Abstract
Neurostimulation, e.g. transcranial direct current stimulation (tDCS), shows promise as an effective cognitive intervention. In spite of low spatial resolution, limited penetration, and temporary influence, evidence highlights tDCS-linked cognitive benefits in a range of cognitive domains. The left posterior parietal cortex (PPC) is an accessible node in frontoparietal networks engaged during long-term memory (LTM). Here, we tested the hypothesis that tDCS can facilitate LTM by pairing LTM encoding and retrieval with PPC stimulation. Healthy young adults performed a verbal LTM task (California Verbal Learning Task: CVLT) with four different stimulation parameters. In Experiment 1, we applied tDCS to left PPC during LTM encoding. In Experiment 2, we applied tDCS just prior to retrieval to test the temporal specificity of tDCS during a LTM task. In later Experiments, we tested hemispheric specificity by replicating Experiment 1 while stimulating the right PPC. Experiment 1 showed that tDCS applied during LTM encoding improved the pace of list learning and enhanced retrieval after a short delay. Experiment 2 indicated anodal left PPC tDCS only improved LTM when applied during encoding, and not during maintenance. Experiments 3 and 4 confirmed that tDCS effects were hemisphere specific and that no effects were found after right PPC stimulation during encoding. These findings indicate that anodal tDCS to the PPC helps verbal LTM in healthy young adults under certain conditions. First, when it is applied to the left, not the right, PPC and second, when it is applied during encoding.
Keywords: long-term memory, neurostimulation, transcranial direct current stimulation, posterior parietal lobe
Introduction
With aging concerns grow regarding cognitive performance, often with regard to declines in long-term memory (LTM). These concerns apply not only to healthy aging but also to those with brain injury or other neurodegenerative disorders as they often present with LTM difficulties (Charlton, Barrick, Markus, & Morris, 2010, 2013; Ishihara, Gondo, & Poon, 2002; Mitchell & Schmitt, 2006; Wais & Gazzaley, 2014). With the increasing aging baby boomer population, the rate of Alzheimer’s and other memory disorders is on the rise. Due to this increase, finding ways in which LTM deficits can be ameliorated is vital to this growing threat. One such technique is neurostimulation, such as transcranial direct current stimulation (tDCS).
TDCS has practical translational potential because it is safe (Nitsche, Liebetanz, et al., 2003), well-tolerated (Kessler, Turkeltaub, Benson, & Hamilton, 2012), and more affordable than other techniques, such as transcranial magnetic stimulation (TMS). TDCS involves applying small amounts (1–2 mA) of electric current to scalp electrodes to modulate the excitability of underlying neurons (Antal, Kincses, Nitsche, Bartfai, & Paulus, 2004; Nitsche & Paulus, 2000; Paulus, 2011; Rosenkranz, Nitsche, Tergau, & Paulus, 2000; Stagg & Nitsche, 2011). Anodal tDCS is associated with depolarizing the resting state of neuron membrane potentials directly under the electrode and cathodal tDCS is associated with the opposite, hyperpolarizing neurons (T. Wagner, Valero-Cabre, & Pascual-Leone, 2007). Importantly, tDCS has been used in patient populations and has shown benefits in treating depression (Brunoni et al., 2011; Fregni et al., 2006), reducing episodic memory deficits in Alzheimer’s (Ferrucci et al., 2008) and Parkinson’s diseases (Boggio et al., 2006), ameliorating aphasia (Baker, Rorden, & Fridriksson, 2010; Fridriksson, Richardson, Baker, & Rorden, 2011; Monti et al., 2008), and improving post-stroke motor function (Fregni et al., 2005; D. Y. Kim, Ohn, Yang, Park, & Jung, 2009; Suzuki et al., 2012).
Recent studies show that LTM can benefit from tDCS, specifically when applied to the dorsolateral prefrontal cortex (DLPFC). For example, Javadi and colleagues applied anodal (excitatory current), cathodal (inhibitory current), and sham tDCS to the left DLPFC to test modulation of LTM encoding, consolidation, and retrieval. They first reported that short pulses of anodal tDCS during encoding improved LTM, whereas cathodal tDCS during encoding impaired LTM (Javadi, Cheng, & Walsh, 2012). These benefits were specific to application during encoding, as tDCS applied at retrieval resulted in no change (anodal tDCS) or impaired LTM performance (cathodal tDCS). This finding was extended in a second verbal LTM study during which participants encoded word lists and 3 hours later performed an old-new recognition test (Javadi & Cheng, 2013). During this recognition test, participants received sham, anodal, or cathodal tDCS and after a delay of 5 hours, completed a second old-new recognition test. The group that received anodal stimulation performed significantly better than the cathodal or sham groups. Furthermore, a second control group did not participate in the second old-new recognition session after 3 hours and showed no benefit of anodal stimulation. Thus, recent studies pairing tDCS with LTM studies demonstrate memory improvement.
As discussed above, the DLPFC has been often studied for its contribution to LTM through the use of tDCS. However, across a range of tasks and experimental techniques, converging evidence also supports the functional involvement of the posterior parietal cortex (PPC) in episodic or long-term memory (reviewed in: (Berryhill, 2012; Cabeza, 2008; Cabeza, Ciaramelli, & Moscovitch, 2012; Cabeza, Ciaramelli, Olson, & Moscovitch, 2008; Ciaramelli, Grady, & Moscovitch, 2008; Olson & Berryhill, 2009; Vilberg & Rugg, 2008; A. D. Wagner, Shannon, Kahn, & Buckner, 2005). One particularly robust example from the fMRI literature is known as the parietal old/new effect due to stronger PPC activity following the endorsement of a probe item as ‘old’, when compared to ‘new’ endorsements (reviewed in: (Cabeza, 2008; Naghavi & Nyberg, 2005; A. D. Wagner et al., 2005). Yet, the nature and extent of PPC contributions to memory remain unclear. Investigations have reported PPC involvement during various stages of LTM, including encoding (Daselaar, Prince, & Cabeza, 2004; H. Kim, 2011; Uncapher, Hutchinson, & Wagner, 2011; Uncapher & Wagner, 2009), and retrieval (Dobbins, Rice, Wagner, & Schacter, 2003; Elman, Cohn-Sheehy, & Shimamura, 2012; Elman, Klostermann, Marian, Verstaen, & Shimamura, 2012; Herron, Henson, & Rugg, 2004; Konishi, Wheeler, Donaldson, & Buckner, 2000; Seibert, Gimbel, Hagler, & Brewer, 2011; Sestieri, Corbetta, Romani, & Shulman, 2011; Tulving, 1985; Xue et al., 2010). These neuroimaging findings point towards the PPC having a role throughout LTM encoding and retrieval.
Neurostimulation methodologies, such as tDCS, are well situated to evaluate the role of the PPC in LTM. Importantly, tDCS avoids the problems with neuropsychological research and the correlative nature of fMRI. It is of no surprise that the PPC has been targeted in LTM paradigms with tDCS previously. Jacobson and colleagues applied tDCS to bilateral PPC sites during verbal LTM encoding and found clear modulatory effects that depended on the direction of current flow. When the montage was anodal-left/cathodal-right, they observed improved LTM encoding; whereas the opposite montage worsened LTM performance (Jacobson, Koslowsky, & Lavidor, 2012). One other study found that anodal tDCS to either the left or right PFC or PPC applied during LTM retrieval facilitated verbal LTM in younger adults (Manenti, Brambilla, Petesi, Ferrari, & Cotelli, 2013). In older adults, LTM only benefited from tDCS applied to left PFC or PPC, but not when it was applied to the right-sided homologues. Taken together, these findings show that anodal tDCS to multiple regions can improve LTM performance.
Here, we investigated novel inter-related questions regarding the temporal and spatial sensitivity of tDCS effects on LTM. First, we tested whether unilateral anodal tDCS to the left PPC would also modulate LTM encoding, as predicted by earlier results showing benefits after bilateral tDCS (Jacobson et al., 2012). Second, we tested whether positive effects were temporally constrained to encoding or whether they extended to LTM retrieval. Finally, we tested the polarity specificity of tDCS effects by applying anodal and cathodal stimulation to the right PPC during the verbal LTM task. Across studies, we used a well-known verbal LTM test: the California Verbal Learning Test (CVLT, (Delis, Kramer, Kaplan, & Ober, 2000). The CVLT evaluates participants’ ability to learn and retain a structured list of items (four exemplars of four semantic categories) tested with and without interference and probed after various delay durations. We also included a visuospatial n-back task as a filler task, drawing on right-lateralized neural activity (Courtney, Ungerleider, Keil, & Haxby, 1996; McCarthy et al., 1996; Smith et al., 1995) that would be unlikely to interfere with verbal LTM.
Experiment 1
Experiment 1 tested whether anodal tDCS to the left PPC would improve LTM, in particular LTM encoding and/or retrieval. We included two testing sessions: sham and anodal tDCS based on several studies reporting improved performance only after anodal tDCS (Javadi & Cheng, 2013; Javadi et al., 2012; Javadi & Walsh, 2012). We predicted that LTM encoding would benefit from anodal stimulation to the left PPC. We remained agnostic as to effects at retrieval.
Methods
Participants
20 neurotypical, native English speaking, right-handed University of Nevada students (mean age: 23.40, standard deviation (SD): 3.33, 15 females) participated. Participants were screened for use of neuroleptic, hypnotic, or seizure medications. Participants reported no history of neurological or psychiatric symptoms or head injuries. All procedures were conducted in accordance with the University of Nevada Institutional Review Board. Participants were compensated $15/hour and signed informed consent documents.
TDCS Protocol
There were two counterbalanced sessions: anodal tDCS (active) and sham (control condition). In both conditions, one electrode was placed over the left parietal cortex at the left PPC (P3, International 10–20 EEG system) and the reference electrode was placed on the contralateral cheek. This reference site has been successfully used in a number of studies (Berryhill & Jones, 2012; Berryhill, Wencil, Branch Coslett, & Olson, 2010; Elmer, Burkard, Renz, Meyer, & Jancke, 2009; Hsu et al., 2011; Jones & Berryhill, 2012; Marshall, Molle, Siebner, & Born, 2005; Tanoue, Jones, Peterson, & Berryhill, 2012; Tseng et al., 2012; Zaehle, Sandmann, Thorne, Jancke, & Herrmann, 2011). Anodal stimulation consisted of 15 minutes of continuous direct current (1.5 mA) delivered by a battery-driven continuous stimulator (Eldith MagStim, GmbH, Ilmenau, Germany). We previously applied 10 minutes of stimulation in our studies using tDCS (Berryhill and Jones 2012; Berryhill et al. 2010; Jones and Berryhill 2012; Tanoue et al. 2012), however in the current study we increased the length to 15 minutes. This was done to allow for more time to overlap with the list-learning portion of the study (Experiments 1, 3, 4) as well as fill more of the maintenance stage in Experiment 2.
Current was delivered through two 5 × 7 cm2 electrodes housed in saline-soaked sponges. During sham stimulation, participants received 20 seconds of stimulation at the start and end of the 15-minute stimulation period. This provides sensory stimulation due to current change without sufficient stimulation to alter cortical excitability. This is an effective method for keeping participants blind to the condition (Gandiga, Hummel, & Cohen, 2006). During the stimulation/delay period, participants were given task instructions. After ten minutes of stimulation, the CVLT task began, although five minutes of stimulation remained. Previous tDCS research employs concurrent stimulation with experimental tasks and found beneficial results (Boggio et al., 2006; de Vries et al., 2010; Floel et al., 2012; Javadi & Walsh, 2012; Ross, McCoy, Wolk, Coslett, & Olson, 2010; Wirth et al., 2011). The effects of tDCS last are thought to last approximately one hour (Nitsche, Liebetanz, et al., 2003; Nitsche, Nitsche, et al., 2003; Nitsche & Paulus, 2000, 2001; Ohn et al., 2008). All tDCS sessions included a wash-out period of at least 24 hours.
California Verbal Learning Test (CVLT)
Testing Protocol
The CVLT (2nd edition, San Antonio, Texas (Delis et al., 2000) proceeds in stages, summarized as follows. The same experimenter presented all verbal lists at the same rate (1 word per second) and inflection for each participant. Participants first heard List A (16 words) spoken aloud by the experimenter followed by a verbal free recall test. These two steps comprised a trial and were repeated for a total of 5 trials with List A. Participants then heard a new list, List B one time and performed free recall for List B words. Participants then were asked to report all items from List A only without hearing it repeated. This test served as a test of short delay free recall (SDFR). Following a delay period of 20 minutes, participants performed a test of long delay free recall (LDFR) for List A items. Participants then completed a yes/no recognition test for List A in which 16 of 48 items were target words. A maximum free recall score for any of these components (trials 1-5, SDFR, LDFR) was 16 items. Finally, in Experiment 1, we also included the 2-alternative forced-choice recognition task with 16 pairs of words. The two normed versions of the CVLT were used in counterbalanced order across participants.
During the post-SDFR (20 minutes) and post-LDFR (10 minutes) delay periods of the CVLT, participants completed a spatial 2-back working memory task (Post-SDFR: 495 trials, 330 non-targets and 165 targets; post-LDFR: 225 trials, 150 non-targets and 75 targets); see Figure 1. The spatial 2-back task was chosen because it was unlikely to interfere with CVLT performance. Previous research has found that right PPC anodal tDCS increases visual search and attention skills (Bolognini, Fregni, Casati, Olgiati, & Vallar, 2010), which should be seen in tDCS-related changes to 2-back performance in the current study (Experiment 3 and 4). The first 2-back task phase took place during the delay periods within the CVLT. The spatial 2-back used uniform green circles (1.5° of visual angle) as stimuli. Circles marked to-be-remembered locations (500 ms) and were followed by a blank ISI (3000 ms). Participants made speeded button press responses to indicate whether the current location matched the location presented two items previously.
Fig. 1.
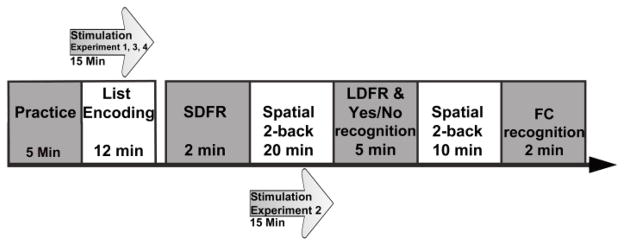
Experimental protocol of Experiments 1, 2, 3 and 4. Experiments 1, 3 and 4 had participants receive stimulation (15 minutes) before and during the word list-encoding portion of the CVLT. Participants completed spatial 2-back tasks during the CVLT delay periods, which followed the short delay free, recall (SDFR) and long delay free recall (LDFR). Experiment 2 had participants receive stimulation (15 minutes) during the 20 minute spatial 2-back before the LDFR. The forced-choice (FC) recognition portion of the CVLT was not included in Experiments 2, 3 and 4 because participants performed uniformly at ceiling in Experiment 1.
Analysis
The CVLT provides many measures of which only a subset were calculated. To assess the effect of tDCS during encoding, the most appropriate measure was the rate of learning as measured by learning slope for trials 1-5. Greater slopes indicate a faster learning rate and the formula was taken from the CVLT manual ((sum # list A trials x total number words recalled) – ((sum # list A trials) x (total number of words recalled)/number of trials)/(sum of number of trials2) – ((sum number of trials)2)/number of trials))). We also compared total number of words recalled for trials 1-5, as well as on trial 5 to determine if overall LTM achievement differed. Because the effects of tDCS at encoding might have also lead to later performance changes we also investigated several measures of recall: short delay free recall (SDFR), long delay free recall (LDFR) and yes/no recognition; see Figure 1. Finally, we also examined the 2-back WM task performance to provide an indication of the specificity of any tDCS effects.
Results
The first question was to identify whether anodal tDCS to the left PPC during encoding enhanced verbal LTM. We assessed the rate of learning by examining the learning slope across the first five trials. A paired-samples t-test indicated that the learning slope after anodal tDCS (mean (standard deviation): 1.79 (.46)) was significantly greater than the learning slope after sham stimulation (Figure 2; 1.36 (.51), t19 = 3.07, p < .01, r2 = .33). In other words, participants acquired List A items more quickly following anodal tDCS to the left PPC than when they received sham stimulation. Another analysis assessed whether tDCS to the left PPC enhanced LTM capacity. However, participants did not achieve a higher overall level of LTM performance in terms of the total number of words recalled on List A after 5 trials (sham: 56.25 (9.25), anodal: 56.55 (7.88), t19 = .17, p = .87, r2 = .01). There was also no difference between the numbers of words recalled during trial 5 (sham: 13.44 (2.35), anodal: 14.05 (1.73), t19 = 1.34, p = .19, r2 = .07).
Fig. 2.
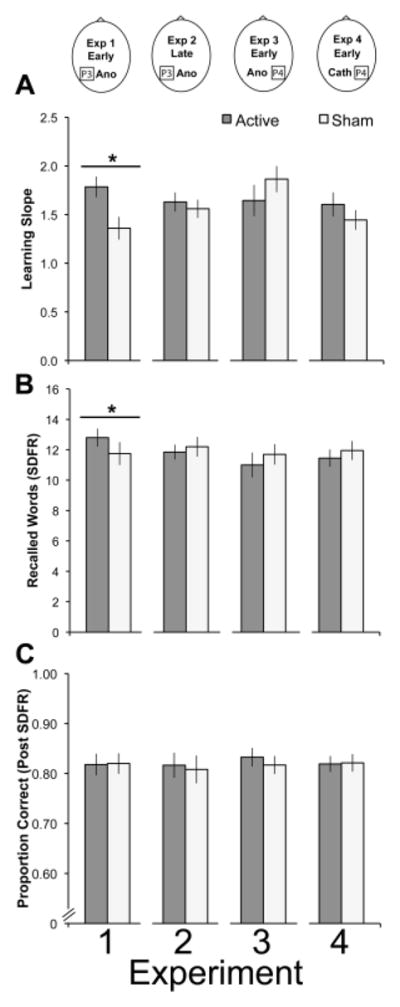
Results from Experiments 1–4. The top panel shows the stimulation site and phase of stimulation, per experiment. Each panel includes the results for Experiments 1, 2, 3, and 4. Learning slope (A) and SDFR (B) for word lists significantly improved only after early anodal tDCS to the left PPC in Experiment 1.
Subsequent analyses investigated tDCS effects on early or late retrieval. Early retrieval, measured by the SDFR, was measured shortly after tDCS ended. There was a significant benefit of tDCS on SDFR (sham: 11.75 (3.27); anodal: 12.80 (2.61); t19 = 2.47, p = .02, r2 = .24). In contrast, late retrieval was tested after the 20-minute delay using the LDFR measure. There was no significant difference between sham and anodal LDFR scores (anodal: 12.00 (2.58), sham: 11.30 (3.10), t19 = 1.38, p = .18, r2 = .09). A final late retrieval measure was yes/no recognition. There was no significant effect of tDCS condition (anodal: 14.90 (1.29); sham: 14.50 (1.93), t19 = 1.02, p = .32, r2 = .06), however participants were so close to ceiling level performance (16) on this measure we discontinued its subsequent use.
Finally, we investigated WM performance on the spatial 2-back working memory task performed during each delay period. Performance was no different for either the post-SDFR (anodal: .82 (.09), sham .82 (.09), t19 = .03, p = .97, r2 = .00) or post-LDFR delay phases (anodal: .82 (.10), sham .83 (.07), t19 = .77, p = .45, r2 = .03). In short, anodal tDCS to the left PPC did not elicit generalized modulatory effects, as it did not alter WM performance.
Discussion
Experiment 1 showed that anodal tDCS applied to the left PPC during encoding modulated several measures of LTM. Anodal tDCS enhanced the pace of list learning. Yet, there were no differences in the total number of words recalled on List A. In other words, the anodal tDCS accelerated the acquisition of word list items, but it did not change the end point. Effects extended to retrieval, as well. During anodal, and not during sham tDCS, encoding showed improved performance on short-delay free recall (SDFR). These benefits were time limited and did not extend to LFDR. One limitation of the yes/no recognition task is that participants’ performance was at ceiling. These data provide support for the view that anodal tDCS to the left PPC enhances LTM by helping participants reach their maximal performance with fewer trials.
Experiment 2
To test the temporal specificity of tDCS effects we next applied left PPC tDCS at a later time point after encoding, but before LDFR. One possibility we considered was that we might have missed tDCS-linked changes to LTM if the effects of tDCS wore off before they were tested. If left PPC tDCS effects are specific to the encoding process, we expected to see no change in LDFR. In contrast, if tDCS simply provides some general LTM enhancement due to increased excitability, we expect to see improved LDFR performance when tDCS is applied later.
Methods
Participants
20 new neurotypical, native English speaking, right-handed University of Nevada students (mean age 22.20, SD 2.46, 14 females) participated following the same screening and approval procedures described above.
TDCS Protocol
Experiment 2 followed the protocol described in Experiment 1, with an alteration in the timing of tDCS administration. TDCS was administered during the delay period after the SDFR phase; see Figure 1. The tDCS setup time and 15 minutes of stimulation filled the 20-minute delay period before participants performed the LDFR, while they performed the 2-back WM task. We also eliminated the 2-alternative forced-choice procedure at the end of the CVLT because our healthy participants performed at ceiling.
Results
Here, we were interested in testing whether tDCS effects were temporally constrained to the encoding phase, or whether general benefits would emerge. Applying tDCS after encoding but before LDFR resulted in no change in performance (anodal: 12.60 (2.33), sham: 12.15 (2.56), t19 = .87, p = .39, r2 = .04). As control analyses, we compared the learning rate and SDFR that were significant in Experiment 1. We found no differences because tDCS occurred after these phases (learning rates: anodal: 1.63 (.43), sham: 1.56 (.41), t19 = .46, p = .65, r2 = .01; SDFR: anodal: 11.85 (2.11), sham: 12.20 (2.82), t19 = .62, p = .54, r2 = .02). Finally, we compared anodal and sham performance in the spatial 2-back spatial WM task and found no difference between sham and anodal conditions (anodal: .82 (.03), sham: .81 (.12), t19 = .62, p = .54, r2 = .02).
Discussion
Experiment 2 showed that tDCS to the left PPC must be applied during encoding to provide a LTM benefit. The data revealed no effect of later tDCS on LTM retrieval. In other words, the effects were temporally constrained to encoding and did not generalize to other phases of LTM. Thus, these data point toward a temporally specific influence of tDCS. We next investigated hemispheric specificity.
Experiment 3
A potential concern when using tDCS is that current flows diffusely through the brain and it can be difficult to associate the location of stimulation with behavioral effects. The purpose of Experiment 3 was to test whether the LTM benefits observed in Experiment 1 were due to general stimulation, or whether they were specific to stimulation in the left PPC. As such, we replicated the CVLT but stimulated the right PPC during encoding. If the findings in Experiment 1 were simply due to stimulation in general, then we predicted we would replicate the enhanced learning rate and SDFR findings previously observed. However, if the Experiment 1 effects were due to left PPC stimulation, stimulating the right PPC should not alter LTM performance.
Participants
20 new neurotypical, native English speaking, right-handed University of Nevada students (mean age 21.05, SD 1.61, 14 females) participated following the same screening and approval procedures described above.
Methods
Experiment 3 replicated the procedure described for Experiment 1 with one change. The anodal electrode placement was on the right PPC (P4) with the cathodal electrode placed on the contralateral cheek.
Results
We replicated the analyses conducted in Experiment 1, beginning with calculating learning rate and SDFR. There were no significant differences in learning rate (anodal: 1.65 (.70), sham: 1.87 (.58), t19 = 1.283, p = .22, r2 = .08) or in the SDFR (anodal: 11.00 (3.58), sham: 11.70 (2.92), t19 = .836, p = .41, r2 = .04). There were also no significant differences in the LDFR (anodal: 11.15 (3.48), sham: 11.35 (3.01), t19 = .307, p = .76, r2 = .02). In short, anodal tDCS to the right PPC during encoding had no significant effect on LTM.
We also analyzed the performance on the spatial 2-back and found that WM performance did not improve following anodal tDCS during the post-SDFR (anodal: .83 (.08), sham: .82 (.07), t19 = 1.03, p = .32, r2 = .05) and post-LDFR delay phase (anodal: .84 (.08), sham: .85 (.06), t19 = 1.14, p = .27, r2 = .06). In summary, anodal tDCS to the right PPC did not enhance performance on the spatial 2-back task and had no effect on LTM.
Discussion
Experiment 3 tested whether the tDCS effects observed in Experiment 1 were due to a general effect of tDCS, or whether they were specific to left PPC stimulation. The results revealed that anodal tDCS to the right PPC during encoding did not modulate verbal LTM as was the case with left PPC stimulation in Experiment 1. Consequently, the previous positive findings were due hemisphere-specific stimulation as only left hemisphere stimulation improved LTM encoding in the current results and other studies (Javadi & Cheng, 2013; Javadi et al., 2012; Javadi & Walsh, 2012).
Experiment 4
One unknown with using tDCS in cognitive tasks of different types is whether the direction of current flow will pose equal and opposite effects, as in motor cortex, or more variable patterns, as has been previously noted (Jacobson et al., 2012). The purpose of Experiment 4 was to test whether the LTM benefits observed in Experiment 1 were specific to anodal tDCS, or whether the same pattern could be elicited by cathodal tDCS to the opposite hemisphere due to interhemispheric competition (see relevant review on tDCS in aphasia (Hamilton, Chrysikou, & Coslett, 2011). As such, we changed the stimulation at the right PPC to cathodal, rather than anodal stimulation. If the anodal left PPC protocol is essential to eliciting improved CVLT performance, we expected to see no beneficial effect of cathodal right PPC stimulation.
Participants
20 new neurotypical, native English speaking, right-handed University of Nevada students (mean age 21.40, SD 2.39, 13 Females) participated following the same screening and approval procedures described above.
Methods
Experiment 4 replicated the procedure described for Experiment 3 with one change. The electrode placement was the same (P4), however the electrodes switched locations. The cathodal electrode was over the right PPC and the anodal electrode was placed on the contralateral cheek. The same experimenter read the CVLT lists across all four Experiments.
Results
We replicated the analyses conducted in the other Experiments, beginning with calculating learning rate and SDFR. There were no significant differences in learning rate (cathodal: 1.61 (.54), sham: 1.45 (.44), t19 = 1.23, p = .23, r2 = .07) or in the SDFR (cathodal: 11.45 (2.50), sham: 11.95 (2.70), t19 = .95, p = .35, r2 = .05). There were also no significant differences in the LDFR (cathodal: 11.35 (2.87), sham: 12.05 (3.03), t19 = 1.14, p = .27, r2 = .06). In short, cathodal tDCS to the right PPC during encoding had no effect on LTM. Finally, on the WM task, effects were mixed. TDCS had no significant effect on the spatial 2-back performance for the post-SDFR phase (cathodal: .82 (.07), sham: .82 (.07), t19 = .19, p = .85, r2 < .01), but WM performance during the post-LDFR delay phase significantly improved (cathodal: .84 (.06), sham: .82 (.09), t19 = 2.13, p = .048, r2 = .19).
Discussion
Experiment 4 tested whether the tDCS effects observed in Experiments 1 and 3 were due to a general effect of tDCS, or whether they were specific to stimulation type and location. The results revealed that cathodal tDCS to the right PPC during encoding did not modulate verbal LTM. These data provide additional supporting control data demonstrating that the effects observed in Experiment 1 were specific to anodal left PPC tDCS during encoding and could not be replicated by suppressing the contralateral homologue. Cathodal stimulation had no effect on the post-SDFR spatial 2-back performance however it improved performance on the post-LDFR, which took place last in testing.
General Discussion
Here, we tested whether tDCS applied unilaterally to the left PPC would improve verbal LTM. Experiment 1 showed that stimulation applied during encoding accelerated list learning and short-delay free recall. Thus, both encoding and early retrieval stages of LTM improved with a single session of anodal tDCS to the left PPC. In contrast, tDCS did not raise the ceiling on LTM performance; it simply allowed participants to reach peak performance faster. This may be helpful for expediting verbal learning paradigms of all types because it reduces the length of training required to reach maximal performance. It also may be relevant in clinical populations showing memory problems that require additional mnemonic support to maintain function, for instance those with mild cognitive impairment or traumatic brain injury. Future work is also needed to determine whether special populations garner greater benefits than healthy young adults because they are further from ceiling. In addition, there was no difference in LTM retrieval after a long delay. Importantly, the encoding-benefits induced by left PPC stimulation were temporally limited and hemisphere specific. Experiment 2 tested whether stimulation just prior to the long-delay would enhance LTM retrieval. There was no effect of tDCS on long-delay free recall. In other words, delaying the application of tDCS did not enhance later retrieval, suggesting that any tDCS-linked LTM benefits were temporally limited to the encoding phase. Experiment 3 and 4 showed that the effect of tDCS on LTM encoding was specific to left PPC stimulation because stimulating the right PPC had no effect on LTM encoding rate or SDFR, regardless of stimulation type. The current findings demonstrate that the left PPC is involved in verbal LTM encoding through the use of targeted anodal tDCS. Furthermore, the results of Experiment 2 demonstrate that the PPC is sensitive to neurostimulation during encoding, and not later retrieval. To summarize, only the left anodal PPC tDCS during encoding, and not retrieval, had any effect on the various LTM tasks in the CVLT.
TDCS and LTM
Neurostimulation shows potential for cognitive maintenance (reviewed in: (Jacobson et al., 2012; Reis et al., 2008) because of its strong safety record (Agnew & McCreery, 1987; Nitsche, Liebetanz, et al., 2003; Nitsche & Paulus, 2011) and relatively low cost. In spite of this potential, tDCS has several limitations including, low spatial specificity, generally temporary effects, and an underlying mechanism that remains unclear. However, in spite of these limitations: researchers have moved forward and found tDCS-related LTM benefits after bilateral PPC (Jacobson et al., 2012; Manenti et al., 2013), bilateral DLPFC (Javadi & Walsh, 2012; Manenti et al., 2013), unilateral DLPFC (Javadi & Walsh, 2012; Manenti et al., 2013), unilateral PPC (Manenti et al., 2013; Meinzer et al., 2014), and unilateral anterior temporal lobe stimulation (Ross, McCoy, Coslett, Olson, & Wolk, 2011; Ross et al., 2010). All of these studies include a verbal component – primarily list learning, with the exception of the Ross studies, which tested associative memory for face/name and place/name pairs. We extend these findings by demonstrating that unilateral anodal left PPC tDCS applied at encoding also enhances LTM by speeding the rate at which items are encoded. We would not expect a facilitatory effect of left PPC stimulation on spatial WM to the extent we see in verbal LTM here. However when we consider state-dependent effects of tDCS, a change in spatial WM is also expected with stimulation of the right PPC (Experiment 4).
One of the unexpected aspects of these results was that unilateral left PPC stimulation facilitated two distinct aspects of LTM: encoding and short-delay retrieval. This is surprising for several reasons. First, with regard to PPC activations during LTM encoding, fMRI shows that stronger superior PPC activity predicted retrieval success, but inferior PPC activity predicted retrieval failure (Daselaar, Prince, & Cabeza, 2004; Kim, 2011; Uncapher, Hutchinson, & Wagner, 2011; Uncapher & Wagner, 2009). One possibility is that our stimulation sites (left PPC: P3, right PPC: P4) provided more stimulation to the superior parietal lobe than to inferior parietal regions. However during LTM retrieval, fMRI shows that activation of inferior parietal regions are associated with retrieval success (reviewed in: (Cabeza, 2008; Naghavi & Nyberg, 2005; Rugg & Vilberg, 2013; Vilberg & Rugg, 2008; A. D. Wagner et al., 2005), and confidence judgments (as reviewed in (Olson & Berryhill, 2009). However, this interpretation is difficult to endorse because tDCS is diffuse and cannot precisely target subregions of the PPC. There is much to learn regarding how various stimulation paradigms and electrode montages elicit LTM benefits. TDCS stimulates broad networks such that stimulating one node elicits distal stimulation as well (Shahid, Wen, & Ahfock, 2013). This may explain why frontoparietal stimulation to either the DLPFC or PPC improves LTM, as both regions are connected to each other and with medial temporal lobe structures, which are essential to LTM (e.g. cingulum bundle, inferior longitudinal fasciculus (Seltzer & Pandya, 1984). Thus, although tDCS has a limited direct spatial reach it may be able to modulate deeper structures based on brain connectivity. Continued refinement of the tDCS technique will clarify these unknowns.
It is also important to note the advances in tDCS protocols that do show clinical and long-term benefits. For tDCS to serve a translational role, interventions must be longer lasting. As discussed in the introduction, tDCS treatments show efficacy in treating aphasia (reviewed in: (Hamilton et al., 2011), depression (Brunoni et al., 2011; Fregni et al., 2006), and motor function after stroke (Hummel et al., 2008; D. Y. Kim et al., 2010; D. Y. Kim et al., 2009; Suzuki et al., 2012). Indeed, in several cases, a single session of tDCS has been associated with performance benefits lasting 1 month in cases of fibromyalgia (Valle et al., 2009) and Alzheimer’s disease (Boggio et al., 2012) as well as 1 year in healthy adults (Dockery, Hueckel-Weng, Birbaumer, & Plewnia, 2009) and patients with aphasia (Chrysikou & Hamilton, 2011). Longitudinal studies show that multiple sessions of tDCS can improve performance in a variety of tasks for a longer period of time. For example, 5 sessions of anodal tDCS to the left PPC improved verbal LTM at follow up a week later (Meinzer et al., 2014), and 10 sessions of bilateral PFC tDCS paired with working memory training sustained benefits a month later (Park, Seo, Kim, & Ko, 2014). These emerging findings suggest that tDCS may be useful in eliciting long-term benefits, with no known negative side effects. Future work combining tDCS with other methods will be needed to clarify the underlying mechanism(s) of these effects and how they can best apply on an individual basis, based on cognitive task basis.
Hemispheric effects: PPC involvement in verbal LTM and spatial WM
In the first two experiments we expected a benefit of anodal tDCS to the left PPC on the verbal LTM task, but not on the spatial 2-back WM task. This WM task was chosen to occupy the delay periods in the CVLT without providing verbal interference. In these left-lateralized stimulation experiments there was no effect of tDCS on WM performance. We predicted that the right PPC anodal stimulation used in Experiment 3 would reveal WM improvement due to the spatial aspect of the task. However, there was no improvement during both the post-SDFR and post LDFR phase. Cathodal stimulation to right PPC showed increased performance on the WM task in Experiment 4. Interestingly, only post LDFR phase WM task performance was benefited from the stimulation. Better spatial performance improvements have been shown with only right PPC anodal tDCS (Bolognini et al., 2010), while worse visual WM performance have been shown with right PPC cathodal tDCS (Berryhill et al. 2010). These studies suggest that there is certainly a relationship between visual WM performance and right PPC. Our results are consistent with the Bolognini results since we also did not observe spatial WM improvement with left PPC stimulation. However we failed to see improved spatial WM performance with anodal right PPC stimulation. Other studies also have demonstrated the involvement of the right PPC in spatial WM by applying inhibitory TMS, which increased spatial WM reaction times (Hamidi, Tononi, & Postle, 2008; Kessels, d’Alfonso, Postma, & de Haan, 2000; Oliveri et al., 2001; Yamanaka, Yamagata, Tomioka, Kawasaki, & Mimura, 2010). Our results contradict with previous studies since we observed spatial WM improvement with only cathodal stimulation to right PPC. One important difference between previous studies and current result is the temporal characteristics of the stimulation. Both of post-SDFR and post LDFR phases, in which the spatial WM task was given, were 10 and 30 minutes after the stimulation in Experiments 3 and 4. The non-significant performance difference between anodal and sham right PPC stimulation result in Experiment 3 may be due to this temporal delay since tDCS effects are known to be short-lived and temporal specific (Nitsche, Liebetanz, et al., 2003; Nitsche, Nitsche, et al., 2003; Nitsche & Paulus, 2000, 2001; Ohn et al., 2008). On the other hand the significant performance difference between cathodal and sham right PPC stimulation in Experiment 4 may be a result of state-dependent effects of stimulation as seen in TMS studies (Cattaneo, Rota, Vecchi, & Silvanto, 2008). The finding of only right PPC tDCS affecting the spatial 2-back task also supports the assumed specialization of the right hemisphere in spatial processing. While we did not find an effect of anodal tDCS, previous studies have found cathodal benefit on WM following right PPC stimulation in some participants (Jones & Berryhill, 2012).
Given the finding that only left PPC anodal tDCS during encoding affected LTM performance, a question arises as to how tDCS applied to Broca’s area would affect performance. Several studies have already applied tDCS to Broca’s area in patients following stroke and found beneficial effects in language recovery (Fiori et al., 2013; Marangolo & Caltagirone, 2014; Marangolo et al., 2014; Monti et al., 2008), however one study found no benefit (Polanowska, Lesniak, Seniow, & Czlonkowska, 2013). These studies, taken with the current findings, support the use of anodal tDCS to both the left PFC and PPC to enhance LTM skills. Furthermore, stimulation of one region may also result in facilitation effects in the other region due to connectivity between the PFC and PPC (Geschwind, 1965). The benefit will certainly be larger in patient populations, such as those cited here, as compared to neurotypical individuals. Continuing to pair rehabilitation techniques with neurostimulation will likely have the greatest beneficial impact on patient populations who suffer from aphasia.
Limitations
Finally, we note several limitations of the current study. First, the CVLT is a standardized neuropsychological test designed for use in patient populations. As such, some of the measures (e.g. old/new recognition) were too easy for our healthy graduate and undergraduate student participants. Previous work suggests that tDCS effects can matter most when tasks are difficult (Jones & Berryhill, 2012). Consequently, more difficult LTM tasks might be essential to developing tDCS-based interventions designed to maintain LTM. Second, one potential confound is that enhanced verbal WM may contribute to the enhanced encoding rate and retrieval gains on the SDFR. There were no general effects on the spatial 2-back WM task to suggest a general WM benefit and we deliberately selected a spatial (non-verbal) task to prevent interference with the list-learning focus of the CVLT. Even if WM were facilitated, it would be difficult to account for the SDFR findings because participants would have had to actively rehearse the list A items in WM for at least two minutes. This strategy, while onerous, remains a possibility. Another concern is the use of a spatial WM task as a filler task. Active stimulation may have affected the state of PPC during the spatial WM task, as seen in TMS studies (Cattaneo et al., 2008). However, only Experiment 2 had tDCS administered concurrently with the spatial 2-back task. In the other three experiments, the spatial WM task was administered after the encoding and SDFR portions of the CVLT and the application of tDCS. Still, we must be careful and take into account these state-dependent effects of PPC, as we cannot know how tDCS alters the state of PPC.
Future investigations will need to more carefully parse the underlying contribution of tDCS left PPC to verbal LTM encoding, and right PPC to working memory. Future research in LTM enhancement should continue to develop tDCS paradigms that show benefits. Furthermore, understanding the LTM contributions of specific regions within the PPC will allow for more targeted neurostimulation aimed at improving performance in healthy and clinical populations.
Acknowledgments
We would like to thank Dwight Peterson, Jaclyn Stephens, and Sierra Kreamer-Hope. This work was supported by an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the NIH P20GM103650 (PI Michael Webster, Project Leader Marian Berryhill), NEI R15EY022775 (to Marian Berryhill and Gideon Caplovitz), and faculty startup funds generously provided by the University of Nevada, Reno. The content is solely the responsibility of the authors and does not represent the official views of the NIGMS, or the NEI.
Footnotes
Conflict of Interest
The authors declare that they have no conflict of interest.
References
- Agnew WF, McCreery DB. Considerations for safety in the use of extracranial stimulation for motor evoked potentials. Neurosurgery. 1987;20(1):143–147. doi: 10.1097/00006123-198701000-00030. [DOI] [PubMed] [Google Scholar]
- Antal A, Kincses TZ, Nitsche MA, Bartfai O, Paulus W. Excitability changes induced in the human primary visual cortex by transcranial direct current stimulation: direct electrophysiological evidence. Invest Ophthalmol Vis Sci. 2004;45(2):702–707. doi: 10.1167/iovs.03-0688. [DOI] [PubMed] [Google Scholar]
- Baker JM, Rorden C, Fridriksson J. Using transcranial direct-current stimulation to treat stroke patients with aphasia. Stroke. 2010;41(6):1229–1236. doi: 10.1161/STROKEAHA.109.576785. STROKEAHA.109.576785 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berryhill ME. Insights from neuropsychology: pinpointing the role of the posterior parietal cortex in episodic and working memory. Front Integr Neurosci. 2012;6:31. doi: 10.3389/fnint.2012.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berryhill ME, Jones KT. tDCS selectively improves working memory in older adults with more education. Neurosci Lett. 2012;521(2):148–151. doi: 10.1016/j.neulet.2012.05.074. S0304-3940(12)00772-0 [pii] [DOI] [PubMed] [Google Scholar]
- Berryhill ME, Wencil EB, Branch Coslett H, Olson IR. A selective working memory impairment after transcranial direct current stimulation to the right parietal lobe. Neurosci Lett. 2010;479(3):312–316. doi: 10.1016/j.neulet.2010.05.087. S0304-3940(10)00716-0 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boggio PS, Ferrucci R, Mameli F, Martins D, Martins O, Vergari M, Priori A. Prolonged visual memory enhancement after direct current stimulation in Alzheimer’s disease. Brain Stimul. 2012;5(3):223–230. doi: 10.1016/j.brs.2011.06.006. S1935-861X(11)00088-X [pii] [DOI] [PubMed] [Google Scholar]
- Boggio PS, Ferrucci R, Rigonatti SP, Covre P, Nitsche M, Pascual-Leone A, Fregni F. Effects of transcranial direct current stimulation on working memory in patients with Parkinson’s disease. J Neurol Sci. 2006;249(1):31–38. doi: 10.1016/j.jns.2006.05.062. S0022-510X(06)00280-2 [pii] [DOI] [PubMed] [Google Scholar]
- Bolognini N, Fregni F, Casati C, Olgiati E, Vallar G. Brain polarization of parietal cortex augments training-induced improvement of visual exploratory and attentional skills. Brain Res. 2010;1349:76–89. doi: 10.1016/j.brainres.2010.06.053. S0006-8993(10)01445-9 [pii] [DOI] [PubMed] [Google Scholar]
- Brunoni AR, Ferrucci R, Bortolomasi M, Vergari M, Tadini L, Boggio PS, Priori A. Transcranial direct current stimulation (tDCS) in unipolar vs. bipolar depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35(1):96–101. doi: 10.1016/j.pnpbp.2010.09.010. S0278-5846(10)00361-1 [pii] [DOI] [PubMed] [Google Scholar]
- Cabeza R. Role of parietal regions in episodic memory retrieval: the dual attentional processes hypothesis. Neuropsychologia. 2008;46(7):1813–1827. doi: 10.1016/j.neuropsychologia.2008.03.019. S0028-3932(08)00112-7 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R, Ciaramelli E, Moscovitch M. Cognitive contributions of the ventral parietal cortex: an integrative theoretical account. Trends in Cognitive Sciences. 2012;16(6):338–352. doi: 10.1016/j.tics.2012.04.008. S1364-6613(12)00101-5 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R, Ciaramelli E, Olson IR, Moscovitch M. The parietal cortex and episodic memory: an attentional account. Nat Rev Neurosci. 2008;9(8):613–625. doi: 10.1038/nrn2459. nrn2459 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattaneo Z, Rota F, Vecchi T, Silvanto J. Using state-dependency of transcranial magnetic stimulation (TMS) to investigate letter selectivity in the left posterior parietal cortex: a comparison of TMS-priming and TMS-adaptation paradigms. European Journal of Neuroscience. 2008;28(9):1924–1929. doi: 10.1111/J.1460-9568.2008.06466.X. [DOI] [PubMed] [Google Scholar]
- Charlton RA, Barrick TR, Markus HS, Morris RG. The relationship between episodic long-term memory and white matter integrity in normal aging. Neuropsychologia. 2010;48(1):114–122. doi: 10.1016/j.neuropsychologia.2009.08.018. S0028-3932(09)00335-2 [pii] [DOI] [PubMed] [Google Scholar]
- Charlton RA, Barrick TR, Markus HS, Morris RG. Verbal working and long-term episodic memory associations with white matter microstructure in normal aging investigated using tract-based spatial statistics. Psychol Aging. 2013;28(3):768–777. doi: 10.1037/a0032668. 2013-29219-001 [pii] [DOI] [PubMed] [Google Scholar]
- Chrysikou EG, Hamilton RH. Noninvasive brain stimulation in the treatment of aphasia: exploring interhemispheric relationships and their implications for neurorehabilitation. Restor Neurol Neurosci. 2011;29(6):375–394. doi: 10.3233/RNN-2011-0610. 102464WN6M147606 [pii] [DOI] [PubMed] [Google Scholar]
- Ciaramelli E, Grady CL, Moscovitch M. Top-down and bottom-up attention to memory: a hypothesis (AtoM) on the role of the posterior parietal cortex in memory retrieval. Neuropsychologia. 2008;46(7):1828–1851. doi: 10.1016/j.neuropsychologia.2008.03.022. S0028-3932(08)00110-3 [pii] [DOI] [PubMed] [Google Scholar]
- Courtney SM, Ungerleider LG, Keil K, Haxby JV. Object and spatial visual working memory activate separate neural systems in human cortex. Cerebral Cortex. 1996;6(1):39–49. doi: 10.1093/Cercor/6.1.39. [DOI] [PubMed] [Google Scholar]
- Daselaar SM, Prince SE, Cabeza R. When less means more: deactivations during encoding that predict subsequent memory. NeuroImage. 2004;23(3):921–927. doi: 10.1016/j.neuroimage.2004.07.031. S1053-8119(04)00403-3 [pii] [DOI] [PubMed] [Google Scholar]
- de Vries MH, Barth AC, Maiworm S, Knecht S, Zwitserlood P, Floel A. Electrical stimulation of Broca’s area enhances implicit learning of an artificial grammar. J Cogn Neurosci. 2010;22(11):2427–2436. doi: 10.1162/jocn.2009.21385. [DOI] [PubMed] [Google Scholar]
- Delis D, Kramer J, Kaplan E, Ober BA. California Verbal Learning Test. 2. Psychological Corporation; San Antonio, TX: 2000. [Google Scholar]
- Dobbins IG, Rice HJ, Wagner AD, Schacter DL. Memory orientation and success: separable neurocognitive components underlying episodic recognition. Neuropsychologia. 2003;41(3):318–333. doi: 10.1016/s0028-3932(02)00164-1. S0028393202001641 [pii] [DOI] [PubMed] [Google Scholar]
- Dockery CA, Hueckel-Weng R, Birbaumer N, Plewnia C. Enhancement of planning ability by transcranial direct current stimulation. J Neurosci. 2009;29(22):7271–7277. doi: 10.1523/JNEUROSCI.0065-09.2009. 29/22/7271 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elman JA, Cohn-Sheehy BI, Shimamura AP. Dissociable parietal regions facilitate successful retrieval of recently learned and personally familiar information. Neuropsychologia. 2012;51(4):573–583. doi: 10.1016/j.neuropsychologia.2012.12.013. S0028-3932(12)00544-1 [pii] [DOI] [PubMed] [Google Scholar]
- Elman JA, Klostermann EC, Marian DE, Verstaen A, Shimamura AP. Neural correlates of metacognitive monitoring during episodic and semantic retrieval. Cogn Affect Behav Neurosci. 2012;12(3):599–609. doi: 10.3758/s13415-012-0096-8. [DOI] [PubMed] [Google Scholar]
- Elmer S, Burkard M, Renz B, Meyer M, Jancke L. Direct current induced short-term modulation of the left dorsolateral prefrontal cortex while learning auditory presented nouns. Behav Brain Funct. 2009;5:29. doi: 10.1186/1744-9081-5-29. 1744-9081-5-29 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrucci R, Mameli F, Guidi I, Mrakic-Sposta S, Vergari M, Marceglia S, Priori A. Transcranial direct current stimulation improves recognition memory in Alzheimer disease. Neurology. 2008;71(7):493–498. doi: 10.1212/01.wnl.0000317060.43722.a3. 01.wnl.0000317060.43722.a3 [pii] [DOI] [PubMed] [Google Scholar]
- Fiori V, Cipollari S, Di Paola M, Razzano C, Caltagirone C, Marangolo P. tDCS stimulation segregates words in the brain: evidence from aphasia. Front Hum Neurosci. 2013;7:269. doi: 10.3389/fnhum.2013.00269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floel A, Suttorp W, Kohl O, Kurten J, Lohmann H, Breitenstein C, Knecht S. Non-invasive brain stimulation improves object-location learning in the elderly. Neurobiol Aging. 2012;33(8):1682–1689. doi: 10.1016/j.neurobiolaging.2011.05.007. S0197-4580(11)00176-X [pii] [DOI] [PubMed] [Google Scholar]
- Fregni F, Boggio PS, Mansur CG, Wagner T, Ferreira MJ, Lima MC, Pascual-Leone A. Transcranial direct current stimulation of the unaffected hemisphere in stroke patients. Neuroreport. 2005;16(14):1551–1555. doi: 10.1097/01.wnr.0000177010.44602.5e. 00001756-200509280-00004 [pii] [DOI] [PubMed] [Google Scholar]
- Fregni F, Boggio PS, Nitsche MA, Marcolin MA, Rigonatti SP, Pascual-Leone A. Treatment of major depression with transcranial direct current stimulation. Bipolar Disord. 2006;8(2):203–204. doi: 10.1111/j.1399-5618.2006.00291.x. BDI291 [pii] [DOI] [PubMed] [Google Scholar]
- Fridriksson J, Richardson JD, Baker JM, Rorden C. Transcranial direct current stimulation improves naming reaction time in fluent aphasia: a double-blind, sham-controlled study. Stroke. 2011;42(3):819–821. doi: 10.1161/STROKEAHA.110.600288. STROKEAHA.110.600288 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandiga PC, Hummel FC, Cohen LG. Transcranial DC stimulation (tDCS): a tool for double-blind sham-controlled clinical studies in brain stimulation. Clin Neurophysiol. 2006;117(4):845–850. doi: 10.1016/j.clinph.2005.12.003. S1388-2457(05)00507-9 [pii] [DOI] [PubMed] [Google Scholar]
- Geschwind N. Disconnexion syndromes in animals and man. I. Brain. 1965;88(2):237–294. doi: 10.1093/brain/88.2.237. [DOI] [PubMed] [Google Scholar]
- Hamidi M, Tononi G, Postle BR. Evaluating frontal and parietal contributions to spatial working memory with repetitive transcranial magnetic stimulation. Brain research. 2008;1230:202–210. doi: 10.1016/j.brainres.2008.07.008. S0006-8993(08)01657-0 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton RH, Chrysikou EG, Coslett B. Mechanisms of aphasia recovery after stroke and the role of noninvasive brain stimulation. Brain Lang. 2011;118(1–2):40–50. doi: 10.1016/j.bandl.2011.02.005. S0093-934X(11)00037-X [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herron JE, Henson RN, Rugg MD. Probability effects on the neural correlates of retrieval success: an fMRI study. NeuroImage. 2004;21(1):302–310. doi: 10.1016/j.neuroimage.2003.09.039. S105381190300586X [pii] [DOI] [PubMed] [Google Scholar]
- Hsu TY, Tseng LY, Yu JX, Kuo WJ, Hung DL, Tzeng OJ, Juan CH. Modulating inhibitory control with direct current stimulation of the superior medial frontal cortex. NeuroImage. 2011;56(4):2249–2257. doi: 10.1016/j.neuroimage.2011.03.059. S1053-8119(11)00338-7 [pii] [DOI] [PubMed] [Google Scholar]
- Hummel FC, Celnik P, Pascual-Leone A, Fregni F, Byblow WD, Buetefisch CM, Gerloff C. Controversy: Noninvasive and invasive cortical stimulation show efficacy in treating stroke patients. Brain Stimul. 2008;1(4):370–382. doi: 10.1016/j.brs.2008.09.003. S1935-861X(08)00336-7 [pii] [DOI] [PubMed] [Google Scholar]
- Ishihara O, Gondo Y, Poon LW. The influence of aging on short-term and long-term memory in the continuous recognition paradigm. Shinrigaku Kenkyu. 2002;72(6):516–521. doi: 10.4992/jjpsy.72.516. [DOI] [PubMed] [Google Scholar]
- Jacobson L, Koslowsky M, Lavidor M. tDCS polarity effects in motor and cognitive domains: a meta-analytical review. Exp Brain Res. 2012;216(1):1–10. doi: 10.1007/s00221-011-2891-9. [DOI] [PubMed] [Google Scholar]
- Javadi AH, Cheng P. Transcranial Direct Current Stimulation (tDCS) Enhances Reconsolidation of Long-Term Memory. Brain Stimul. 2013;6(4):668–674. doi: 10.1016/j.brs.2012.10.007. S1935-861X(12)00199-4 [pii] [DOI] [PubMed] [Google Scholar]
- Javadi AH, Cheng P, Walsh V. Short duration transcranial direct current stimulation (tDCS) modulates verbal memory. Brain Stimul. 2012;5(4):468–474. doi: 10.1016/j.brs.2011.08.003. S1935-861X(11)00117-3 [pii] [DOI] [PubMed] [Google Scholar]
- Javadi AH, Walsh V. Transcranial direct current stimulation (tDCS) of the left dorsolateral prefrontal cortex modulates declarative memory. Brain Stimul. 2012;5(3):231–241. doi: 10.1016/j.brs.2011.06.007. S1935-861X(11)00089-1 [pii] [DOI] [PubMed] [Google Scholar]
- Jones KT, Berryhill ME. Parietal contributions to visual working memory depend on task difficulty. Front Psychiatry. 2012;3:81. doi: 10.3389/fpsyt.2012.00081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessels RP, d’Alfonso AA, Postma A, de Haan EH. Spatial working memory performance after high-frequency repetitive transcranial magnetic stimulation of the left and right posterior parietal cortex in humans. Neuroscience letters. 2000;287(1):68–70. doi: 10.1016/s0304-3940(00)01146-0. S0304-3940(00)01146-0 [pii] [DOI] [PubMed] [Google Scholar]
- Kessler SK, Turkeltaub PE, Benson JG, Hamilton RH. Differences in the experience of active and sham transcranial direct current stimulation. Brain Stimul. 2012;5(2):155–162. doi: 10.1016/j.brs.2011.02.007. S1935-861X(11)00024-6 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DY, Lim JY, Kang EK, You DS, Oh MK, Oh BM, Paik NJ. Effect of transcranial direct current stimulation on motor recovery in patients with subacute stroke. Am J Phys Med Rehabil. 2010;89(11):879–886. doi: 10.1097/PHM.0b013e3181f70aa7. 00002060-201011000-00004 [pii] [DOI] [PubMed] [Google Scholar]
- Kim DY, Ohn SH, Yang EJ, Park CI, Jung KJ. Enhancing motor performance by anodal transcranial direct current stimulation in subacute stroke patients. Am J Phys Med Rehabil. 2009;88(10):829–836. doi: 10.1097/PHM.0b013e3181b811e3. 00002060-200910000-00008 [pii] [DOI] [PubMed] [Google Scholar]
- Kim H. Neural activity that predicts subsequent memory and forgetting: a meta-analysis of 74 fMRI studies. NeuroImage. 2011;54(3):2446–2461. doi: 10.1016/j.neuroimage.2010.09.045. S1053-8119(10)01226-7 [pii] [DOI] [PubMed] [Google Scholar]
- Konishi S, Wheeler ME, Donaldson DI, Buckner RL. Neural correlates of episodic retrieval success. NeuroImage. 2000;12(3):276–286. doi: 10.1006/nimg.2000.0614. S1053-8119(00)90614-1 [pii] [DOI] [PubMed] [Google Scholar]
- Manenti R, Brambilla M, Petesi M, Ferrari C, Cotelli M. Enhancing verbal episodic memory in older and young subjects after non-invasive brain stimulation. Front Aging Neurosci. 2013;5:49. doi: 10.3389/fnagi.2013.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marangolo P, Caltagirone C. Options to enhance recovery from aphasia by means of non-invasive brain stimulation and action observation therapy. Expert Rev Neurother. 2014;14(1):75–91. doi: 10.1586/14737175.2014.864555. [DOI] [PubMed] [Google Scholar]
- Marangolo P, Fiori V, Campana S, Calpagnano MA, Razzano C, Caltagirone C, Marini A. Something to talk about: enhancement of linguistic cohesion through tdCS in chronic non fluent aphasia. Neuropsychologia. 2014;53:246–256. doi: 10.1016/j.neuropsychologia.2013.12.003. S0028-3932(13)00425-9 [pii] [DOI] [PubMed] [Google Scholar]
- Marshall L, Molle M, Siebner HR, Born J. Bifrontal transcranial direct current stimulation slows reaction time in a working memory task. BMC Neurosci. 2005;6:23. doi: 10.1186/1471-2202-6-23. 1471-2202-6-23 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy G, Puce A, Constable RT, Krystal JH, Gore JC, GoldmanRakic P. Activation of human prefrontal cortex during spatial and nonspatial working memory tasks measured by functional MRI. Cerebral Cortex. 1996;6(4):600–611. doi: 10.1093/Cercor/6.4.600. [DOI] [PubMed] [Google Scholar]
- Meinzer M, Jahnigen S, Copland DA, Darkow R, Grittner U, Avirame K, Floel A. Transcranial direct current stimulation over multiple days improves learning and maintenance of a novel vocabulary. Cortex. 2014;50:137–147. doi: 10.1016/j.cortex.2013.07.013. S0010-9452(13)00185-8 [pii] [DOI] [PubMed] [Google Scholar]
- Mitchell DB, Schmitt FA. Short- and long-term implicit memory in aging and Alzheimer’s disease. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn. 2006;13(3–4):611–635. doi: 10.1080/13825580600697616. X3762410X0772G4Q [pii] [DOI] [PubMed] [Google Scholar]
- Monti A, Cogiamanian F, Marceglia S, Ferrucci R, Mameli F, Mrakic-Sposta S, Priori A. Improved naming after transcranial direct current stimulation in aphasia. J Neurol Neurosurg Psychiatry. 2008;79(4):451–453. doi: 10.1136/jnnp.2007.135277. jnnp.2007.135277 [pii] [DOI] [PubMed] [Google Scholar]
- Naghavi HR, Nyberg L. Common fronto-parietal activity in attention, memory, and consciousness: shared demands on integration? Conscious Cogn. 2005;14(2):390–425. doi: 10.1016/j.concog.2004.10.003. S1053-8100(04)00117-5 [pii] [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Liebetanz D, Lang N, Antal A, Tergau F, Paulus W. Safety criteria for transcranial direct current stimulation (tDCS) in humans. Clin Neurophysiol. 2003;114(11):2220–2222. doi: 10.1016/s1388-2457(03)00235-9. author reply 2222-2223 S1388245703002359 [pii] [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Nitsche MS, Klein CC, Tergau F, Rothwell JC, Paulus W. Level of action of cathodal DC polarisation induced inhibition of the human motor cortex. Clin Neurophysiol. 2003;114(4):600–604. doi: 10.1016/s1388-2457(02)00412-1. S1388245702004121 [pii] [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Paulus W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J Physiol. 2000;527(Pt 3):633–639. doi: 10.1111/j.1469-7793.2000.t01-1-00633.x. PHY_1055 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitsche MA, Paulus W. Sustained excitability elevations induced by transcranial DC motor cortex stimulation in humans. Neurology. 2001;57(10):1899–1901. doi: 10.1212/wnl.57.10.1899. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Paulus W. Transcranial direct current stimulation--update 2011. Restor Neurol Neurosci. 2011;29(6):463–492. doi: 10.3233/RNN-2011-0618. 252R1135VU705H53 [pii] [DOI] [PubMed] [Google Scholar]
- Ohn SH, Park CI, Yoo WK, Ko MH, Choi KP, Kim GM, Kim YH. Time-dependent effect of transcranial direct current stimulation on the enhancement of working memory. Neuroreport. 2008;19(1):43–47. doi: 10.1097/WNR.0b013e3282f2adfd. 00001756-200801080-00008 [pii] [DOI] [PubMed] [Google Scholar]
- Oliveri M, Turriziani P, Carlesimo GA, Koch G, Tomaiuolo F, Panella M, Caltagirone C. Parieto-frontal interactions in visual-object and visual-spatial working memory: evidence from transcranial magnetic stimulation. Cerebral cortex. 2001;11(7):606–618. doi: 10.1093/cercor/11.7.606. [DOI] [PubMed] [Google Scholar]
- Olson IR, Berryhill M. Some surprising findings on the involvement of the parietal lobe in human memory. Neurobiol Learn Mem. 2009;91(2):155–165. doi: 10.1016/j.nlm.2008.09.006. S1074-7427(08)00169-X [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SH, Seo JH, Kim YH, Ko MH. Long-term effects of transcranial direct current stimulation combined with computer-assisted cognitive training in healthy older adults. Neuroreport. 2014;25(2):122–126. doi: 10.1097/WNR.0000000000000080. [DOI] [PubMed] [Google Scholar]
- Paulus W. Transcranial electrical stimulation (tES - tDCS; tRNS, tACS) methods. Neuropsychol Rehabil. 2011;21(5):602–617. doi: 10.1080/09602011.2011.557292. [DOI] [PubMed] [Google Scholar]
- Polanowska KE, Lesniak M, Seniow JB, Czlonkowska A. No effects of anodal transcranial direct stimulation on language abilities in early rehabilitation of post-stroke aphasic patients. Neurol Neurochir Pol. 2013;47(5):414–422. doi: 10.5114/ninp.2013.38221. 21554 [pii] [DOI] [PubMed] [Google Scholar]
- Reis J, Robertson E, Krakauer JW, Rothwell J, Marshall L, Gerloff C, Cohen LG. Consensus: “Can tDCS and TMS enhance motor learning and memory formation?”. Brain Stimul. 2008;1(4):363–369. doi: 10.1016/j.brs.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenkranz K, Nitsche MA, Tergau F, Paulus W. Diminution of training-induced transient motor cortex plasticity by weak transcranial direct current stimulation in the human. Neurosci Lett. 2000;296(1):61–63. doi: 10.1016/s0304-3940(00)01621-9. S0304-3940(00)01621-9 [pii] [DOI] [PubMed] [Google Scholar]
- Ross LA, McCoy D, Coslett HB, Olson IR, Wolk DA. Improved proper name recall in aging after electrical stimulation of the anterior temporal lobes. Front Aging Neurosci. 2011;3:16. doi: 10.3389/fnagi.2011.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross LA, McCoy D, Wolk DA, Coslett HB, Olson IR. Improved proper name recall by electrical stimulation of the anterior temporal lobes. Neuropsychologia. 2010;48(12):3671–3674. doi: 10.1016/j.neuropsychologia.2010.07.024. S0028-3932(10)00319-2 [pii] [DOI] [PubMed] [Google Scholar]
- Rugg MD, Vilberg KL. Brain networks underlying episodic memory retrieval. Curr Opin Neurobiol. 2013;23(2):255–260. doi: 10.1016/j.conb.2012.11.005. S0959-4388(12)00167-5 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seibert TM, Gimbel SI, Hagler DJ, Jr, Brewer JB. Parietal activity in episodic retrieval measured by fMRI and MEG. NeuroImage. 2011;55(2):788–793. doi: 10.1016/j.neuroimage.2010.11.078. S1053-8119(10)01556-9 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seltzer B, Pandya DN. Further observations on parieto-temporal connections in the rhesus monkey. Exp Brain Res. 1984;55(2):301–312. doi: 10.1007/BF00237280. [DOI] [PubMed] [Google Scholar]
- Sestieri C, Corbetta M, Romani GL, Shulman GL. Episodic memory retrieval, parietal cortex, and the default mode network: functional and topographic analyses. J Neurosci. 2011;31(12):4407–4420. doi: 10.1523/JNEUROSCI.3335-10.2011. 31/12/4407 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahid S, Wen P, Ahfock T. Numerical investigation of white matter anisotropic conductivity in defining current distribution under tDCS. Comput Methods Programs Biomed. 2013;109(1):48–64. doi: 10.1016/j.cmpb.2012.09.001. S0169-2607(12)00216-7 [pii] [DOI] [PubMed] [Google Scholar]
- Smith EE, Jonides J, Koeppe RA, Awh E, Schumacher EH, Minoshima S. Spatial Versus Object Working-Memory - Pet Investigations. Journal of Cognitive Neuroscience. 1995;7(3):337–356. doi: 10.1162/Jocn.1995.7.3.337. [DOI] [PubMed] [Google Scholar]
- Stagg CJ, Nitsche MA. Physiological basis of transcranial direct current stimulation. Neuroscientist. 2011;17(1):37–53. doi: 10.1177/1073858410386614. 17/1/37 [pii] [DOI] [PubMed] [Google Scholar]
- Suzuki K, Fujiwara T, Tanaka N, Tsuji T, Masakado Y, Hase K, Liu M. Comparison of the after-effects of transcranial direct current stimulation over the motor cortex in patients with stroke and healthy volunteers. Int J Neurosci. 2012;122(11):675–681. doi: 10.3109/00207454.2012.707715. [DOI] [PubMed] [Google Scholar]
- Tanoue RT, Jones KT, Peterson DJ, Berryhill ME. Differential frontal involvement in shifts of internal and perceptual attention. Brain Stimul. 2012 doi: 10.1016/j.brs.2012.11.003. S1935-861X(12)00204-5 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng P, Hsu TY, Chang CF, Tzeng OJ, Hung DL, Muggleton NG, Juan CH. Unleashing Potential: Transcranial Direct Current Stimulation over the Right Posterior Parietal Cortex Improves Change Detection in Low-Performing Individuals. J Neurosci. 2012;32(31):10554–10561. doi: 10.1523/JNEUROSCI.0362-12.2012. 32/31/10554 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulving E. Memory and Consciousness. Canadian Psychology. 1985;26(1):1–12. [Google Scholar]
- Uncapher MR, Hutchinson JB, Wagner AD. Dissociable effects of top-down and bottom-up attention during episodic encoding. J Neurosci. 2011;31(35):12613–12628. doi: 10.1523/JNEUROSCI.0152-11.2011. 31/35/12613 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uncapher MR, Wagner AD. Posterior parietal cortex and episodic encoding: insights from fMRI subsequent memory effects and dual-attention theory. Neurobiol Learn Mem. 2009;91(2):139–154. doi: 10.1016/j.nlm.2008.10.011. S1074-7427(08)00204-9 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valle A, Roizenblatt S, Botte S, Zaghi S, Riberto M, Tufik S, Fregni F. Efficacy of anodal transcranial direct current stimulation (tDCS) for the treatment of fibromyalgia: results of a randomized, sham-controlled longitudinal clinical trial. J Pain Manag. 2009;2(3):353–361. [PMC free article] [PubMed] [Google Scholar]
- Vilberg KL, Rugg MD. Memory retrieval and the parietal cortex: a review of evidence from a dual-process perspective. Neuropsychologia. 2008;46(7):1787–1799. doi: 10.1016/j.neuropsychologia.2008.01.004. S0028-3932(08)00015-8 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner AD, Shannon BJ, Kahn I, Buckner RL. Parietal lobe contributions to episodic memory retrieval. Trends in Cognitive Sciences. 2005;9(9):445–453. doi: 10.1016/j.tics.2005.07.001. S1364-6613(05)00207-X [pii] [DOI] [PubMed] [Google Scholar]
- Wagner T, Valero-Cabre A, Pascual-Leone A. Noninvasive human brain stimulation. Annu Rev Biomed Eng. 2007;9:527–565. doi: 10.1146/annurev.bioeng.9.061206.133100. [DOI] [PubMed] [Google Scholar]
- Wais PE, Gazzaley A. Distractibility during retrieval of long-term memory: domain-general interference, neural networks and increased susceptibility in normal aging. Front Psychol. 2014;5:280. doi: 10.3389/fpsyg.2014.00280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirth M, Rahman RA, Kuenecke J, Koenig T, Horn H, Sommer W, Dierks T. Effects of transcranial direct current stimulation (tDCS) on behaviour and electrophysiology of language production. Neuropsychologia. 2011;49(14):3989–3998. doi: 10.1016/j.neuropsychologia.2011.10.015. S0028-3932(11)00480-5 [pii] [DOI] [PubMed] [Google Scholar]
- Xue G, Dong Q, Chen C, Lu Z, Mumford JA, Poldrack RA. Greater neural pattern similarity across repetitions is associated with better memory. Science. 2010;330(6000):97–101. doi: 10.1126/science.1193125. science.1193125 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka K, Yamagata B, Tomioka H, Kawasaki S, Mimura M. Transcranial magnetic stimulation of the parietal cortex facilitates spatial working memory: near-infrared spectroscopy study. Cerebral cortex. 2010;20(5):1037–1045. doi: 10.1093/cercor/bhp163. bhp163 [pii] [DOI] [PubMed] [Google Scholar]
- Zaehle T, Sandmann P, Thorne JD, Jancke L, Herrmann CS. Transcranial direct current stimulation of the prefrontal cortex modulates working memory performance: combined behavioural and electrophysiological evidence. BMC Neurosci. 2011;12:2. doi: 10.1186/1471-2202-12-2. 1471-2202-12-2 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]


