker for the development of a new clinical contraindication (e.g. hepatic congestion or liver function derangements), or was an inadvertent prescribing error.26,28
Consistent with IOM guidance, prescribed interventions in patients approaching death should promote the main goal of care.1Prior studies report that over 90% of proxies of NH residents with advanced dementia state that their goal of care is comfort.3,4 Statinsdo not promote comfort and thus for the residents with advanced dementia, their role in the treatment plan is not substantiated. Even for the minority of residents with advanced dementia whose goal of care remains life prolongation, it is not clear that statins promote that goal given the very limited life expectancy in advanced dementia. While we were unable to specifically identify goals of care in this study, 64% of our statin users had a DNR order, 9.9% had a DNH order, and 14.1% were enrolled in hospice. While hospice enrollment was associated with lower statin use at cohort entry, none of these factors independently increased the likelihood of statin discontinuation after baseline. While our prior study did show that inappropriate medication use was lower with the presence of a DNH order, we did not observe this finding in the current analysis.10
We note several limitations of this study. First, we acknowledge that by creating an inception cohort based on decline to advanced dementia, we may have selected for patients who had recent hospitalizations because new ‘change of status’ MDS forms are typically completed following a return to the nursing home from the hospital. Therefore, it is unclear whether hospitalizations are truly independent predictors of statin discontinuation, or whether they are just a marker for late stage disease. Second, we calculated overall change in number of medications at time of statin discontinuation, and not the actual number of changes (new starts and discontinuations), because overall medication use is the best measure of polypharmacy, a well-known independent risk factor for adverse drug events and poor outcomes. Third, we did not measure prn medications in change of overall medication burden with statin discontinuation. We acknowledge that the number of chronic disease drug discontinuations, and new starts for hospice related medications, would better allow us to understand whether statins were stopped as part of an overall shift in care to ‘comfort measures only’, but this was outside the scope of this study. Further, we did not include changes in advance directives or resident characteristics in this analysis as a time-varying covariate, and this may explain the lack of association for factors such as DNH ordersand the outcome of statin discontinuation. Mitigating these limitations is the strength of the population-based sampling from 5 large states in the U.S. and inclusion of clinical data in attempting to understand statin use in advanced illness.
In summary, while statin use in late life remains controversial, we find evidence of a practice of discontinuation with clinical status decline. Whether these discontinuations are associated with improved quality of life or poorer prognosis has yet to be determined, but are the subject of a randomized clinical trial by the Palliative Care Research Cooperative Group examining whether statin discontinuation affects survival and quality of life in patients with advanced life-limiting illness.29[ClinicalTrials.gov identifier NCT01415934] While our paper highlights some reasons for discontinuations, further investigation to tie changes in goals of care with changes in medication prescribing deserve attention.
ACKNOWLEDGMENTS
Funding/Support: This study was supported by the Agency for Healthcare Research and Quality grant (R21 HS19579; PI: Tjia). Dr. Cutrona is supported by Award Number KL2TR000160 from the National Center for Research Resources (NCRR). Dr. Mitchell is support by NIH-NIA K24AG033640. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NCRR or the National Institutes of Health.
Sponsor's Role: The funding organizations did not participate in the design or conduct of the study, in the collection, analysis or interpretation of the data, or in the preparation, review, or approval of the manuscript.
Footnotes
Author Contributions: Jennifer Tjia was responsible for the study concept and design, acquisition of data, analysis and interpretation of data, and preparation and critical review of the manuscript. Sarah L. Cutrona was responsible for interpretation of data, and preparation and critical review of the manuscript. Daniel Peterson and George Reed was responsible for analysis and critical review of the manuscript. Susan E. Andrade and Susan L. Mitchell were responsible for study design, interpretation of data, and preparation and critical review of the manuscript.
Conflict of Interest: The editor in chief has reviewed the conflict of interest checklist provided by the authors and has determined that the authors have no financial or any other kind of personal conflicts with this paper.
REFERENCES
- 1.Field M, Cassell C. Approaching death: Improving care at the end of life. National Academy Press; Washington DC: 1997. Institute of Medicine Report. [PubMed] [Google Scholar]
- 2.Holmes HM, Sachs GA, Shega JW, et al. Integrating palliative medicine into the care of persons with advanced dementia: Identifying appropriate medication use. J Am Geriatr Soc. 2008;56:1306–1311. doi: 10.1111/j.1532-5415.2008.01741.x. [DOI] [PubMed] [Google Scholar]
- 3.Mitchell SL, Teno JM, Kiely DK, et al. The clinical course of advanced dementia. N Engl J Med. 2009;361:1529–1538. doi: 10.1056/NEJMoa0902234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Luchins DJ, Hanrahan P. J Am Geriatr Soc. 1993;What Is Appropriate health care for end-stage dementia?41:25–30. doi: 10.1111/j.1532-5415.1993.tb05943.x. [DOI] [PubMed] [Google Scholar]
- 5.Vollrath AM, Sinclair C, Hallenbeck J. Discontinuing cardiovascular medications at the end of life: Lipid-lowering agents. J Pall Med. 2005;8:876–881. doi: 10.1089/jpm.2005.8.876. [DOI] [PubMed] [Google Scholar]
- 6.Bayliss E, Bronsert MR, Reifler LM, et al. Statin prescribing patterns in a cohort of cancer patients with poor prognosis. J Pall Med. 2013;16:1–7. doi: 10.1089/jpm.2012.0158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Silveira MJ, Kazanis AS, Shevrin MP. Statins in the last six months of life: A recognizable, life-limiting condition does not decrease their use. J Pall Med. 2008;11:685–693. doi: 10.1089/jpm.2007.0215. [DOI] [PubMed] [Google Scholar]
- 8.Currow DC, Stevenson JP, Abernethy AP, et al. prescribing in palliative care as death approaches. J Am Geriatr Soc. 2007;55:590–595. doi: 10.1111/j.1532-5415.2007.01124.x. [DOI] [PubMed] [Google Scholar]
- 9.Blass DM, Black BS, Phillips H, et al. Medication use in nursing home residents with advanced dementia. Int J Geriatr Psychiatry. 2008;23:490–496. doi: 10.1002/gps.1921. [DOI] [PubMed] [Google Scholar]
- 10.Tjia J, Rothman MR, Kiely DK, et al. Daily medication use in nursing home residents with advanced dementia. J Am Geriatr Soc. 2010;58:880–888. doi: 10.1111/j.1532-5415.2010.02819.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tanvetyanon T, Choudhury AM. Physician practice in the discontinuation of statins among patients with advanced lung cancer. J Palliat Care. 2006;22:281–285. [PubMed] [Google Scholar]
- 12.Morris JR, Hawes C, Murphy K, et al. Resident Assessment Instrument Training Manual and Resource Guide. Eliot Press; Natick, MA: 1991. [Google Scholar]
- 13.Rahman AN, Applebaum RA. The nursing home minimum data set assessment instrument: manifest functions and unintended consequences - Past, present, and future. Gerontologist. 2009;49:727–735. doi: 10.1093/geront/gnp066. [DOI] [PubMed] [Google Scholar]
- 14.Morris JN, Fries BE, Mehr DR, et al. MDS Cognitive Performance Scale. J Gerontol. 1994;49:M174–182. doi: 10.1093/geronj/49.4.m174. [DOI] [PubMed] [Google Scholar]
- 15.Avorn J, Gurwitz JH. Drug use in the nursing home. Ann Intern Med. 1995;123:195–204. doi: 10.7326/0003-4819-123-3-199508010-00007. [DOI] [PubMed] [Google Scholar]
- 16.Doshi JA, Shaffer T, Briesacher BA. National estimates of medication use in nursing homes: Findings from the 1997 Medicare current beneficiary survey and the 1996 medical expenditure survey. J Am Geriatr Soc. 2005;53:438–443. doi: 10.1111/j.1532-5415.2005.53161.x. [DOI] [PubMed] [Google Scholar]
- 17.Liperoti R, Mor V, Lapane KL, et al. The use of atypical antipsychotics in nursing homes. J Clin Psychiatry. 2003;64:1106–1112. doi: 10.4088/jcp.v64n0918. [DOI] [PubMed] [Google Scholar]
- 18.Snowden M, Sato K, Roy-Byrne P. Assessment and treatment of nursing home residents with depression or behavioral symptoms associated with dementia: A review of the literature. J Am Geriatr Soc. 2003;51:1305–1317. doi: 10.1046/j.1532-5415.2003.51417.x. [DOI] [PubMed] [Google Scholar]
- 19.Brauner DJ, Muir JC, Sachs GA. Treating nondementia illnesses in patients with dementia. JAMA. 2000;283:3230–3235. doi: 10.1001/jama.283.24.3230. [DOI] [PubMed] [Google Scholar]
- 20.Sampson EL, Blanchard MR, Jones L, et al. Dementia in the acute hospital: prospective cohort study of prevalence and mortality. Br J Psychiatry. 2009;195:61–66. doi: 10.1192/bjp.bp.108.055335. [DOI] [PubMed] [Google Scholar]
- 21.Teno JM, Mitchell SL, Gozalo PL, et al. Hospital characteristics associated with feeding tube placement in nursing home residents with advanced cognitive impairment. JAMA. 2010;303:544–550. doi: 10.1001/jama.2010.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morrison RS, Siu AL. Mortality from pneumonia and hip fractures in patients with advanced dementia. JAMA. 2000;284:2447–2448. [PubMed] [Google Scholar]
- 23.Gaugler JE, Mittelman MS, Hepburn K, et al. Predictors of change in caregiver burden and depressive symptoms following nursing home admission. Psychol Aging. 2009;24:385–396. doi: 10.1037/a0016052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Epstein-Lubow G, Gaudiano B, Darling E, et al. Differences in depression severity in family caregivers of hospitalized individuals with dementia and family caregivers of outpatients with dementia. Am J Geriatr Psychiatry. 2011;20:815–819. doi: 10.1097/JGP.0b013e318235b62f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wright AA, Zhang B, Ray A, et al. Associations between end of life discussion, patient mental health, medical care near death, and caregiver bereavement adjustment. JAMA. 2008;300:1665–1673. doi: 10.1001/jama.300.14.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bell CM, Brener SS, Gunraj N, et al. Association of ICU or hospital admission with unintentional discontinuation of medications for chronic diseases. JAMA. 2011;306:840–847. doi: 10.1001/jama.2011.1206. [DOI] [PubMed] [Google Scholar]
- 27.Shaffer T, Simoni-Wastila L, Toler W, et al. Changing patterns in medication use with increasing probability of death for older Medicare beneficiaries. J Am Geriatr Soc. 2010;58:1549–1555. doi: 10.1111/j.1532-5415.2010.02957.x. [DOI] [PubMed] [Google Scholar]
- 28.Boockvar K, Fishman E, Kyriacou CK, et al. Adverse events due to discontinuations in drug use and dose changes in patients transferred between acute and long-tetrm care facilities. Arch Int Med. 2004;164:545–550. doi: 10.1001/archinte.164.5.545. [DOI] [PubMed] [Google Scholar]
- 29.Abernethy AP, Aziz NM, Basch E, et al. A strategy to advance the evidence base in palliative medicine: Formation of a palliative care research cooperative group. J Palliat Med. 2010;13:1407–1413. doi: 10.1089/jpm.2010.0261. [DOI] [PMC free article] [PubMed] [Google Scholar]