Abstract
Dyslexia is a complex neurodevelopmental disorder characterized by impaired reading ability despite normal intellect, and is associated with specific difficulties in phonological and rapid auditory processing, visual attention, and working memory. Genetic variants in DCDC2 have been associated with dyslexia, impairments in phonological processing, and in short term/working memory. The purpose of this study was to determine whether sensory and behavioral impairments can result directly from mutation of the Dcdc2 gene in mice. Several behavioral tasks, including a modified pre-pulse inhibition paradigm (to examine auditory processing), a 4/8 radial arm maze (to assess/dissociate working versus reference memory), and rotarod (to examine sensorimotor ability and motor learning) were used to assess the effects of Dcdc2 mutation. Behavioral results revealed deficits in rapid auditory processing, working memory, and reference memory in Dcdc2del2/del2 mice as compared to matched wild types. Current findings parallel clinical research linking genetic variants of DCDC2 with specific impairments of phonological processing and memory ability.
Keywords: Dyslexia, Dcdc2, Rapid auditory processing, working memory, Language impairment
INTRODUCTION
DCDC2 (Doublecortin domain containing protein 2; chromosome 6p22) was first identified as a candidate gene for dyslexia, a multifactorial neurobehavioral disorder associated with multiple gene and environmental risk factors (Meng et al., 2005). Expressed in neural areas critical to reading (e.g., inferior and medial temporal cortex; Cope et al., 2012; Meng et al., 2011), DCDC2 variants have also specifically been linked to behavioral deficits related to reading disability, including phonological processes, as well as single letter forward and backward span tasks (short term and working memory, respectively; Marino et al., 2011; Berninger et al., 2008). Overall, several independent research groups have associated DCDC2 with impaired reading related measures in divergent populations (Meng et al., 2005; Marino et al., 2011; Schumacher et al., 2006; Scerri et al., 2011; Lind et al., 2010; Powers et al., 2013).
Although the molecular function of DCDC2 remains unclear, clues to its neurobiological function have been inferred through its relationship to the DCX superfamily of genes that contain doublecortin peptide domains, and are related to microtubule organization and assembly (Coquelle et al., 2006). In addition, DCX is a widely studied gene known for its function in neuronal migration and cortical development (Gleeson et al., 1999). To further examine the function of DCDC2 and its potential role in neuronal migration, studies using in utero electroporation of RNAi against Dcdc2 in rats revealed that neurons transfected with Dcdc2 RNAi migrated a shorter distance from the ventricular zone compared to neurons transfected with control plasmid (Meng et al., 2005; Burbridge et al., 2008). In addition, Dcdc2 protein is localized in primary cilia of rat primary hippocampal neurons with alterations in expression leading to changes in primary cilia length and cell signaling – processes critical to appropriate neuronal migration and cortical development (Massinen et al., 2011; Lee & Gleeson, 2010). These findings suggest that Dcdc2 play a key role in early brain development. Interestingly, studies of Dcdc2 knockout mice (Dcdc2del2/del2) reveal no gross neuromorphological anomalies or evidence of migrational disruption (Wang et al., 2011). Nonetheless, neurophysiological recordings of layer 2/3 and layer 4 pyramidal neurons in somatosensory cortex of mutant mice revealed that Dcdc2 is required to maintain normal neuronal excitability and temporally precise patterns of action potential firing rates (Che, Girgenti & LoTurco, 2013).
With regard to specific behavioral assessments of Dcdc2del2/del2 mice, one report using a modified Hebb-Williams maze and visual discrimination task reported that subjects were unimpaired when tested on “easier” cognitive tasks. However, a visuo-spatial learning/memory impairment emerged when Dcdc2del2/del2 mice were required to perform more cognitively demanding tasks (i.e., more complex Hebb Williams maze configurations and longer inter-trial intervals; Gabel et al., 2011).
The current series of experiments were designed to examine the role of Dcdc2 function using behavioral paradigms that have been adapted to model basic nonverbal behaviors that may be dysfunctional in language and reading impaired populations – specifically auditory processing, working and reference memory ability, and motor learning. Given recent evidence that Dcdc2del2/del2 in mice exhibit atypical neuronal spike timing (indicating imprecise temporal encoding of input), along with human findings implicating DCDC2 in memory ability and phonological processing, we predicted that behavioral assessment of Dcdc2del2/del2 mice would yield evidence of impairment on some/all tasks.
MATERIALS AND METHODS
Subjects
Dcdc2 knockout mice (Dcdc2del2/del2) carried a constitutive homozygous deletion of exon 2 (del2) within the Dcdc2 gene region of a 129SJ x C57BL/6J hybrid background backcrossed to C57BL/6J for 10 generations (Wang et al., 2011 for detailed Dcdc2 gene targeting and RT-PCR analysis). All subjects were generated from the Dcdc2 colony maintained by AC/JJL at the University of Connecticut using a heterozygous-heterozygous (Dcdc2wt/del2 × Dcdc2wt/del2) mating scheme with resultant genotypes recovered in the expected mendelian ratios (1:2:1). Two separate cohorts of Dcdc2del2/del2 and Dcdc2 wild type (WT) were generated from these breedings. Cohort 1 included 6 WT and 13 Dcdc2del2/del2 mice, with behavioral assessment starting at 20 weeks. Cohort 2 included 11 WT and 9 Dcdc2del2/del2 mice, with behavioral assessment starting at 11 weeks. Both cohorts (from a developmental stand point) were sexually mature young adults based on literature describing sexual maturity occurring around 4–5 weeks of age in male C57BL/6J mice (Qui et al., 2013). Only male subjects were assessed across behavioral measures. All subjects were single-housed in standard mouse tubs (12 h/12 h light/dark cycle), with food and water ad lib. In addition, all behavioral testing occurred during the light cycle. Procedures were performed blind to genotype and in compliance with the National Institutes of Health and University of Connecticut’s Institutional Animal Care and Use Committee (IACUC).
Auditory Processing - Silent Gap and Embedded Tone
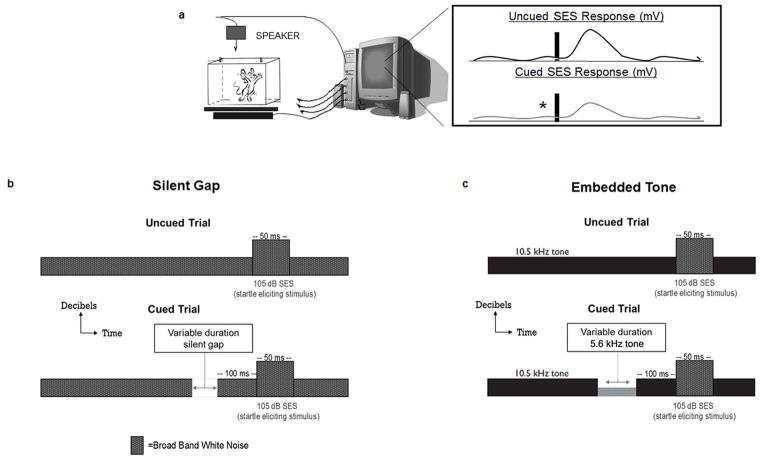
Subjects in cohorts 1 and 2 (17 WT and 22 Dcdc2del2/del2 mice) were examined for auditory processing ability using a modified pre-pulse inhibition paradigm (see Fitch et al., 2008 for review; Fig. 1a). Subjects were placed on individual load-cell platforms (MED Associates, St. Albans, VT) and presented with auditory stimuli generated using RPvdsEx on a Dell Pentium D PC and RX6 multifunction processor (Tucker Davis Technologies, Alachua, FL). Sounds were amplified using a Niles SI-1260 Integration Amplifier (Niles Audio Corp., Carlsbad, CA) and delivered through powered Yamaha YH-M100 speakers (Buena Park, CA). The acoustic startle reflex (ASR; a reflexive response elicited by an unexpected, intense stimulus) was recorded on an iMac 7.1 running Acknowledge 4.1 and obtained via the voltage output from each load cell platform through a linear amplifier (PHM-250U; Med Associates, St. Albans, VT) connected to a Biopac MP150 acquisition system (Biopac Systems, Goleta, CA). The modified pre-pulse inhibition paradigm measured differences in ASR to a loud startle eliciting stimulus (SES) when presented with/without a preceding acoustic cue. The ASR difference on cued versus uncued trials provided a measure of cue detection and/or discrimination. If the auditory cue was detected, a reduction (attenuation) in the ASR was expected relative to the ASR elicited when the auditory cue was not present (or not detected). This phenomenon was quantified using an “attenuation score” (ATT) that compared the average amplitude of the ASR from the cued trial to the average ASR of the uncued trial ([average cued ASR/average uncued ASR]*100). First, a Silent Gap (SG) task was used to assess the ability to detect breaks in continuous white noise (Fig. 1b). A session included 300 trials with a continuous 75 dB broadband white noise background. Cued and uncued trials occurred pseudorandomly. On cued trials, a silent gap of variable duration (2–100 ms) was presented 100 ms before the SES (50 ms, 105 dB, white noise burst). Uncued trials lacked a silent gap cue (0 ms). Next, the variable duration Embedded Tone (EBT) task was administered (with 300 sequential pseudorandom trials), but this task assessed ability to detect a change in tone frequency relative to a standard background tone (Fig. 1c; cue was a variable duration 5.6 kHz pure tone embedded in a 10.5 kHz background pure tone). On cued trials, the cue was presented 100 ms before the SES (50 ms, 105 dB), while uncued trials used a tone “cue” of 0 ms. Two EBT tasks were used in this study – a long-duration EBT (0 ms to 100 ms), and a short-duration EBT (0 ms to 10 ms).
Figure 1.
Auditory processing assessments. (a) Auditory processing in mice was examined using a modified pre-pulse inhibition (PPI) paradigm. A reduction in the acoustic startle reflex (ASR) to a startle eliciting stimulus (SES) is expected if a cue is presented prior to the SES. A reduction in the ASR during cued trials suggests auditory discrimination. Silent gap (b) examines the subjects’ ability to detect discontinuity (varying in duration from 2–100 ms) in a continuous broadband white noise background. The embedded tone (c) task assesses the subjects’ ability to detect a variable duration (2–100 ms for long, 2–10 ms for short) 5.6 kHz tone embedded within a constant 10.5 kHz pure tone.
Water maze assessment – Visible platform and 4/8 radial water maze
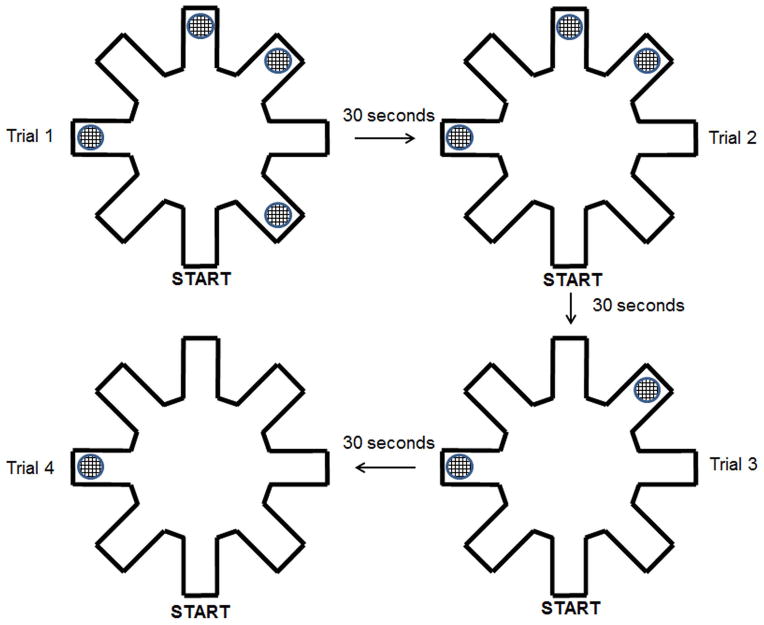
Subjects in Cohort 21 (11 WT and 9 Dcdc2del2/del2 mice) were tested on a visible platform control task (also called “water escape”) prior to radial water maze assessment, to ascertain any underlying impairments that might confound further maze testing (i.e., deficits in motivation, swimming, or visual acuity). Subjects were placed in the far end of an oval tub (103 cm × 55.5 cm) filled with room temperature water, and were given 45 seconds to swim to a visible escape platform (8.5 cm in diameter; 1 cm above water surface) located at the opposite end of the tub. Swim latencies were recorded for assessment. One Dcdc2del2/del2 mouse was dropped due to impaired swimming ability. Remaining subjects (11 WT and 8 Dcdc2del2/del2 mice) were then tested on a water version of the 4/8 radial arm maze (adapted from Hyde, Hoplight & Denenberg, 1998). This task measured spatial reference and working memory ability simultaneously, using a standard 8 arm radial maze with 4 arms baited (i.e., containing submerged goal platform), and 4 arms open but never baited (Fig. 2). Configurations of goal and start arm locations remained fixed across all test sessions. In addition, high contrast extra maze cues were present around the maze. Locations for these cues remained static for the entire experiment.
Figure 2.
Schematic of the 4/8 radial arm water maze and general testing protocol utilized in the experiment to assess both working and reference memory performance. As hidden goal platforms were found by the subject, the recently located goal was removed from the maze prior to the subsequent trial until all 4 platforms were found.
The day prior to testing (Day 1), subjects were given a training session where they were released from the start arm and allowed 120 seconds or 10 incorrect arm entries (errors) to locate one of four hidden goal platforms. If the subject failed to find a platform in this window, they were guided to the nearest available goal. Once on the platform, subjects remained on the platform for 20 seconds and then removed from the maze to a heated home cage (30 second inter-trial interval; ITI). During the ITI, the recently located platform was removed and the entrance to that arm was blocked so that the subject could no longer enter for the remainder of the training session. On trial 2, the subject was then placed back in the start arm, and this procedure was repeated until all four platforms were located (four trials total).
Testing began on Day 2, and continued for an additional 14 consecutive days (15 total days including training). The test session followed training procedures, but instead of blocking the goal arm of the most recently located platform, the goal platform was removed during the 30 second ITI, with the arm remaining open and unbaited for the remainder of the test session. Test sessions were recorded using a Sony camera integrated with the SMART video tracking program (Panlab, Barcelona, Spain). An arm entry was counted for a subject when all four paws entered an arm. Three types of errors were quantified for analysis. 1) Working memory errors consisted of the number of initial and repeat entries into arms from which a platform had been removed during that test session. 2) Initial reference memory errors consisted of the total number of first entries into arms that never contained a goal platform. 3) Working memory incorrect errors were the total number of repeat entries (following the initial entry) into arms that never contained escape platforms. Total errors per test session in each category were tabulated, averaged within genotype, and used for analysis across days of testing.
Finally, in order to determine whether subjects utilized a spatial or chaining (swimming to successive adjacent arms) strategy to solve the water maze, angles of arm choices were analyzed. Video tracking data obtained from the SMART system was reviewed and turn angle entry was tabulated to determine 1) the percentage of each angle type per session and 2) the average turn angle utilized across sessions. A higher percentage of 45° turns suggest that subjects preferred adjacent arm choices to solve the maze. Alternatively, higher percentage of turns 90° and greater and/or an average turn angle around 90° would suggest a preference for more spatial strategies to solve the maze.
Sensorimotor/motor learning
Subjects from Cohort 2 (11 WT and 9 Dcdc2del2/del2 mice) were assessed for sensorimotor ability and motor learning using the rotarod task. Subjects were placed on a rotating cylindrical drum that gradually accelerated from 4 to 40 rotations per minute across a span of 2 minutes. Four trials were administered per test day across five consecutive days. For analysis, latency to fall from the rotating drum was measured and averaged across the four trials for each day.
Statistical Analysis
All behavioral data was analyzed using a mixed factorial design. A 2 × 9 repeated measures analysis of variance (ANOVA) with Genotype (2 levels: WT and Dcdc2del2/del2) as the between measure and Gap (9 levels) as the within measure was conducted to analyze SG auditory processing performance. A 2 × 9 × 2 and 2 × 9 × 4 repeated measures ANOVA was performed on EBT 0–100 ms and EBT 0–10 ms, respectively, with Genotype (2 levels: WT and Dcdc2del2/del2) as the between measure and Duration (9 levels) and Day (2 levels for EBT 0–100 ms; 4 levels for EBT 0–10 ms) as within measures. In addition, subjects were assessed for auditory cue discrimination using a paired samples t-test comparing mean cued and uncued ASR. Average total, working memory correct, reference memory, and working memory incorrect errors on the 4/8 radial arm maze were independently examined using a 2 × 14 repeated measures ANOVA, with Genotype (2 levels: WT and Dcdc2del2/del2) as the between measure and Test Session (14 levels) as the within measure. Within session performance across trials was conducted using a 2 × 4 × 14 repeated measures ANOVA with Genotype (2 levels: WT and Dcdc2del2/del2) as the between measure and Trial (4 levels) and Session (14 levels) as within measures. In addition, a univariate ANOVA and a paired samples t-test was used to assess between- and within-group differences, respectively, in turn angle preference and average turn angle on the 4/8 radial arm water maze. Finally, group differences in rotarod performance were analyzed using a repeated measures ANOVA with Genotype (2 levels: WT and Dcdc2del2/del2) as the between measure and Day (5 levels) as the within measure. All statistical analyses were conducted using SPSS 19 with an alpha criterion of 0.05.
RESULTS
Dcdc2 deletion does not affect silent gap (SG) detection, but impairs tone detection
WT performance across Cohorts 1 and 2 were compared to ensure comparable performance before pooling data. Within genotype analysis across cohorts demonstrated that WT controls performed comparably on the SG 0–100 ms [F(1,15)=0.27, N.S], EBT 0–100 ms [F(1,15)=0.02, N.S.], and EBT 0–10 ms [F(1,15)=3.56, N.S.] paradigms. Thus data from cohorts 1 and 2 were pooled, for a total n of WT n=17; Dcdc2del2/del2 n=22.
Subjects were initially administered a gap detection task using silent gaps between 0 and 100 ms. Performance on this task was poor (mean values for each group shown in Fig. 3a), and there was no overall Genotype effect [F(1,37)=3.14, N.S], nor Genotype × Duration interaction [F(8,296)=0.966, N.S.]. Next, we administered an embedded tone task, also at 0–100 ms durations. Overall performance on this task was better than SG (mean ATT 75 Dcdc2del2/del2, 69 WT; Fig. 3b), and a Genotype × Duration interaction was seen [F(8,296)=3.45, P<0.001] with significant differences at 20 ms (P<0.05) and marginally significant differences at 75 and 100 ms (Dcdc2del2/del2 worse than WT; P<0.1). However, no main effect of Genotype was observed on the EBT 0–100 ms task [F(1,37)=2.39, N.S.]. Finally, we performed an embedded tone task using 0–10 ms stimuli. Although this task was more difficult than 0–100 (as supported by a large psychophysical literature on gap detection in humans and rodents; see Fitch et al., 2008 for review), subjects overall performed roughly comparable to the 0–100 ms task, reflecting a counteracting contribution of ongoing experience (with experience enhancing gap acuity on this task; Fitch et al., 2008). Interestingly, on the 0–10 ms EBT task, a significant overall effect of Genotype was found, with Dcdc2del2/del2 performing worse overall as compared to WT [F(1,37)=5.86, P<0.05] (Fig. 3c).
Figure 3.
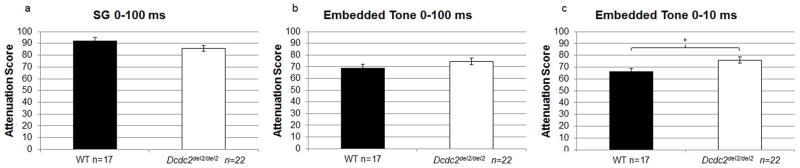
Dcdc2del2/del2 subjects show impaired embedded tone performance. (a) rapid auditory processing performance on the silent gap 0–100 ms paradigm were comparable between Dcdc2del2/del2 subjects and WT controls. (b) Analysis of embedded tone performance on the 0–100 ms paradigm also saw no significant difference in auditory processing ability, but (c) Dcdc2del2/del2 subjects did show impaired auditory processing performance on the embedded tone 0–10 ms task. Note that higher attenuation scores indicate poorer auditory discrimination of the cue. *P<0.05.
Dcdc2 mutant mice show impaired working and reference memory
Prior to spatial water maze testing, a visible platform control task was conducted to assess for underlying impairments that could confound water maze performance (e.g. swim, see, motivation). A univariate ANOVA on latencies found no main effect of Genotype [F(1,17)=0.01, N.S.] indicating that Dcdc2del2/del2 subjects showed no impairments on underlying aspects of the water task (Supporting Information 1; Fig. S1). One Dcdc2del2/del2 mouse was dropped due to impaired swimming ability, thus 11 WT and 8 Dcdc2del2/del2 mice were studied for analysis.
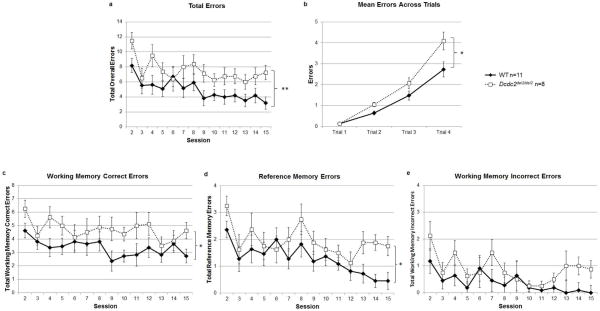
The 4/8 radial arm water maze was used to simultaneously measure spatial working and reference memory ability. Analysis of the average number of total errors (working memory correct, initial reference, and working memory incorrect) revealed a significant difference between WT and Dcdc2del2/del2 groups [F(1,17)=8.86, P<0.01] via repeated measures ANOVA, with Dcdc2del2/del2 making significantly more errors that WTs (Fig. 4a). A main effect of Session [F(12,221)=3.54, P<0.001] was also observed, confirming that both groups reduced errors across sessions (i.e., showed learning). Within test session analysis of total errors across trials revealed a main effect of Genotype [F(1,17)=7.42, P<0.05] and a Trial × Genotype interaction [F(3,51)=3.62, P<0.05] with Dcdc2del2/del2 subjects making significantly more errors on trials 2 and 4 (P<0.05) as more platforms were removed from the maze (Fig. 4b). Individual analysis of group differences for different error types was also performed, to determine whether impairments in specific memory domains (i.e., working memory correct, reference memory, and working memory incorrect) could be observed. A repeated measure ANOVA on total working memory correct errors (across Sessions) revealed that Dcdc2del2/del2 subjects made significantly more errors than WT subjects [F(1,17)=6.02, P<0.05]. No main effect of Session [F(13,221)=1.41, N.S.] nor Session × Genotype interaction [F(13,221)<1, N.S.] (Fig. 4c) were observed, indicating that working memory errors for both groups did not significantly change over days of testing. Analysis of reference memory errors (repeated measures ANOVA across Sessions) found that Dcdc2del2/del2 subjects made significantly more reference memory errors than WT controls [F(1,17)=4.76, P<0.05]. Within subject analysis of reference memory errors did show a significant main effect of Session [F(13,221)=3.54, P<0.001] (indicating learning), and no Session × Genotype interaction [F(13,221)<1, N.S.] (Fig. 4d), indicating that all subjects showed learning (as indicated by error reduction). However, the main effect of Genotype suggests that the Dcdc2del2/del2 group maintained a deficit (despite learning) relative to WTs. Next, examination of working memory incorrect errors (repeated measures ANOVA across sessions) indicated no differences in errors made between Dcdc2del2/del2 and WT subjects [F(1,17)=2.94, N.S.] (Fig. 4e). There was a significant main effect of Session [F(13,221)=2.66, P<0.01] indicating that all subjects significantly reduced the number of working memory incorrect errors over time.
Figure 4.
General memory impairment in mice with a mutation of Dcdc2. (a) Analysis of total errors (working correct, reference, and working incorrect) made across 14 test sessions indicate that Dcdc2del2/del2 mice made significantly more errors, overall, across all 14 test sessions. (b) Within test session analysis of total errors across trials reveal that Dcdc2del2/del2 subjects made significantly more errors in the maze across trials than wild type controls. Specifically, as the cognitive load of the task increased (number of remaining escape platforms decreases). (c) Further examination by error type found that Dcdc2del2/del2 mice made significantly more working memory correct (c) and reference memory (d) errors in comparison to WT mice, but were comparable in the amount of working memory incorrect errors performed (e). *P<0.05; **P<0.01
Finally, a univariate ANOVA examining the percent frequency of successive angle entries averaged across sessions revealed that Dcdc2del2/del2 had a greater preference for 45° (adjacent) arm entries in comparison to WT [F(1,17)=5, P<0.05] (Fig. 5a), and paired samples analysis within the Dcdc2del2/del2 group indicated that Dcdc2del2/del2 subjects made significantly more 45° arm entries than arm angles greater than 90° [t(7)=3.91, P<0.01]. However analysis of average turn angle for each session showed no main effect of Genotype [F(1,17)=0.55, N.S.] (Fig. 5b). Furthermore, there was no main effect of Test Session [F(13,221)=1.38, N.S] (Fig. 5b). This suggests that despite Dcdc2del2/del2 making more adjacent arm choices, they still utilized larger turn angles in their search strategy so that their average turn angle per test session was similar to WTs.
Figure 5.
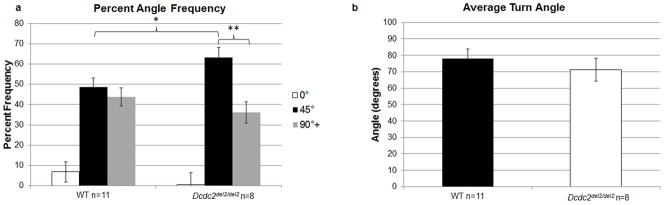
Strategy analysis on the 4/8 radial arm maze. (a) Dcdc2del2/del2 mice showed a greater preference for making 45° (adjacent) arm entries in comparison to WT. Further within subject analysis of Dcdc2del2/del2 turn angles also revealed that subjects made significantly more 45° turns, overall, than turns 90° or more. (b) However, examination of average turn angle indicate that both Dcdc2del2/del2 and WT mice had comparable mean turn angles, suggesting that Dcdc2del2/del2 also had to make a number of larger turn angles greater than 90° together with the increased incidence of 45° turn angles. *P<0.05; **P<0.01
Sensorimotor ability unaffected by Dcdc2 deletion
A repeated measures ANOVA examining average latency on the rotarod across 5 days of testing found no main effect of Genotype [F(1,18)=2.47, N.S.] (Fig. 6), nor a main effect of Session for either WT [F(4,40)=1.11, N.S.] and Dcdc2del2/del2 [F(4,32)=0.972, N.S.]. Furthermore, there was no significant Genotype × Session interaction between WT and Dcdc2del2/del2 subjects [F(4,72)=1.19, N.S.], therefore individual analysis at specific time points could not be conducted. These results indicate that WT and Dcdc2del2/del2 subjects had comparable sensorimotor ability and motor learning for this task.
Figure 6.

Sensorimotor ability in Dcdc2del2/del2 mice. No differences in sensorimotor performance between Dcdc2del2/del2 and WT subjects were observed on the rotarod task. Both groups were comparable in their latency to remain on the rotating cylinder across five days of testing.
DISCUSSION
Current results provide the first evidence (of which we are aware) for behavioral effects from manipulations of Dcdc2 in rodents, as evidenced across domains of auditory processing and working and reference memory ability. Specific auditory results showed poor overall performance on the initial Silent Gap task, with no significant Genotype difference. On the subsequent EBT 0–100 ms task, we observed better overall performance, with a Genotype × Duration interaction (Dcdc2del2/del2 worse than WT at 20ms, 75 ms and 100 ms). Finally, we administered a 0–10 ms EBT task, and here we found a main effect of Genotype (Dcdc2del2/del2 worse than WT). These combined findings likely reflect co-occurring influences of altered task difficulty together with ongoing experience. However, it is nonetheless interesting that strong Dcdc2del2/del2 deficits emerged primarily on the shortest-duration version of the task, and this finding appears consistent with literature showing acoustic processing deficits specific to short stimulus durations (i.e., rapid auditory processing) in language-impaired populations (Benasich & Tallal, 2002; Tallal, 1980; Choudhury et al., 2007). Alternately, results may reflect enhanced effects of prior experience in WT mice, which would suggest a failure of beneficial experience to improve acoustic acuity in Dcdc2del2/del2 mice. Future studies could parse these different interpretations by administering counter-balanced task batteries in subsets of both Dcdc2del2/del2 and WT mice.
Persistent memory impairments were also observed in mice with a mutation of Dcdc2. Across 14 consecutive days of testing on the 4/8 radial arm water maze, Dcdc2del2/del2 subjects consistently made more working and reference memory errors. Lack of a motor learning impairment in Dcdc2del2/del2 subjects suggests that Dcdc2 function is not associated with gross sensorimotor learning (at least on the rotarod task), thus supporting the view that behavioral deficiencies observed on the 4/8 spatial water maze task were not confounded by underlying sensorimotor deficits. Analysis of turn angles to examine maze solving strategy indicated that Dcdc2del2/del2 subjects more frequently utilized 45° turns (adjacent arm entries) in their search strategy, however, overall average turn angle within and across test sessions were similar between Dcdc2del2/del2 and WT subjects. Although both Dcdc2del2/del2 and WT subjects were capable of learning the water maze task (as evidenced by the significant reduction in total errors), this dichotomy likely reflects the fact that Dcdc2del2/del2 were less able to recall and identify remaining baited goal arms, and therefore employed a next-arm search strategy, whereas WTs were more likely to utilize extra maze cues to more efficiently navigate the maze. In addition, as the cognitive load of the task increased within a test session (as number of remaining escape platforms decreased), Dcdc2del2/del2 subjects made considerably more errors suggesting that Dcdc2del2/del2 had greater difficulty recalling the locations of remaining goal locations.
Previous research has shown that the hippocampus is required to acquire both spatial working and reference memory on different maze tasks, including the 4/8 radial arm maze (Jarrard, 1978; Nadel & MacDonald, 1980; Olton & Papas, 1979). Therefore, it is interesting that impairments in water maze performance were observed despite absence of gross hippocampal anomalies in Dcdc2del2/del2 mice (Wang et al., 2011). However, it remains unknown whether Dcdc2 may directly mediate hippocampal function and circuitry, which may ultimately disrupt memory consolidation. It could also be that abnormalities in other areas relevant to working and reference memory ability (e.g. prefrontal cortex and parietal cortex, respectively) could also contribute to the poor performance observed in mice with a mutation of Dcdc2, since Dcdc2del2/del2 subjects were capable in reducing total errors made across test sessions, suggesting that at least some aspects of task acquisition were retained (Soblosky et al., 1996; Goldman-Rakic, 1995).
Also consistent with our findings is a recent report showing that deletion of the Dcdc2 gene in mice degrades neural spike timing (Che, Girgenti & LoTurco, 2013), a neuronal parameter likely critical to efficient population coding of rapidly occurring changes in sensory stimuli. Our concurrent results also indicate (at the behavioral level) that mice with Dcdc2 deletion have difficulty encoding rapid sequential sensory information within the auditory domain. In addition, memory disruptions observed could reflect direct alterations in the neural circuitry subserving memory, for example in the accurate encoding of initial sensory input critical to effective memory formation. In fact, GENSAT images indicate high Dcdc2 promoter activity in layer 4 neurons of sensory cortices (particularly somatosensory, visual, and auditory cortex), as well as moderate expression in ventral hippocampus (an area of the brain implicated in spatial working memory; Brady, Saul & Wiest, 2010; Tseng et al., 2008; Gong et al., 2003). Currently, the mechanistic relationship between Dcdc2 and the manifestation of the altered spike timing phenotype remains unclear, but evidence shows that changes in synaptic transmission observed in Dcdc2del2/del2 subjects are driven by elevated NMDA receptor activity (Che, Girgenti & LoTurco, 2013). Interestingly, aberrant NMDA receptor activity has widely been implicated in impaired memory performance, specifically in working memory ability (Wang et al., 2013; Korotkova et al., 2010).
The current findings must also be taken in the context of evidence using RNAi in rats (Burbridge et al., 2008). For example, other reports have documented RAP deficits associated with disruptions of both Kiaa0319 and Dyx1c1 (rodent homologs of candidate dyslexia susceptibility genes), as well as working memory impairments associated with disruption of Dyx1c1 (Szalkowski et al., 2013; Szalkowski et al., 2012; Szalkowski et al., 2011; Threlkeld et al., 2007). Rodent models have also found a correlation between cortical spike timing response patterns to human speech stimuli and behavioral discrimination on an operant conditioning style task (Centanni, Engineer & Kilgard, 2013). Interestingly, RNAi of Kiaa0319 in auditory cortex has been shown to cause unstable neural representation of human speech sounds in primary auditory cortex using this paradigm (Centanni et al., 2013), a finding consistent with impaired auditory gap detection in Kiaa0319 knockdown rats as noted above (Szalkowski et al., 2012).
Overall, our current findings correspond to clinical evidence from fMRI and ERP data that suggest impaired auditory temporal processing and/or memory encoding co-occurs with dysfunction in language related neural networks and associated microcircuitry – all of which may contribute to difficulties in speech perception, language learning, and reading ability (Lehongre et al., 2011; McAnally & Stein, 1996; Temple et al., 2000; Raschle et al., 2013; Beneventi et al., 2010a; Beneventi et al., 2010b; Schulte-Körne et al., 2001; Hornickel & Kraus, 2013; Gou, Choudhury & Benasich, 2011). Alterations in cortical morphology and neocortical activity associated with reading and language related areas are in fact seen in dyslexic individuals with genetic variants of dyslexia risk gene DCDC2 and/or KIAA0319 (Cope et al., 2012; Meda et al., 2008; Darki et al., 2012; Pinel et al., 2012).
In conclusion, the current findings taken together with previous clinical and rodent research suggest that Dcdc2 function may mediate various basic aspects of neurobiological function and behavior including synaptic firing, auditory processing, and memory ability. Although our understanding of the molecular function of Dcdc2 is still under investigation, concurrent electrophysiological and RNA expression data offer clues to the potential relationships between Dcdc2, altered neural activity, and behavioral outcomes. Overall, these findings offer potential targets for both future behavioral and biological research to further understand DCDC2 function, as well as providing an opportunity to study and better understand the genetic and biological substrates that contribute to disruptions of language and reading dysfunction in humans.
Supplementary Material
Acknowledgments
This research is supported by the National Institutes of Health grant P01HD057853.
Footnotes
Cohort 1 subjects were tested for 12 weeks on a delayed match to sample radial arm maze that proved too difficult and no subjects evidenced learning, so the data was not reported.
The authors report no biomedical financial interests or conflicts of interest.
References
- Benasich AA, Tallal P. Infant discrimination of rapid auditory cues predicts later language impairment. Behav Brain Res. 2002;136:31–49. doi: 10.1016/s0166-4328(02)00098-0. [DOI] [PubMed] [Google Scholar]
- Beneventi H, Tønnessen FE, Ersland L, Hugdahl K. Executive working memory processes in dyslexia: Behavioral and fMRI evidence. Scand J Psychol. 2010a;51:192–202. doi: 10.1111/j.1467-9450.2010.00808.x. [DOI] [PubMed] [Google Scholar]
- Beneventi H, Tønnessen FE, Ersland L, Hugdahl K. Working Memory Deficit in Dyslexia: Behavioral and fMRI Evidence. Int J Neurosci. 2010b;120:51–59. doi: 10.3109/00207450903275129. [DOI] [PubMed] [Google Scholar]
- Berninger VW, Raskind W, Richards T, Abbott R, Stock P. A Multidisciplinary Approach to Understanding Developmental Dyslexia Within Working-Memory Architecture: Genotypes, Phenotypes, Brain, and Instruction. Dev Neuropsychol. 2008;33:707–744. doi: 10.1080/87565640802418662. [DOI] [PubMed] [Google Scholar]
- Brady AM, Saul RD, Wiest MK. Selective deficits in spatial working memory in the neonatal ventral hippocampal lesion rat model of schizophrenia. Neuropharmacology. 2010;59:605–611. doi: 10.1016/j.neuropharm.2010.08.012. [DOI] [PubMed] [Google Scholar]
- Burbridge TJ, Wang Y, Volz AJ, Peschansky VJ, Lisann L, Galaburda AM, Lo Turco JJ, Rosen GD. Postnatal analysis of the effect of embryonic knockdown and overexpression of candidate dyslexia susceptibility gene homolog Dcdc2 in the rat. Neuroscience. 2008;152:723–733. doi: 10.1016/j.neuroscience.2008.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centanni TM, Booker AB, Sloan AM, Chen F, Maher BJ, Carraway RS, Khodaparast N, Rennaker R, LoTurco JJ, Kilgard MP. Knockdown of the Dyslexia-Associated Gene Kiaa0319 Impairs Temporal Responses to Speech Stimuli in Rat Primary Auditory Cortex. Cereb Cortex. 2013 doi: 10.1093/cercor/bht028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centanni TM, Engineer CT, Kilgard MP. Cortical speech-evoked response patterns in multiple auditory fields are correlated with behavioral discrimination ability. J Neurophysiol. 2013;110:177–189. doi: 10.1152/jn.00092.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Che A, Girgenti M, LoTurco JJ. The dyslexia-associated gene Dcdc2 is required for spike-timing precision in mouse neocortex. Biol Psychiatry. 2013 doi: 10.1016/j.biopsych.2013.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhury N, Leppanen PHT, Leevers HJ, Benasich AA. Infant information processing and family history of specific language impairment: converging evidence for RAP deficits from two paradigms. Dev Sci. 2007;10:213–236. doi: 10.1111/j.1467-7687.2007.00546.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cope N, Eicher JD, Meng H, Gibson CJ, Hager K, Lacadie C, Fulbright RK, Constable RT, Page GP, Gruen JR. Variants in the DYX2 locus are associated with altered brain activation in reading-related brain regions in subjects with reading disability. NeuroImage. 2012;63:148–156. doi: 10.1016/j.neuroimage.2012.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coquelle FM, Levy T, Bergmann S, Wolf SG, Bar-El D, Sapir T, Brody Y, Orr I, Barkai N, Eichele G, Reiner O. The DCX Superfamily 1: Common and Divergent Roles for Members of the Mouse DCX Superfamily. Cell Cycle. 2006;5:976–983. doi: 10.4161/cc.5.9.2715. [DOI] [PubMed] [Google Scholar]
- Darki F, Peyrard-Janvid M, Matsson H, Kere J, Klingberg T. Three Dyslexia Susceptibility Genes, DYX1C1, DCDC2, and KIAA0319, Affect Temporo-Parietal White Matter Structure. Biol Psychiatry. 2012;72:671–676. doi: 10.1016/j.biopsych.2012.05.008. [DOI] [PubMed] [Google Scholar]
- Fitch RH, Threlkeld SW, McClure MM, Peiffer AM. Use of a modified prepulse inhibition paradigm to assess complex auditory discrimination in rodents. Brain Res Bull. 2008;76:1–7. doi: 10.1016/j.brainresbull.2007.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabel LA, Marin I, LoTurco JJ, Che A, Murphy C, Manglani M, Kass S. Mutation of the dyslexia-associated gene Dcdc2 impairs LTM and visuo-spatial performance in mice. Gene Brain Behav. 2011;10:868–875. doi: 10.1111/j.1601-183X.2011.00727.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleeson JG, Lin PT, Flanagan LA, Walsh CA. Doublecortin Is a Microtubule-Associated Protein and Is Expressed Widely by Migrating Neurons. Neuron. 1999;23:257–271. doi: 10.1016/s0896-6273(00)80778-3. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Cellular basis of working memory. Neuron. 1995;14:477–485. doi: 10.1016/0896-6273(95)90304-6. [DOI] [PubMed] [Google Scholar]
- Gong S, Zheng C, Doughty ML, Losos K, Didkovsky N, Schambra UB, Nowak NJ, Joyner A, Leblanc G, Hatten ME, Heintz N. A gene expression atlas of the central nervous system based on bacterial artificial chromosomes. Nature. 2003;425:917–925. doi: 10.1038/nature02033. [DOI] [PubMed] [Google Scholar]
- Gou Z, Choudhury N, Benasich AA. Resting frontal gamma power at 16, 24 and 36 months predicts individual differences in language and cognition at 4 and 5 years. Behav Brain Res. 2011;220:263–270. doi: 10.1016/j.bbr.2011.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornickel J, Kraus N. Unstable representation of sound: a biological marker of dyslexia. J Neurosci. 2013;33:3500–3504. doi: 10.1523/JNEUROSCI.4205-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde LA, Hoplight BJ, Denenberg VH. Water version of the radial-arm maze: Learning in three inbred strains of mice. Brain Res. 1998;785:236–244. doi: 10.1016/s0006-8993(97)01417-0. [DOI] [PubMed] [Google Scholar]
- Jarrard LE. Selective hippocampal lesions: differential effects on performance by rats of a spatial task with preoperative versus postoperative training. J Comp Physiol Psychol. 1978;92:1119–1127. doi: 10.1037/h0077516. [DOI] [PubMed] [Google Scholar]
- Korotkova T, Fuchs EC, Ponomarenko A, von Engelhardt J, Monyer H. NMDA Receptor Ablation on Parvalbumin-Positive Interneurons Impairs Hippocampal Synchrony, Spatial Representations, and Working Memory. Neuron. 2010;68:557–569. doi: 10.1016/j.neuron.2010.09.017. [DOI] [PubMed] [Google Scholar]
- Lee JH, Gleeson JG. The role of primary cilia in neuronal function. Neurobiol Dis. 2010;38:167–172. doi: 10.1016/j.nbd.2009.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehongre K, Ramus F, Villiermet N, Schwartz D, Giraud A. Altered Low-Gamma Sampling in Auditory Cortex Accounts for the Three Main Facets of Dyslexia. Neuron. 2011;72:1080–1090. doi: 10.1016/j.neuron.2011.11.002. [DOI] [PubMed] [Google Scholar]
- Lind PA, Luciano M, Wright MJ, Montgomery GW, Martin NG, Bates TC. Dyslexia and DCDC2: normal variation in reading and spelling is associated with DCDC2 polymorphisms in an Australian population sample. Eur J Hum Genet. 2010;18:668–673. doi: 10.1038/ejhg.2009.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marino C, Meng H, Mascheretti S, Rusconi M, Cope N, Giorda R, Molteni M, Gruen JR. DCDC2 genetic variants and susceptibility to developmental dyslexia. Psychiatr Genet. 2011;22:25–30. doi: 10.1097/YPG.0b013e32834acdb2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massinen S, Hokkanen M, Matsson H, Tammimies K, Tapia-Paez I, Dahlstrom-Heuser V, Kuja-Panula J, Burghoorn J, Jeppsson KE, Swoboda P, Peyrard-Janvid M, Toftgard R, Castren E, Kere J. Increased Expression of the Dyslexia Candidate Gene DCDC2 Affects Length and Signaling of Primary Cilia in Neurons. PLoS ONE. 2011;6:e20580. doi: 10.1371/journal.pone.0020580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAnally KI, Stein JF. Auditory temporal coding in dyslexia. Proc Biol Sci. 1996;263:961–965. doi: 10.1098/rspb.1996.0142. [DOI] [PubMed] [Google Scholar]
- Meda SA, Gelernter J, Gruen JR, Calhoun VD, Meng H, Cope NA, Pearlson GD. Polymorphism of DCDC2 Reveals Differences in Cortical Morphology of Healthy Individuals-A Preliminary Voxel Based Morphometry Study. Brain Imaging Behav. 2008;2:21–26. doi: 10.1007/s11682-007-9012-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng H, Powers NR, Ling T, Cope NA, Ping-Xia Z, Fuleihan R, Gibson C, Page GP, Gruen JR. A Dyslexia-Associated Variant in DCDC2 Changes Gene Expression. Behav Genet. 2011;41:58–66. doi: 10.1007/s10519-010-9408-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng H, Smith SD, Hager K, Held M, Liu J, Olson RK, Pennington BF, DeFries JC, Gelernter J, O’Reilly-Pol T, Somlo S, Skudlarski P, Shaywitz SE, Shaywitz BA, Marchione K, Wang Y, Paramasivam M, LoTurco JJ, Page GP, Gruen JR. DCDC2 is associated with reading disability and modulates neuronal development in the brain. Proc Natl Acad Sci USA. 2005;102:17053–17058. doi: 10.1073/pnas.0508591102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadel L, MacDonald L. Hippocampus: cognitive map or working memory? Behav Neural Biol. 1980;29:405–409. doi: 10.1016/s0163-1047(80)90430-6. [DOI] [PubMed] [Google Scholar]
- Olton DS, Papas BC. Spatial memory and hippocampal function. Neuropsychologia. 1979;17:669–682. doi: 10.1016/0028-3932(79)90042-3. [DOI] [PubMed] [Google Scholar]
- Pinel P, Fauchereau F, Moreno A, Barbot A, Lathrop M, Zelenika D, Le Bihan D, Poline J, Bourgeron T, Dehaene S. Genetic Variants of FOXP2 and KIAA0319/TTRAP/THEM2 Locus Are Associated with Altered Brain Activation in Distinct Language-Related Regions. J Neurosci. 2012;32:817–825. doi: 10.1523/JNEUROSCI.5996-10.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers N, Eicher J, Butter F, Kong Y, Miller L, Ring S, Mann M, Gruen J. Alleles of a Polymorphic ETV6 Binding Site in DCDC2 Confer Risk of Reading and Language Impairment. Am J Hum Genet. 2013;93:19–28. doi: 10.1016/j.ajhg.2013.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qui X, Dowling AR, Marino JS, Faulkner LD, Bryant B, Brüning JC, Elias CF, Hill JW. Delayed puberty but normal fertility in mice with selective deletion of insulin receptors from Kiss1 cells. Endocrinology. 2013;154:1337–1348. doi: 10.1210/en.2012-2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raschle NM, Stering PL, Meissner SN, Gaab N. Altered Neuronal Response During Rapid Auditory Processing and Its Relation to Phonological Processing in Prereading Children at Familial Risk for Dyslexia. Cereb Cortex. 2013 doi: 10.1093/cercor/bht104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scerri TS, Morris AP, Buckingham L, Newbury DF, Miller LL, Monaco AP, Bishop DVM, Paracchini S. DCDC2, KIAA0319 and CMIP Are Associated with Reading-Related Traits. Biol Psychiatry. 2011;70:237–245. doi: 10.1016/j.biopsych.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte-Körne G, Deimel W, Bartling J, Remschmidt H. Speech perception deficit in dyslexic adults as measured by mismatch negativity (MMN) Int J Psychophysiol. 2001;40:77–87. doi: 10.1016/s0167-8760(00)00152-5. [DOI] [PubMed] [Google Scholar]
- Schumacher J, Anthoni H, Dahdouh F, König IR, Hillmer AM, Kluck N, Manthey M, Plume E, Warnke A, Remschmidt H, Hülsmann J, Cichon S, Lindgren CM, Propping P, Zucchelli M, Ziegler A, Peyrard-Janvid M, Schulte-Körne G, Nöthen MM, Kere J. Strong Genetic Evidence of DCDC2 as a Susceptibility Gene for Dyslexia. Am J Hum Genet. 2006;78:52–62. doi: 10.1086/498992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soblosky JS, Tabor SL, Matthews MA, Davidson JF, Chorney DA, Carey ME. Reference memory and allocentric spatial localization deficits after unilateral cortical brain injury in the rat. Behav Brain Res. 1996;80:185–194. doi: 10.1016/0166-4328(96)00034-4. [DOI] [PubMed] [Google Scholar]
- Szalkowski CE, Booker AB, Truong DT, Threlkeld SW, Rosen GD, Fitch RH. Knockdown of the candidate dyslexia susceptibility gene homolog Dyx1c1 inrodents: Effects on auditory processing, visual attention, and cortical and thalamic anatomy. Dev Neurosci. 2013;35:50–68. doi: 10.1159/000348431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szalkowski CE, Hinman JR, Threlkeld SW, Wang Y, LePack A, Rosen GD, Chrobak JJ, LoTurco JJ, Fitch RH. Persistent spatial working memory deficits in rats following in utero RNAi of Dyx1c1. Gene Brain Behav. 2011;10:244–252. doi: 10.1111/j.1601-183X.2010.00662.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szalkowski CE, Fiondella CG, Galaburda AM, Rosen GD, LoTurco JJ, Fitch RH. Neocortical disruption and behavioral impairments in rats following in utero RNAi of candidate dyslexia risk gene Kiaa0319. Int J Dev Neurosci. 2012;30:293–302. doi: 10.1016/j.ijdevneu.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallal P. Auditory temporal perception, phonics, and reading disabilities in children. Brain Lang. 1980;9:182–198. doi: 10.1016/0093-934x(80)90139-x. [DOI] [PubMed] [Google Scholar]
- Temple E, Poldrack RA, Protopapas A, Nagarajan S, Salz T, Tallal P, Merzenich MM, Gabrieli JD. Disruption of the neural response to rapid acoustic stimuli in dyslexia: evidence from functional MRI. Proc Natl Acad Sci USA. 2000;97:13907–13912. doi: 10.1073/pnas.240461697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Threlkeld SW, McClure MM, Bai J, Wang Y, LoTurco JJ, Rosen GD, Fitch RH. Developmental disruptions and behavioral impairments in rats following in utero RNAi of Dyx1c1. Brain Res Bull. 2007;75:508–514. doi: 10.1016/j.brainresbull.2006.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng KY, Lewis BL, Hashimoto T, Sesack SR, Kloc M, Lewis DA, O’Donnell P. A Neonatal Ventral Hippocampal Lesion Causes Functional Deficits in Adult Prefrontal Cortical Interneurons. J Neurosci. 2008;28:12691–12699. doi: 10.1523/JNEUROSCI.4166-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Yang Y, Wang C, Gamo N, Jin L, Mazer J, Morrison J, Wang X, Arnsten AT. NMDA Receptors Subserve Persistent Neuronal Firing during Working Memory in Dorsolateral Prefrontal Cortex. Neuron. 2013;77:736–749. doi: 10.1016/j.neuron.2012.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Yin X, Rosen G, Gabel L, Guadiana SM, Sarkisian MR, Galaburda AM, LoTurco JJ. Dcdc2 knockout mice display exacerbated developmental disruptions following knockdown of doublecortin. Neuroscience. 2011;190:398–408. doi: 10.1016/j.neuroscience.2011.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.