Abstract
Purpose
To explore use of the Magnetohydrodynamic Voltage (VMHD), observed in intra-MRI 12-lead Electrocardiograms (ECG), to indicate the timing of the onset of left-ventricular mechanical activation (LVMA) and the orientation of the aortic-arch (AAO).
Theory
Blood flow through the aortic arch during systole, in the presence of the MRI magnetic field (B0), generates VMHD. Since the magnitude and direction of VMHD are determined by the timing and directionality of blood flow relative to B0, we hypothesized that clinically useful measures, LVMA and AAO, could be extracted from temporal and vectorial VMHD characteristics.
Methods
VMHD signals were extracted from 12-lead ECG traces by comparing traces obtained inside and outside the MRI scanner. VMHD was converted into the Vectorcardiogram frame of reference. LVMA was quantified in 1 subject at 1.5T and 3 subjects at 3T, and the result compared to CINE MRI. AAO was inferred for 4 subjects at 3T and compared to anatomical imaging of the aortic arch orientation in the transverse plane.
Results and Conclusions
A <10% error was observed in LVMA measurements, while a <3° error was observed in aortic arch orientation measurements. The temporal and vectorial nature of VMHD is useful in estimating these clinically relevant parameters.
Keywords: 12-Lead Electrocardiogram, Cardiac MRI, High-field MRI, ECG, MHD, mechanical activation, orientation, Magnetohydrodynamic
INTRODUCTION
The Electrocardiogram (ECG) is a clinical standard for monitoring patient cardiac activity. Its use for imaging synchronization and for physiological monitoring within Magnetic Resonance Imaging (MRI) scanners is complicated due to the presence of the MRI static magnetic field (B0), which creates Magnetohydrodynamic (MHD) distortions caused by interactions between B0 and blood-plasma electrolytes ejected into the aortic arch during early systole (1). MHD distortions induce a voltage (VMHD) overlaid onto ECGs obtained within the bore of the MRI. In (≥1.5 Tesla) MRI, and in large-diameter vessels where the direction of the blood flow and B0 is are approximately orthogonal, the large-amplitude VMHD may obscure the ECG’s QRS complex (2,3).
VMHD overlay in ECG signal traces can result in intermittent QRS detection for cardiac MRI synchronization/gating, leading to motion artifacts in the acquired images and longer scan times (4). Traditional methods for cardiac MRI gating treat the MHD signal as an artifact and attempt to remove it from the real ECG (5,6). Methods of QRS complex detection in the presence of high-field MRI include Vectorcardiogram-based (VCG) approaches (7,8), signal pre-processing (9), independent component analysis (10,11), and cross-correlation based methods (12).
Previous work has demonstrated that VMHD can be mathematically extracted from intra-MRI ECG traces, through subtraction of ECGs recorded inside (ECGreal + VMHD) and outside the MRI (ECGreal) (13,14). The relationship between the temporal variations in VMHD and blood flow velocity and directionality (5,6) suggest that information regarding the heart’s mechanical performance can be extracted from the magnitude and direction of the MHD voltage detected at each of the 10 surface electrodes used in the 12-lead ECG acquisition. Due to the strong relationship between cardiovascular blood-flow and cardiovascular function, there is a potential to derive clinically relevant metrics derived from further analysis of the vectorial nature of VMHD. Several theoretical studies have discussed its clinical potential in depth (15,16) but there is a scarcity of experimental study and verification.
Monitoring of the vectorial VMHD may allow for non-invasive assessments of cardiovascular physiology through the use of a strong magnetic field. In the current study, we investigate two potential applications of the MHD effect using the static magnetic field of an MRI scanner, allowing for non-invasive cardiovascular information to be obtained continuously during each cardiac cycle. Determination of these metrics using MRI imaging is possible, but it (i) requires a much longer acquisition time, which prevents beat-to-beat comparisons to be performed and (ii) it requires dedication of the entire imaging session for this purpose, which prohibits the use of the MRI for obtaining a host of other physiological and anatomical data.
1) Left Ventricular Mechanical Activation
The onset of cardiac Left-Ventricular Mechanical Activation (LVMA) is a measure of the elapsed time between the R-wave peak (Rpeak) in the QRS complex and the initial mechanical contraction of the left ventricle (LV) lateral-wall. LVMA is typically evaluated using ultrasound tissue Doppler Imaging (17) and MRI gradient echo (GRE) CINE imaging (18–20) and Displacement Encoding with Stimulated Echoes (DENSE) CINE (18,21). These methods typically assess levels of ventricular dyssynchrony, the timing of such initial contraction in several cardiac regions, in an effort to optimize pacing lead placement for cardiac resynchronization therapy (CRT) (22). Average dyssynchrony levels across the ventricle wall can be used as an estimation of LVMA, providing a method of comparison between patients as an effort to assess need and potential for positive response to CRT. Abnormal LVMA and associated ventricular behavior have also been shown to be an important factor in the prediction of cardiac events (23). We hypothesize that a method of post-processing ECG and extracted VMHD signals recorded in the presence of a magnetic field can be used to quantify LVMA and averaged LV dyssynchrony levels.
2) Aortic Arch Orientation
The orientation of the aortic arch relative to the human chest, specifically in the Anterior-Posterior (A-P) direction, has been shown to be correlated with risk of atherosclerosis, and hypertrophic obstructive cardiomyopathy (24,25). Longitudinal monitoring of this feature over the course of such diseases or after surgery may provide a tool to non-invasively assess risk and patient condition.
MATERIALS AND METHODS
12-lead ECG traces were recorded during 20-second breath holds in the presence of a strong magnetic field using an MRI-compatible GE (Waukesha, WI) digital Cardiolab-IT 16-bit ECG recorder with a standard 12-lead ECG chest placement at a sampling frequency of 2 kHz. MRI Gradient Echo (GRE) CINE data was obtained to validate VMHD-based metrics for each test subject.
Study Population
The study was conducted with Institutional Review Board (IRB) approval. Subjects in poor physical condition before the procedure or at any stage during (such as unable to hold their breath) were immediately excused from the procedure. LVMA was quantified in four healthy subjects: one subject at 1.5T, and three subjects at 3T. Quantification of Aortic Arch Orientation was performed using two patients diagnosed with Atrial Fibrillation (AF) at 3T, and two healthy subjects at 3T (healthy subjects #3 and #4 from the LVMA study).
ECG Signal Preprocessing
ECGs were recorded using an FDA-cleared GE Cardiolab digital-IT system with default pass-band filters of 0.05 Hz – 100 Hz (14). Post-processing of the 12-lead ECG traces was performed using custom Matlab (MathWorks, Natick, MA) routines. ECG traces were analyzed in the Vectorcardiogram (VCG) domain and synthesized into VCGs using an inverse Dower Transform (26) (iDT). The iDT utilizes all 8 independent traces obtained from the 12-lead ECG recordings (Equations 1, 2). iDT is an experimentally determined transform matrix, calculated from simultaneous measurements of 12-lead ECGs and a standard VCG.
| (1) |
| (2) |
Time stamps for occurrences of the QRS complex were recorded using the 3DQRS method (27), and then verified by a cardiologist; S-T segment detection was performed by a cardiologist. Time-integration of systolic VMHD was performed for deduction of aortic arch orientation during the S-T segment and calculated for each recorded cardiac cycle (R-R interval) during the 20-second breath-hold. An average of time-integrated systolic VMHD was taken over the 20 second period.
The three VCG directions, X, Y, and Z, respectively, denote the Left-Right, Anterior-Posterior and Superior-Inferior directions in the anatomical reference frame. In terms of body planes, X-Z describes the frontal plane, X-Y the transverse plane, and Y-Z the median or mid-sagittal plane (Fig. 1b).
Figure 1. Gregory, T. Stan.
Visualization of the first MHD peak in the VCG domain. (a) MRI Coordinate plane. (b) Body planes shown in MRI coordinate system: X–Y is shown to be the transverse plane, X–Z is the coronal plane, and Y–Z is the sagittal plane for human subjects in the MRI bore. (c) ECG obtained inside the MRI transformed into the VCG domain. Blue and Red curves, show the true VCG (same as outside MRI) and MHD, respectively, as a function of time in the cardiac cycle, with dark blue and dark red lines, respectively, showing the Rpeak and MHD1 (d) Pre-cordial ECG leads and the X, Y, and Z VCG leads are shown as a function of time, with Rpeak and MHD1 indicated.
1) Left Ventricular Mechanical Activation
VMHD-based Approach
VMHD-based LVMA estimation was performed through the measurement of the timing delay between the Rpeak in the QRS complex (electrical) and the onset of blood flow (mechanical contraction) marked by the first recorded VMHD peak (MHD1), both in the VCG domain, where the VCG is presented by its X, Y, and Z traces as a function of time. Rpeak and VMHD peak were identified in the VCG domain, with the corresponding time stamps then retrieved and utilized towards the measurement of the time interval.
MHD1 was deemed to be the start of mechanical contraction due to the strong correlation between the MHD signal and aortic blood flow (5,6).
Due to the sensitive nature of the timing measurements, a specific protocol was used to determine when Rpeak and MHD1 occurred. The Rpeak was calculated by finding the maximum R-wave vector length in the VCG domain, using methods previously described (7,8). MHD1 was subsequently measured to be the first vector after the J-point that deviated from the Rpeak vector by a magnitude of 30 degrees, shown to be a reliable threshold for differentiating blood-flow potentials and Rpeak in a majority of patient orientations(7); due to the typical orthogonal relationship between VMHD and the QRS complex in 3T MRI, as seen in the VCG domain (7,28). This can be illustrated through the viewing of VCGs obtained inside the MRI scanner (Fig.1).
The time at which Rpeak and MHD1 occurred was measured using this method for each cardiac cycle over the course of each 20-second breath hold. The time difference between Rpeak and MHD1 recorded during each cardiac cycle was averaged for each subject and used to represent VMHD-based subject LVMA levels.
MRI-based Validation
MRI-based LVMA levels were obtained by analyzing ECG-gated short-axis GRE CINE MRI images over the various time delays (cardiac phases) after the Rpeak. LVMA was determined to occur when 10% LV lateral wall displacement occurred (29). The time to 10% LV lateral wall displacement was measured by tracking changes in the maximum LV short-axis diameter over successive Cine images of the same slice. Displacements between Cine images were estimated using a calculated linear interpolation between images. Temporal resolution of this method was approximately 20 ms for Subjects 1, 2, and 3, and 6 ms for Subject 4.
This analysis was performed for four healthy subjects: one at 1.5T, and three at 3T.
2) Aortic Arch Orientation
For the purposes of this study, the aortic arch orientation was defined as the angle, located in the transverse plane, formed between the aortic-arch and the patient’s coronal (Left-Right) chest plane, oriented using thoracic vertebrae.
VMHD-based Approach
Due to the sensitive nature of these measurements, electrode position will affect VCG synthesis. Electrode position is expected to vary with subject chest size (30) and repeated applications (31), subsequently causing ECG variations. Variations have been shown to be limited when specific electrode placement procedures were followed (30,32) and trained electrocardiography technicians were used to place the electrodes (31), whereas the Rpeak amplitude was shown to only experience a maximum error of 200µV for up to 2 inches of placement error, generally keeping the error levels to be <7% in the ECG domain, assuming a Rpeak of <3mV (33). Variations in the amplitude of the MHD signal due to these issues have not been thoroughly studied at this time.
Several techniques in the synthesis of MHD-based arch orientation were employed to reduce error levels present in the final calculation. These techniques involved operating in the VCG domain transformed to the MRI coordinate plane (Fig. 1a), averaging of error levels, and signal normalization in order to obtain a more precise level of measurement. This method, an improvement on prior work (14), can increase the estimation accuracy for blood flow directionality by utilizing all 12-lead ECGs traces through VCG domain processing.
In order to estimate the aortic-arch orientation, the VMHD vector was extracted from VCG traces synthesized from ECG traces obtained inside the MRI. This was performed in three steps. The first step (1) was to integrate the VCG trace obtained inside and outside the MRI in each of the three directions (X, Y, and Z) over the S-T segment (Equation 3), to produce two time-integrated S-T segments, SToutside and STinside, respectively. The signal was only integrated over the S-T segment due to the dominance of the MHD signal during early systole, as compared to T-wave contributions, which was after the blood has left the aortic arch and entered other vasculature(1). The STinside contained the MHD signal and was thereby proportional to the blood flow, while SToutside contained only contributions from the subject’s true ECG (e.g. the actual S-T elevation, which is subject specific).
| (3) |
The second step (2) involved subtracting SToutside from STinside in each of the three VCG directions, X, Y, and Z, where X denotes the Left-Right, Y the Anterior-Posterior and Z the Superior-Inferior directions, in order to obtain the time-integrated MHD signal in the VCG domain. This relied on the assumption that the true ECG signal during the ST segment contribution does not change between measurements made inside and outside of the MRI bore. This may not always be true as Rpeak may sometimes shift inside the MRI bore as compared to recordings taken outside the MRI bore. In order to minimize such errors, each ECG cycle was scaled to 60 beats per minute to ensure a proper subtraction of STinside and SToutside, and subsequently scaled back to the original heart rate after cardiac cycle subtraction was performed (14). This equal lengthening allowed obtaining an accurate estimation of beat-to-beat aortic arch orientation. An average value of aortic arch orientation was then taken based on calculated metrics from each cardiac cycle.
Due to the strong correlation between the MHD signal and aortic blood flow, this summed blood flow potential over the S-T segment recorded inside the MRI (STinside) should be proportional to Stroke Volume (SV), thereby deemed a Stroke Volume Index (SVI) after the baseline ECG levels (SToutside) had been removed, isolating the MHD signal contribution from the contribution of the true time-integrated ECG (Equation 4).
| (4) |
The third step (3) involved the assumption that the majority of the SVI was due to blood flowing within the aortic arch, thereby suggesting that directionality measures of SVI in the transverse plane of the human torso should be equal to directionality measures of the aortic arch (Equation 5).
| (5) |
MHD-based aortic orientation was calculated during each cardiac cycle over the course of the 20-second breath hold. Due to the potential for variability in the MHD signal in healthy patients (34), an averaged MHD-based orientation measure was obtained for each 20-second breath hold. A similar method could then be applied to deduce the aortic orientation in the sagittal and coronal planes by altering Eqn. 5 to calculate alternate angles. This method was not applied in the sagittal and coronal planes.
MRI-based Validation of Aortic-Arch Orientation
Method validation was performed through high-resolution paired subject MRI scan data using the following GRE MRI scan parameters: TR/TE 28.8 msec, 1.31 msec, slice thickness 5mm, and sequence variations of segmented k-space (SK), spoiled (SP), and oversampling phase (OSP). Orientation was calculated from a transverse MRI slice, using thoracic vertebral bodies as a point of axis alignment, by drawing a vector from the intersection of the coronal and sagittal planes through the ascending and descending aorta. The angle between the vector and the body plane was then measured for assessment of MRI-based orientation.
This analysis was performed in four subjects at 3T: two subjects diagnosed with Atrial Fibrillations (AF), and two healthy subjects.
Evaluation of Methodology Sensitivity
To evaluate the sensitivity of the method to changes in magnetic field direction, 12-lead ECG recordings were obtained while healthy subject #3 was positioned in three test configurations: (i) feet first with the heart at MRI isocenter; (ii) horizontal in the supine orientation while lying directly outside and parallel to the MRI bore entrance; and (iii) horizontal on their side while lying directly outside and parallel to the MRI bore entrance.
RESULTS
1) Left Ventricular Mechanical Activation
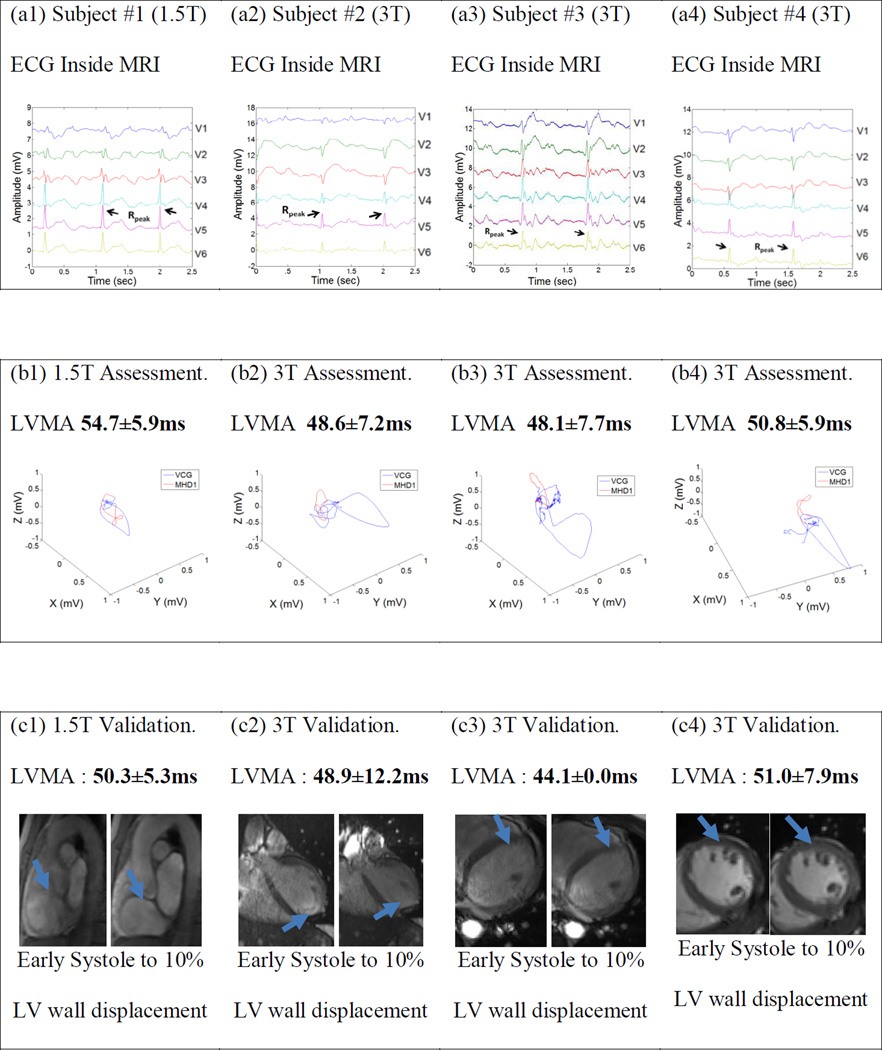
The 12-lead ECG traces were recorded (Fig. 2a1–a4) and the associated LVMA was calculated for each volunteer subject using both VMHD (Fig. 2b1–b4) and MRI-based (Fig. 2c1–c4) approaches. LVMA is clearly visible using each technique (Fig. 2 b, c).
Figure 2. Gregory, T. Stan.
Figure 2: ECGs (a) obtained inside 1.5T and 3T MRI scanners, with ensuing (b) detection of the onset of mechanical motion (MHD1), from which LVMA was assessed. LVMA was also detected using cine MRI methods (c), with blue arrows denoting the region where wall displacement was evaluated. Both mean (μ) and standard deviation (s) of the LVMA are reported.
Mean LVMA values were determined using VMHD and MRI-based methods, with percent deviation from MRI measured values calculated (Table 1). The discrepancy in VMHD-based relatively to the MRI-based measurements were consistently <10%.
Table 1.
Results of LVMA Measurements
| ECG (VMHD) | MRI | Deviation (%) from MRI measurement |
|||
|---|---|---|---|---|---|
| µ (ms) | s (ms) | µ (ms) | s (ms) | ||
| Healthy Subject #1 (1.5T) | 54.7 | 5.9 | 50.3 | 5.3 | 8.75% |
| Healthy Subject #2 (3T) | 48.6 | 7.2 | 48.9 | 12.2 | 0.61% |
| Healthy Subject #3 (3T) | 48.1 | 7.7 | 44.1 | 0.0 | 9.07% |
| Healthy Subject #4 (3T) | 50.8 | 5.9 | 51.0 | 7.9 | 0.39% |
µ denotes mean and s denotes standard deviation
These results are consistent with levels reported for healthy adults (23,29), which are typically less than 80ms, whereas certain disorders can increase this delay to up to 140ms (35).
2) Aortic Arch Orientation
Due to variations in the direction of the magnetic field outside of the MRI bore, (Siemens) manufacturer specified magnetic field information was obtained to measure the subject rotations relative to changes in the magnetic field direction(36). VMHD vectors were extracted from recorded 12-lead ECGs using techniques described in the Methods section and then rotated to the MRI reference plane (Fig. 1a) for direction comparison (Fig. 3d).
Figure 3. Gregory, T. Stan.
Assessment of the VMHD vector’s dependence on magnetic field direction and the angle of blood flow using 12-lead ECG monitoring with a single subject placed in various orientations within or outside the MRI bore. (d) Derived MHD vector directions for the orientations shown in a–c.
For each approximately 90-degree subject rotation, a similar rotation of the extracted VMHD vector was observed (Figure 3d). This illustrates the dependence of VMHD on both magnetic field and blood flow. Some of the measurement errors can be explained by the shift in the heart’s position within the chest as the subject was positioned on their side, as well as on relatively low magnetic-field homogeneity outside the MRI bore.
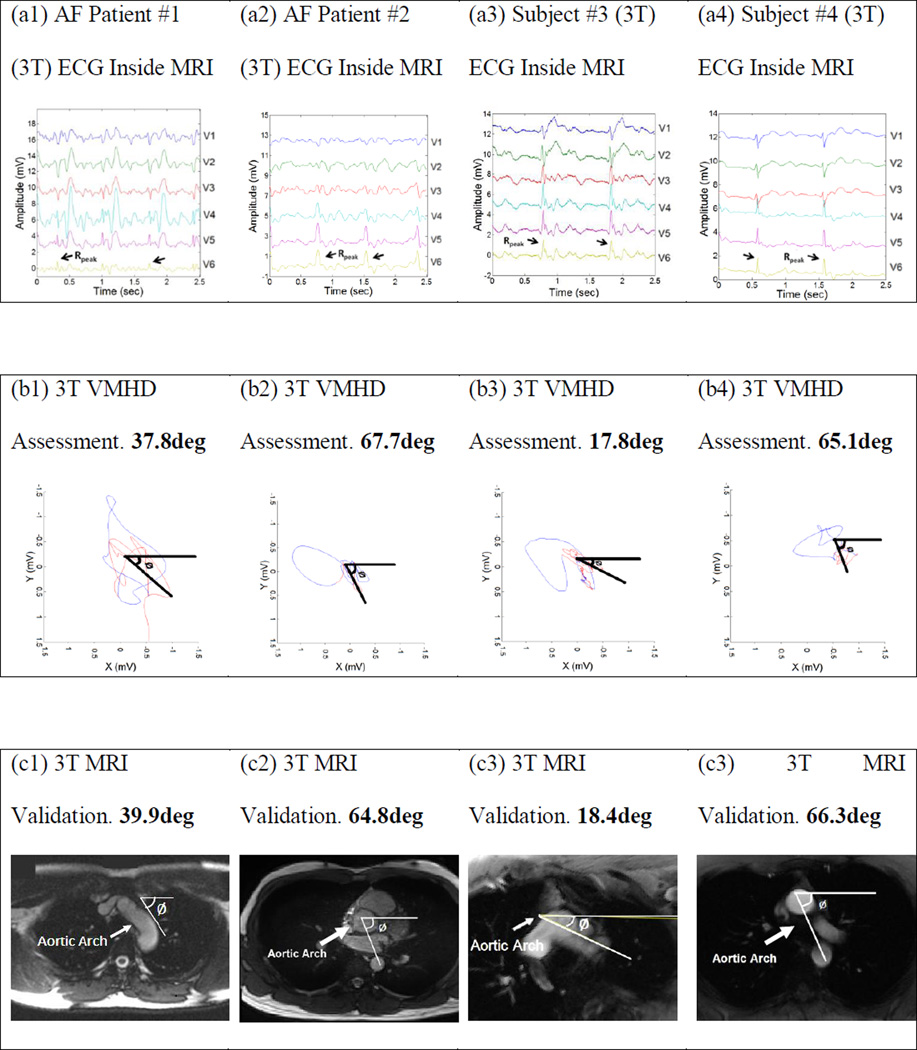
Orientation was calculated in the transverse plane for each subject using both ECG and MRI based techniques (Fig. 4). Resultant orientation measurements were, using ECG vs. MRI methods; AF patient #1: 37.8° versus 39.9°, AF patient #2: 67.7° versus 64.8°, healthy subject #3: 17.8° versus 18.4°, and healthy subject #4: 65.1° versus 66.3°. The error was < 3.0° for all subjects.
Figure 4. Gregory, T. Stan.
3T Assessment of Aortic Arch Angulation
DISCUSSION
The MHD voltage was utilized to estimate two clinically relevant parameters through post-processing of standard 12-lead ECG signal traces. This information can be easily integrated into cardiac MRI routines, since it comes “for free” when cardiac MRI with ECG gating is performed. Compared to the duration of a normal cardiac scan, approximately 45 minutes, an average of 5 minutes was required for patient preparation and application of 12-lead electrodes, with an additional 3 minutes needed for breath-holding instruction training. While in this study, data was acquired during breath-holds, ECG traces enabling VCG calculation can be acquired without breath-holding, with appropriate filtration of respiratory artifacts. In this feasibility study, manual S-T segment identification was required for accurate metric computation, a process which could be automated performed in the future for more efficient integration into the clinical workflow (37).
VMHD with the heart at magnet isocenter was deemed to be primarily generated from aortic arch blood flow for three reasons: (i) the blood flow velocity is greatest in the aortic arch immediately following left ventricular ejection, relative to other vasculature, with pulmonary flow velocity typically ≤ 50% that of aortic velocity (38), (ii) the aorta is the largest diameter blood vessel in the region of interest (1,5,6), and (iii) the blood flow through the aorta is approximately perpendicular to the MRI main magnetic field direction as compared to other vasculature (1,5,6), which will increase the aortic VMHD contribution comparatively (iv) the magnitude and direction of the MHD voltage observed can be explained entirely based on the aortic-arch contributions (1,10). Branches of the pulmonary arteries were excluded from consideration due to the bilateral symmetry of the vasculature, causing induced VMHD to be of an opposite polarity in branch, resulting in negligible net induced VMHD. In order to further validate the source of VMHD, beyond the present literature, a comprehensive simulation of VMHD contributions by both systemic and pulmonary arterial systems should be performed.
The LVMA measurement resolution using the MRI imaging method was limited by its own temporal resolution and sequence repetition time, while the proposed VMHD method was able to consistently maintain a high level of resolution due to the higher (2 kHz) sampling rate of the ECG signals, which was shorter than the temporal resolution (20 msec) of the cine scans. VMHD based LVMA calculations can be performed beat-to-beat with a 1Hz update rate once the subject is located within the bore of the MRI, while MRI based LVMA requires a longer acquisition time. This tool can potentially be used as an indicator of overall levels of LV dyssynchrony, providing clinicians with the ability to rapidly compare relative levels in patients and evaluate treatment plans, and serving as a global indicator of ventricular behavior to alert clinicians that a higher resolution MRI scan should be performed.
Rapid estimation of the aortic arch orientation was performed through analysis of the MHD signal and validated through MRI measurements, proving to be a potential assessment and diagnostic tool.
In conclusion, this feasibility study demonstrated that the MHD voltage can provide important insight into the function and anatomy of the heart, providing a quicker diagnostic tool than conventional MRI scanning. Methodologies presented in this body of work can be implemented in the form of standalone devices that feature strong magnetic fields which allow for bio-potential measurements.
Future work includes further validation of these methods in a larger population of patients and subjects as an effort to determine sensitivity in patient diagnosis.
Limitations
Due to the sensitive nature of the aortic arch orientation measurements presented in this body of work, electrode position will affect VCG synthesis and is expected to vary during repeated applications and patient anthropometry as previously discussed. This presents a limitation for repeatability and accuracy of this method, generally <7% error in the ECG domain, assuming a Rpeak of <3mV (33). A second limitation lies in the assumption that the signal contributions of the true ECG recordings taken inside and outside the MRI bore are equal, allowing for extraction of a pure MHD signal, which will not always be the case. The magnetic field outside the MRI scanner can, experience a variation of up to 7.3%, subject to the specific MRI scanner type utilized (36), so the results reported in Figure 3 may have large associated errors.
While it was demonstrated that VMHD can characterize the time to contraction and the aortic arch orientation, more work is required in order to prove the clinical application to specific diseases. VMHD derived aortic arch orientation was found only in the transverse plane and characterization of the arch in the coronal and sagittal planes is necessitated to further study related pathologies.
Modeling of the total MHD signal, including contributions from other vasculature, such as the pulmonary arteries, may be necessary in order to further understand the temporal and spatial information that can be obtained, potentially allowing the MHD signal to become a clinical tool for diagnosis and monitoring.
The small number of subjects studied presents a limitation to the method efficacy. Beat-to-Beat variation in both measured parameters may also affect the accuracy of each method, although the metrics reported herein were averaged over 20 cardiac cycles to minimize error.
Acknowledgments
FUNDING SOURCES
NIH U41-RR019703, NIH R03 EB013873-01A1, SBIR-1 R43 HL110427-01
Footnotes
DISCLOSURES
None
REFERENCES
- 1.Gupta A, Weeks AR, Richie SM. Simulation of elevated T-waves of an ECG inside a static magnetic field (MRI) IEEE transactions on bio-medical engineering. 2008;55:1890–1896. doi: 10.1109/TBME.2008.919868. [DOI] [PubMed] [Google Scholar]
- 2.Blandford R, Thorne K. Applications of Classical Physics. CA: CalTech; 2004. Magnetohydrodynamics. [Google Scholar]
- 3.Krug J, Rose G. Magnetohydrodynamic distortions of the ECG in different MR scanner configurations. Computing in Cardiology. 2011:769–772. [Google Scholar]
- 4.Birkholz T, Schmid M, Nimsky C, Schuttler J, Schmitz B. ECG artifacts during intraoperative high-field MRI scanning. Journal of neurosurgical anesthesiology. 2004;16:271–276. doi: 10.1097/00008506-200410000-00002. [DOI] [PubMed] [Google Scholar]
- 5.Nijm G, Swiryn S, Larson A, Sahakian A. Characterization of the magnetohydrodynamic effect as a signal from the surface electrocardiogram during cardiac magnetic resonance imaging. IEEE; 2006. [Google Scholar]
- 6.Nijm G, Swiryn S, Larson A, Sahakian A. Extraction of the magnetohydrodynamic blood flow potential from the surface electrocardiogram in magnetic resonance imaging. Medical & biological engineering & computing. 2008;46:729–733. doi: 10.1007/s11517-008-0307-1. [DOI] [PubMed] [Google Scholar]
- 7.Fischer SE, Wickline SA, Lorenz CH. Novel real-time R-wave detection algorithm based on the vectorcardiogram for accurate gated magnetic resonance acquisitions. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine. 1999;42:361–370. doi: 10.1002/(sici)1522-2594(199908)42:2<361::aid-mrm18>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 8.Chia JM, Fischer SE, Wickline SA, Lorenz CH. Performance of QRS detection for cardiac magnetic resonance imaging with a novel vectorcardiographic triggering method. Journal of Magnetic Resonance Imaging. 2000;12:678–688. doi: 10.1002/1522-2586(200011)12:5<678::aid-jmri4>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 9.Kohler BU, Hennig C, Orglmeister R. The principles of software QRS detection. IEEE engineering in medicine and biology magazine : the quarterly magazine of the Engineering in Medicine & Biology Society. 2002;21:42–57. doi: 10.1109/51.993193. [DOI] [PubMed] [Google Scholar]
- 10.Oster J, Pietquin O, Abacherli R, Kraemer M, Felblinger J. Independent component analysis-based artefact reduction: application to the electrocardiogram for improved magnetic resonance imaging triggering. Physiological measurement. 2009;30:1381–1397. doi: 10.1088/0967-3334/30/12/007. [DOI] [PubMed] [Google Scholar]
- 11.Krug J, Rose G, Stucht D, Clifford G, Oster J. Filtering the Magnetohydrodynamic Effect from 12-lead ECG Signals using Independent Component Analysis. Computing in Cardiology. 2012:589–592. [Google Scholar]
- 12.Tse Z, Dumoulin C, Clifford G, Jerosch-Herold M, Kacher D, Kwong R, Stevenson W, Schmidt E. Improved R-wave detection for enhanced cardiac Gating using an MRI-compatible 12-lead ECG and multi-channel analysis. Journal of Cardiovascular Magnetic Resonance. 2011;13:P3. [Google Scholar]
- 13.Tse Z, Dumoulin G, Clifford G, Jerosch-Herold M, Kacher D, Kwong R, Stevenson W, Schmidt E. Real-ECG extraction and stroke volume from MR-Compatible 12-Lead ECGs; testing during stress, in PVC and in AF patients. Journal of Cardiovascular Magnetic Resonance. 2011;13:6. [Google Scholar]
- 14.Tse ZT, Dumoulin CL, Clifford GD, Schweitzer J, Qin L, Oster J, Jerosch-Herold M, Kwong RY, Michaud G, Stevenson WG, Schmidt EJ. A 1.5T MRI-conditional 12-lead electrocardiogram for MRI and intra-MR intervention. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine. 2013 doi: 10.1002/mrm.24744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abi-Abdallah D, Drochon A, Robin V, Fokapu O. Effects of Static Magnetic Field Exposure on Blood Flow. The European Physical Journal Applied Physics. 2009;45:1–27. [Google Scholar]
- 16.Abi-Abdallah D, Robin V, Drochon A, Fokapu O. Alterations in human ECG due to the MagnetoHydroDynamic effect: a method for accurate R peak detection in the presence of high MHD artifacts; Conference proceedings : Annual International Conference of the IEEE Engineering in Medicine and Biology Society IEEE Engineering in Medicine and Biology Society Conference; 2007. pp. 1842–1845. [DOI] [PubMed] [Google Scholar]
- 17.Penicka M, Bartunek J, De Bruyne B, Vanderheyden M, Goethals M, De Zutter M, Brugada P, Geelen P. Improvement of left ventricular function after cardiac resynchronization therapy is predicted by tissue Doppler imaging echocardiography. Circulation. 2004;109:978–983. doi: 10.1161/01.CIR.0000116765.43251.D7. [DOI] [PubMed] [Google Scholar]
- 18.Epstein FH. MRI of left ventricular function. Journal of nuclear cardiology. 2007;14:729–744. doi: 10.1016/j.nuclcard.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 19.Wyman BT, Hunter WC, Prinzen FW, McVeigh ER. Mapping propagation of mechanical activation in the paced heart with MRI tagging. American Journal of Physiology-Heart and Circulatory Physiology. 1999;276:H881–H891. doi: 10.1152/ajpheart.1999.276.3.H881. [DOI] [PubMed] [Google Scholar]
- 20.Helm RH, Lardo AC. Cardiac magnetic resonance assessment of mechanical dyssynchrony. Current opinion in cardiology. 2008;23:440–446. doi: 10.1097/HCO.0b013e32830b3865. [DOI] [PubMed] [Google Scholar]
- 21.Aletras AH, Balaban RS, Wen H. High-resolution strain analysis of the human heart with fast-DENSE. Journal of magnetic resonance. 1999;140:41–57. doi: 10.1006/jmre.1999.1821. [DOI] [PubMed] [Google Scholar]
- 22.Abraham WT. Cardiac resynchronization therapy. Progress in cardiovascular diseases. 2006;48:232–238. doi: 10.1016/j.pcad.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 23.Cho GY, Kim HK, Kim YJ, Choi DJ, Sohn DW, Oh BH, Park YB. Electrical and mechanical dyssynchrony for prediction of cardiac events in patients with systolic heart failure. Heart. 2010;96:1029–1032. doi: 10.1136/hrt.2009.167585. [DOI] [PubMed] [Google Scholar]
- 24.Kwon DH, Smedira NG, Popovic ZB, Lytle BW, Setser RM, Thamilarasan M, Schoenhagen P, Flamm SD, Lever HM, Desai MY. Steep left ventricle to aortic root angle and hypertrophic obstructive cardiomyopathy: study of a novel association using three-dimensional multimodality imaging. Heart. 2009;95:1784–1791. doi: 10.1136/hrt.2009.166777. [DOI] [PubMed] [Google Scholar]
- 25.Nishimura RA, Holmes DR. Hypertrophic Obstructive Cardiomyopathy. New England Journal of Medicine. 2004;350:1320–1327. doi: 10.1056/NEJMcp030779. [DOI] [PubMed] [Google Scholar]
- 26.Dower GE. The ECGD - a Derivation of the Ecg from Vcg Leads. Journal of electrocardiology. 1984;17:189–191. doi: 10.1016/s0022-0736(84)81094-8. [DOI] [PubMed] [Google Scholar]
- 27.Gregory TS, Schmidt EJ, Zhang SH, Ho Tse ZT. 3DQRS: A method to obtain reliable QRS complex detection within high field MRI using 12-lead electrocardiogram traces. Magnet Reson Med. 2014;71:1374–1380. doi: 10.1002/mrm.25078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krug J, Rose G, Stucht D, Clifford G, Oster J. Limitations of VCG based gating methods in ultra high field cardiac MRI. Journal of Cardiovascular Magnetic Resonance. 2013;15:19. doi: 10.1186/1532-429X-15-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Westenberg JJ, Lamb HJ, van der Geest RJ, Bleeker GB, Holman ER, Schalij MJ, de Roos A, van der Wall EE, Reiber JH, Bax JJ. Assessment of Left Ventricular Dyssynchrony in Patients With Conduction Delay and Idiopathic Dilated Cardiomyopathy Head-to-Head Comparison Between Tissue Doppler Imaging and Velocity-Encoded Magnetic Resonance Imaging. J Am Coll Cardiol. 2006;47:2042–2048. doi: 10.1016/j.jacc.2006.01.058. [DOI] [PubMed] [Google Scholar]
- 30.Rautaharju PM, Park L, Rautaharju FS, Crow R. A standardized procedure for locating and documenting ECG chest electrode positions: consideration of the effect of breast tissue on ECG amplitudes in women. Journal of electrocardiology. 1998;31:17–29. doi: 10.1016/s0022-0736(98)90003-6. [DOI] [PubMed] [Google Scholar]
- 31.Wenger W, Kligfield P. Variability of precordial electrode placement during routine electrocardiography. Journal of electrocardiology. 1996;29:179–184. doi: 10.1016/s0022-0736(96)80080-x. [DOI] [PubMed] [Google Scholar]
- 32.Soliman EZ. A simple measure to control for variations in chest electrodes placement in serial electrocardiogram recordings. Journal of electrocardiology. 2008;41:378–379. doi: 10.1016/j.jelectrocard.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 33.Finlay DD, Nugent CD, Nelwan SP, Bond RR, Donnelly MP, Guldenring D. Effects of electrode placement errors in the EASI-derived 12-lead electrocardiogram. Journal of electrocardiology. 2010;43:606–611. doi: 10.1016/j.jelectrocard.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 34.Frauenrath T, Fuchs K, Dieringer MA, Ozerdem C, Patel N, Renz W, Greiser A, Elgeti T, Niendorf T. Detailing the use of magnetohydrodynamic effects for synchronization of MRI with the cardiac cycle: a feasibility study. Journal of magnetic resonance imaging : JMRI. 2012;36:364–372. doi: 10.1002/jmri.23634. [DOI] [PubMed] [Google Scholar]
- 35.Sweeney M, Prinzen F. Advances in Arrhythmia and Electrophysiology. Circulation. 2008:120–126. doi: 10.1161/CIRCEP.108.777904. [DOI] [PubMed] [Google Scholar]
- 36.Kännälä S, Toivo T, Alanko T, Jokela K. Occupational exposure measurements of static and pulsed gradient magnetic fields in the vicinity of MRI scanners. Physics in medicine and biology. 2009;54:2243. doi: 10.1088/0031-9155/54/7/026. [DOI] [PubMed] [Google Scholar]
- 37.Bulusu SC, Faezipour M, Ng V, Nourani M, Tamil LS, Banerjee S. Transient ST-segment episode detection for ECG beat classification. IEEE; 2011. pp. 121–124. [Google Scholar]
- 38.Wilson N, Goldberg S, Dickinson D, Scott O. Normal intracardiac and great artery blood velocity measurements by pulsed Doppler echocardiography. British heart journal. 1985;53:451–458. doi: 10.1136/hrt.53.4.451. [DOI] [PMC free article] [PubMed] [Google Scholar]