Abstract
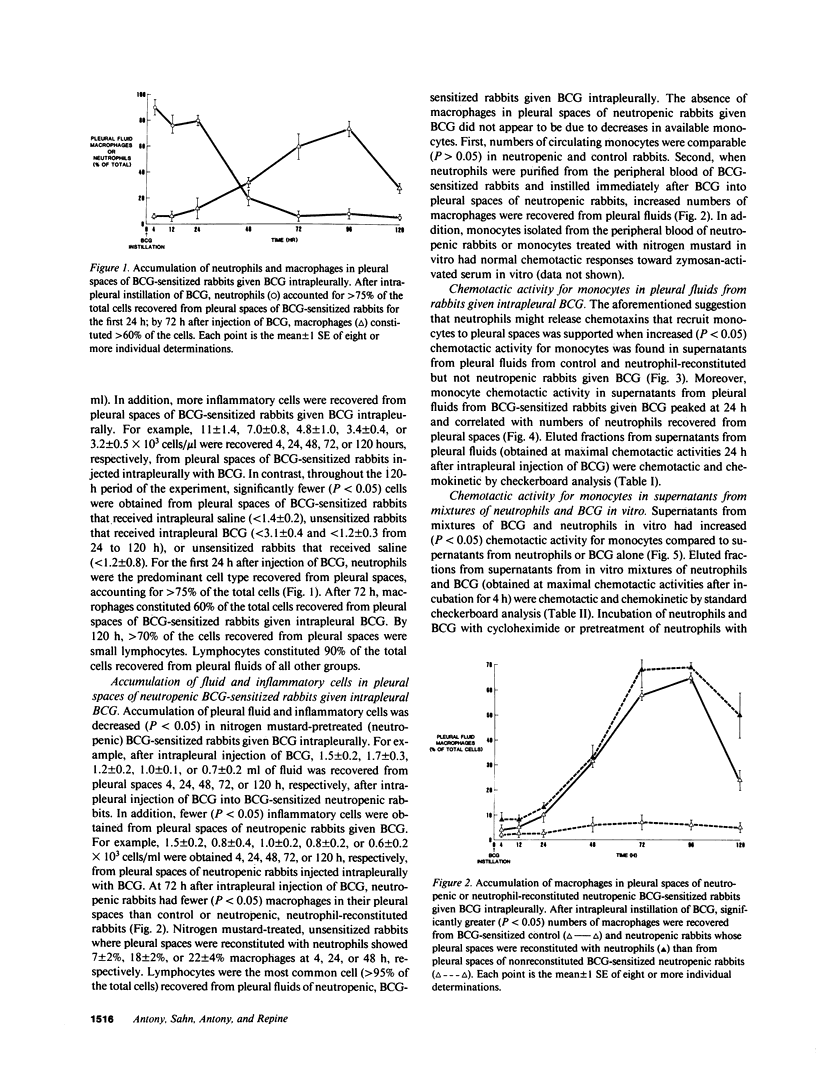
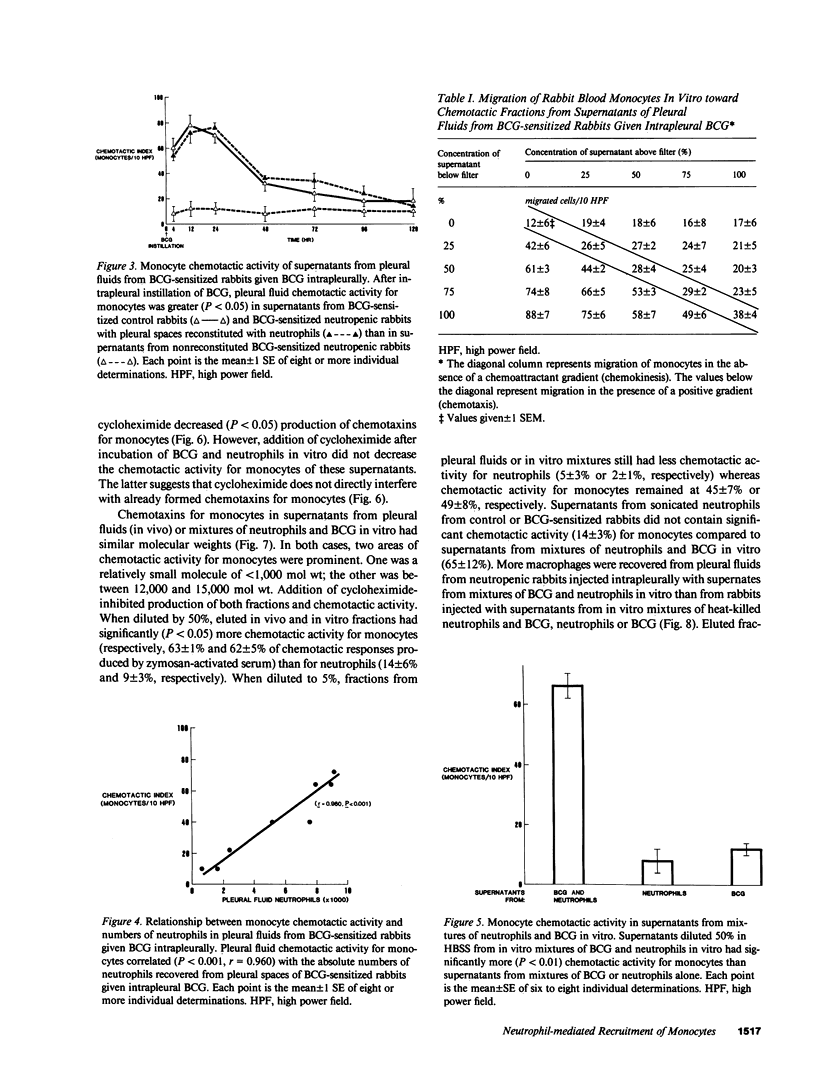
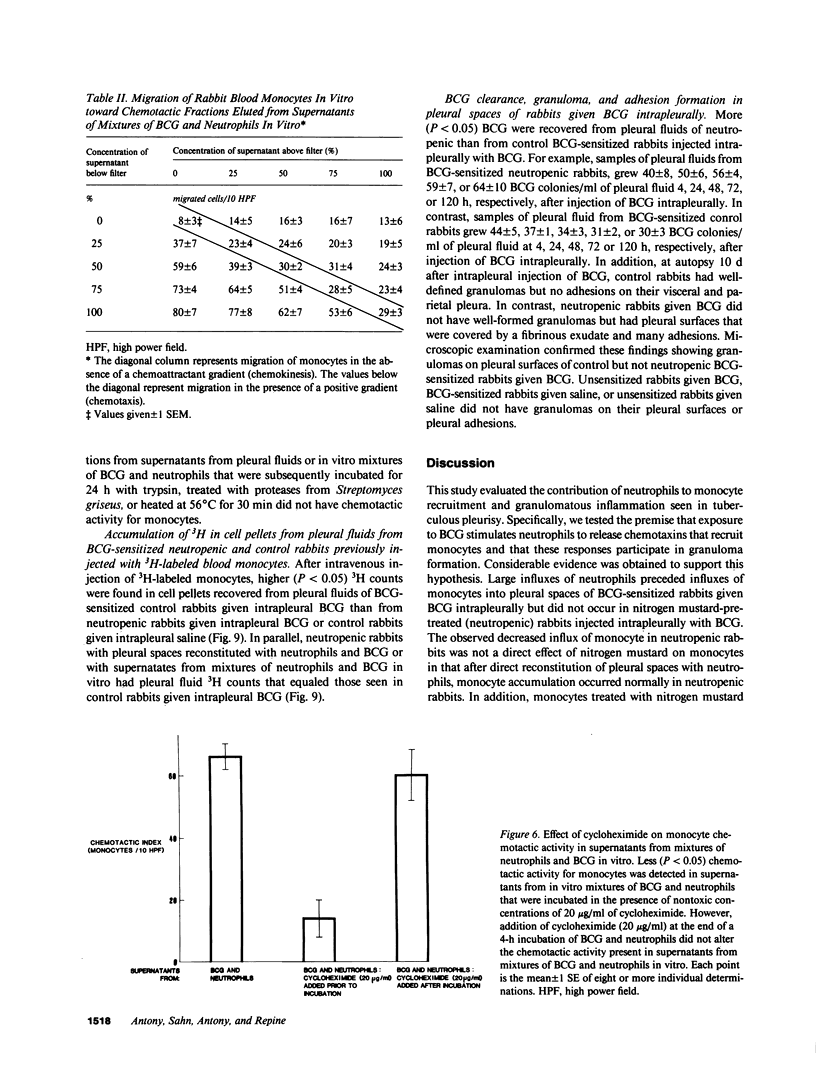
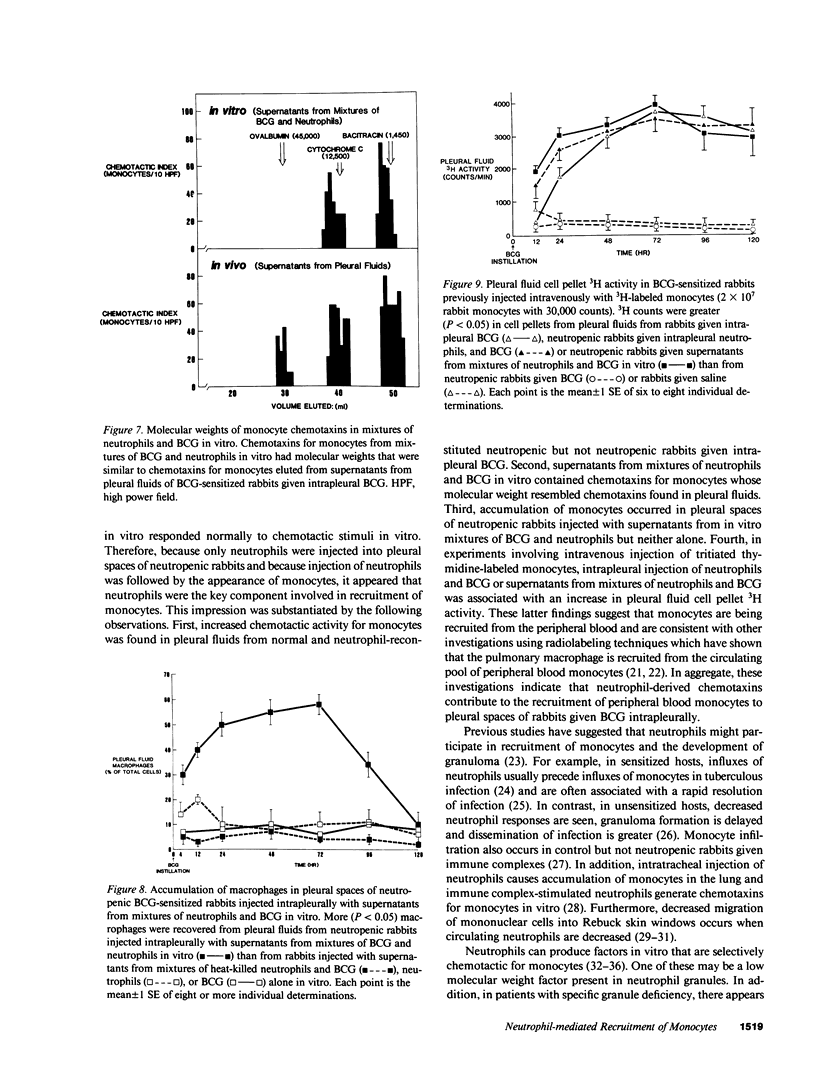
Neutrophils are often seen first at sites of granulomatous inflammation but their contribution to monocyte recruitment and granuloma formation is unknown. We tested the hypothesis that neutrophils release chemotaxins which attract monocytes. We found that rapid accumulations of fluid and influxes of neutrophils followed by monocytes occurred in bacillus Calmette--Guérin (BCG)-sensitized rabbits given BCG intrapleurally but did not occur in nitrogen mustard-treated (neutropenic) BCG-sensitized rabbits given BCG intrapleurally--unless the rabbits were also given intrapleural injections of neutrophils. We also found monocyte chemotaxins in pleural spaces of control and neutrophil-reconstituted neutropenic but not in neutropenic rabbits given BCG intrapleurally. Moreover, pleural fluid monocyte chemotaxins had molecular weights (12,000-15,000 and 1,000) that were similar to molecular weights of monocyte chemotaxins present in supernatants from mixtures of neutrophils and BCG in vitro. In addition, intrapleural injection of neutrophils and BCG or supernatants from in vitro mixtures of neutrophils and BCG (but not neutrophils or BCG alone) increased the numbers of monocytes and 3H cell pellet activity in pleural fluids from untreated neutropenic rabbits or neutropenic rabbits previously injected intravenously with 3[H]methyl thymidine-labeled monocytes. Furthermore, fewer BCG were recovered from pleural fluids of BCG-sensitized control compared to neutropenic rabbits given BCG, and at autopsy 10 d after instillation of BCG, control but not neutropenic rabbits had well-defined granulomas without adhesions on their pleural surfaces. Our results suggest that BCG stimulates neutrophils to release chemotaxins that recruit monocytes, and that these responses might contribute to granuloma formation in tuberculous pleurisy.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. O. The granulomatous inflammatory response. A review. Am J Pathol. 1976 Jul;84(1):164–192. [PMC free article] [PubMed] [Google Scholar]
- Antony V. B., Repine J. E., Harada R. N., Good J. T., Jr, Sahn S. A. Inflammatory responses in experimental tuberculosis pleurisy. Acta Cytol. 1983 May-Jun;27(3):355–361. [PubMed] [Google Scholar]
- Blussé van Oud Alblas A., van der Linden-Schrever B., Van Furth R. Origin and kinetics of pulmonary macrophages during an inflammatory reaction induced by intra-alveolar administration of aerosolized heat-killed BCG. Am Rev Respir Dis. 1983 Aug;128(2):276–281. doi: 10.1164/arrd.1983.128.2.276. [DOI] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Clawson C. C., Repine J. E. Quantitation of maximal bactericidal capability in human neutrophils. J Lab Clin Med. 1976 Aug;88(2):316–327. [PubMed] [Google Scholar]
- Dale D. C., Wolff S. M. Skin window studies of the acute inflammatory responses of neutropenic patients. Blood. 1971 Aug;38(2):138–142. [PubMed] [Google Scholar]
- Gallin J. I., Fletcher M. P., Seligmann B. E., Hoffstein S., Cehrs K., Mounessa N. Human neutrophil-specific granule deficiency: a model to assess the role of neutrophil-specific granules in the evolution of the inflammatory response. Blood. 1982 Jun;59(6):1317–1329. [PubMed] [Google Scholar]
- Goetzl E. J., Klickstein L. B., Watt K. W., Wintroub B. U. The preferential human mononuclear leukocyte chemotactic activity of the substituent tetrapeptides of angiotensin II. Biochem Biophys Res Commun. 1980 Dec 16;97(3):1097–1102. doi: 10.1016/0006-291x(80)91488-6. [DOI] [PubMed] [Google Scholar]
- Good J. T., Jr, Taryle D. A., Hyers T. M., Sahn S. A. Clotting and fibrinolytic activity of pleural fluid in a model of pleural adhesions. Am Rev Respir Dis. 1978 Nov;118(5):903–908. doi: 10.1164/arrd.1978.118.5.903. [DOI] [PubMed] [Google Scholar]
- Hunninghake G. W., Gallin J. I., Fauci A. S. Immunologic reactivity of the lung: the in vivo and in vitro generation of a neutrophil chemotactic factor by alveolar macrophages. Am Rev Respir Dis. 1978 Jan;117(1):15–23. doi: 10.1164/arrd.1978.117.1.15. [DOI] [PubMed] [Google Scholar]
- Jungi T. W., McGregor D. D. Dissociation of macrophage accumulation and local chemotactic activity in delayed inflammatory sites. Cell Immunol. 1979 Oct;47(2):274–284. doi: 10.1016/0008-8749(79)90337-x. [DOI] [PubMed] [Google Scholar]
- PAGE A. R., GOOD R. A. Studies on cyclic neutropenia; a clinical and experimental investigation. AMA J Dis Child. 1957 Dec;94(6):623–661. doi: 10.1001/archpedi.1957.04030070035006. [DOI] [PubMed] [Google Scholar]
- Repine J. E., Clawson C. C., Friend P. S. Influence of a deficiency of the second component of complement on the bactericidal activity of neutrophils in vitro. J Clin Invest. 1977 May;59(5):802–809. doi: 10.1172/JCI108702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repine J. E., Clawson C. C. Influence of surface proteins and separation techniques on neutrophil unstimulated and stimulated locomotion in vitro. J Reticuloendothel Soc. 1978 Sep;24(3):217–226. [PubMed] [Google Scholar]
- Repine J. E., White J. G., Clawson C. C., Holmes B. M. Effects of phorbol myristate acetate on the metabolism and ultrastructure of neutrophils in chronic granulomatous disease. J Clin Invest. 1974 Jul;54(1):83–90. doi: 10.1172/JCI107752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahn S. A., Potts D. E. Turpentine pleurisy in rabbits: a model of pleural fluid acidosis and low pleural fluid glucose. Am Rev Respir Dis. 1978 Nov;118(5):893–901. doi: 10.1164/arrd.1978.118.5.893. [DOI] [PubMed] [Google Scholar]
- Sahn S. A., Taryle D. A., Good J. T., Jr Experimental empyema. Time course and pathogenesis of pleural fluid acidosis and low pleural fluid glucose. Am Rev Respir Dis. 1979 Aug;120(2):355–361. doi: 10.1164/arrd.1979.120.2.355. [DOI] [PubMed] [Google Scholar]
- Shasby D. M., Fox R. B., Harada R. N., Repine J. E. Reduction of the edema of acute hyperoxic lung injury by granulocyte depletion. J Appl Physiol Respir Environ Exerc Physiol. 1982 May;52(5):1237–1244. doi: 10.1152/jappl.1982.52.5.1237. [DOI] [PubMed] [Google Scholar]
- Spector W. G. The granulomatous inflammatory exudate. Int Rev Exp Pathol. 1969;8:1–55. [PubMed] [Google Scholar]
- Spilberg I., Mandell B., Wochner R. D. Studies on crystal-induced chemotactic factor. I. Requirement for protein synthesis and neutral protease activity. J Lab Clin Med. 1974 Jan;83(1):56–63. [PubMed] [Google Scholar]
- Spilberg I., Mehta J. Demonstration of a specific neutrophil receptor for a cell-derived chemotactic factor. J Clin Invest. 1979 Jan;63(1):85–88. doi: 10.1172/JCI109282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stauffer J. L., Potts D. E., Sahn S. A. Cellular content of the normal rabbit pleural space. Acta Cytol. 1978 Nov-Dec;22(6):570–574. [PubMed] [Google Scholar]
- Ward P. A. Chemotoxis of mononuclear cells. J Exp Med. 1968 Nov 1;128(5):1201–1221. doi: 10.1084/jem.128.5.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward P. A., Hill J. H. C5 chemotactic fragments produced by an enzyme in lysosomal granules of neutrophils. J Immunol. 1970 Mar;104(3):535–543. [PubMed] [Google Scholar]
- Wintroub B. U., Klickstein L. B., Kaempfer C. E., Austen K. F. A human neutrophil-dependent pathway for generation of angiotensin II: purification and physicochemical characterization of the plasma protein substrate. Proc Natl Acad Sci U S A. 1981 Feb;78(2):1204–1208. doi: 10.1073/pnas.78.2.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]


