Summary
Relapse to cocaine use necessitates remodeling excitatory synapses in the nucleus accumbens, and synaptic reorganization requires matrix metalloproteinase (MMP) degradation of the extracellular matrix proteins. We found enduring increases in MMP-2 activity in rats after withdrawal from self-administered cocaine and transient increases in MMP-9 during cue-induced cocaine relapse. Cue-induced heroin and nicotine relapse increased MMP activity, and increased MMP activity was required for both cocaine relapse and relapse-associated synaptic plasticity.
Vulnerability to relapse is a defining characteristic of drug addiction, and controlling relapse a primary therapeutic goal in treating addiction1. The inability to control drug use is associated with neuropathologies in cortical regulation of the striatal circuitry, including constitutive potentiation of cortical glutamatergic synapses in the nucleus accumbens core (NAcore)2, 3, and further transient synaptic potentiation (t-SP) when relapse is initiated by cocaine injection or cocaine-associated cues4, 5. Although these studies show that synaptic potentiation at glutamatergic synapses in NAcore is required for relapse to cocaine seeking, it is not understood how the long-lasting potentiation after withdrawal is stabilized, or how relapse-associated t-SP is initiated.
Synaptic remodeling depends on the extracellular matrix (ECM), which is a proteinacious network ensheathing synapses that is regulated by Zn2+-dependent endopeptidases called matrix metalloproteinases (MMPs)6. MMP-2 and -9 make up the gelatinase subfamily7 that regulates synaptic structure and physiology by proteolytically processing ECM glycoproteins to initiate glutamate receptor trafficking and actin polymerization6, 8. Using a relapse model of cocaine, heroin and nicotine self-administration and reinstatement in rats, we tested the hypothesis that MMP-2 and -9 are required for both cue-induced reinstatement and associated synaptic plasticity.
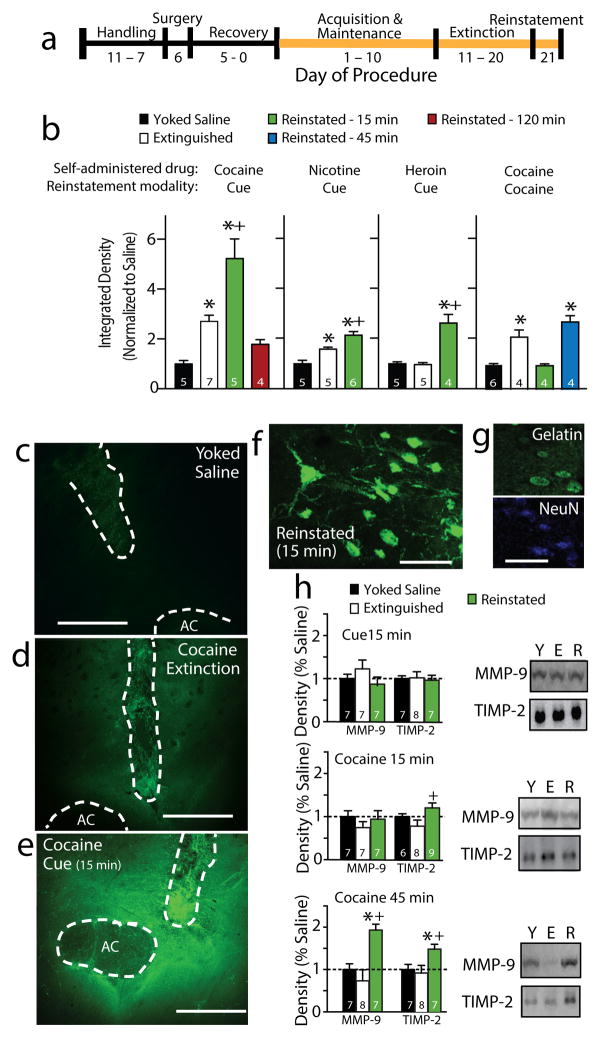
MMP-2 and -9 proteolytic activity within the NAcore was quantified using a FITC-quenched gelatin peptide that fluoresces following cleavage by MMP-2 or -9 9 in a linear manner over 60 min (Fig. S1). Rats were trained to self-administer cocaine, heroin or nicotine and lever pressing was extinguished (Fig. 1a & S2). FITC-gelatin was microinjected into NAcore, and 15 minutes later rats were sacrificed before or at various times after initiating drug-seeking by restoring drug-associated conditioned cues (tone/light) to reinstate active lever pressing (Fig. S2). Gelatinase activity was increased in NAcore of cocaine-extinguished compared with yoked-saline control rats, and 15 min of cue-induced reinstatement caused a further increase (Fig. 1b–e). The increase in MMP activity returned to pre-reinstatement levels by 120 min (Fig. 1b). Rats trained to self-administer nicotine showed constitutively increased MMP activity after extinction, and both nicotine- and heroin-trained rats showed increases after 15 min of cued-reinstatement. When cocaine-trained rats were reinstated using a noncontingent cocaine injection, the constitutive increase in MMP activity was eliminated at 15 min after injection, but rebounded by 45 min. The increase in fluorescence was localized to the soma and dendrites of NAcore neurons (Fig. 1f, g). No increases in MMP activity were measured in the dorsal striatum or accumbens shell after 15 min of cue-induced reinstatement (Fig. S3a, b).
Figure 1. Cocaine extinction and reinstatement elevated MMP activity in the NAcore.
a) Outline of the self-administration/reinstatement protocol. b) Gelatinase activity was increased following extinction from cocaine and nicotine self-administration, and further increased 15 minutes following cue-induced cocaine (F(3,17)=17.80, p<0.001), nicotine (F(2,13)=19.70, p<0.001) or heroin reinstatement (F(2,11)=25.19, p<0.001), or 45 min after cocaine-induced reinstatement (F(3,14)=23.42, p<0.001). c,d,f) Examples of FITC-gelatin fluorescence in NAcore of yoked-saline, extinguished and cue-reinstated rats. AC-anterior commissure, dashed line outlines AC and injection site that were masked-out for quantification (data are average of 4 NAcore slices/animal). Bar=500 μm. f,g) Representative micrographs showing MMP activity over neurons (NeuN- neuronal marker). Bar=100 μm. h) The level of TIMP-2 was elevated in cocaine-reinstated rats compared to rats after extinction at 15 min (F (2,20)=3.76, p<0.05), and both MMP-9 and TIMP-2 were elevated compared to extinguished and yoked-saline rats at 45 min (TIMP-F(2,19)= 4.306, p< 0.05; MMP-9-F(2,19)=10.35, p<0.001). Full-length gels are shown in figure S10. Data shown as mean ± sem. * p< 0.05 compared to yoked-saline using a Newman-Kuels test for multiple comparisons; + p< 0.05 compared to extinction.
Neither the enduring increase in gelatinase activity after extinction nor the increase elicited by 15 min of cue-induced reinstatement were accompanied by a change in NAcore protein content of MMP-2 or MMP-9, or the MMP-2/9 inhibitory protein TIMP-2, although TIMP-2 was elevated in reinstated compared to rats after extinction (Fig. 1h and S4a). Furthermore, no difference was detected in MMP-2 or MMP-9 mRNA between yoked-saline and reinstated rats (Fig. S4b), indicating that the increase in MMP-2 and MMP-9 activity likely results from protein activation rather than protein synthesis6. In contrast, both MMP-9 and TIMP-2 protein content were elevated 45 min after reinstating cocaine-seeking (Fig. 1h).
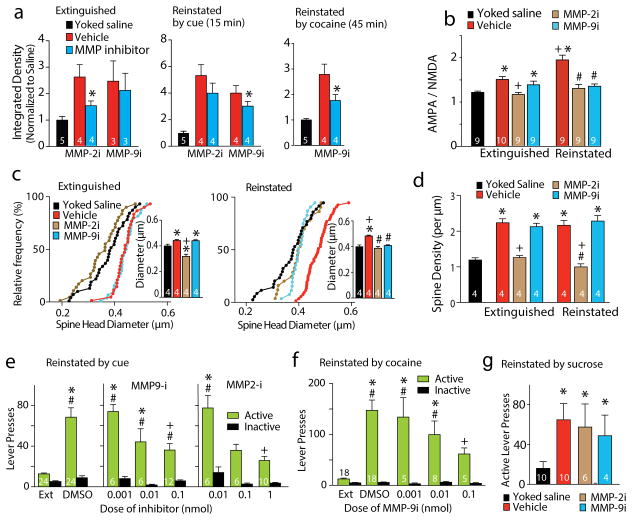
We used pharmacological inhibitors of MMP-2 and -9 to determine which MMP was mediating the increased fluorescence10. The constitutive increase in fluorescence in rats after extinction was abolished by intra-NAcore microinjection of an MMP-2, but not MMP-9 inhibitor (MMP-9i; Fig. 2a). Conversely, the increase in fluorescence after 15 min of cue-induced reinstatement was reduced by an MMP-9i, but not MMP-2i (Fig. 2a). Increased MMP activity after 45 min after cocaine-induced reinstatement was also prevented by an MMP-9i (Fig. 2a). This pattern of gelatinase expression is consistent with work showing that the brain constitutively expresses MMP-2 activity, while MMP-9 is transiently induced by external stimuli11.
Figure 2. Constitutively induced MMP-2 following extinction, and transient increases in MMP-9 by reinstatement mediate t-SP.
a) MMP-2i (1 nmol/side) decreased gelatinase activity following extinction compared to vehicle injection into the contralateral NAcore, t(3)=3.72, p=0.034, while MMP-9i (0.1 nmol/side) was without effect. Yoked-saline data shown for comparison are from panel 1b. MMP-9i, not MMP-2i, reduced gelatinase activity 15 minutes following cue-induced reinstatement. t(3)=3.47, p=0.040. MMP-9 inhibition reduced fluorescence induced 45 minutes after a cocaine-priming injection t(3)=3.77, p=0.037. N inside bar is number of rats. *p< 0.05, comparing vehicle with inhibitor using a paired Student’s t-test. b) A/N elevated after cocaine extinction was reduced by MMP-2i, while transiently increased A/N during reinstatement was reduced by either MMP-2i or MMP-9i. N in bars is number of neurons recorded from >3 rats in each condition, F(6,57)=13.08, p<0.001. c) The enduring elevation of dh by cocaine extinction was reduced by MMP-2i, while transient increases in dh during reinstatement were reduced by either MMP-2i or MMP-9i. N in bars is the number of rats quantified (6–12 neurons/rat), F(8, 27) =11.68, p<0.001. d) The increase in spine density produced in rats after extinction was blocked by MMP-2i, not MMP-9i. F(8, 27)=13.47, p<0.001. * p<0.05, compared to yoked-saline vehicle, using a post-hoc Newman-Keuls test. + p<0.05 compared to extinguished vehicle, # p<0.05, compared to reinstated vehicle. e) Bilateral microinjection of MMP-2i or MMP-9i into NAcore decreased active lever pressing in response to cocaine-conditioned cues over a two-hour reinstatement session. A randomized cross-over deign over 3 sessions was used, and 3 reinstatement sessions yields equivalent active lever pressing (Fig. S9), interaction F(7,172)=8.02, p<0.001. f) Bilateral microinjection of MMP-9i decreased active lever pressing in response to a cocaine priming injection, interaction F(4, 57)=11.28, p<0.001. g) Intra-NAcore microinjection of either MMP-2i (1 nmol/side) or MMP-9i (0.1 nmol/side) failed to reduce cue-induced reinstatement of sucrose seeking. Kruskal-Wallis(4,30)=10.61, p=0.014. N inside bar is number of rats. Data shown as mean±s.e.m.
* p<0.05 compared to extinction, + p<0.05 compared to vehicle, # p<0.05 compared to paired inactive responding.
Withdrawal from cocaine self-administration is associated with constitutive synaptic potentiation in NAcore excitatory synapses12–14, and after 15 min of reinstated lever pressing NAcore synapses undergo t-SP4, 5. We assessed synaptic potentiation morphologically as spine density and head diameter (dh), and electrophysiologically as the ratio of AMPA to NMDA currents (A/N). Whole-cell patch clamp measurement of A/N in medium spiny neurons (MSN) revealed that following vehicle microinjection into the NAcore, A/N in cocaine-extinguished rats was elevated compared to yoked-saline rats, and was further elevated 15 min after initiating cue-induced reinstatement (Fig. 2b). The increase in A/N in rats after extinction was restored to yoked-saline levels by the MMP-2i, not MMP-9i. However, either inhibitor prevented the elevated A/N initiated by 15 min of reinstatement (Fig. S5 for current traces, NMDA decay time, sEPSC frequency and amplitude). Diolistic labeling of MSNs with lipophilic DiI (Fig. S6a–c for examples) revealed that following vehicle injection into the NAcore, dh was increased after extinction from cocaine self-administration compared to yoked-saline, and was further increased 15 min after cue-induced reinstatement of cocaine seeking (Fig. 2c). The constitutive increase in dh in rats after extinction depended on MMP-2 activity, and the increase after 15 minutes of reinstatement depended on both MMP-2 and -9 activity. Spine density was elevated in cocaine-extinguished compared to yoked-saline rats, but no further elevation was produced by cue-induced reinstatement (Fig. 2d & S6d). Spine density in both extinguished and reinstated rats was normalized to yoked-saline levels by the MMP-2i, but not MMP-9i. Combined with the measure of dh, these data indicate that reinstatement is associated with transiently increasing the size (dh) of existing spines, not creating new spines, and that MMP-2 activity supports the increase in spine number in rats after extinction that are enlarged by MMP-9 activity during cued reinstatement. Neither MMP inhibitor affected A/N, dh or spine density in yoked-saline rats (Fig. S7).
Given that reinstated behavior requires synaptic potentiation in accumbens MSNs5, and the dependence of synaptic potentiation on MMP-2 and MMP-9 activity, we hypothesized that MMP-2i and MMP-9i would reduce cue-induced reinstatement. Microinjection of either inhibitor into the NAcore dose-dependently reduced in cue-induced reinstatement compared to control (Fig. 2e; Fig. S8 for histology). MMP-9i also reduced cocaine-induced reinstatement (Fig. 2f). Consistent with the lack of synaptic potentiation in the NAcore during cue-induced sucrose reinstatement5, neither inhibitor reduced cue-induced reinstatement of lever pressing for sucrose pellets (Fig. 2g).
A role for MMPs in addiction is indicated by MMP-9 gene expression being altered in the brain of cocaine addicts15, the serum of heroin addicts16, and an MMP-9 gene polymorphism being associated with alcohol dependence17. Also, intra-cerebroventricular injection of nonselective MMP inhibitors reduces drug seeking in animal models18, 19. Our data show a specific and necessary role for MMP-2 and MMP-9 in the enduring vulnerability to relapse, and open study of ECM signaling as a research theme for understanding and treating addiction.
ONLINE METHODS
Animal Housing and Surgery
Male Sprague-Dawley Rats (250g; Charles River) were individually housed with a 12:12 hour dark/light cycle. All experimentation occurred during the dark phase, and animals were allowed to acclimate to the vivarium environment for 4 days prior to surgery. Rats were ~65 days old when they were anesthetized with a combination of ketamine HCl and xylazine, and received ketorolac for analgesia. All rats received intrajugular catheters, and rats for microinjection experiments received intracranial cannula targeted 2 mm above the NAcore, dorsolateral striatum or NAshell 20. Rats were food restricted to 25g of rat chow per day. All methods used comply with the NIH Guide for the Care and Use of Animals and were approved by the Institutional Animal Care and Use Committee at the Medical University of South Carolina.
Drugs Used
Cocaine HCl was supplied by the National Institute of Drug Abuse. Inhibitors used include the MMP-2 inhibitor Oleoyl-N-Hydroxylamide (OA-Hy) (EMD4BioScience MMP-2 Ki = 1.07 μM, MMP-9 Ki > 50μM,), and MMP-9 inhibitor C27H33N3O5S (EMD4Bioscience; MMP-9 Ki=5nM, MMP-1 Ki=1.05mM, and MMP-13 Ki=113nM 21). The MMP inhibitors were dissolved in a maximum concentration of 2% DMSO.
Drug Self-Administration and Reinstatement
After 5 days of recovery from surgery, rats underwent one overnight (12 hour) food training session. The next day, animals began daily 2-hour self-administration (SA) sessions for either cocaine or nicotine, or 3-hour self-administration session for heroin5, 22, 23. During SA, drug was delivered using an FR1 schedule with a 20 s timeout following each infusion. Active lever presses that resulted in cocaine (cocaine hydrochloride; 0.2 mg/infusion; donated by the National Institute on Drug Abuse), heroin (heroin-hydrochloride; 100 μg/infusion for day 1–2, 50 μg/infusion for day 3–4, 25 μg/infusion for day 5–10; donated by National Institute on Drug Abuse), or nicotine (0.02 mg/kg/infusion) infusion simultaneously resulted in presentation of a compound light (above the active lever) and tone (2900 Hz) conditioning stimulus. An inactive lever was also provided to control for non-motivated responding. Following ≥ 10 SA sessions at 10 infusions/day, rats began extinction training, during which all programmed consequences were removed from lever pressing. Extinction training lasted at least 10 days, or until two consecutive days ≤ 25 active lever presses. Reinstatement was induced by presentation of light/tone cues following an active lever press. Microinjections of an MMP inhibitor or vehicle were given 15 minutes prior to beginning reinstatement in most cases, or 15 minutes prior to gel infusion for zymography experiments. For behavioral experiments (Figure 6), a within-subjects crossover design was used. In this paradigm, each rat received each condition (MMP-2i, MMP-9i, or Vehicle) according to a Latin square design. Rats were required to meet extinction criteria prior to each reinstatement test. Reinstatement sessions lasted 120 minutes in the behavioral experiment, and for zymography, spine morphology, and A:N experiments reinstatement sessions were 15, 45, or 120 minutes long, at which point rats were sacrificed for further measurements. When rats were assigned to different drug versus yoked-saline groups, they were randomly assigned. When rats were assigned to extinguished or reinstated groups they were assigned in order to maintain equal variance and mean number of drug infusions during the last 3 days of self-administration.
In Vivo Zymography
Because MMPs are secreted in inactive pro-forms and catalytically activated within the ECM, activity assays are preferable to immunoblotting for protein content for assessing changes in MMP function 24. We used an in vivo zymography assay to directly measure MMP activity. Dye-quenched gelatin is an MMP-2/9 substrate containing intra-molecularly quenched FITC fluorophores that cannot fluoresce until proteolytically processed by MMP-2 or MMP-9 9. The amount of fluorescence produced forms a linear relationship with incubation time and MMP activity (Figure S1). Dye-quenched FITC-Gelatin (Molecular Probes, Eugene, OR) was reconstituted in PBS at 1 mg/ml pH 7.2–7.4. 3.0μl of gel (1.5μl/side) was microinjected 15 minutes prior administering an overdose of pentobarbital (100 mg/kg, ip) and beginning transcardial perfusion of 4% paraformaldehyde (PFA). Brains were removed, placed in 4% PFA for 90 minutes for additional fixation, a vibratome was used to obtain 50 μm sections through the NAc. Sections were mounted and coverslipped. Fluorescence was excited with a 488nm Argon laser, emissions filtered to 515–535nm, and images obtained through a 10x objective with a 0.3 numerical aperture (Leica confocal microscope). Only slices in which the injection site and anterior commissure could be visualized in the same frame were imaged. ImageJ (NIH) was used to quantify images. All quantified images contained the anterior commissure, which was masked to prevent being quantified, but provided a landmark for the NAcore. MMP activity is induced as part of the acute inflammatory response to tissue damage from the microinjector, and thus the microinjector tract was readily visible in all quantified sections due to equivalent high fluorescence in all treatment groups (Figure 1). This tract was also masked to eliminate quantifying any MMP activity caused by microinjection-induced acute damage. Fluorescence was quantified bilaterally as integrated density from four sections per rat, and the integrated densities were averaged within each rat and normalized to yoked-saline control values. Quantification of density was conducted by an individual blinded to the treatment group.
Western Blotting
Rats were rapidly decapitated after extinction of cocaine self-administration or yoked-saline, or following 15 or 45 minutes following cued or cocaine-primed reinstatement.. The NAcore was dissected and homogenized in RIPA lysis buffer containing 1.0% SDS and protease/phosphatase inhibitors. Homogenate was centrifuged at 4°C for 5 minutes at 10,000 x g. Supernatant was collected and protein concentration was determined via a biconchinic acid assay (Thermo Scientific). 30μg protein was added to each lane of 10% Bis-Tris gels (Bio-Rad), and transferred to nitrocellulose membranes via the Invitrogen iBlot transfer system. Primary antibodies were used for MMP-2 (1:1500, Abcam ab79781), MMP-9 (1:500, Millipore AB6001), and TIMP2 (1:1000, Abcam ab53730) and HRP-conjugated Goat anti-Rabbit secondary was used at 1:10,000. GAPDH was used as a loading control for MMPs-2 and -9, and Calnexin was used for TIMP-2. A Kodak Image Station was used to visualize and quantify protein expression. Each Western blot was repeated twice.
Semi-quantitative RT-PCR
NAcore brain tissue was dissected from cocaine or saline animals sacrificed on the final day of extinction training. Total RNA was extracted from NAcore tissue using the Qiagen RNeasy mini kit with Qiashredder homogenization (Qiagen). Reverse transcription was performed at 37°C for one hour using 1μg of total RNA for each sample in 20μl reactions using the High Capacity RNA-to-cDNA kit (Applied Biosystems). PCR reactions were assembled in taq PCR master mix (Qiagen) with 35pmol of each primer set detailed below and 6μl of cDNA as template yielding a final reaction volume of 20μl. PCR reactions were run in a MyCycler thermal cycler (Bio-Rad) with a protocol consisting of a single 94ºC step for 3 minutes followed by 35 repetitions of 94ºC for 30 seconds, 51ºC for 30 seconds and 72ºC for 1 minute cycle, ending with a final 5 min extension at 72ºC. 35 cycles was selected following optimization experiments performed prior to mRNA measurement indicating that 35 cycles resulted in levels of product formation that remained in the linear range for each set of primers and thus was suitable for semi-quantitative RT-PCR (data not shown). The following primers were used 25, MMP2 forward 5′-GATCTGCAAGCAAGACATTGTCTT-3′ MMP2 reverse 5′-GCCAAATAAACCGATCCTTGAA-3′ MMP9 forward 5′-GTAACCCTGGTCACCGGACTT-3′ MMP9 reverse 5′-ATACGTTCCCGGCTGATCAG TIMP-2 forward 5′-AGGGAAGGCGGAAGGAGAT-3′ TIMP-2 reverse 5′-CCAGGGCACAATAAAGTCACAGA-3′ TIMP-3 forward 5′-AGCATCAGCAATGCCACAGA-3′ and Cyclophilin forward 5′-GGGGAGAAAGGATTTGGCTA-3′ Cyclophilin reverse 5′-ACATGCTTGCCATCCAGCC-3′ 26. PCR reactions were separated by 1.5% agarose gel electrophoresis and densitometry values were determined using Fiji (imagej Version 1.47) software. During each PCR run no template negative controls were run and contained no products, in addition minus RT samples were also run and show no product formation (data not shown). Relative amounts of MMP2, MMP9, TIMP-2 and TIMP3 mRNA were calculated as a ratio of the density value of amplicon for MMP2, MMP9, TIMP-2 or TIMP3 that of the corresponding cyclophilin control amplicon.
Quantification of dendritic spine head morphology
Rats were deeply anesthetized with ketamine HCl (87.5 mg/kg, i.p.) and xylazine (5 mg/kg, i.p.). Transcardial perfusions with phosphate buffered saline (PBS) followed by 1.5% paraformaldehyde (PFA) in PBS. Brains were removed and post-fixed in the same fixative for 30 minutes, then coronally sectioned at 200 μm in PBS on a vibratome. Tungsten particles (1.3 μm diameter, Bio-Rad) were coated with the lipophilic carbocyanine dye DiI (Invitrogen). DiI-coated particles were delivered diolistically into the tissue at 80 PSI using a Helios Gene Gun system (Bio-Rad) fitted with a polycarbonate filter with a 3.0 μm pore size (BD Biosciences). DiI was allowed to diffuse along neuron axons and dendrites in PBS for 24 hours at 4° C, and then fixed again in a 4% PFA for 1 hour at room temperature. After a brief PBS wash, tissue was mounted onto slides in aqueous medium Prolong Gold (Invitrogen).
Spine morphology was performed as described in detail elsewhere 27. Briefly, images of DiI-labeled sections were taken on a confocal microscope (Zeiss) using a Helium/Neon 543 nm laser line. Optimal sampling frequency was calculated using the Nyquist-Shannon sampling theorem. Images of dendrites were taken through a 63x oil immersion objective (Plan-Apochromat, Zeiss; NA = 1.4, WD = 90 μm) with pixel size 0.07 μm in the XY-plane and 0.10 μm intervals along the Z-axis at 0.1 μm intervals. Images were deconvoluted via Autoquant prior to analysis (Media Cybernetics, Bethesda, MD), and a 3-D perspective was rendered by the Surpass module of Imaris software package (Bitplane; Saint Paul, MN). The smallest quantifiable diameter spine head was 0.143μm. Only spines on dendrites beginning >75 μm and ending <200 μm distal to the soma and after the first branch point were quantified on cells localized to the NAcore. The length of quantified segments was 45–55 μm. One segment from each neuron was quantified, and the minimum spine head diameter was set at 0.15 μm. Between 6 and 12 neurons were imaged in each animal (the number of neurons per treatment group: yoked saline/vehicle- 32 neurons; yoked saline/MMP-2i- 34 neurons; yoked saline/MMP-9i- 29 neurons; extinguished/vehicle- 29 neurons; extinguished/MMP-21- 33 neurons; extinguished/MMP-9i- 33 neurons; reinstated/vehicle- 48 neurons; reinstated/MMP-2i- 25 neurons; reinstated/MMP-9i- 25 neurons. Morphological measurements were conducted by an individual unaware of the treatment groups.
Slice preparation for electrophysiology
Rats were anesthetized with ketamine HCl (100 mg/kg Ketaset, Fort Dodge Animal Health, Iowa) and decapitated. The brain was removed from the skull and 220μm thick coronal NAc sections were obtained using a vibratome (VT1200S Leica vibratome; Leica Microsystems, Wetzlar, Germany). Slices were immediately placed into a vial containing artificial cerebrospinal fluid (aCSF) (in mM: 126 NaCl, 1.4 NaH2PO4, 25 NaHCO3, 11 glucose, 1.2 MgCl2, 2.4 CaCl2, 2.5 KCl, 2.0 NaPyruvate, 0.4 ascorbic acid, bubbled with 95% O2 and 5% CO2) and a mixture of 5 mM kynurenic acid and 50 μM D-(−)-2-Amino-5-phosphonopentanoic acid (D-AP5). Slices were incubated at room temperature until recording.
In vitro whole cell recording
All recordings were collected at 32°C (controlled by TC-344B, Warner Instrument Corporation, Hamden, Connecticut) in the dorsomedial NAcore. Inhibitory synaptic transmission was blocked with picrotoxin (50 μM). Multiclamp 700B (Axon Instruments, Union City, CA) was used to record excitatory postsynaptic currents (EPSCs) in whole cell patch-clamp configuration. Glass microelectrodes (1–2 MΩ) were filled with cesium-based internal solution (in mM: 124 cesium methanesulfonate, 10 HEPES potassium, 1 EGTA, 1 MgCl2, 10 NaCl, 2.0 MgATP, and 0.3 NaGTP, 1 QX-314, pH 7.2–7.3, 275 mOsm). Data were acquired at 10 kHz, and filtered at 2 kHz using AxoGraph X software (AxoGraph Scientific, Sydney, Australia). To evoke EPSCs a bipolar stimulating electrode (FHC, Bowdoin, Maine) was placed ~300 μm dorsomedial to the recorded cell to maximize chances of stimulating prelimbic afferents. The stimulation intensity was set to evoke an EPSC of 200–500 pA which was usually 30–70% of maximal EPSC. Recordings were collected every 20 sec. Series resistance (Rs) measured with a 2 mV hyperpolarizing step (10 ms) given with each stimulus and holding current were always monitored online. Recordings with unstable Rs, or when Rs exceeded 10 MΩ were aborted.
Measuring the AMPA/NMDA ratio
Recordings started no earlier than 10 min after the cell membrane was ruptured, to allow diffusion of the internal solution into the cell. AMPA currents were first measured at −80 mV to ensure stability of response. The membrane potential was then gradually increased to +40 mV. Recording of currents resumed 5 min after reaching +40 mV to allow stabilization of cell parameters. Currents composed of both AMPA and NMDA currents were then obtained. Then D-AP5 was bath-applied (50 μM) to block NMDA currents and recording of AMPA currents at +40 mV was started after 2 min. NMDA currents were obtained by subtracting the AMPA currents from the total current at +40 mV. Electrophysiological measurements were conducted by an individual unaware of the treatment groups.
Statistical Analysis
Sample size for each experiment was determined by power analysis by G*Power and from analysis of power in similar experiments previously published from our laboratory. All statistics were done using GraphPad Prism Version 6. Zymography measurements were analyzed using either a one-way ANOVA followed by a Newman-Kuels post hoc test (figure 1) or paired Student’s t-test (figure 2) when comparisons were made between different treatments in each brain hemisphere. Protein (figure 1h, S4a) and mRNA (figure S4b) measurements were made using a one-way ANOVA followed by a Newman-Kuels post hoc test or an unpaired Student’s t-test, respectively. Electrophysiological data (figures 2b, S5, S7) and dendritic spine data (figures 2c, d, S6, S7) were analyzed using a one-way ANOVA with a Newman-Kuels post hoc test. Reinstatement of cocaine seeking behavior in figure 2e, f was analyzed via a two-Way ANOVA using lever and dose of inhibitor as factors and a Newman-Kuels post hoc. In contrast, sucrose reinstatement behavior in figure 2g that was compared using a Kruskal-Wallis nonparametric test because the data were not normally distributed according to a D’Agostino-Pearson omnibus normality test.
Supplementary Material
Acknowledgments
This research was support by NIH grants DA003906, DA012513 and DA015369.
Footnotes
Author Contributions: ACWS conducted the behavioral and zymography studies, analyzed data and wrote the manuscript, YMK conducted the electrophysiological experiments, MDS conducted the Western blotting and contributed to the spine analysis, CDG contributed to spine analysis, AW developed the in vivo zymography assay to generate preliminary data for this study, PWK oversaw the project, analyzed data and wrote the manuscript.
Disclosures: The authors have no competing interests to declare in publishing this manuscript.
The authors have no conflicts of interest to disclose.
References
- 1.Vocci F, Ling W. Pharmacol Ther. 2005;108:94–108. doi: 10.1016/j.pharmthera.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 2.Russo SJ, et al. Tr Neurosciences. 2010;33:267–276. doi: 10.1016/j.tins.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Conrad KL, et al. Nature. 2008;454:118–121. doi: 10.1038/nature06995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anderson SM, et al. Nat Neurosci. 2008;11:344–353. doi: 10.1038/nn2054. [DOI] [PubMed] [Google Scholar]
- 5.Gipson CD, et al. Neuron. 2013;77:867–872. doi: 10.1016/j.neuron.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huntley GW. Nature Rev Neurosci. 2012;13:743–757. doi: 10.1038/nrn3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sternlicht M, Werb Z. Annu Rev Cell Dev Biol. 2001;17:463–516. doi: 10.1146/annurev.cellbio.17.1.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cingolani LA, et al. Neuron. 2008;58:749–762. doi: 10.1016/j.neuron.2008.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bozdagi O, Nagy V, Kwei KT, Huntley GW. J Neurophysiol. 2007;98:334–344. doi: 10.1152/jn.00202.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Levin JI, et al. Bioorg Med Chem Lett. 2001;11:2189–2192. doi: 10.1016/s0960-894x(01)00419-x. [DOI] [PubMed] [Google Scholar]
- 11.Verslegers M, Lemmens K, Van Hove I, Moons L. Prog Neurobiology. 2013 doi: 10.1016/j.pneurobio.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 12.Wolf ME, Ferrario CR. Neurosci Biobehav Rev. 2011;35:185–211. doi: 10.1016/j.neubiorev.2010.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moussawi K, et al. Nat Neurosci. 2009;12:182–189. doi: 10.1038/nn.2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Robinson TE, Kolb B. Neuropharmacology. 2004;47(Suppl 1):33–46. doi: 10.1016/j.neuropharm.2004.06.025. [DOI] [PubMed] [Google Scholar]
- 15.Mash D, French-Mullen J, Adi N, Qin Y, Buck A, Pablo J. PLoS One. 2007;2:1187. doi: 10.1371/journal.pone.0001187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kovatsi L, et al. Tox Mech Meth. 2013;23:377–381. doi: 10.3109/15376516.2012.758681. [DOI] [PubMed] [Google Scholar]
- 17.Samochowiec A, et al. Brain Res. 2010;1327:103–106. doi: 10.1016/j.brainres.2010.02.072. [DOI] [PubMed] [Google Scholar]
- 18.Brown T, et al. Learning & Memory. 2007;14:214–223. doi: 10.1101/lm.476207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Van Den Oover M, et al. Neuropsychopharm. 2010;35:2120–2133. doi: 10.1038/npp.2010.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Elsevier Academic Press; Burlington: 2007. [Google Scholar]
- 21.Levin JI, et al. Bioorg Med Chem Lett. 2001;11:2189–2192. doi: 10.1016/s0960-894x(01)00419-x. [DOI] [PubMed] [Google Scholar]
- 22.Shen H, Moussawi K, Zhou W, Toda S, Kalivas PW. Proc Nat Acad Sci. 2011;108:19407–19412. doi: 10.1073/pnas.1112052108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gipson CD, et al. Proc Nat Acad Sci. 2013;110:9124–9129. doi: 10.1073/pnas.1220591110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kupai K, et al. J Pharmacol Toxicol Methods. 2010;61:205–209. doi: 10.1016/j.vascn.2010.02.011. [DOI] [PubMed] [Google Scholar]
- 25.Liu W, Furuichi T, Miyake M, Rosenberg GA, Liu KJ. J Neurosci Res. 2007;85:829–836. doi: 10.1002/jnr.21179. [DOI] [PubMed] [Google Scholar]
- 26.Morales-Mulia M, de Gortari P, Amaya MI, Mendez M. J Mol Neuroscience : MN. 2013;49:289–300. doi: 10.1007/s12031-012-9823-4. [DOI] [PubMed] [Google Scholar]
- 27.Shen H, Sesack SR, Toda S, Kalivas PW. Brain Struct Funct. 2008;213:149–158. doi: 10.1007/s00429-008-0184-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.