Abstract
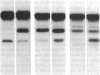
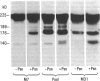
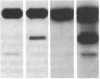
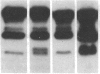
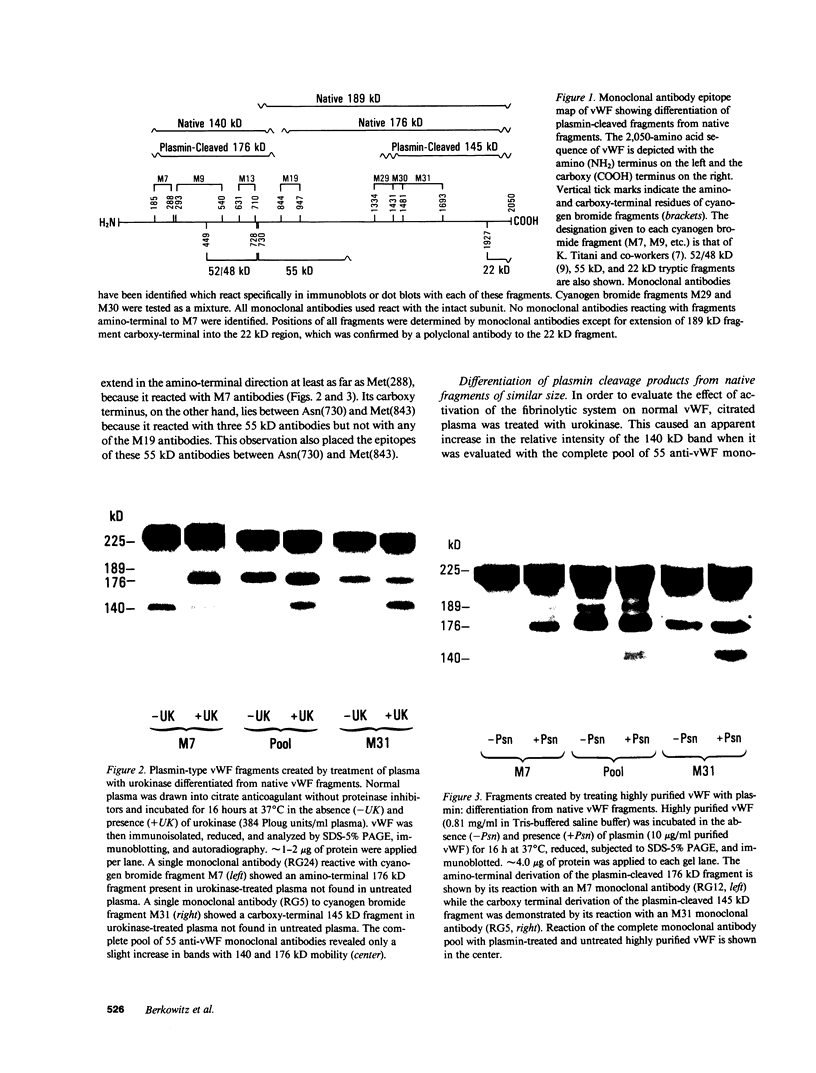
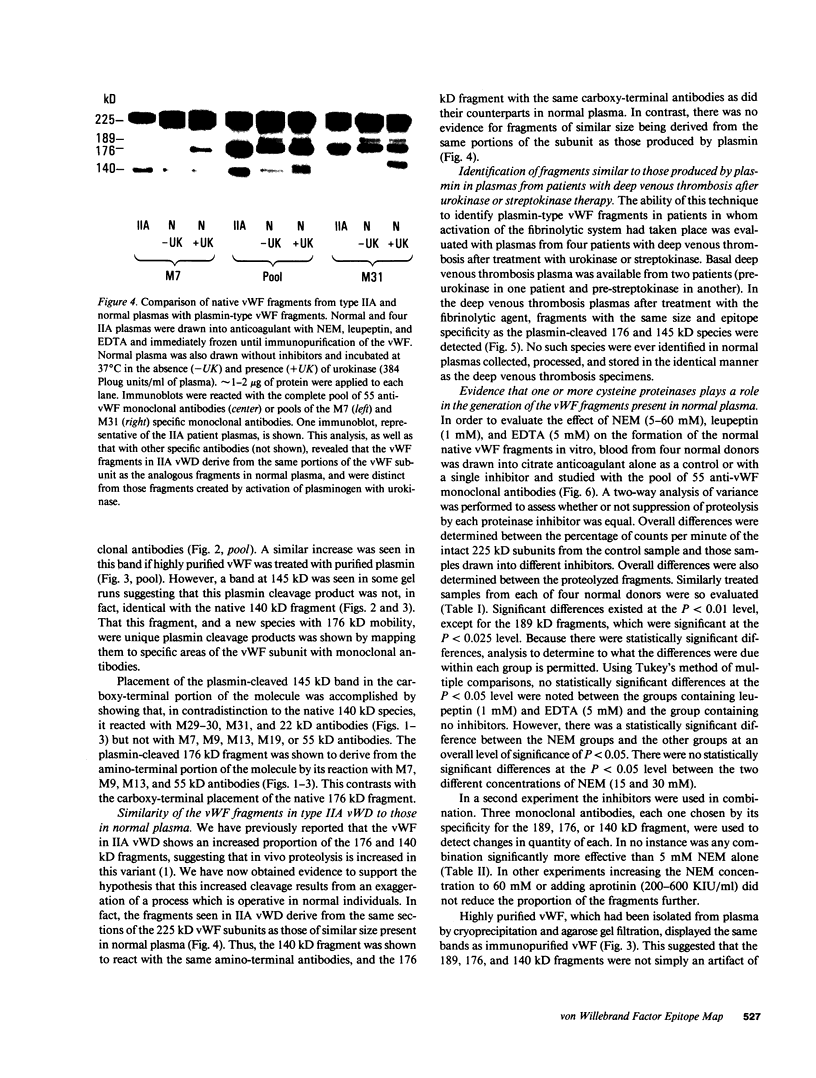
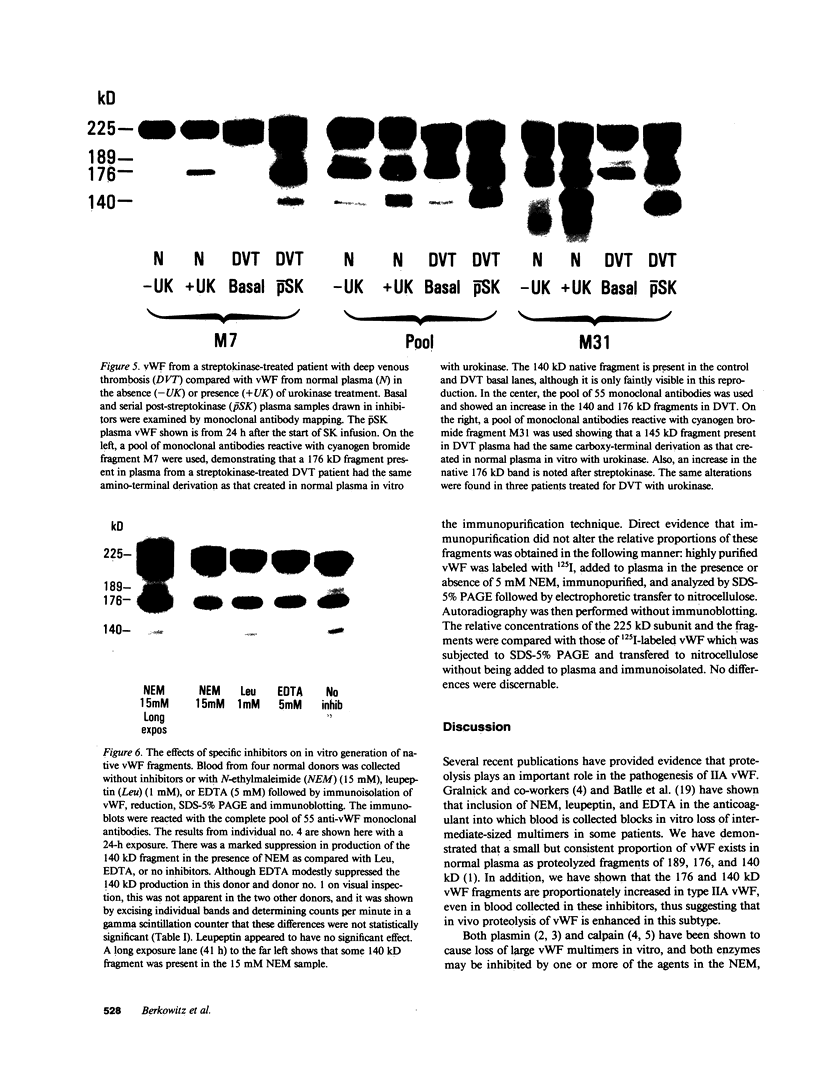
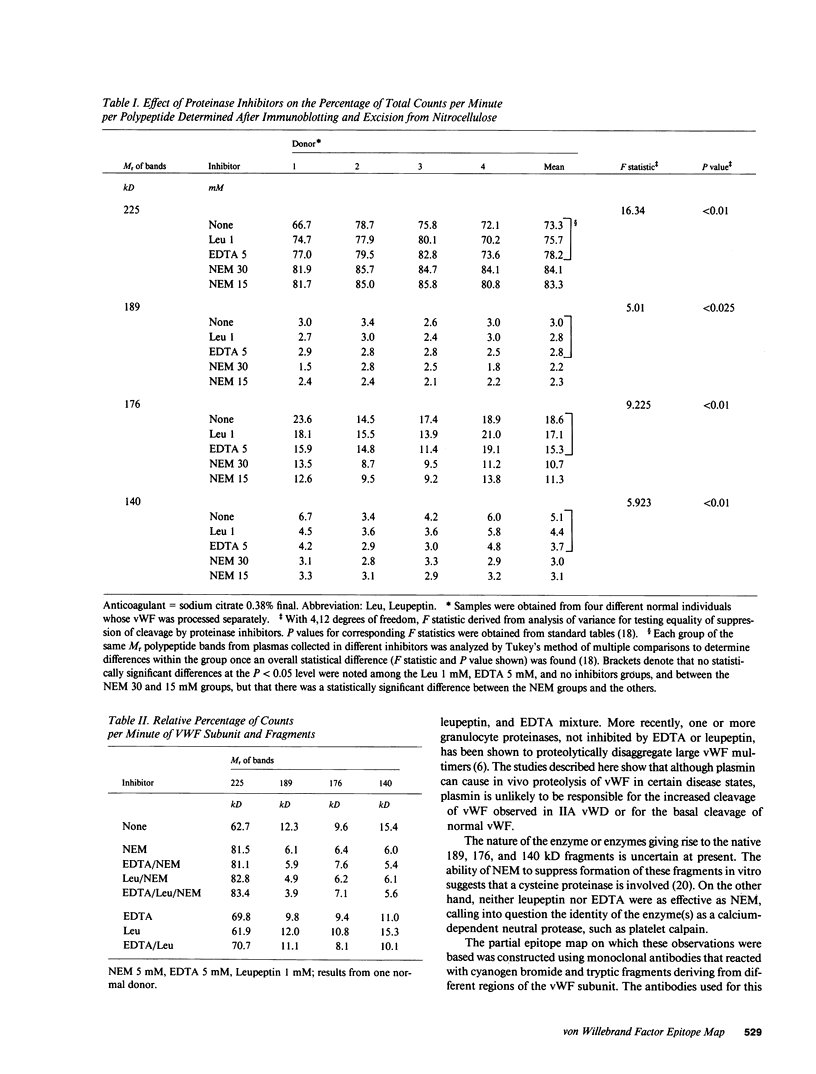
A small but consistent proportion of the von Willebrand factor (vWF) in normal plasma is composed of 189, 176, and 140 kD fragments cleaved from the 225 kD subunit. A monoclonal antibody map of vWF, based on the reactivity of individual antibodies with cyanogen bromide and tryptic fragments of known carboxy and/or amino termini, showed that in normal and IIA von Willebrand disease (vWD) plasmas the 140 kD fragment was derived from the amino-terminal region, whereas the 176 kD fragment was derived from the carboxy-terminal region of the subunit. In type IIA vWD, however, the fragments comprised a greater proportion of circulating vWF. In contrast, plasmin cleaved a 176 kD fragment from the amino terminus and a 145 kD fragment from the carboxy terminus of the subunit. Species similar to these plasmin-cleaved fragments were demonstrated in plasmas from four patients treated with fibrinolytic agents, but not in IIA vWD.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Deutsch D. G., Mertz E. T. Plasminogen: purification from human plasma by affinity chromatography. Science. 1970 Dec 4;170(3962):1095–1096. doi: 10.1126/science.170.3962.1095. [DOI] [PubMed] [Google Scholar]
- Federici A. B., Elder J. H., De Marco L., Ruggeri Z. M., Zimmerman T. S. Carbohydrate moiety of von Willebrand factor is not necessary for maintaining multimeric structure and ristocetin cofactor activity but protects from proteolytic degradation. J Clin Invest. 1984 Dec;74(6):2049–2055. doi: 10.1172/JCI111628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimura Y., Titani K., Holland L. Z., Russell S. R., Roberts J. R., Elder J. H., Ruggeri Z. M., Zimmerman T. S. von Willebrand factor. A reduced and alkylated 52/48-kDa fragment beginning at amino acid residue 449 contains the domain interacting with platelet glycoprotein Ib. J Biol Chem. 1986 Jan 5;261(1):381–385. [PubMed] [Google Scholar]
- Fulcher C. A., Zimmerman T. S. Characterization of the human factor VIII procoagulant protein with a heterologous precipitating antibody. Proc Natl Acad Sci U S A. 1982 Mar;79(5):1648–1652. doi: 10.1073/pnas.79.5.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gralnick H. R., Williams S. B., McKeown L. P., Maisonneuve P., Jenneau C., Sultan Y., Rick M. E. In vitro correction of the abnormal multimeric structure of von Willebrand factor in type IIa von Willebrand's disease. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5968–5972. doi: 10.1073/pnas.82.17.5968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton K. K., Fretto L. J., Grierson D. S., McKee P. A. Effects of plasmin on von Willebrand factor multimers. Degradation in vitro and stimulation of release in vivo. J Clin Invest. 1985 Jul;76(1):261–270. doi: 10.1172/JCI111956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkes R., Niday E., Gordon J. A dot-immunobinding assay for monoclonal and other antibodies. Anal Biochem. 1982 Jan 1;119(1):142–147. doi: 10.1016/0003-2697(82)90677-7. [DOI] [PubMed] [Google Scholar]
- Kunicki T. J., Montgomery R. R., Schullek J. Cleavage of human von Willebrand factor by platelet calcium-activated protease. Blood. 1985 Feb;65(2):352–356. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Newman J., Johnson A. J., Karpatkin M. H., Puszkin S. Methods for the production of clinically effective intermediate- and high-purity factor-VIII concentrates. Br J Haematol. 1971 Jul;21(1):1–20. doi: 10.1111/j.1365-2141.1971.tb03413.x. [DOI] [PubMed] [Google Scholar]
- Ruggeri Z. M., De Marco L., Gatti L., Bader R., Montgomery R. R. Platelets have more than one binding site for von Willebrand factor. J Clin Invest. 1983 Jul;72(1):1–12. doi: 10.1172/JCI110946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheele G. A. Two-dimensional gel analysis of soluble proteins. Charaterization of guinea pig exocrine pancreatic proteins. J Biol Chem. 1975 Jul 25;250(14):5375–5385. [PubMed] [Google Scholar]
- Switzer M. E., McKee P. A. Studies on human antihemophilic factor. Evidence for a covalently linked subunit structure. J Clin Invest. 1976 Apr;57(4):925–937. doi: 10.1172/JCI108369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson E. A., Howard M. A. Proteolytic cleavage of human von Willebrand factor induced by enzyme(s) released from polymorphonuclear cells. Blood. 1986 May;67(5):1281–1285. [PubMed] [Google Scholar]
- Titani K., Kumar S., Takio K., Ericsson L. H., Wade R. D., Ashida K., Walsh K. A., Chopek M. W., Sadler J. E., Fujikawa K. Amino acid sequence of human von Willebrand factor. Biochemistry. 1986 Jun 3;25(11):3171–3184. doi: 10.1021/bi00359a015. [DOI] [PubMed] [Google Scholar]
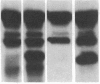
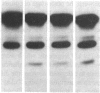
- Zimmerman T. S., Dent J. A., Ruggeri Z. M., Nannini L. H. Subunit composition of plasma von Willebrand factor. Cleavage is present in normal individuals, increased in IIA and IIB von Willebrand disease, but minimal in variants with aberrant structure of individual oligomers (types IIC, IID, and IIE). J Clin Invest. 1986 Mar;77(3):947–951. doi: 10.1172/JCI112394. [DOI] [PMC free article] [PubMed] [Google Scholar]