Abstract
Purpose
Parathyroid hormone (PTH) 1-34 is known to enhance fracture healing. Tendon repair is analogous to bone healing in its dependence on the proliferation and differentiation of mesenchymal stem cells, matrix formation, and tissue remodeling.1-3 We hypothesized that PTH 1-34 enhances tendon healing in a flexor digitorum longus (FDL) tendon repair model.
Methods
C57Bl/6J mice were treated with either intraperitoneal PTH 1-34 or vehicle-control (PBS). Tendons were harvested at 3-28 days for histology, gene expression, and biomechanical testing.
Results
The metatarsophalangeal joint range of motion was reduced 1.5-2-fold in PTH 1-34 mice compared to control mice. The gliding coefficient, a measure of adhesion formation, was 2-3.5-fold higher in PTH 1-34 mice. At 14 days post-repair, the tensile strength was 2-fold higher in PTH 1-34 specimens, but at 28 days there were no differences. PTH 1-34 mice had increased fibrous tissue deposition that correlated with elevated expression of collagens and fibronectin as seen on quantitative PCR.
Conclusions
PTH 1-34 accelerated the deposition of reparative tissue but increased adhesion formation.
Keywords: PTH 1-34, forteo, tendon healing, adhesions, biomechanics
Introduction
The flexor muscles of the forearm exert their actions in the hand through their tendinous extensions. In the hand, flexor tendons enter synovial sheaths that provide lubrication and remarkably low friction that permit tendon gliding.4 Lacerations of flexor tendons in Zone II of the hand are common and healing complications associated with these injuries remain a challenging clinical problem.5 Despite advances in surgical technique and physical therapy, adhesion formation occurs in up to 25% of patients.4 Given the current limitations of treatment, growth factor based therapies have the potential to improve the tendon healing response.4,6
Previous studies7-9 have demonstrated that PTH 1-34, a biologically active peptide fragment of parathyroid hormone, stimulates mesenchymal stem cell populations. Both in vivo10,11 and in vitro8,9 studies have demonstrated the osteogenic effect of PTH 1-34, and recent work suggests that it also promotes chondrogenesis.12-16 As PTH 1-34 is a systemic hormone, it is important to consider the effects it may have on other cells in the mesenchymal lineage, including tendon cells. A stimulatory role for PTH 1-34 on tendon-to-bone healing was first suggested by Rodeo et al.17 who reported increased bone and fibrocartilage formation after recombinant parathyroid hormone (rhPTH) treatment in a rat rotator cuff model. Given the anabolic effect of PTH 1-34 on fracture healing and tendon-to-bone healing, it may also enhance extracellular matrix deposition and organization during tendon-to-tendon healing.
In these experiments, we used a mouse model of primary flexor digitorum longus (FDL) tendon repair to test the hypothesis that PTH 1-34 enhances flexor tendon healing. We performed histological analyses to examine the remodeling process in and around the repair as well as real-time RT-PCR to analyze the temporal changes in gene expression involved in the healing process. In addition, we assessed the biomechanical properties of the repaired tendon including the metatarsophalangeal (MTP) joint flexion and breaking tensile force as measures of adhesion formation and repair strength, respectively.
Materials and Methods
Tendon Repair Surgery and PTH 1-34 Treatment
All animal procedures were approved by the University Committee on Animal Research. Six-to-eight-week-old male C57BL/6J mice (Charles River Laboratories, Wilmington, MA) were operated on as previously described.3,6 Mice were anesthetized and a longitudinal incision was made at the plantar surface of the metatarsal bones to expose the right deep digital flexor digitorum longus (FDL) tendon.18 The tendon was transected and immediately repaired using 8-0 nylon sutures in a modified Kessler pattern.3,6 The myotendinous junction was released to prevent the transmission of active forces against the repair. Following surgery, the mice were allowed free passive motion of their lower extremities. Ninety-seven mice received daily subcutaneous injections of 40 μg/kg of PTH 1-34 (Sigma-Aldrich, St. Louis, MO) and ninety-seven control mice were treated with PBS (vehicle-control) for a total of 194 mice (n=97 per treatment group). The dose of PTH 1-34 was selected based on literature establishing that 40 μg/kg was the lowest effective dose to promote anabolic effects in mesenchymal tissue, including bone and cartilage.14,19,20 No side effects were noted with this dose of PTH 1-34. PTH 1-34 and PBS treatments were initiated at the time of surgery and continued for the duration of the experiment. Limbs were harvested on days 3, 7, 14, 21 and 28 days post-repair for histological analysis (n=4 mice per treatment per time-point), immunohistochemistry (n=4 mice per treatment per time-point) and RNA extraction for real-time RT-PCR (n=5 mice per treatment per time point). Additionally, limbs were harvested at 0, 14, 21, and 28 days post-repair for adhesion and biomechanical testing (n=8 mice per treatment per time point).
Histology
The harvested samples were fixed in 10% neutral buffered formalin for 48 h, washed in PBS, and decalcified for 21 days in 14% EDTA (pH 7.2). The tissues were processed and embedded in paraffin. 3-μm thick sections were prepared and stained with alcian blue/hematoxylin/Orange G or picrosirius red. The picrosirius red-stained sections were illuminated with monochromatic polarized light to provide a qualitative assessment of collagen fiber organization. Increased brightness was interpreted as better collagen organization and fiber alignment whereas increased darkness was associated with different extinction angles and more disorganized collagen.21
RNA Extraction and Real-Time RT-PCR
RNA extraction and real-time RT-PCR were performed as previously described.3,6 FDL tendons were harvested and immediately frozen in liquid nitrogen. Five repaired tendons per time point were homogenized in Trizol reagent (Invitrogen Corporation, Carlsbad, CA) using the Ultra Turrax T8 homogenizer (IKA Works, Wilmington, NC) and RNA was extracted using the TRIzol protocol. RNA was reverse-transcribed into single-stranded cDNA using a reverse transcription kit (Invitrogen). cDNA was used as a template for real-time PCR with SYBR Green (Applied Biosystems, Foster City, CA) and gene specific primers in a Rotor-Gene 2000 thermocycler (Corbett Research, Sydney, Australia). Gene expression levels were standardized to the internal control β-actin and normalized to expression in day 3 control tendons.
Immunohistochemistry (IHC)
PTHR1 was localized immunohistochemically using 3-μm sections from the same blocks used for histologic analysis. Sections were deparaffinized and rehydrated. Epitope retrieval was performed in a 0.1% proteinase K solution (Invitrogen, Carlsbad, CA) for 10 min at room temperature. The following primary antibodies were used: mouse monoclonal anti-PTH/PTHrP receptor (Upstate Cell Signaling Solutions, Charlottesville, VA, 1:100 dilution). Slides were treated with biotinylated secondary antibodies and developed with the avidin-biotin peroxidase complex and 3,3′-diaminobenzidine (DAB) chromagen (Vector Laboratories, Burlingame, CA) and then counterstained with hematoxylin.
Adhesion and Biomechanical Testing
MTP joint flexion angle testing was performed as previously described.3,6 The lower hind limb was fixed in an apparatus while the FDL tendon was incrementally loaded along the axis of the flexor muscle line of force. The loading was accomplished using dead weights (0–19g) that were suspended for 30 s before the digital image was taken to avoid creep effects. For each increment of load, a digital picture was taken to quantify the MTP flexion angle relative to the neutral position. The MTP flexion angles were determined using ImageJ software (http://rsb.info.nih.gov/ij/) and plotted against their corresponding loads. The flexion data were fitted to a single-phase exponential equation where MTP flexion angle = β[1–exp(–m/α)]; where m is the applied load (Prism Graphpad 5.0a, GraphPad Software, San Diego, CA). The curve fit was constrained to the maximum flexion angle (β) for normal tendons that was previously determined to be 75° for the maximum applied load of 19g.3,6 Nonlinear regression was used to determine the gliding coefficient (α), a measure of the resistance to MTP joint flexion due to impaired gliding.3 Following adhesion testing, biomechanical testing was performed as previously described.3,6 The FDL tendon was mounted on the Instron 8841 DynaMight™ axial servohydraulic testing system (Instron, Norwood, MA) and loaded at a rate of 30 mm/min until failure. The maximum tensile force was determined from the force-displacement curves.
Statistical Analyses
Real-time RT-PCR (n=5 mice per treatment per time point) and biomechanical data (n=8 mice per treatment per time point) were analyzed using a two-way analysis of variance (ANOVA) followed by Bonferroni's multiple comparisons at the α = 0.05 significance level using Prism GraphPad 5.0a statistical software. Data are presented as mean ± SD.
Results
PTH 1-34 Increased Fibrous Tissue Formation and Improved Collagen Fiber Organization
On day 7, the tendon ends adjacent to the repair site underwent catabolism and invasion by the granulation tissue in the PTH 1-34 mice, while less granulation tissue filled in the repair site in the control mice (Fig. 1A). At 14 days, there was an increased granulation tissue response in the PTH 1-34 mice that bridged the tendons ends more rapidly relative to the control mice. At 21 and 28 days, there was less native tendon tissue in the PTH 1-34 mice, and more remodeled tendon tissue in the PTH 1-34 mice by 28 days.
Figure 1.
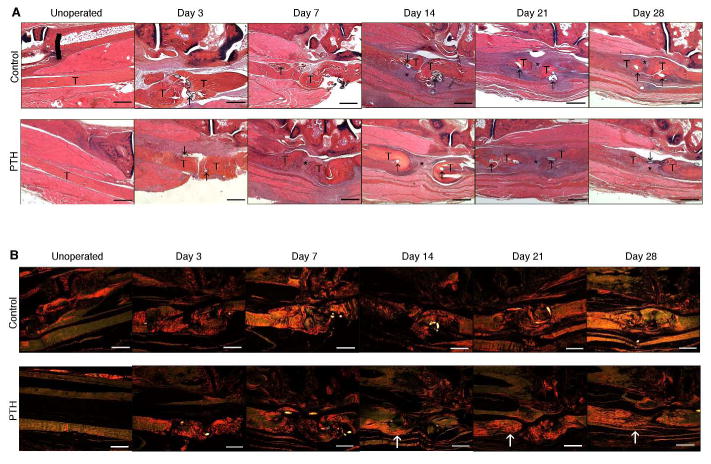
Representative histologic sections of control and PTH 1-34 un-operated flexor digitorum longus (FDL) tendons and FDL repair tendons at days 3, 7, 14, 21 and 28 post-repair. Tendon ends (T), sutures (↑), and fibroblastic granulation tissue (*) are marked. Sections were stained with (A) alcian blue/hematoxylin and orange G and (B) and picrosirius red illumination by monochromatic polarized light. White arrows indicate areas of increased brightness in PTH 1-34 repairs relative to time-matched controls. Scale bars represent 200 μm. Images are shown at 5X magnification.
Under polarized light, the control and PTH 1-34 specimens showed no observable differences in collagen fiber organization on days 3 and 7 (Fig. 1B). On days 14, 21 and 28, the PTH 1-34 specimens had increased collagen fiber organization compared to control specimens as evidenced by the increased brightness of their collagen fibers.
PTH 1-34 Increased Pthr1 Expression
Expression of the PTH receptor 1 gene, Pthr1, was observed in control tendons throughout healing and was increased in response to PTH 1-34 treatment as healing progressed. PTH 1-34 treatment resulted in a significant increase in Pthr1 expression at 14 (2-fold, p<0.05) and 21 (2.5-fold, p<0.001) days. Pthr1 expression was not significantly different during healing in control tendons (Fig. 2A). Likewise, immunostaining showed an increase in PTHR1 expressing cells in the granulation tissue of PTH 1-34 treated mice (Fig. 2B).
Figure 2.
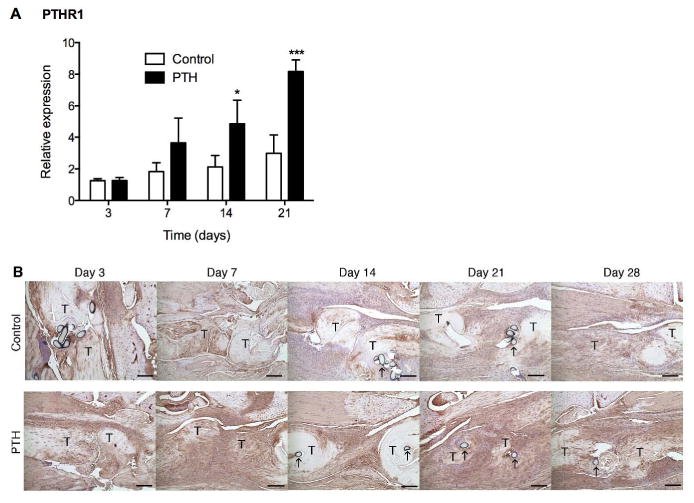
(A) Gene expression of Pthr1 in control and PTH 1-34 FDL repair tendons on post-repair days 3, 7, 14 and 21. Gene expression was standardized with the internal β-actin control. Data are presented as the mean fold induction (over control day 3 repairs) ± SD. * and ** indicate significant differences of p<0.05 and p<0.01, respectively, between control and PTH 1-34. (B) Immunohistochemistry of PTHR1 receptor in control and PTH 1-34 FDL repair tendons on post-repair days 3, 7, 14, 21 and 28. Tendon ends (T) and sutures (↑) are marked. Scale bars represent 25 μm. Images are shown at 10X magnification.
PTH 1-34 Increased Fn1, Col1a1, and Col3a1 Expression
PTH 1-34 up-regulated Fn1 gene expression 1.5-2.2-fold on days 7, 14 and 21, although the differences were only significant on day 21 (p<0.01, Fig. 3A). Col1a1 expression exhibited a similar trend, with 2-2.5-fold increases at days 7, 14, and 21, with significance at days 14 and 21 (p<0.05, Fig. 3B). PTH 1-34 treatment also up-regulated Col3a1 gene expression by approximately 50% at days 7, 14 and 21, but significance was only achieved at day 21 (p<0.05, Fig. 3C).
Figure 3.
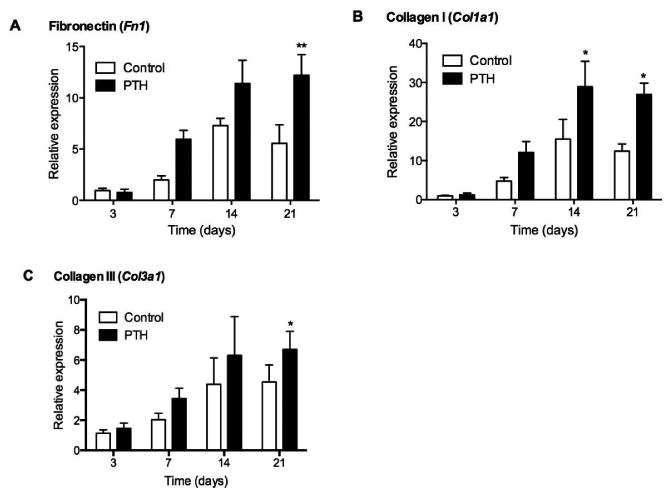
Gene expression of (A) Fn1, (B) Col1a1 and (C) Col3a1 in control and PTH 1-34 FDL repair tendons on post-repair days 3, 7, 14 and 21. Gene expression was standardized with the internal β-actin control. Data are presented as the mean fold induction (over control day 3 repairs) ± SD. * and ** indicate significant differences of p<0.05 and p<0.01, respectively, between control and PTH 1-34.
PTH 1-34 Increased Mmp2 and Mmp14 Expression but Decreased Mmp9 Expression
Expression of Mmp2 and Mmp14, which are associated with remodeling of granulation tissue,4 was significantly elevated in PTH 1-34 treated mice compared to control mice at all time points (Fig. 4A and B). Interestingly, the expression of Mmp9 was greater in control tendons (Fig. 4C). On day 7, Mmp9 expression was 1.9-fold greater in control than in PTH 1-34 repair tendons (p<0.05). While Mmp9 expression declined at 14 days for both control and PTH 1-34 specimens, it was still 4 times greater in control than in PTH 1-34 mice (p<0.01). At 21 days post-repair, there were no significant differences in Mmp9 expression between control and PTH 1-34 samples.
Figure 4.
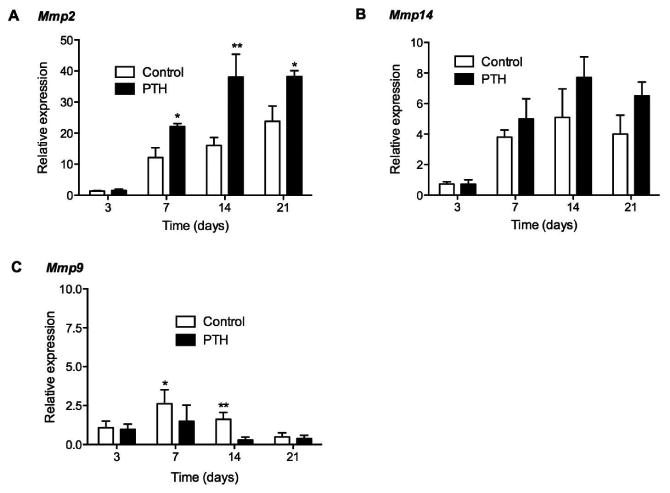
Gene expression of (A) Mmp2, (B) Mmp14 and (C) Mmp9 in control and PTH 1-34 FDL repair tendons on post-repair days 3, 7, 14 and 21. Gene expression was standardized with the internal β-actin control. Data are presented as the mean fold induction (over control day 3 repairs) ± SD. * and ** indicate significant differences of p<0.05 and p<0.01, respectively, between control and PTH 1-34.
PTH 1-34 Reduced MTP Joint Range of Motion
The ex-vivo measurement of MTP joint range of motion (ROM) with a defined force pulling on the flexor tendon proximal to the repair site is a sensitive way to biomechanically measure tendon gliding.3,6 The MTP joint ROM was defined as the flexion angle upon the application of the maximum excursion load of 19 g. On day 14, the MTP joint ROM was significantly reduced in mice treated with PTH 1-34 (27.2° ± 3.9) compared to control mice (43.5° ± 8.0, p<0.001, Fig. 5A). The calculated gliding coefficient for PTH 1-34 treated mice (42.4 ± 12.0) was significantly higher than that of control mice (21.0 ± 7.7) on day 14 (p<0.05, Fig. 5B). PTH 1-34 mice continued to have significantly lower MTP joint ROM and higher gliding coefficients compared to control mice for the duration of the experiment.
Figure 5.
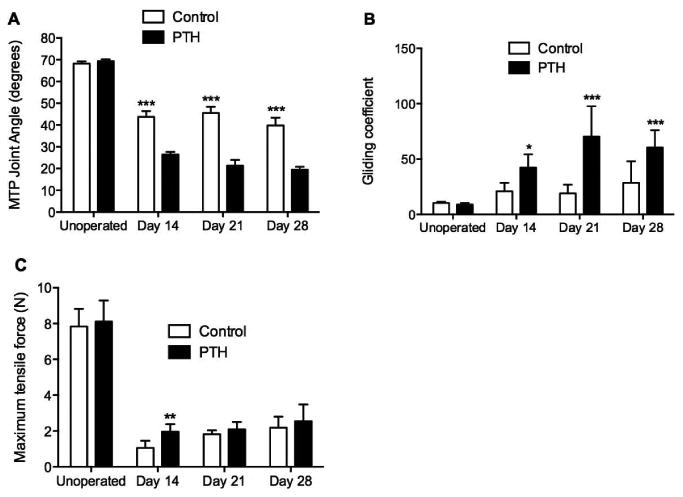
Un-operated tendons and 14, 21 and 28 day post-repair tendons from control and PTH 1-34 were analyzed for (A) metatarsophalangeal (MTP) joint flexion range of motion (ROM) at the maximum applied load of 19 g, (B) gliding coefficient, and (C) maximum tensile force. Data are presented as mean ± SD. *, **, and *** indicate significant differences of p<0.05, p<0.01, and p<0.001 respectively, between control and PTH 1-34.
PTH 1-34 Transiently Increased the Tensile Strength of Tendon Repairs
The tensile strength of un-operated tendons from control and PTH 1-34 treated mice was not significantly different (Fig. 5C). The tensile strength for PTH 1-34 tendon repairs was significantly greater than that of control tendon repairs at 14 days (1.96 ± 0.4 N and 1.0 ± 0.4 N, respectively; p<0.01). Maximum tensile force increased over time for both control and PTH 1-34 mice, and reached 27.8% and 31.4% of the strength of un-operated tendons by 28 days after repair, respectively. There were no significant differences in maximum tensile force at 21 and 28 days post-repair.
Discussion
The present study indicates that PTH 1-34 exerts an anabolic effect on murine flexor tendon healing. Histology of the repair site demonstrated earlier and more robust accumulation of reparative tissue, and this was associated with increased expression of genes involved in tissue repair, including Col1a1, Col3a1, and Fn1. Interestingly, treatment with PTH 1-34 increased gene and protein expression of PTHR1, the cell surface receptor for PTH. This suggests that activation of PTH signaling in soft tissue repair could trigger a feedback-loop, which further increases receptor-mediated cell and tissue responses to PTH.
PTH 1-34 modulated the expression of genes involved in matrix deposition and remodeling. The key matrix components involved in tissue repair are fibrillar collagens, type I and type III, and fibronectin.22 Evidence from this and other studies3,6 indicates that maximal adhesion occurs early in the healing response during granulation tissue formation and matrix deposition. Later, adhesions decrease with matrix remodeling and formation of a more organized tissue that resembles normal tendon morphology. PTH 1-34 led to modest increases in the expression and deposition of all matrix components that were analyzed, suggesting that PTH 1-34 augments the normal granulation tissue response and remodeling phase of tendon healing.
As both tensile strength and gliding ability are essential for normal tendon function, we used biomechanical tests that have been designed to address both aspects of tendon function. We performed a biomechanical test of digit motion in situ to measure digit ROM and computed the gliding coefficient, which is a measure of the resistance to tendon gliding due to the presence of fibrotic adhesions.3 Although there were no significant differences in tensile strength four weeks post-repair, PTH 1-34 treatment resulted in a 2-fold decrease in ROM compared to control repairs. Similarly, PTH 1-34 specimens displayed a 2-4-fold increase in the gliding coefficient compared to their control counterparts. Taken together, these data suggest that PTH 1-34 maintains the biomechanics of the healing tendon while simultaneously increasing deposition of reparative tissue resulting in increased adhesion formation. This finding agrees with recent work where recombinant human parathyroid hormone (rhPTH) resulted in increased fibrocartilage formation in a rat model of rotator cuff tendon repair.17
Our findings showed that PTH 1-34 differentially regulated the expressions of Mmp9, Mmp2, and Mmp14. PTH 1-34 reduced expression of Mmp9 and increased expressions of Mmp2 and Mmp14. Previously, we examined the differential expression of MMPs in tendon healing and observed that in contrast to Mmp2 and Mmp14, which are most highly expressed during the period of tissue remodeling and reduction of adhesions, Mmp9 was expressed early in healing when adhesions are developing.3 Using a model that involved lethal irradiation following bone marrow transplantation, it was established that Mmp9 expression is related to the recruitment of circulating cells from the bone marrow in early tendon repair.6 In contrast to Mmp2 and Mmp14, Mmp9 may be involved in the catabolism of the damaged tendon tissue at the repair site resulting in extension of the zone of injury. Extension of the zone of injury resulted in increased removal of native tendon and replacement with a larger area of granulation tissue. This was supported by the finding that Mmp9-/- mice had reduced adhesions and that transplantation of bone marrow from Mmp9-/- mice into wild type mice resulted in reduced adhesions similar to that observed in the Mmp9-/- mice.6 In the present study, while the reduction in Mmp9 and stimulation of Mmp2 and Mmp14 by PTH 1-34 would tend to lead to reduced adhesions, this is likely counterbalanced by the marked anabolic response with robust increases in type I and III collagens and fibronectin.
Prior studies show that PTH 1-34 stimulates stem cell populations. Di Bernardo et al.7 showed that PTH treatment increased proliferation of mesenchymal stem cells (MSCs) in an in vitro model. It is known that PTH 1-34 induces a phenotypic shift of MSCs towards the osteogenic lineage8,9, and prior in vivo have shown that PTH 1-34 increases fracture callus bone mineral density and strength.10,11 Recent work suggests that PTH 1-34 also stimulates chondrogenesis.12,13 PTH 1-34 enhances chondrogenesis during the early stages of femoral fracture repair14,15 and promotes chondrogenic repair in a full-thickness articular cartilage defect model.16 In a mouse model of injury-induced knee osteoarthritis, systemic PTH 1-34 treatment demonstrated significant chondroregenerative effects in degenerating cartilage.19 These observations suggest that PTH 1-34 stimulates the proliferation and differentiation of MSCs, raising the possibility that it could have similar positive effects on tenogenesis.
Although these findings support the hypothesis that PTH 1-34 exerts an anabolic effect on tendon healing, these data do not address the molecular mechanism(s) through which PTH 1-34 modulates this response. It has been shown that PTH signaling is mediated through PTHR1, a G-protein coupled receptor that regulates multiple pathways, including cAMP/protein kinase A (PKA).23,24 PKA signaling is a key mediator of regenerative responses in a number of tissues and organs.25 Notably, it is the dominant mechanism for the PTH-induced anabolic effect in bone.23,24 Wang et al.13 showed that PTH 1-34-induced chondrogenesis was mediated by Runx1 through activation of PKA signaling. Taken together, these data suggest that PTH 1-34 may stimulate tenogenesis at least in part by mediating cAMP/PKA signaling. However, the mechanism by which PTH 1-34 exerts its effect in tendon is still unknown and warrants further investigation.
As with any animal study, the healing response in murine flexor tendon injury may not reflect all aspects of the repair process in humans. A limitation of our model is the release of the tendon at the myotendinous junction. Release of the myotendinous junction is necessary to protect the tendon from large forces that would be transmitted due to normal ambulation following surgery that could rupture the repair.3,6 Mice typically regain active flexion between 21 and 28 days in this model.
In conclusion, our findings demonstrate that PTH 1-34 exerts an anabolic response on tendon healing by increasing the deposition of repair tissue. However, this anabolic effect did not translate to greater overall mechanical strength of repairs, as tensile strength was superior at only 14 days post-repair in the PTH 1-34 mice and came at the price of increased adhesions. For this reason, PTH 1-34 may not be suitable for flexor tendon repair as it may compromise tendon gliding. At the same time, the robust anabolic effect of PTH 1-34 may positively influence tendon healing in certain contexts where the early post-surgical strength of repairs is a major concern. Given the frequency with which PTH 1-34 is administered for bone repair and the possibility of concurrent bone and tendon injuries, it is important to elucidate the effect of PTH 1-34 on flexor tendon healing and further studies are warranted to explore its potential clinical applications.
Acknowledgments
This work was supported by PHS award P50AR954041 and Grant R01AR056696 from the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Youssef Farhat is a trainee in the Medical Scientist Training Program funded by National Institutes of Health T32 GM07356.
Contributor Information
Daniel J. Lee, The Center for Musculoskeletal Research, University of Rochester Medical Center, 601 Elmwood Avenue, Box 665, Rochester, NY 14642.
Richard D. Southgate, Email: richard_southgate@urmc.rochester.edu, Department of Orthopaedic Surgery, University of Rochester Medical Center, 601 Elmwood Avenue, Box 665, Rochester, NY 14642.
Youssef M. Farhat, The Center for Musculoskeletal Research, University of Rochester Medical Center, 601 Elmwood Avenue, Box 665, Rochester, NY 14642.
Alayna E. Loiselle, The Center for Musculoskeletal Research, University of Rochester Medical Center, 601 Elmwood Avenue, Box 665, Rochester, NY 14642.
Warren C. Hammert, Email: warren_hammert@urmc.rochester.edu, Department of Orthopaedic Surgery, University of Rochester Medical Center, 601 Elmwood Avenue, Box 665, Rochester, NY 14642.
Hani A. Awad, The Center for Musculoskeletal Research, University of Rochester Medical Center, 601 Elmwood Avenue, Box 665, Rochester, NY 14642.
Regis J. O'Keefe, Email: regis_okeefe@urmc.rochester.edu, The Center for Musculoskeletal Research, University of Rochester Medical Center, 601 Elmwood Avenue, Box 665, Rochester, NY 14642.
References
- 1.Bi Y, Ehirchiou D, Kilts TM, et al. Identification of tendon stem/progenitor cells and the role of the extracellular matrix in their niche. Nat Med. 2007;13:1219–1227. doi: 10.1038/nm1630. [DOI] [PubMed] [Google Scholar]
- 2.Zhang J, Wang JH. Mechanobiological response of tendon stem cells: implications of tendon homeostasis and pathogenesis of tendinopathy. J Orthop Res. 2010;28:639–643. doi: 10.1002/jor.21046. [DOI] [PubMed] [Google Scholar]
- 3.Loiselle AE, Bragdon GA, Jacobson JA, et al. Remodeling of murine intrasynovial tendon adhesions following injury: MMP and neotendon gene expression. J Orthop Res. 2009;27:833–840. doi: 10.1002/jor.20769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amadio PC. Gliding resistance and modifications of gliding surface of tendon: clinical perspectives. Hand Clin. 2013;29:159–166. doi: 10.1016/j.hcl.2013.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gelberman RH, Manske PR, Akeson WH, et al. Flexor tendon repair. J Orthop Res. 1986;4:119–128. doi: 10.1002/jor.1100040116. [DOI] [PubMed] [Google Scholar]
- 6.Loiselle AE, Frisch BJ, Wolenski M, et al. Bone marrow-derived matrix metalloproteinase-9 is associated with fibrous adhesion formation after murine flexor tendon injury. PLoS One. 2012;7:e40602. doi: 10.1371/journal.pone.0040602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Di Bernardo G, Galderisi U, Fiorito C, et al. Dual role of parathyroid hormone in endothelial progenitor cells and marrow stromal mesenchymal stem cells. J Cell Physiol. 2010;222:474–480. doi: 10.1002/jcp.21976. [DOI] [PubMed] [Google Scholar]
- 8.Yu B, Zhao X, Yang C, et al. Parathyroid hormone induces differentiation of mesenchymal stromal/stem cells by enhancing bone morphogenetic protein signaling. J Bone Miner Res. 2012;27:2001–2014. doi: 10.1002/jbmr.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hollnagel A, Ahrens M, Gross G. Parathyroid hormone enhances early and suppresses late stages of osteogenic and chondrogenic development in a BMP-dependent mesenchymal differentiation system (C3H10T1/2) J Bone Miner Res. 1997;12:1993–2004. doi: 10.1359/jbmr.1997.12.12.1993. [DOI] [PubMed] [Google Scholar]
- 10.Alkhiary YM, Gerstenfeld LC, Krall E, et al. Enhancement of experimental fracture-healing by systemic administration of recombinant human parathyroid hormone (PTH 1-34) J Bone Joint Surg Am. 2005;87:731–741. doi: 10.2106/JBJS.D.02115. [DOI] [PubMed] [Google Scholar]
- 11.Andreassen TT, Ejersted C, Oxlund H. Intermittent parathyroid hormone (1-34) treatment increases callus formation and mechanical strength of healing rate fractures. J Bone Miner Res. 1999;14:960–968. doi: 10.1359/jbmr.1999.14.6.960. [DOI] [PubMed] [Google Scholar]
- 12.Crabb ID, O'Keefe RJ, Puzas JE, et al. Differential effects of parathyroid hormone on chick growth plate and articular chondrocytes. Calcif Tissue Int. 1992;50:61–66. doi: 10.1007/BF00297299. [DOI] [PubMed] [Google Scholar]
- 13.Wang J, Wang X, Holz JD, et al. Runx1 is critical for PTH-induced onset of mesenchymal progenitor cell chondrogenic differentiation. PLoS One. 2013;8:e74255. doi: 10.1371/journal.pone.0074255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kakar S, Einhorn TA, Vora S, et al. Enhanced chondrogenesis and Wnt signaling in PTH-treated fractures. J Bone Miner Res. 2007;22:1903–1912. doi: 10.1359/jbmr.070724. [DOI] [PubMed] [Google Scholar]
- 15.Nakazawa T, Nakajima A, Shiomi K, et al. Effects of low-dose, intermittent treatment with recombinant human parathyroid hormone (1-34) on chondrogenesis in a model of experimental fracture healing. Bone. 2005;37:711–719. doi: 10.1016/j.bone.2005.06.013. [DOI] [PubMed] [Google Scholar]
- 16.Kudo S, Mizuta H, Takagi K, et al. Cartilaginous repair of full-thickness articular cartilage defects is induced by the intermittent activation of PTH/PTHrP signaling. Osteoarthritis Cartilage. 2011;19:886–894. doi: 10.1016/j.joca.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 17.Hettrich CM, Beamer BS, Bedi A, et al. The effect of rhPTH on the healing of tendon to bone in a rat model. J Orthop Res. 2012;30:769–774. doi: 10.1002/jor.22006. [DOI] [PubMed] [Google Scholar]
- 18.Wong J, Bennett W, Ferguson MW, McGrouther DA. Microscopic and histological examination of the mouse hindpaw digit and flexor tendon arrangement with 3D reconstruction. J Anat. 209:533–545. doi: 10.1111/j.1469-7580.2006.00625.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sampson ER, Hilton MJ, Tian Y, et al. Teriparatide as a chondroregenerative therapy for injury-induced osteoarthritis. Sci Transl Med. 2011;3:101ra93. doi: 10.1126/scitranslmed.3002214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaback LA, Soung DY, Naik A, et al. Teriparatide (1-34 human PTH) regulation of osterix during fracture repair. J Cell Biochem. 2008;105:219–226. doi: 10.1002/jcb.21816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Whittaker P, Canham PB. Demonstration of quantitative fabric analysis of tendon collagen using two-dimensional polarized light microscopy. Matrix. 1991;11:56–62. doi: 10.1016/s0934-8832(11)80227-1. [DOI] [PubMed] [Google Scholar]
- 22.Williams IF, McCullagh KG, Silver IA. The distribution of types I and III collagen and fibronectin in the healing equine tendon. Connect Tissue Res. 1984;12:211–227. doi: 10.3109/03008208409013684. [DOI] [PubMed] [Google Scholar]
- 23.Yang D, Singh R, Diveti P, et al. Contributions of parathyroid hormone (PTH)/PTH-related peptide receptor signaling pathways to the anabolic effect of PTH on bone. Bone. 2007;40:1453–1461. doi: 10.1016/j.bone.2007.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rixon RH, Whitfeld JF, Gagnon L, et al. Parathyroid hormone fragments may stimulate bone growth in ovariectomized rats by activating adenylyl cyclase. J Bone Miner Res. 1994;9:1179–1189. doi: 10.1002/jbmr.5650090807. [DOI] [PubMed] [Google Scholar]
- 25.Goessling W, North TE, Loewer S, et al. Genetic interaction of PGE2 and Wnt signaling regulates developmental specification of stem cells and regeneration. Cell. 2009;136:1136–1147. doi: 10.1016/j.cell.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]


