Abstract
Background
Two recent randomized controlled trials of type 2 diabetes mellitus (T2DM) patients with history of, or at high risk for, cardiovascular disease (CVD) showed no risk of ischemic cardiovascular events associated with dipeptidyl peptidase-4 inhibitors (DPP4i) but an increased risk of heart failure (HF) with saxagliptin. We evaluated the risk of cardiovascular disease (CVD) including myocardial infarction (MI), stroke, coronary revascularization, and HF associated with DPP4i in T2DM patients with and without baseline CVD as used in the community.
Methods
Using US commercial insurance claims data (2005–2012), we conducted a cohort study that included initiators of DPP4i and non-DPP4i treatment. Composite CVD endpoints including MI, stroke, coronary revascularization and HF, were defined with a hospital discharge diagnosis or procedure code. Cox proportional hazards models compared the risk of composite and individual CVD endpoints in propensity score (PS) matched initiators of DPP4 vs. non-DPP4i.
Results
We included 79,538 (18% with baseline CVD) persons in PS-matched pairs of DPP4i and non-DPP4i initiators. The incidence rate per 1,000 person-years for composite CVD was 30.30 (95%CI 28.24–32.51) in DPP4i and 34.76 (95%CI 32.34–37.36) in non-DPP4i. The PS-matched hazard ratio (HR) for composite CVD was 0.87 (95%CI 0.79–0.96) in DPP4i vs. non-DPP4i. The PS-matched HR for HF was 0.81 (95%CI 0.70–0.94) in DPP4i vs. non-DPP4i. Among patients with baseline CVD, there was no increased risk for CVD or HF associated with DPP4i use.
Conclusions
Among T2DM patients initiating DPP4i was not associated with a greater risk of CVD or HF compared to non-DPP4i initiators.
Keywords: dipeptidyl peptidase-4 inhibitor, type 2 diabetes, cardiovascular disease, heart failure
BACKGROUND
Dipeptidyl peptidase-4 inhibitors (DPP4i), such as alogliptin, sitagliptin, saxagliptin and linagliptin, are newly available glucose-lowering drugs that can be used as monotherapy or combination therapy with other oral hypoglycemic agents for treating type 2 diabetes mellitus (T2DM).[1–5] While a potential risk of acute pancreatitis related to use of DPP4i was suggested, more recent data do not find such risk.[6, 7] These drugs are otherwise generally well-tolerated with limited specific contraindication.
It is well-known that T2DM is a major risk factor for cardiovascular disease including coronary heart disease, cardiomyopathy, and stroke.[8–10] Recently, two large randomized controlled trials (RCT) in T2DM patients who had a history of, or were at risk for, cardiovascular events at baseline showed no excess risk of ischemic cardiovascular events associated with saxagliptin use for a median follow-up time of 2.1 years or alogliptin use for a median followup time of 1.5 years.[11, 12] However, an increased risk of hospitalization for heart failure (HF) was observed in the saxagliptin group, and a trend of similar magnitude, albeit not statistically significant, in the smaller RCT of alogliptin.[11–13] Another RCT showed a significantly decreased risk of major cardiovascular events in linagliptin users compared to glimepiride in T2DM patients inadequately controlled on metformin.[4]
The objectives of this study were to evaluate the risk of composite CVD including MI, stroke, coronary revascularization, and HF associated with initiation of DPP4i compared to initiation of other diabetes drugs in ‘real world’ patients with and without baseline CVD, to determine the risk of CVD associated with initiation of DPP4i vs. non-DPP4i in patients with baseline diagnosis of CVD, and to examine the risk of HF associated with initiation of DPP4i vs. initiation of other diabetes drugs in patients with and without established CVD.
METHODS
Data Source
We conducted a cohort study using the claims data for the period January 1, 2005 to December 31, 2012, from United HealthCare, a commercial U.S. health plan, which insures primarily working adults and their family members. This database contains longitudinal claims information including medical diagnoses, procedures, hospitalizations, physician visits, and pharmacy dispensing on its approximately 14 million subscribers across the U.S. on a yearly basis. Patient informed consent was not required as the dataset was de-identified to protect subject confidentiality. The study protocol was approved by the Institutional Review Board of the Brigham and Women’s Hospital.
Study Cohort
Patients who had ≥1 dispensing for an oral hypoglycemic agent any time during the study period with a visit coded with the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD 9-CM) code, 250.xx, for DM were first identified for the study cohort. To avoid selecting patients with type 1 diabetes, we excluded patients aged <40 years and those who used insulin. Two mutually exclusive exposure groups were defined: 1) initiators of DPP4i monotherapy or combotherapy and 2) initiators of non-DPP4i monotherapy or combotherapy. For combotherapy, patients were required to have metformin plus a DPP4i or a non-DPP4i, as metformin is recommended as first line therapy[14] and to exclude imbalance for renal disease where metformin would be relatively contraindicated. DPP4i drugs include linagliptin (approved in 2011), saxagliptin (approved in 2009) and sitagliptin (approved in 2006), as these are FDA approved and marketed in the US at the time of study. Non-DPP4i drugs include metformin, sulfonylureas, thiazolidinediones (TZD), and meglitinides.
For monotherapy initiators, the index date was the first dispensing of a DPP4i or a non-DPP4i drug. For combotherapy initiators, the index date was defined as the earliest date of starting a DPP4i drug with concurrent use of metformin for the DPP4i group and the earliest date of adding a second non-DPP4i drug with concurrent use of metformin for the non-DPP4i group. All patients (mono- and combination initiators) were required to be naïve to DPP4i in the 180 days prior to the index date. For non-DPP4i combotherapy initiators, patients were required to have ≥180 days without using multiple oral hypoglycemic drugs prior to their index date. Furthermore, patients were required to have ≥365 days of continuous insurance enrollment before the index date. Patients with and without CVD at baseline were included. Patients with end-stage renal disease, renal transplantation, HIV, cancer, and use of glucagon-like peptide 1 agonists in the 365 days prior to the index date were excluded.
For a subgroup analysis, we selected DPP4i and TZD initiators who were naïve to both DPP4i and TZD in the 180 days prior to the index date. The TZD group was chosen based on the known risk of HF associated with TZD[15].
Follow-up began on the day after the index date. Patients were followed up to the first of any of the following censoring events: discontinuation or switching of study drugs (i.e. ‘as treated’), occurrence of CVD events (any CVD events for the composite endpoint and separately for the individual components of CVD), disenrollment from the health plan, December 31, 2012, or death. Patients were allowed to have gaps of up to 30 days between prescription fill dates in the calculation of continuous therapy. In the case of drug discontinuation or switching, the exposure risk window for each patient treatment episode extended until 30 days after the expiration of the supply of the last fill. Patients were only allowed to enter the study cohort once.
Study Outcome
The primary outcome was a composite CVD endpoint including non-fatal MI, non-fatal stroke, coronary revascularization, and HF, defined with a hospital discharge diagnosis and/or procedure code. The secondary outcomes were the individual components of the primary endpoint and all-cause death. In prior studies, the positive predictive values of these claims-based algorithms for CVD events were at least 80%.[16–19] Hospital admission or procedure dates were used as the date of outcome occurrence. To capture patients with HF not requiring a hospitalization, we also assessed incident use of loop diuretics as an outpatient.
Covariates
Variables potentially related to development of CVD were assessed using data from the 365-day baseline period before the index date. These baseline variables (see Table 1) were age, sex, year of the index date, DM-related comorbidities, CVD, other comorbidities, medications, and health care utilization factors, and laboratory test ordered for serum BUN, creatinine, and HbA1c. To further quantify patients’ comorbidities at baseline, we also calculated a comorbidity score that combined 20 medical conditions included in both the Charlson Index and the Elixhauser system based on ICD-9.[20] To characterize diabetes treatment intensity, the number of oral hypoglycemic drugs taken at the index date was also determined. Baseline serum BUN, creatinine, and HbA1c levels were available in a subgroup of the study cohort.
Table 1.
Patient characteristics in the 365-day baseline period: 1:1 propensity score matched DPP4i and non-DPP4i initiators
| DPP4i (N=39,769) | Non-DPP4i (N=39,769) | |
|---|---|---|
| Mean ± SD or percentage | ||
| Demographics | ||
| Age | 55.4 ± 8.6 | 55.4 ± 8.7 |
| Male | 57 | 58 |
| Monotherapy | 18 | 18 |
| Combotherapy | 82 | 82 |
| Index year | ||
| 2006 | 1 | 1 |
| 2007 | 15 | 15 |
| 2008 | 18 | 18 |
| 2009 | 14 | 14 |
| 2010 | 15 | 15 |
| 2011 | 18 | 18 |
| 2012 | 19 | 19 |
| Comorbidities | ||
| Hypertension | 76 | 76 |
| Cardiovascular disease a | 18 | 18 |
| Coronary artery disease | 14 | 15 |
| Stroke | 5 | 5 |
| Heart failure | 3 | 3 |
| Dyslipidemia b | 80 | 80 |
| Peripheral vascular disease | 3 | 3 |
| Pulmonary disease | 12 | 12 |
| Chronic kidney disease | 4 | 4 |
| Liver disease | 5 | 5 |
| Smoking | 7 | 8 |
| Obesity | 15 | 15 |
| Combined comorbidity score c | 0.05 ± 1.3 | 0.04 ± 1.3 |
| Diabetes-related | ||
| DM nephropathy | 3 | 3 |
| DM neuropathy | 6 | 6 |
| DM retinopathy | 7 | 7 |
| No. of diabetic drugs at the index date | 2.0 ± 0.7 | 2.0 ± 0.6 |
| Type of oral hypoglycemic drugs at the | ||
| index date | ||
| Metformin | 82 | 95 |
| Sulfonylureas | 14 | 68 |
| Thiazolidinediones | 7 | 38 |
| DPP4i | 100 | 0 |
| Meglitinides | 1 | 2 |
| Medications | ||
| Calcium channel blockers | 19 | 19 |
| Beta blockers | 19 | 19 |
| Loop diuretics | 7 | 7 |
| Thiazides | 9 | 9 |
| ACEI/ARB | 56 | 56 |
| Digoxin | 1 | 1 |
| Statins | 51 | 51 |
| Other lipid lowering drugs | 22 | 22 |
| Anticoagulants | 3 | 3 |
| Antiplatelets | 6 | 6 |
| Opioids | 30 | 30 |
| Oral corticosteroids | 11 | 12 |
| Non-steroidal anti-inflammatory drugs | 21 | 21 |
| Cyclooxygenase-2 inhibitors | 3 | 3 |
| Health care utilization | ||
| No. of any physician visits | 6.9 ± 5.2 | 6.9 ± 5.8 |
| Visits to primary care physicians | 5.0 ± 4.1 | 5.0 ± 4.6 |
| Visit to cardiology | 0.8 ± 2.4 | 0.8 ± 2.6 |
| Visit to endocrinology | 0.3 ± 1.1 | 0.3 ± 1.3 |
| No. of emergency room visits | 0.2 ± 0.7 | 0.2 ± 0.7 |
| Acute hospitalizations | 11 | 11 |
| No. of prescription drugs | 9.6 ± 5.4 | 9.6 ± 5.5 |
| Laboratory tests | ||
| BUN test ordered | 63 | 63 |
| BUN level available | 35 | 32 |
| BUN level, mg/dL d | 15.6 ± 5.5 | 15.6 ± 5.3 |
| Creatinine test ordered | 63 | 64 |
| Creatinine level available | 35 | 33 |
| Creatinine level, mg/dL d | 0.9 ± 1.2 | 0.9 ± 1.1 |
| HbA1c test ordered | 80 | 80 |
| HbA1c level available | 32 | 30 |
| HbA1c level, % d | 8.1 ± 1.9 | 8.1 ± 3.3 |
DPP4i: dipeptidyl peptidase-4 inhibitor, SD: standard deviation
Includes coronary artery disease, stroke and heart failure
defined as a diagnosis of hyperlipidemia or use of lipid-lowering drugs
The range of combined comorbidity score is −2 to 26.
In a subgroup of patients with laboratory results available
Statistical Analysis
We compared the baseline characteristics between DPP4i and non-DPP4i groups. To control for potential confounders, we used the propensity score (PS) matching method.[21] Multivariable logistic regression including all the baseline covariates listed in Table 1 was used to estimate the PS, defined as the predicted probability of a patient receiving therapy with DPP4i versus non-DPP4i. For PS-matched analysis, we used nearest neighbor matching without replacement within a “caliper” of 0.025 on the PS at a fixed ratio of 1:1.[22, 23] Matching was stratified on two factors: baseline CVD (yes/no) and monotherapy (yes/no) so that, for example, a DPP4i combotherapy initiator with baseline CVD was matched to a non-DPP4i combotherapy initiator with baseline CVD. Incidence rates and hazard ratio (HR) of CVD outcomes with 95% confidence intervals (CI) were calculated in DPP4i initiators versus non-DPP4i. Kaplan-Meier curves were plotted for the cumulative incidence of each outcome in the PS-matched DPP4i and non-DPP4i cohorts. Among patients with baseline CVD, separate incidence rates and hazard ratio (HR) of CVD outcomes with 95% CI were calculated.
In patients with no loop diuretics at baseline, separate incidence rates and HRs of receiving a new prescription for loop diuretics with 95% CI were calculated in DPP4i initiators versus their PS-matched non-DPP4i initiators. All these analyses were repeated for the subgroup analyses comparing DPP4i versus TZD initiators. Additional subgroup analysis compared initiators of DPP4i monotherapy to initiators of non-DPP4i monotherapy. The proportional hazards assumption was assessed by testing the significance of the interaction term between exposure and time and was not violated.[24] All analyses were done using SAS 9.2 Statistical Software (SAS Institute Inc., Cary, NC).
RESULTS
Cohort Selection
There were 4,353,160 dispensings for DPP4i or non-DPP4i drugs between 2006 and 2012 in the study database. After applying the inclusion and exclusion criteria, there were 43,583 patients with T2DM who started DPP4i monotherapy or combotherapy and 370,245 who started non-DPP4i monotherapy or combotherapy. Matching on PS with a 1:1 ratio further selected a total of 79,538 individuals as 39,769 pairs of DPP4i and non-DPP4i initiators (eFigure 1).
Patient Characteristics
The mean age of the propensity score matched patients was 55.5 years. 57% of the DPP4i group and 58% of the non-DPP4i group were male (Table 1). Of the DPP4i group, 83% were on sitagliptin, 15% on saxagliptin and 3% on linagliptin. 82% were combotherapy users and 18% had established cardiovascular disease at baseline. Overall, baseline characteristics were well-balanced in the PS-matched DPP4i and non-DPP4i groups. The mean (SD) follow-up was 0.7 (0.8) years for DPP4i and 0.5 (0.7) years for non-DPP4i initiators. The median followup was 0.3 years for both groups with 42,628 individuals with over 0.3 years of followup. Of these, 13,297 individuals had over 1 year of followup. The majority of patients were censored due to drug discontinuation (71%), followed by disenrollment (16%) and the end of study period (11%).
Risk of CVD in T2DM with and without baseline CVD
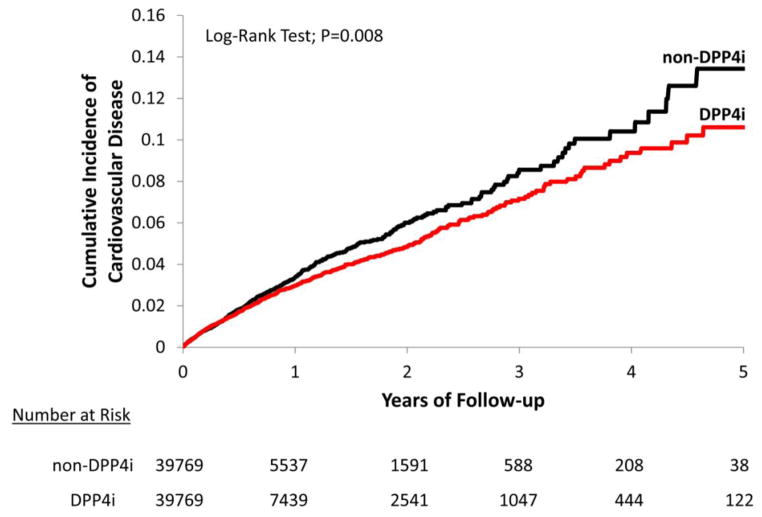
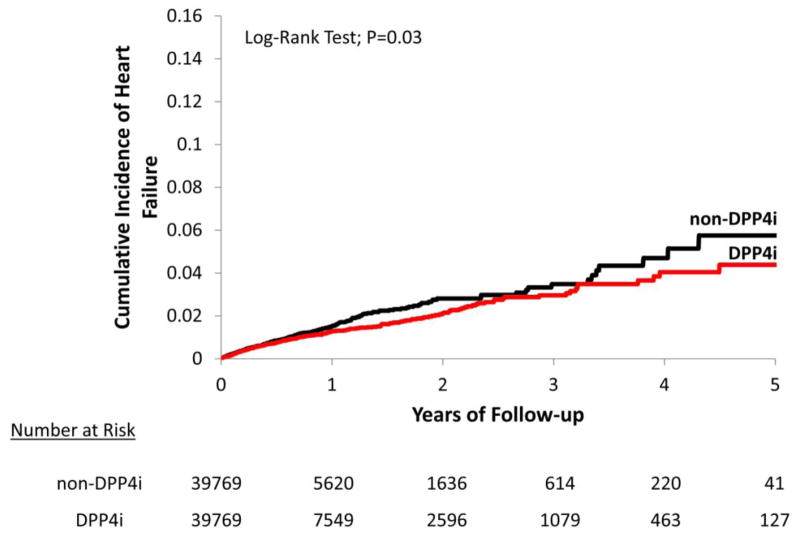
During the follow-up period, 775 composite CVD events occurred with 25,578 person-years of follow-up in DPP4i initiators and 740 composite CVD events with 21,291 person-years of follow-up in non-DPP4i initiators. The incidence rate for composite CVD was 30.3 per 1,000 person-years in the DPP4i group and 34.8 per 1,000 person-years in the non-DPP4i group. The incidence rate for HF was 12.8 per 1,000 person-years in the DPP4i group and 15.9 per 1,000 person-years in the non-DPP4i group. Incidence rates for other individual CVD endpoints were slightly higher in the non-DPP4i compared to DPP4i initiators. The PS- matched HR of DPP4i compared to non-DPP4i was 0.87 (95% CI 0.79–0.96) for composite CVD and 0.81 (95%CI 0.70–0.94) for HF. No significant association between DPP4i and the components of the composite CVD endpoint, including MI, coronary revascularization and stroke, was noted (Table 2). Figures 1 and 2 display the Kaplan-Meier curves comparing the cumulative incidence of composite CVD between the PS-matched DPP4i and non-DPP4i groups.
Table 2.
Risk of CVD associated with dipeptidyl peptidase-4 inhibitor (DPP4i) versus non-DPP4i: PS matched analysis
| DPP4i (N=39,769) | Non-DPP4i (N=39,769) | ||||||
|---|---|---|---|---|---|---|---|
| Cases | Person-years | IR a (95% CI) | Cases | Person-years | IR a (95% CI) | HR b(95% CI) | |
| CVD | 775 | 25,578 | 30.30 (28.24–32.51) | 740 | 21,291 | 34.76 (32.34–37.36) | 0.87 (0.79–0.96) |
| MI | 115 | 25,985 | 4.43 (3.69–5.32) | 112 | 21,594 | 5.19 (4.31–6.25) | 0.85 (0.66–1.10) |
| Coronary revascularization | 377 | 25,809 | 14.61 (13.21–16.16) | 335 | 21,448 | 15.62 (14.03–17.39) | 0.94 (0.81–1.09) |
| Stroke | 139 | 25,975 | 5.35 (4.53–6.32) | 132 | 21,597 | 6.11 (5.15–7.25) | 0.88 (0.69–1.12) |
| Heart failure | 332 | 25,854 | 12.84 (11.53–14.30) | 342 | 21,508 | 15.90 (14.30–17.68) | 0.81 (0.70–0.94) |
| CVD without heart failure | 547 | 25,716 | 21.27 (19.56–23.13) | 489 | 21,390 | 22.86 (20.92–24.98) | 0.93 (0.82–1.05) |
| All-cause death | 59 | 26,050 | 2.26 (1.75–2.92) | 62 | 21,651 | 2.86 (2.23–3.67) | 0.78 (0.54–1.11) |
DPP4i: dipeptidyl peptidase-4 inhibitor, IR: incidence rate, HR: hazard ratio, CI: confidence interval, CVD: cardiovascular disease, PS: propensity score
Per 1,000 person-years
Non-DPP4i as a referent group
Figure 1. Propensity score-matched Kaplan-Meier curves for cumulative incidence of cardiovascular disease.
DPP4i and non-DPP4i cohorts are propensity score-matched.
Figure 2. Propensity score-matched Kaplan-Meier curves for cumulative incidence of heart failure.
DPP4i and non-DPP4i cohorts are propensity score-matched.
Risk of CVD in T2DM with baseline CVD
Among 14,586 individuals contributing to 7,293 PS-matched pairs of DPP4i and non-DPP4i initiators who had baseline CVD, the incidence rates of all the CVD endpoints were higher compared to those in the overall study population (Table 3). The HR of DPP4i compared to the PS-matched non-DPP4i initiators was 0.88 (95%CI 0.77–1.01) for composite CVD and 0.84 (95%CI 0.70–1.01) for HF. The risk of other CVD endpoints was not significantly different for DPP4i compared to non-DPP4i initiators. Among 28,922 PS-matched pairs of DPP4i and non-DPP4i initiators with no baseline use of loop diuretics, 400 DPP4i and 464 non-DPP4i initiators received a new prescription for loop diuretics during the follow-up. The risk of getting a new prescription for loop diuretics was lower in DPP4i initiators compared to non-DPP4i (HR 0.74, 95%CI 0.65–0.84).
Table 3.
Risk of CVD associated with dipeptidyl peptidase-4 inhibitor (DPP4i) in patients with baseline CVD compared to non-DPP4i: PS matched analysis
| DPP4i (N=7,293) | Non-DPP4i (N=7,293) | ||||||
|---|---|---|---|---|---|---|---|
| Cases | Person-years | IR a (95% CI) | Cases | Person-years | IR a (95% CI) | HR b(95% CI) | |
| CVD | 427 | 4,527 | 94.33 (85.79–103.72) | 433 | 3,864 | 112.05 (101.98–123.12) | 0.88 (0.77–1.01) |
| MI | 48 | 4,761 | 10.08 (7.60–13.38) | 49 | 4,049 | 12.10 (9.14–16.01) | 0.84 (0.56–1.25) |
| Coronary revascularization | 195 | 4,660 | 41.85 (36.37–48.16) | 184 | 3,962 | 46.44 (40.19–53.66) | 0.93 (0.76–1.14) |
| Stroke | 63 | 4,762 | 13.23 (10.34–16.94) | 66 | 4,048 | 16.30 (12.81–20.75) | 0.85 (0.60–1.20) |
| Heart failure | 217 | 4,654 | 46.63 (40.82–53.27) | 232 | 3,970 | 58.44 (51.38–66.47) | 0.84 (0.70–1.01) |
| CVD without heart failure | 271 | 4,626 | 58.58 (52.00–65.99) | 261 | 3,935 | 66.32 (58.74–74.87) | 0.91 (0.77–1.08) |
| All-cause death | 34 | 4,789 | 7.10 (5.07–9.94) | 31 | 4,073 | 7.61 (5.35–10.82) | 0.90 (0.55–1.46) |
DPP4i: dipeptidyl peptidase-4 inhibitor, IR: incidence rate, HR: hazard ratio, CI: confidence interval, CVD: cardiovascular disease, PS: propensity score
Per 1,000 person-years
Non-DPP4i as a referent group
Subgroup Analysis
In the subgroup analysis comparing 21,068 DPP4i initiators to their PS matched TZD initiators (eTable 1), HR of DPP4i was 0.93 (95%CI 0.81–1.07) for composite CVD and 0.82 (95%CI 0.66–1.02) for HF (eTable 2). Among 3,592 PS-matched pairs of DPP4i and TZD subgroup patients with baseline CVD, the HR of DPP4i initiators versus TZD was 0.97 (95%CI 0.80–1.17) for composite CVD and 1.01 (95%CI 0.77–1.34) for HF. Among the DPP4i and TZD subgroup patients who had no baseline use of loop diuretics (n=16,222 pairs), the risk of getting a new prescription for loop diuretics was lower in DPP4i initiators compared to TZD (HR 0.59, 95%CI 0.50–0.69). Among the initiators of DPP4i monotherapy with baseline CVD (n=1,720 pairs), the HR was 0.85 (95%CI 0.66–1.09) for composite CVD and 0.88 (95%CI 0.65–1.19) for HF. Among the initiators of DPP4i monotherapy without baseline CVD (n=5,630 pairs), the HR was 0.99 (95%CI 0.69–1.43) for composite CVD and 0.92 (95%CI 0.50–1.67) for HF.
DISCUSSION
In this large cohort of T2DM patients, initiating DPP4i was not associated with an increased risk of CVD or its components including HF compared with those initiating non-DPP4i anti-diabetic drugs. In fact, we found a modestly lower risk for composite CVD and HF among DPP4i initiators compared with non-DPP4i initiators. Even among patients with established CVD, the risks for composite CVD or HF were not higher in DPP4i initiators. Subgroup analysis comparing DPP4i to TZD showed a decreased, albeit not statistically significant, risk for HF. Furthermore, the risk of being newly prescribed loop diuretics was significantly lower in DPP4i compared with TZD initiators as expected given the association of TZD with fluid retention and HF.
This study cohort consists of ‘real-world’ patients on either monotherapy or combotherapy of oral anti-diabetic drugs. Our finding of no increased CVD risk in DPP4i initiators as used in the community is consistent with RCT data on linagliptin, saxagliptin and alogliptin.[4, 11, 12, 25] Compared to glimepiride, linagliptin reduced the risk of cardiovascular death, MI, stroke, or hospitalization for unstable angina (relative risk 0.46, 95%CI 0.23–0.91) in T2DM patients free of recent CVD events. In an RCT of saxagliptin versus placebo that included 16,492 T2DM patients with a history of CVD or multiple cardiovascular risk factors at baseline, saxagliptin did not increase or decrease the risk of cardiovascular death, MI or ischemic stroke (HR 1.00, 95%CI 0.89–1.12).[11] The risk of the secondary endpoint in this trial- cardiovascular death, MI, stroke, hospitalization for unstable angina, coronary revascularization or HF - was also not higher (HR 1.02, 95%CI 0.94–1.11) with saxagliptin. Another recent RCT of alogliptin included 5,380 T2DM patients with acute coronary syndrome of recent onset did not find a higher risk of cardiovascular death, MI, or stroke in the alogliptin group compared to placebo.[12] A pooled analysis of 11 RCTs of alogliptin versus placebo also did not find an increased CVD risk including HF.[25]
An unexpected observation in the Saxagliptin Assessment of Vascular Outcomes Recorded in Patients with Diabetes Mellitus – Thrombolysis in Myocardial Infarction 53 (SAVOR-TIMI 53) trial was a higher risk of hospitalization for HF (HR 1.27, 95%CI 107–1.51).[11] A smaller trial of alogliptin also noted a non-significant increase in the risk of HF in patients on active therapy (HR 1.19, 95%CI 0.90–1.58). [11, 13] The mechanism by which saxagliptin or alogliptin might increase the rates of HF are not known. Vildaglipitin, only in Europe, increased left ventricle end-diastolic volume and left ventricle end-systolic volume, as well as stroke volume in an RCT setting, but did not alter ejection fraction.[26] B-type natriuretic peptides, which play key physiologic roles in the regulation of renal sodium and fluid balance, are cleaved by DPP4 in plasma. Some investigators have hypothesized that the disruption of this neurohormonal regulatory mechanism may be one explanation for the observation of increased HF in patients on saxagliptin in SAVOR-TIMI 53.[27] We did not observe a higher risk of hospitalization for HF or incident use of diuretics in initiators of DPP4i therapy. This discrepancy could be explained by several differences between our study and the SAVOR-TIMI 53 trial. First, our study had a shorter follow-up time and included sitagliptin and linagliptin as well as saxagliptin. Second, the mean age of our patients is younger than that in the SAVOR-TIMI 53 cohort (55.4 v. 65.1 years, respectively). Third, the severity of T2DM and CVD risk in our cohort might be lower compared to the SAVOR-TIMI 53 cohort. We included patients with and without baseline CVD, but performed an a priori secondary analysis on patients that appeared similar to the SAVOR-TIMI 53 study cohort. Additionally, we excluded patients with insulin use at baseline in order to exclude all potential type 1 diabetic patients. Fourth, a treating physician’s threshold for admitting a patient for HF might be different when a patient is enrolled in an RCT even if blinded to the treatment assignment.
This present study also makes important contributions to our understanding of challenges in evaluating comparative cardiovascular safety of antidiabetic medications for T2DM using observational data. As seen in a relatively short period of follow-up in this study, patients’ adherence to medication was generally suboptimal and switching to a new drug or adding a new drug for T2DM was common. Second, even though we used rigorous pharmacoepidemiologic approaches in the study design and analysis including a new user design, active comparator, and PS matched analysis to simultaneously account for more than 45 potential confounders,[21, 28, 29] residual confounding can still be an issue as in any observational studies. For example, in attempt to use TZD as ‘a positive control’ based on the known risk of HF in TZD users, we conducted a subgroup analysis comparing DPP4i to TZD initiators. There was a lower, albeit not statistically significant, risk for HF and a significantly lower risk for incident use of loop diuretics in DPP4i initiators compared to TZD. However, it is important to note that the observed risk of CVD or HF related to use of TZD in our cohort is likely underestimated as physicians correctly do not prescribe TZD to those with or at increased risk for HF. Similarly, if prescribing physicians were uncertain about cardiovascular safety of DPP4i and therefore more careful upon selecting patients for DPP4i compared to non-DPP4i, it is possible that our study underestimates the risk of CVD and HF associated with DPP4i. Finally, if risk of HF occurs with DPP4i in similar magnitude to TZD then no increased risk with TZD would be observed, as seen in our analysis.
This study has limitations. First, the primary outcome of a composite CVD endpoint does not include cardiovascular deaths, as the causes of death are not available in the study database. Second, no data were available on race, ethnicity, socioeconomic status, duration of diabetes, family history of CVD, physical activity, dietary factors, and body mass index. Third, even though we used previously validated claims-based algorithms to define CVD outcomes with a positive predictive value at least 84%, [16–19] there is a potential for outcome misclassification. It is also possible that we did not capture mild HF treated as an outpatient because HF outcomes were based on inpatient diagnoses. However, in a subgroup analysis, DPP4i was associated with a decreased risk for incident use of loop diuretics during the follow-up (HR 0.74, 95%CI 0.65–0.84) versus non-DPP4i. Fourth, as the mean follow-up time was relatively short mainly due to drug discontinuation in the study cohort, the long-term effect of DPP4i could not be assessed reliably. Reasons for drug discontinuation were not available in the study database. However, this study still includes 13,297 patients with at least one year of followup on treatment. Fifth, our results may not be generalizable to patients with different insurance types or no insurance coverage as having a commercial health insurance is likely related to patients’ socioeconomic status, medication adherence, and other risk factors for CVD. It is, however, unlikely the biological effect of DPP4i on CVD differs by the insurance status. Lastly, we assessed a number of potential confounders using the claims data from the 12 months prior to the index date; however, it is possible that the 12-month baseline period was not long enough to capture all the information on potential confounders and that there was incomplete ascertainment of some variables in the claims data.
CONCLUSIONS
In this large cohort study, initiation of DPP4i did not appear to be associated with a higher risk of composite CVD including HF compared to the initiation of non-DPP4i diabetes drugs. There was no increased risk of CVD or HF associated with the initiation of DPP4i in patients with baseline CVD as used in the community setting.
Supplementary Material
Acknowledgments
Kim is supported by the NIH grant K23 AR059677. Goldfine is supported by the NIH grants R56 DK095451, P50 HL083813, R01 DK088214, U01 HL101422, P30-DK03836, and American Diabetes Association 7-13-CE-17.
List of Abbreviations
- CVD
cardiovascular disease
- DM
diabetes mellitus
- DPP4i
dipeptidyl peptidase-4 inhibitors
- HbA1c
glycated hemoglobin
- HF
heart failure
- HR
hazard ratio
- ICD-9 CM
International Classification of Diseases, Ninth Revision, Clinical Modification 9th edition
- PS
propensity score
- RCT
randomized controlled trial
- T2DM
type 2 diabetes
- TZD
thiazolidinedione
Footnotes
Author Contributions
Kim had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. She is the guarantor for the study. All authors conceived and designed the study, analysed and interpreted the data, and critically revised the manuscript for important intellectual content. Kim drafted the paper.
Prior Presentation
Parts of this study were presented in abstract form at the ICE/ENDO 2014 meeting in Chicago, IL, 21–24 June 2014.
Statement of Human Rights
The study protocol was approved by the Institutional Review Board of the Brigham and Women’s Hospital. All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008.
Statement of Informed Consent
Patient informed consent was not required as the dataset was de-identified to protect subject confidentiality.
Conflict of Interest
Kim received research support from Pfizer, Inc.
Glynn received research grants from AstraZeneca and Novartis.
Liu has no conflict of interest.
Everett receives research support from Roche Diagnostics and Novartis.
Goldfine receives research support in the form of materials and supplies from Amneal Pharmaceuticals; Lifescan, a Division of Johnson and Johnson; Novo Nordisk; Mercodia and Nestle, Inc
Contributor Information
Seoyoung C. Kim, Email: skim62@partners.org.
Robert J. Glynn, Email: rglynn@rics.bwh.harvard.edu.
Jun Liu, Email: JLIU3@PARTNERS.ORG.
Brendan M. Everett, Email: BEVERETT@PARTNERS.ORG.
Allison B. Goldfine, Email: Allison.Goldfine@joslin.harvard.edu.
References
- 1.Amori RE, Lau J, Pittas AG. Efficacy and safety of incretin therapy in type 2 diabetes: systematic review and meta-analysis. JAMA. 2007;298(2):194–206. doi: 10.1001/jama.298.2.194. [DOI] [PubMed] [Google Scholar]
- 2.Karagiannis T, Paschos P, Paletas K, Matthews DR, Tsapas A. Dipeptidyl peptidase-4 inhibitors for treatment of type 2 diabetes mellitus in the clinical setting: systematic review and meta-analysis. BMJ. 2012;344:e1369. doi: 10.1136/bmj.e1369. [DOI] [PubMed] [Google Scholar]
- 3.Aschner P, Kipnes MS, Lunceford JK, Sanchez M, Mickel C, Williams-Herman DE Sitagliptin Study 021 Group. Effect of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy on glycemic control in patients with type 2 diabetes. Diabetes Care. 2006;29(12):2632–2637. doi: 10.2337/dc06-0703. [DOI] [PubMed] [Google Scholar]
- 4.Gallwitz B, Rosenstock J, Rauch T, Bhattacharya S, Patel S, von Eynatten M, Dugi KA, Woerle HJ. 2-year efficacy and safety of linagliptin compared with glimepiride in patients with type 2 diabetes inadequately controlled on metformin: a randomised, double-blind, non-inferiority trial. Lancet. 2012;380(9840):475–483. doi: 10.1016/S0140-6736(12)60691-6. [DOI] [PubMed] [Google Scholar]
- 5.DeFronzo RA, Fleck PR, Wilson CA, Mekki Q. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor alogliptin in patients with type 2 diabetes and inadequate glycemic control: a randomized, double-blind, placebo-controlled study. Diabetes Care. 2008;31(12):2315–2317. doi: 10.2337/dc08-1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Faillie JL, Azoulay L, Patenaude V, Hillaire-Buys D, Suissa S. Incretin based drugs and risk of acute pancreatitis in patients with type 2 diabetes: cohort study. BMJ. 2014;348:g2780. doi: 10.1136/bmj.g2780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li L, Shen J, Bala MM, Busse JW, Ebrahim S, Vandvik PO, Rios LP, Malaga G, Wong E, Sohani Z, et al. Incretin treatment and risk of pancreatitis in patients with type 2 diabetes mellitus: systematic review and meta-analysis of randomised and non-randomised studies. BMJ. 2014;348:g2366. doi: 10.1136/bmj.g2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grundy SM, Benjamin IJ, Burke GL, Chait A, Eckel RH, Howard BV, Mitch W, Smith SC, Jr, Sowers JR. Diabetes and cardiovascular disease: a statement for healthcare professionals from the American Heart Association. Circulation. 1999;100(10):1134–1146. doi: 10.1161/01.cir.100.10.1134. [DOI] [PubMed] [Google Scholar]
- 9.Wit MA, de Mulder M, Jansen EK, Umans VA. Diabetes mellitus and its impact on long-term outcomes after coronary artery bypass graft surgery. Acta diabetologica. 2013;50(2):123–128. doi: 10.1007/s00592-010-0223-3. [DOI] [PubMed] [Google Scholar]
- 10.Tarquini R, Lazzeri C, Pala L, Rotella CM, Gensini GF. The diabetic cardiomyopathy. Acta diabetologica. 2011;48(3):173–181. doi: 10.1007/s00592-010-0180-x. [DOI] [PubMed] [Google Scholar]
- 11.Scirica BM, Bhatt DL, Braunwald E, Steg PG, Davidson J, Hirshberg B, Ohman P, Frederich R, Wiviott SD, Hoffman EB, et al. Saxagliptin and Cardiovascular Outcomes in Patients with Type 2 Diabetes Mellitus. The New England journal of medicine. 2013;369(14):1317–1326. doi: 10.1056/NEJMoa1307684. [DOI] [PubMed] [Google Scholar]
- 12.White WB, Cannon CP, Heller SR, Nissen SE, Bergenstal RM, Bakris GL, Perez AT, Fleck PR, Mehta CR, Kupfer S, et al. Alogliptin after Acute Coronary Syndrome in Patients with Type 2 Diabetes. The New England journal of medicine. 2013;369(14):1327–1335. doi: 10.1056/NEJMoa1305889. [DOI] [PubMed] [Google Scholar]
- 13.White WB. Results from EXAMINE. 49th European Association for the Study of Diabetes; September 26 2013; Barcelona, Spain. 2013. [Google Scholar]
- 14.Standards of medical care in diabetes--2014. Diabetes Care. 2014;37 (Suppl 1):S14–80. doi: 10.2337/dc14-S014. [DOI] [PubMed] [Google Scholar]
- 15.Lipscombe LL, Gomes T, Levesque LE, Hux JE, Juurlink DN, Alter DA. Thiazolidinediones and cardiovascular outcomes in older patients with diabetes. JAMA. 2007;298(22):2634–2643. doi: 10.1001/jama.298.22.2634. [DOI] [PubMed] [Google Scholar]
- 16.Andrade SE, Harrold LR, Tjia J, Cutrona SL, Saczynski JS, Dodd KS, Goldberg RJ, Gurwitz JH. A systematic review of validated methods for identifying cerebrovascular accident or transient ischemic attack using administrative data. Pharmacoepidemiology and drug safety. 2012;21 (Suppl 1):100–128. doi: 10.1002/pds.2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saczynski JS, Andrade SE, Harrold LR, Tjia J, Cutrona SL, Dodd KS, Goldberg RJ, Gurwitz JH. A systematic review of validated methods for identifying heart failure using administrative data. Pharmacoepidemiology and drug safety. 2012;21 (Suppl 1):129–140. doi: 10.1002/pds.2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kiyota Y, Schneeweiss S, Glynn RJ, Cannuscio CC, Avorn J, Solomon DH. Accuracy of Medicare claims-based diagnosis of acute myocardial infarction: estimating positive predictive value on the basis of review of hospital records. American heart journal. 2004;148(1):99–104. doi: 10.1016/j.ahj.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 19.Choma NN, Griffin MR, Huang RL, Mitchel EF, Jr, Kaltenbach LA, Gideon P, Stratton SM, Roumie CL. An algorithm to identify incident myocardial infarction using Medicaid data. Pharmacoepidemiology and drug safety. 2009;18(11):1064–1071. doi: 10.1002/pds.1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gagne JJ, Glynn RJ, Avorn J, Levin R, Schneeweiss S. A combined comorbidity score predicted mortality in elderly patients better than existing scores. J Clin Epidemiol. 2011;64(7):749–759. doi: 10.1016/j.jclinepi.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rubin D. Estimating causal effects from large data sets using propensity scores. Ann Intern Med. 1997;127(8 Pt 2):757–763. doi: 10.7326/0003-4819-127-8_part_2-199710151-00064. [DOI] [PubMed] [Google Scholar]
- 22.Austin PC. The performance of different propensity score methods for estimating marginal odds ratios. Statistics in medicine. 2007;26(16):3078–3094. doi: 10.1002/sim.2781. [DOI] [PubMed] [Google Scholar]
- 23.Austin PC. Some methods of propensity-score matching had superior performance to others: results of an empirical investigation and Monte Carlo simulations. Biometrical journal Biometrische Zeitschrift. 2009;51(1):171–184. doi: 10.1002/bimj.200810488. [DOI] [PubMed] [Google Scholar]
- 24.Kleinbaum D, Klein M. Evaluating the Proportional Hazards Assumption. In: Gail M, Krickberg K, Samet J, Tsiatis A, Wong W, editors. Survival Analysis: A Self-Learning Text. 3. Springer; 2012. [Google Scholar]
- 25.White WB, Pratley R, Fleck P, Munsaka M, Hisada M, Wilson C, Menon V. Cardiovascular safety of the dipetidyl peptidase-4 inhibitor alogliptin in type 2 diabetes mellitus. Diabetes Obes Metab. 2013;15(7):668–673. doi: 10.1111/dom.12093. [DOI] [PubMed] [Google Scholar]
- 26.McMurray J. The Vildagliptin in Ventricular Dysfunction Diabetes trial (VIVIDD). European Society of Cardiology Heart Failure Association; May 26 2013; Lisbon, Portugal. 2013. p. 99. [Google Scholar]
- 27.Ussher JR, Drucker DJ. Cardiovascular biology of the incretin system. Endocrine reviews. 2012;33(2):187–215. doi: 10.1210/er.2011-1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johnson ES, Bartman BA, Briesacher BA, Fleming NS, Gerhard T, Kornegay CJ, Nourjah P, Sauer B, Schumock GT, Sedrakyan A, et al. The incident user design in comparative effectiveness research. Pharmacoepidemiol Drug Saf. 2013;22(1):1–6. doi: 10.1002/pds.3334. [DOI] [PubMed] [Google Scholar]
- 29.Ray WA. Evaluating medication effects outside of clinical trials: new-user designs. American journal of epidemiology. 2003;158(9):915–920. doi: 10.1093/aje/kwg231. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.