Abstract
Hedgehog (Hh) ligand production by ballooned hepatocytes drives nonalcoholic steatohepatitis (NASH) progression in mice. The NIDDK-sponsored PIVENS trial (NCT00063622) showed that Vitamin E (VitE) improved NASH. We investigated whether VitE treatment and improvement in NASH were associated with changes in Hh pathway activity. Immunohistochemistry (IHC) was performed on both pre- and post-treatment liver biopsies of 59 PIVENS patients randomized to VitE (n=30) or placebo (n=29). Sonic Hh (Shh) ligand-producing cells and Shh-responsive cells were quantified. The latter was accomplished by triple IHC for gli2+ (marker of Hh signaling), sox-9 (progenitor marker), and α-smooth muscle actin [α-SMA] (myofibroblast marker). Ballooned hepatocytes were quantified by keratin 8/18 and ubiquitin (K8/18/Ub) staining. IHC results were correlated with primary clinical and histologic PIVENS data. Pretreatment clinical, histologic, and IHC parameters did not differ significantly in the two treatment groups. Regardless of treatment arm, the number of Shh+ hepatocytes correlated with K8/18/Ub foci (r2=0.47, p<0.001) and AST (r2=0.15, p=0.002). Treatment-related changes in the numbers of Shh+ hepatocytes correlated with changes in serum AST (partial r2=0.75, p<0.0001), hepatocyte ballooning (p=0.004), the ductular reaction (i.e., numbers of gli2+/sox9+ cells; p=0.03 and α-SMA+ cells; p=0.10), and fibrosis stage (p=0.02). Treatment response was associated with greater decrease in Shh+ hepatocytes than non-response (p=0.007). The VitE group demonstrated a greater reduction in K8/18/Ub+ foci (p<0.08) and Shh+ hepatocytes (p<0.05) than the placebo group, effects that became more significant after correction for baseline differences and multiple linear regression analysis. Conclusion: During PIVENS, treatment response correlated with loss of Shh+ hepatocytes and improvement in Hh-regulated processes that promote NASH progression.
Keywords: nonalcoholic steatohepatitis, ductular reaction, glioblastoma family transcription factor 2, sox-9, hepatocyte ballooning, damage associated molecular patterns
INTRODUCTION
Nonalcoholic steatohepatitis (NASH) is a growing global health problem in adults and children (1, 2). Deeper understanding of the pathogenic mechanisms and potential therapeutic options are necessary. Although the NIDDK-sponsored clinical trial, PIVENS (NCT00063622), demonstrated vitamin E (VitE) to be somewhat efficacious in NASH (3), it is widely accepted that better therapies are needed.
Lipotoxic liver cell injury and death have been identified as key pathobiologic alterations in NASH (4, 5). The prototypical manifestation of hepatic lipotoxicity is hepatocellular ballooning (6, 7), and the numbers of ballooned hepatocytes generally correlate with the severity of liver inflammation and fibrosis in NASH (4, 8, 9). Ballooned hepatocytes exhibit signs of endoplasmic reticulum (ER) and oxidative stress, as evidenced by their reduced expression of keratins 8 and 18 (K8/18) and accumulation of ubiquitinated proteins (Ub) (6, 7, 10). Based on their seminal roles in hepatocyte lipotoxicity, hepatic ER stress and oxidative stress have also been proposed as key drivers of NASH progression (6, 7, 10, 11,12).
Previous work from our group demonstrated that ballooned hepatocytes produce and release Sonic hedgehog (Shh) ligands (13, 14, 15, 16). Studies in cultured hepatocytes and experimental animals with liver injury showed that ER stress and oxidative stress-related mechanisms mediate this response (13, 14). We suspected that these findings might have important implications for NASH progression because in adult Drosophila, dying cells release Shh ligands as damage associated molecular patterns (DAMPs) to trigger wound healing responses, including the outgrowth of progenitors that eventually differentiate to replace the cells that died (12, 17, 18, 19, 20, 21). Hence, the work in flies revealed that adult organisms can reactivate developmental morphogenic signaling pathways, such as Hedgehog (Hh), to regenerate their tissues during injury. This suggested that complex wound healing responses in adults may somewhat recapitulate ontogeny.
In developing embryos, epithelial cells synthesize and release Hh ligands which diffuse away to interact with neighboring stromal cells that express Patched (Ptc), the cell surface receptor for Hh ligands. Ligand-receptor interaction inhibits basal Ptc-mediated suppression of Smoothened (Smo), the signaling competent Hh co-receptor. This initiates intracellular signaling that ultimately results in the nuclear accumulation of glioblastoma family transcription factors (gli1, gli2, gli3) which in turn, regulate the expression of Hh target genes that control the viability, proliferation, migration, and differentiation of Hh-responsive cells. Consequently, paracrine Hh signaling coordinates diverse processes that are necessary for organogenesis in developing embryos, including progenitor outgrowth, cell migration, matrix remodeling, and vasculogenesis (22).
Various types of cells that accumulate in injured adult livers, including alpha smooth muscle actin (α-SMA)-expressing myofibroblastic cells and sex-determining region Y-box 9- (sox9) positive liver progenitors, express receptors for Hh ligands and thus, are Hh responsive (23). Studies in various animal models of liver injury indicate that Hh signaling orchestrates hepatic wound healing responses, including outgrowth of hepatic progenitor cells (HPCs) and myofibroblasts, recruitment of inflammatory cells, vascular remodeling, and extracellular matrix deposition (24). Indeed, it has been demonstrated that the level of Hh pathway activity parallels the severity of hepatocyte ballooning, inflammatory liver injury, and fibrosis in patients with NASH (15). In animal models of diet-induced NASH, pharmacologic agents that antagonize Hh signaling improve NASH and NASH-related fibrosis (25). Whether or not treatments that improve NASH in humans influence hepatic Hh pathway activity is unknown, however. Therefore, the aim of this study was to determine if Hh pathway activity decreased in parallel with diminished hepatocellular injury subsequent to successful VitE therapy in NASH.
PATIENTS AND METHODS
Study Design and Population
We performed a cross-sectional analysis using data and core liver biopsy sections from a representative subset (n = 59) of subjects enrolled in the NIDDK-sponsored PIVENS clinical trial (NCT00063622) (3). The PIVENS trial randomly assigned adult patients (n = 247) with NASH and without diabetes to receive VitE at a dose of 800 IU daily (n = 84), pioglitazone at a dose of 30 mg daily (n = 84), or placebo (n = 83) for 96 weeks. The primary outcome was an improvement in histological features of NASH, as assessed by a composite of standard scores for steatosis, lobular inflammation, hepatocellular ballooning and fibrosis. In the PIVENS trial, VitE therapy was found to be superior to placebo for the treatment of NASH; no benefit was found with pioglitazone over placebo for the primary outcomes. Therefore we evaluated responses in the VitE-treated group and placebo-treated group, and excluded the pioglitazone-treated group, in our analyses.
In order to obtain equivalent data distributions on key clinical and histological features, our study population (VitE arm, n = 30; placebo arm, n = 29) was randomly selected from the larger PIVENS cohort after excluding subjects who did not have follow-up biopsy and using stratifications based on gender and degree of hepatocyte ballooning.
Histologic Evaluation of NAFLD
Liver biopsies from all the cases had been previously stained with hematoxylin and eosin (H&E) and Masson's trichrome, reviewed and scored by the NIDDK-sponsored NASH Clinical Research Network (NASH CRN) Pathology Committee according to the published NASH CRN scoring system (26), in which hepatocyte ballooning was graded as 0 (none), 1 (few), or 2 (many). In addition, fibrosis was staged as 0 (no pathologic fibrosis), 1 (centrilobular or periportal pericellular fibrosis), 2 (centrilobular and periportal pericellular fibrosis), 3 (bridging fibrosis), or 4 (cirrhosis).
Immunohiostochemical Staining and Evaluations
We obtained 3 unstained slides from both the entry and end of study formalin-fixed paraffin-embedded core liver biopsies. Hh ligand expression was evaluated by immunohistochemistry (IHC) for Shh. Response to Hh ligand, gli2 positivity, was evaluated by IHC for Hh-responsive (gli2+) progenitors sox-9-positive cells and myofibroblastic hepatic stellate cells (α-SMA-positive). Ballooning injury was evaluated by K8/18/Ub IHC. Details of the IHC methods and antibodies have been previously published (14, 15, 23).
Using 100x magnification, the total number of high power fields (HPFs) was counted for each biopsy fragment and multiplied by the field of view diameter (2.2 mm) to calculate the total length of tissue available for each subject. All Shh+ hepatocytes, K8/18-negative hepatocytes, and Ub+ foci were counted along the entire biopsy length and normalized to the length of the biopsy to permit comparison among biopsies of different lengths. To quantify and characterize Hh-responsive (i.e., gli2-positive) populations, including liver progenitors (sox9-positive) and myofibroblasts (α-SMA-positive), a relative grading scale was used to capture the intensity and extent (i.e., involved surface area) of gli2/sox9/α-SMA co-staining. The intensity and extent of gli2/sox9 double-staining (indicative of Hh-responsive progenitors) was graded on a relative scale from 0-3; 0 (no staining), 1 (mild staining), 2 (moderate staining), 3 (marked staining). Because our staining approach did not easily distinguish gli2+/α-SMA+ cells from gli2-negative/α-SMA+ cells, the general intensity of α-SMA-staining was graded separately, from 0 (no α-SMA staining) to 4 (diffuse sinusoidal staining plus strong staining in fibrous septa).
Clinical Information
All clinical information was collected within 6 months of the baseline and end-of-study liver biopsy. Age, body mass index (BMI; kg/m2), hypertension (HTN), impaired glucose tolerance (IGT), serum aspartate aminotransferase (AST), serum alanine aminotransferase (ALT), homeostasis model assessment–insulin resistance (HOMA-IR) were evaluated.
Statistical Analyses
Data were reported as the mean ± standard deviation (SD) or median (interquartile range, IQR) for continuous variables and as a percentage for categorical variables. Clinical characteristics at baseline were compared between the treatment groups, VitE vs. placebo, using t-tests or Chi-square tests. For variables with a skewed data distribution, Wilcoxon rank-sum tests were used. To compare baseline immunohistochemistry data between the treatment groups, Wilcoxon rank-sum tests were used.
To assess associations between IHC parameters, serum aminotransferases, and H&E histologic scores, we used a multiple linear (or ordinal logistic) regression model to adjust for baseline values. More specifically, when the association between changes in Shh+ hepatocytes and changes in K8/18-negative/ubiquitin+ foci was assessed, baseline numbers of Shh+ hepatocytes and K8/18-negative/ubiquitin+ foci were included in the model. Similarly, for the associations between changes in serum aminotransferases and changes in Shh+ hepatocytes, baseline values of these were included. For the associations of changes in ballooning and fibrosis stage with changes in Shh+ hepatocytes, the baseline H&E score and Shh+ hepatocytes were included. For the association between changes in the accumulation of Shh-responsive progenitors and changes in Shh+ hepatocytes, baseline grades and numbers of these were included. For the association between changes in α-SMA staining and changes in Shh+ hepatocytes, baseline grades of α-SMA staining and baseline numbers of Shh+ hepatocytes were included. To assess the associations of changes in Shh+ hepatocytes and K8/18-negative/ubiquitin+ foci with the treatment response, we used Wilcoxon rank-sum tests as well as multiple linear regression models to adjust for baseline numbers of Shh+ hepatocytes or K8/18-negative/ubiquitin+ foci.
In addition, we assessed associations between the treatment groups with regards to responders/non-responders and we assessed associations between responders and nonresponders within each treatment group using Wilcoxon rank sum tests. Also, the IHC parameters were compared between patients with NASH resolution (defined as definite steatohepatitis at baseline and borderline steatohepatitis or simple steatosis at the end of study) and those without NASH resolution using Wilcoxon rank sum tests in a sub-cohort of patients who had definite steatohepatitis at baseline.
We used JMP Pro statistical software version 9.02 (SAS institute INC, NC) and considered differences statistically significant when the p-values were less than 0.05. All p-values are two-sides, and have not been adjusted for multiple comparisons.
RESULTS
Clinical, Histological and IHC Characteristics of the Study Population
The clinical and histological characteristics of the study population are summarized in Table 1. There were no statistical differences between the pretreatment placebo and VitE groups with regards to age, gender, race, ethnicity, BMI, HTN, IGT, ALT, AST and HOMA-IR. Likewise, the pre-treatment histological scores for ballooning grade and fibrosis stage were not statistically different between the pretreatment groups. The baseline (pre-treatment) IHC scores are summarized in Table 2. The baseline IHC scores for Shh, ballooned hepatocytes (marked by K8/18/Ub) and Hh-responsive progenitors and myofibroblasts (marked by co-expression of gli2/sox9/α-SMA) were also not statistically different between the placebo and VitE groups. Histological improvement, as defined previously in the PIVENS study design (3), occurred in 27.6% of the placebo group and 43.3% of the VitE group.
Table 1.
Baseline clinical characteristics of the study population.
| Placebo (N=29) | Vitamin E (N=30) | p-value# | |
|---|---|---|---|
| Age, years | 46±11 | 47±10 | 0.60 |
| Gender, female % | 58.6% | 60.0% | 0.91 |
| Race, White % | 82.8% | 80.0% | 0.79 |
| Ethnicity, Hispanic % | 6.9% | 20.0% | 0.13 |
| BMI, kg/m2 | 35.5±7.1 | 35.0±7.5 | 0.80 |
| HTN, % | 58.6% | 66.7% | 0.52 |
| IGT, % | 48.3% | 56.7% | 0.52 |
| Serum ALT, IU/L | 74.9±37.0 | 87.6±47.3 | 0.26 |
| Serum AST, IU/L | 49.4±25.4 | 57.0±29.5 | 0.30 |
| HOMA-IR | 4.9±3.9 | 5.7±4.5 | 0.46* |
| Histological scores | |||
| Hepatocyte ballooning, grade 0, 1, 2 % | 17.2%; 34.5%; 48.3% | 16.7%; 36.7%; 46.7% | 0.98 |
| Fibrosis, stage 0, 1, 2, 3 % | 13.8%; 31.0%; 34.5%; 20.7% | 6.7%; 40.0%; 30.0%; 23.3% | 0.75 |
#: p-values were from t-test or Chi-square test, except for
(Wilcoxon Rank-sum test). BMI: body mass index, IGT: impaired glucose tolerance, HTN: systemic hypertension, HOMA- IR: Homeostasis Model of Assessment - Insulin Resistance
Table 2.
Baseline immunohistochemistry data.
| Placebo (N=29) | Vitamin E (N=30) | p-value# | |
|---|---|---|---|
| Shh-positive hepatocytes, cells/HPF | 4.5 ± 9.1 | 4.4 ± 8.0 | 0.60 |
| K8/18 negative or ubiquitin-positive foci, cells/HPF | 2.8 ± 5.6 | 3.4 ± 6.3 | 0.73 |
| SMA-positive stain score, 0 to 4 | 2(1, 2.8) | 2(2, 3) | 0.34 |
| Gli2+/sox9+ stain score, 0 to 4 | 1(1, 2) | 2(1, 2) | 0.23 |
#: p-values were from Wilcoxon Rank Sum tests. Data were presented as mean ± SD for numeric variables and median (IQR) for ordinal variables.
Changes in Shh expression correlate with changes in K8/18/Ub score, ballooning score, fibrosis stage, and serum AST and ALT values
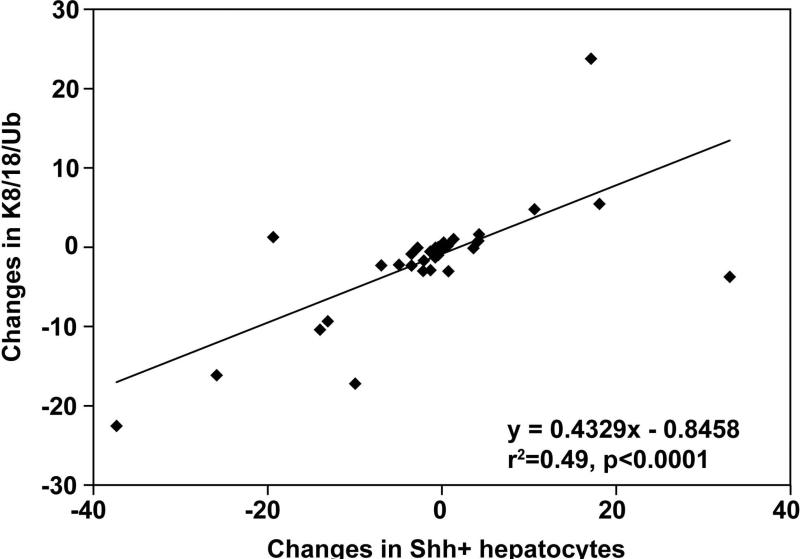
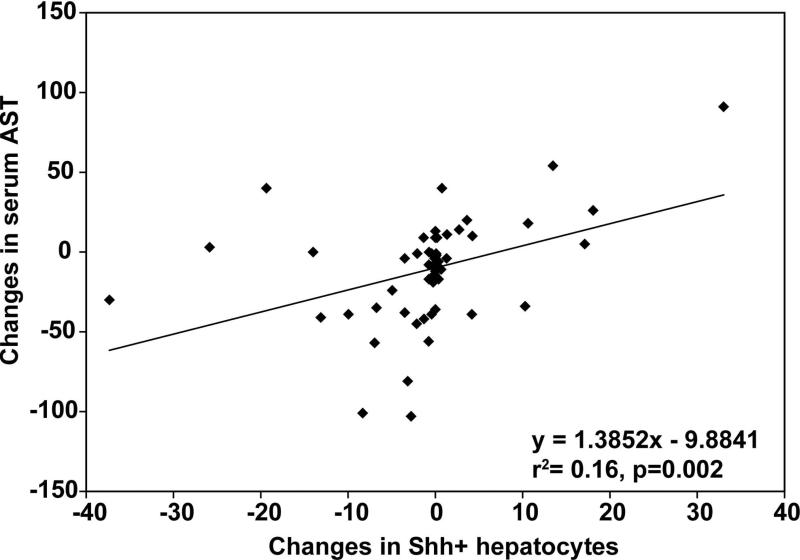
Regardless of the treatment arm, changes in the number of Shh+ hepatocytes were correlated with changes in the severity of liver injury, as reflected by alterations in the number of K8/18/Ub foci (r2=0.49, p<0.001, Figure 1) and the serum AST value (r2=0.16, p=0.002, Figure 2). After adjusting for baseline numbers, changes in Shh+ hepatocytes and the changes in K8/18-negative/ubiquitin+ foci were directly correlated (partial r2= 0.19, p = 0.0031). Changes in serum aminotransferases, AST (partial r2=0.75, p<0.0001) and ALT (partial r2=0.27, p<0.0001), were also directly correlated with changes in the number of Shh+ hepatocytes. These findings imply that in the overall study cohort, the changes in the amount of Shh ligands were directly correlated with changes in severity of liver injury. Changes in ballooning grade and fibrosis stage were also associated with changes in Shh+ hepatocytes; after adjusting for baseline numbers of Shh+ hepatocytes and H&E histologic scores, a decrease in numbers of Shh+ hepatocytes was associated with an increased likelihood of improved hepatocyte ballooning (p=0.004) and fibrosis (p=0.02).
FIGURE 1.
The plot shows results from simple linear regression analysis between the changes in Shh+ ballooned hepatocytes (horizontal axis) and the changes in K8/18-negative hepatocytes/ubiquitin-positive foci (vertical axis). There was significant positive correlation (p<0.0001).
FIGURE 2.
The plot shows simple linear regression between the changes in Shh+ ballooned hepatocytes (horizontal axis) and the changes in serum AST (vertical axis).There was significant positive correlation (p=0.002).
Changes in Shh expression correlate with changes in the number of Shh-responsive progenitors (the ductular reaction)
In NASH, the accumulation of ductular-appearing progenitor cells and associated stromal elements (dubbed the ductular reaction) correlates with fibrosis severity (27, 28). In our study cohorts, changes in the numbers of Shh+ hepatocytes were directly associated with changes in the accumulation of Shh-responsive progenitors (gli2+/sox9+ cells); after adjusting for baseline differences in numbers of Shh+ hepatocytes and Hh-responsive progenitors, the association was statistically significant (p=0.03). These results suggest that decreasing exposure to Shh ligands attenuated the ductular reaction. Consistent with this evidence, a decrease in the numbers of Shh+ hepatocytes also tended to be associated with an increased likelihood of improving the intensity and extent of α-SMA staining, which reflects fibrogenesis (p=0.10).
Response to treatment correlates with a greater decrease in Shh+ hepatocytes compared to non-response
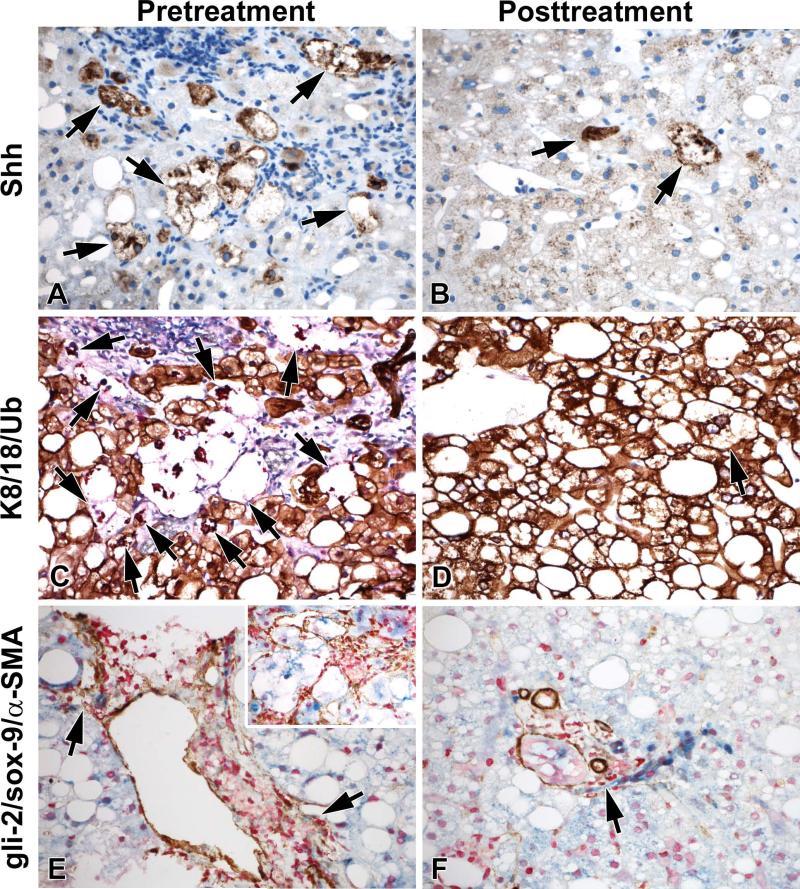
A response to treatment (as established by the PIVENS study design) was associated with a greater decrease in Shh+ hepatocytes compared to non-response (p=0.007). Compared to the placebo group, the VitE group showed a greater reduction in the number of Shh+ hepatocytes (p<0.05) and K/8/18/Ub foci (p=0.08; Table 3). The effect of VitE became more apparent after adjusting for baseline numbers of Shh+ hepatocytes or K8/18/Ub foci by multiple linear regression models (p<0.04 for both decrease in Shh+ hepatocytes and K8/18/Ub foci in VitE group versus placebo-treated controls). Among non-responders, the VitE group also showed a greater reduction of Shh+ hepatocytes compared to the placebo group (p<0.05). Figure 3 shows representative IHC photomicrographs from a VitE responder.
Table 3.
Comparison of changes in immunohistochemical data with and without adjusting for baseline values: Placebo vs. Vitamin E groups.
| Changes in: | Placebo (N=29) | Vitamin E (N=30) | p-value# | β±SE/ OR[95%CI] | p-value |
|---|---|---|---|---|---|
| Shh+ hepatocytes, cells/HPF | 1.2±10.7 | −3.0±8.3 | 0.048 | −4.4±2.1 | 0.037 |
| K8/18 negative cells or ubiquitin-positive foci/HPF | 0.2±1.4 | −2.9±1.3 | 0.082 | −2.6±1.2 | 0.037 |
| α-SMA+ stain score, 0 to 4 | 0 (0, 0) | 0 (−1, 0) | 0.097 | 1.8[0.6, 5.6] | 0.34 |
| Gli2/sox9+ stain score, 0 to 4 | 0 (0, 0) | 0 (−1, 0) | 0.068 | 1.7[0.5, 5.5] | 0.36 |
Data were presented as mean ± SD for numeric variables and median (IQR) for ordinal variables. #: Wilcoxon Rank-Sum tests There were some cases with missing data: Shh-positive hepatocytes (N=2), K8/18 negative or ubiquitin-positive foci (N=14), α-SMA-positive stain score (N=1) and gli2/sox9-positive stain score (N=2). B-coefficients and SE were computed in multiple linear regression models, while cumulative odds ratios were computed in multiple ordinal logistic regression models, both of which were adjusted for baseline values.
FIGURE 3.
Representative photomicrographs of a VitE responder pre- and post-treatment show decreased numbers of Shh+ hepatocytes (A and B, arrows, Shh is brown), decreased numbers of K8/18-negative hepatocytes/Ub+ foci (C and D, arrows, K8/18 is brown, Ub is red), and decreased ductular reaction (E and F, arrows, α-SMA is brown, gli-2 is red, and sox-9 is blue), with marked pericellular fibrosis (inset E) in the pre-treatment biopsy. All photos are at 400× magnification.
Comparison between non-responders and responders
Regardless of the treatment group (VitE or placebo), “responders” (as established by the PIVENS study design) showed statistically significant improvements in steatosis, lobular inflammation, hepatocyte ballooning, Mallory-Denk bodies, AST, ALT, Shh-positive hepatocytes and α-SMA score and tended to show significant improvement in gli-2-positive progenitor score (Table 4). Of note, there was no statistical evidence that VitE responders were more improved than placebo responders with regards to any parameter other than portal inflammation. However, compared to placebo “non-responders”, VitE non-responders had greater improvements in liver enzymes and Shh-positive hepatocytes.
Table 4.
Comparison of non-responders to responders.
| Non-responders | Responders | p-value# | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Combined VitE and Placebo (N=38) | Placebo (N=21) | Vitamin E (N=17) | p-value# | Combined VitE and Placebo (N=21) | Placebo (N=8) | Vitamin E (N=13) | p-value$ | ||
| H&E Histologic changes | |||||||||
| Steatosis | 0[−1, 0.25] | 0[−0.5, 1] | 0[−2, 0] | 0.0624 | −1[−1.5, 0] | −0.5[−1, 0] | −1[−2, 0] | 0.2819 | 0.0104 |
| Lobular inflammation | 0[−0.25, 0] | 0[0, 0.5] | 0[−1, 0] | 0.1238 | −1[−1,0] | −1[−1,0.25]§ | −1[−1, 0] | 0.7186 | 0.0003 |
| Hepatocyte ballooning | 0[0, 1] | 0[0, 1] | 0[0, 1] | 0.8593 | −1[−2, −1] | −1.5[−2 ,−1]§ | −1[−1, −1] | 0.1057 | <0.0001 |
| Mallory-Denk bodies | 0[0, 0] | 0[0, 0] | 0[−1, 0] | 0.2594 | 0[−1, 0] | 0[−0.75, 0] | 0[−1, 0] | 0.5647 | 0.0311 |
| Portal inflammation | 0[0, 0] | 0[0, 0] | 0[0, 0] | 0.9545 | 0[−0.5, 0] | 0[0, 0.75] | 0[−1, 0]§ | 0.0147 | 0.1859 |
| Fibrosis | 0[−1, 0.25] | 0[0, 0] | 0[−1, 1] | 0.9748 | 0[−1, 0] | 0[−1, 0] | 0[−1, 0] | 0.8006 | 0.1140 |
| Biochemical changes | |||||||||
| Serum ALT change, U/L | −11.2±47.8 | 0.2±30.3 | −26.1±61.9 | 0.0215 | −49.1±50.6 | −32.8±40.5§ | −59.2±55.0 | 0.4254 | 0.0078 |
| Serum AST change, U/L | −1.6±34.3 | 9.4±29.8 | −16.1±35.2 | 0.0205 | −24.7±29.2 | −12.9±23.5 | −31.9±30.8 | 0.1282 | 0.0103 |
| IHC parameter changes | |||||||||
| Shh-positive hepatocytes, cells/HPF | 0.74±10.3 | 2.6±12.2 | −1.9±6.2 | 0.0464 | −3.6±8.2 | −2.4±2.9§ | −4.3±10.2 | 0.7705 | 0.0067 |
| K8/18 negative cells or ubiquitin-positive foci/HPF | −0.91±6.8 | 0.4±7.5 | −2.5±5.7 | 0.1451 | −2.5±5.9 | −0.8±0.9 | −3.5±7.3 | 0.5002 | 0.1556 |
| α-SMA-positive stain score | 0[0, 0] | 0[0, 0] | 0[0, 0] | 0.3736 | 0[−1, 0] | 0[0, 0] | 0[−1, 0] | 0.2388 | 0.0468 |
| gli2/sox9-positive stain score | 0[0, 0] | 0[0, 0] | 0[−1, 0] | 0.0876 | 0[−1, 0] | 0[−0.75, 0] | 0[−1, 0] | 0.7050 | 0.0805 |
: p-values for non-responders vs. responders (combined groups: vitamin E and placebo).
: p-values for placebo vs. vitamin E.
: p-value<0.05 vs. non-responders within the same treatment group.
Comparison between those with and without NASH resolution
NASH resolution is defined as a diagnosis of definite NASH on entry biopsy and a diagnosis of borderline NASH or simple steatosis at the end of study biopsy. Forty four patients (20 in the placebo group and 24 in the VitE group) had a diagnosis of definite NASH at the time of the entry biopsy. Ten patients in the placebo group and 12 patients in the vitamin E group showed NASH resolution. Patients who showed NASH resolution tended to show improvement in Shh-positive hepatocytes compared to ones without NASH resolution (p=0.06).
DISCUSSION
The aim of this study was to evaluate the hypothesis that reduced Hh pathway activity would occur with improvement of NASH in humans. Our earlier work in humans examined the relationship between NASH progression and pathway activity (14, 15, 16, 23). Here we evaluate the relationship between human NASH regression and pathway activity. This is important because if the Hh pathway plays a role in human NAFLD severity, it should be more active when disease is more severe (which our earlier work demonstrates) and less active when disease is less severe.
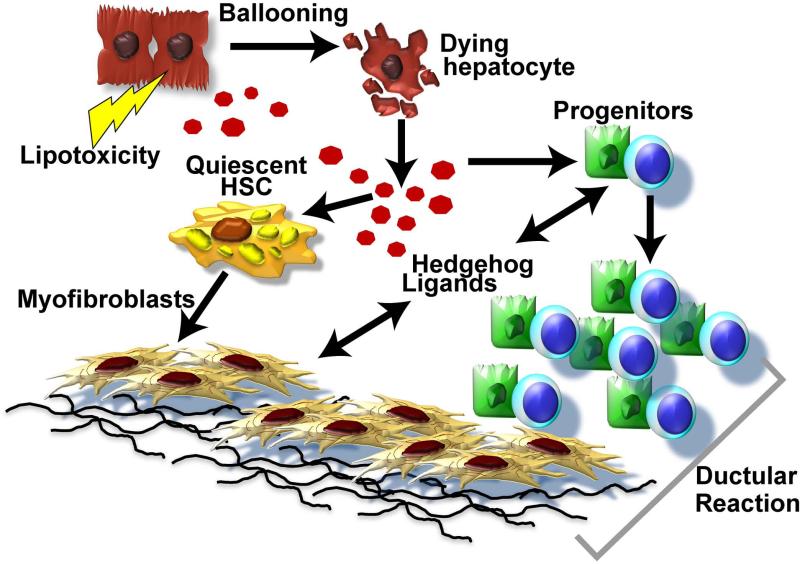
This study shows that improving NASH reduces hepatocyte ballooning and accompanying pathology, including accumulation of progenitors and myofibroblasts. The latter cell types play pivotal roles in liver regeneration but have also been implicated in the pathogenesis of cirrhosis (29). Importantly, our new data link resolution of hepatocyte injury in humans with reduced production of Shh ligands, evolutionarily-conserved DAMPs that orchestrate various regenerative responses in injured adult tissues (12, 17, 18, 19, 20, 21). In our analyses, the histologic responders also showed greater improvement in biochemical, and IHC parameters related to Hh signaling pathway, when compared to the non-responders. Interestingly, VitE nonresponders had greater improvements in liver enzymes and Shh-positive hepatocytes compared to placebo “non-responders” although there was no strong statistical evidence that VitE responders were more improved than placebo responders. It is important to emphasize, however, that our study was not powered for these sub-analyses. Previous studies in experimental animals have shown that deregulated Hh signaling promotes liver fibrosis and cancer (30, 31), and proven that liver progenitor accumulation and fibrogenic repair can be controlled by modulating the intensity of Hh signaling (32) (Figure 4).
FIGURE 4.
Model of Hh mediated, deregulated fibrogenic repair in NASH.
In patients enrolled in the PIVENS trial, we found that numbers of Shh-producing hepatocytes paralleled the severity of liver injury (as reflected by serum aminotransferase levels) and the levels of progenitor and MF accumulation (i.e., the intensity of the ductular reaction). More importantly, comparison of pre- and post-treatment liver biopsies showed that subjects who experienced greater treatment-related reductions in Shh-positive hepatocytes also demonstrated greater improvements in the ductular reaction. Multiple regression analysis revealed that VitE treatment reduced accumulation of Shh-producing hepatocytes and Hh-regulated sequelae significantly more than placebo. Given evidence that the intensity of the ductular reaction predicts fibrosis severity in NASH (27, 28), this insight reveals a potential mechanism to explain why VitE is beneficial in human NASH. In addition, the results justify future studies to determine if long-term VitE therapy will slow progression of NASH-related fibrosis.
The results in this human study complement our prior work in which we identified processes that induce Shh expression in isolated hepatocytes. These processes include apoptotic stress (caused by inhibition of nuclear factor kappa beta function in hepatocytes) (13) and ER stress (as demonstrated in tunicamycin-treated hepatocytes) (14). Other groups have shown that VitE inhibits hepatotoxicity (33-36). Ballooned hepatocytes, a hallmark of lipotoxicity in NASH, have been shown to exhibit apoptotic and ER stress (12, 37). NASH CRN data in humans with NASH enrolled in PIVENS demonstrated that VitE significantly reduced hepatocyte ballooning and serum ALT (a surrogate marker of hepatocyte injury) (3). Therefore, it is reasonable to conclude that VitE reduced hepatocyte production of Shh ligand because it reduced stress-related mechanisms that ordinarily trigger Shh production by hepatocytes.
The new results also support the concept that Shh ligands and other Hh-regulated soluble factors are potential biomarkers of nonalcoholic fatty liver disease (NAFLD) severity in patients. Earlier studies in mice demonstrated that Shh and Osteopontin (OPN), a Hh-regulated fibrogenic factor, are released into the blood during chronic fibrosing liver injury (38, 39). Since those findings were published, reports linking circulating levels of OPN with the severity of liver damage have accumulated rapidly (40, 41). A recently-published microarray analysis of percutaneous liver biopsies from patients on opposite ends of the histologic spectrum of NAFLD provides a mechanism for these observations. Namely, transcripts encoding several Hh-regulated genes, including OPN, were differentially enriched in liver biopsies of patients with subclinical, but histologically-severe, NAFLD relative to biopsies from comparable patients with histologic features of mild NAFLD (23). The gene signature data, in turn, complement and extend earlier reports which used immunohistochemistry to show that accumulation of Shh-producing cells and Shh-responsive cells strongly correlated with NAS score and fibrosis stage in NAFLD patients (15).
Although there is now a preponderance of data demonstrating the relevance of Hh pathway activation to NAFLD outcomes, further research is necessary to directly demonstrate that deregulated Hh signaling per se drives NASH progression in humans, as has been proven to occur in animals (25). Clinical proof-of-concept studies should be feasible because several small molecules inhibitors of the Hh pathway have already been approved for use as cancer chemotherapeutics (42). A number of drugs that have FDA approval for other indications are also capable of blocking Hh signaling and thus, could be re-deployed as therapeutic agents in NASH (43, 44). Finally, aptamers and antibodies that antagonize OPN activity are already in clinical use as treatments for certain rheumatologic conditions (45) and thus, could also be re-purposed to treat NASH patients. Such studies must monitor long-term safety, as well as therapeutic efficacy, because both morphogens are known to have pleitrophic effects and NAFLD is a chronic disease, likely necessitating protracted treatment.
Ultimately, evidence that liver injury in adults reactivates Hh signaling is likely to be most significant because it suggests that related developmental pathways, such as Notch, might be similarly mobilized. During embryogenesis, the Hh and Notch pathways interact to orchestrate tissue construction (46). Thus, factors that control Notch signaling might also prove to be useful therapeutic targets in NAFLD. The general idea of targeting developmental morphogens to improve the outcomes of adult liver injury is paradigm-shifting. The concept is particularly appealing in NASH because recent data demonstrate that Hh and Notch control the fate of wound healing cells by modulating metabolic processes that are known to be deregulated in NAFLD, including glucose and lipid turnover and energy balance (47, 48, 49, 50). Further research will reveal if morphogen mis-regulation is at the root of defective liver repair and demonstrate if the power of these pathways can be harnessed to improve the outcomes of metabolic liver disease in humans.
Acknowledgments
Sources of funding:
Dr. Anna Mae Diehl is supported by NIH (UO1DK061713, R01-DK053792 and R01-DK077794).
Dr. Ayako Suzuki is supported by an American Recovery and Reinvestment Act (ARRA) grant from the NIAAA: 5RC2 AA019399 (Anna Mae Diehl, Principal Investigator).
Dr. Manal Abdelmalek is supported by NIH (UO1DK061713).
List of Abbreviations
- NASH
nonalcoholic steatohepatitis
- VitE
vitamin E
- ER
endoplasmic reticulum
- K
keratin
- Ub
ubiquitin
- Shh
Sonic hedgehog
- DAMPs
damage-associated molecular patterns
- Hh
hedgehog
- Ptc
Patched
- Smo
Smoothened
- Gli
Glioblastoma family transcription factors
- Gli1
Glioblastoma 1 transcription factor
- Gli2
Glioblastoma 2 transcription factor
- Gli3
Glioblastoma 3 transcription factor
- α-SMA
alpha-smooth muscle actin
- Sox9
sex-determining region Y-box 9
- HPCs
hepatic progenitor cells
- H&E
hematoxylin and eosin
- NASH CRN
Nonalcoholic Steatohepatitis Clinical Research Network
- IHC
immunohistochemistry
- HPF
high power field
- BMI
body mass index (weight in kg/height in square meters)
- HTN
hypertension
- IGT
impaired glucose tolerance
- AST
aspartate aminotransferase
- ALT
alanine aminotransferase
- HOMA-IR
homeostasis model of assessment - insulin resistance
- SD
standard deviation
- IQR
interquartile range
- NAFLD
nonalcoholic fatty liver disease
- OPN
osteopontin
Footnotes
Conflict of Interest: The authors report no conflict of interest.
Financial Disclosure: The authors have no financial disclosures.
Author Contributions:
Cynthia D Guy contributed to the generation of the research idea, contributed to the histological evaluations, performed the immunohistochemical evaluations, contributed to the analyses and interpretation of data, contributed to the manuscript writing and critical review of manuscript for final submission.
Ayako Suzuki contributed to the generation of the research idea and study design, performed the analyses, contributed to the interpretation of data, manuscript writing and critical review of manuscript for final submission.
Manal F Abdelmalek contributed to the generation of the research idea, participated in data acquisition, interpretation of data, manuscript writing and critical review of the manuscript for final submission.
James L Burchette optimized and performed the immunohistochemistry of liver biopsy slides and contributed critical review of manuscript for final submission.
Anna Mae Diehl contributed to the generation of the research idea, funded and supervised data acquisition, assisted in data analysis and interpretation, contributed to manuscript writing and critical review and revision of the manuscript for important intellectual content.
REFERENCES
- 1.Loomba R, Sanyal AJ. The global NAFLD epidemic. Nat Rev Gastroenterol Hepatol. 2013;10(11):686–690. doi: 10.1038/nrgastro.2013.171. [DOI] [PubMed] [Google Scholar]
- 2.Roberts EA. Pediatric nonalcoholic fatty liver disease (NAFLD): a “growing” problem? J Hepatol. 2007;46(6):1133–1142. doi: 10.1016/j.jhep.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 3.Sanyal AJ, Chalasani N, Kowdley KV, McCullough A, Diehl AM, Bass NM, Neuschwander-Tetri BA, Lavine JE, Tonascia J, Unalp A, Van Natta M, Clark J, Brunt EM, Kleiner DE, Hoofnagle JH, Robuck PR. NASH CRN. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010;362(18):1675–85. doi: 10.1056/NEJMoa0907929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Matteoni CA, Younossi ZM, Gramlich T, Boparai N, Liu YC, McCullough AJ. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology. 1999;116(6):1413–9. doi: 10.1016/s0016-5085(99)70506-8. [DOI] [PubMed] [Google Scholar]
- 5.Alkhouri N, Dixon LJ, Feldstein AE. Lipotoxicity in nonalcoholic fatty liver disease: not all lipids are created equal. Expert Rev Gastroenterol Hepatol. 2009;3(4):445–51. doi: 10.1586/egh.09.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Herms A, Bosch M, Ariotti N, Reddy BJ, Fajardo A, Fernández-Vidal A, Alvarez-Guaita A, Fernández-Rojo MA, Rentero C, Tebar F, Enrich C, Geli MI, Parton RG, Gross SP, Pol A. Cell-to-cell heterogeneity in lipid droplets suggests a mechanism to reduce lipotoxicity. Curr Biol. 2013;23(15):1489–96. doi: 10.1016/j.cub.2013.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Caldwell S, Ikura Y, Dias D, Isomoto K, Yabu A, Moskaluk C, Pramoonjago P, Simmons W, Scruggs H, Rosenbaum N, Wilkinson T, Toms P, Argo CK, Al-Osaimi AM, Redick JA. Hepatocellular ballooning in NASH. J Hepatol. 2010;53(4):719–23. doi: 10.1016/j.jhep.2010.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gramlich T, Kleiner DE, McCullough AJ, Matteoni CA, Boparai N, Younossi ZM. Pathologic Features Associated with Fibrosis in Nonalcoholic Fatty Liver Disease. Hum Pathol. 2004;35:196–199. doi: 10.1016/j.humpath.2003.09.018. [DOI] [PubMed] [Google Scholar]
- 9.Brunt EM, Kleiner DE, Wilson LA, Belt P. Neuschwnader-Tetri BA; for the NASH CRN. Nonalcoholic fatty liver disease (NAFLD) activity score and the histopathologic diagnosis in NAFLD: distinct clinicopathologic meanings. Hepatology. 2011;53:810–820. doi: 10.1002/hep.24127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lackner C, Gogg-Kamerer M, Zatloukal K, Stumptner C, Brunt EM, Denk H. Ballooned hepatocytes in steatohepatitis: the value of keratin immunohistochemistry for diagnosis. J Hepatol. 2008;48(5):821–888. doi: 10.1016/j.jhep.2008.01.026. [DOI] [PubMed] [Google Scholar]
- 11.Schuppan D, Schattenberg JM. Non-alcoholic steatohepatitis: pathogenesis and novel therapeutic approaches. J Gastroenterol Hepatol. 2013;28(Suppl 1):68–76. doi: 10.1111/jgh.12212. [DOI] [PubMed] [Google Scholar]
- 12.Kakisaka K, Cazanave SC, Werneburg NW, Razumilava N, Mertens JC, Bronk SF, Gores GJ. A hedgehog survival pathway in ‘undead’ lipotoxic hepatocytes. J Hepatology. 2012;57:844–851. doi: 10.1016/j.jhep.2012.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jung Y, Witek RP, Syn WK, Choi SS, Omenetti A, Premont R, Guy CD, Diehl AM. Signals from dying hepatocytes trigger growth of liver progenitors. Gut. 2010;59(5):655– 65. doi: 10.1136/gut.2009.204354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rangwala F, Guy CD, Lu J, Suzuki A, Burchette JL, Abdelmalek MF, Chen W, Diehl AM. Increased Production of Sonic Hedgehog by Ballooned Hepatocytes. J Pathol. 2011;224(3):401–410. doi: 10.1002/path.2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guy CD, Suzuki A, Zdanowicz M, Abdelmalek MF, Burchette J, Unalp A, Diehl AM, NASH CRN. Hedgehog pathway activation parallels histologic severity of injury and fibrosis in human nonalcoholic fatty liver disease. Hepatology. 2012;55(6):1711–21. doi: 10.1002/hep.25559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Swiderska-Syn M, Suzuki A, Guy CD, Schwimmer JB, Abdelmalek MF, Lavine JE, Diehl AM. Hedgehog pathway and pediatric nonalcoholic fatty liver disease. Hepatology. 2013;57(5):1814–1825. doi: 10.1002/hep.26230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martín FA, Peréz-Garijo A, Morata G. Apoptosis in Drosophila: compensatory proliferation and undead cells. Int J Dev Biol. 53(8-10):1341–1347. doi: 10.1387/ijdb.072447fm. [DOI] [PubMed] [Google Scholar]
- 18.Ryoo HD, Gorenc T, Steller H. Apoptotic cells can induce compensatory cell proliferation through the JNK and the Wingless signaling pathways. Dev Cell. 2004;7(4):491–501. doi: 10.1016/j.devcel.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 19.Fan Y, Bergmann A. Distinct mechanisms of apoptosis-induced compensatory proliferation in proliferating and differentiating tissues in the Drosophila eye. Dev Cell. 2008;14(3):399–410. doi: 10.1016/j.devcel.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morata G, Shlevkov E, Pérez-Garijo A. Mitogenic signaling from apoptotic cells in Drosophila. Dev Growth Differ. 2011;53(2):168–176. doi: 10.1111/j.1440-169X.2010.01225.x. [DOI] [PubMed] [Google Scholar]
- 21.Rubartelli A, Lotze MT. Inside, outside, upside down: damage-associated molecular-pattern molecules (DAMPs) and redox. Trends Immunol. 2007;28(10):429–436. doi: 10.1016/j.it.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 22.Briscoe J, Thérond PP. The mechanisms of Hedgehog signaling and its roles in development and disease. Nat Rev Mol Cell Biol. 2013;14(7):416–429. doi: 10.1038/nrm3598. [DOI] [PubMed] [Google Scholar]
- 23.Moylan CA, Pang H, Dellinger A, Suzuki A, Garrett M, Ashley-Koch AE, Choi SS, Michelotti G, Murphy SK, Guy CD, Hampton DD, Chen Y, Tillmann HL, Abdelmalek MF, Hauser MA, Diehl AM. Hepatic Gene Expression Profiles Differentiate Pre-Symptomatic Patients with Mild versus Severe Nonalcoholic Fatty Liver Disease (Severe NAFLD Gene Signature). Hepatology. 2013 Aug 2; doi: 10.1002/hep.26661. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Omenetti A, Choi S, Michelotti G, Diehl AM. Hedgehog signaling in the liver. J Hepatology. 2011;54:366–373. doi: 10.1016/j.jhep.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hirsova P, Ibrahim Sh, Bronk SF, Yagita H, Gores GJ. Vismodegib suppresses TRAIL-mediated liver injury in a mouse model of nonalcoholic steatohepatitis. PLoS One. 2013;8(7):e70599doi. doi: 10.1371/journal.pone.0070599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, Ferrell LD, Liu YC, Torbenson MS, Unalp-Arida A, Yeh M, McCullough AJ, Sanyal AJ. Nonalcoholic Steatohepatitis Clinical Research Network. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41(6):1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 27.Roskams T, Yang SQ, Koteish A, Durnez A, DeVos R, Huang X, Achten R, Verslype C, Diehl AM. Oxidative stress and oval cell accumulation in mice and humans with alcoholic and nonalcoholic fatty liver disease. Am J Pathol. 2003;163(4):1301–11. doi: 10.1016/S0002-9440(10)63489-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Richardson MM, Jonsson JR, Powell EE, Brunt EM, Neuschwander-Tetri BA, Bhathal PS, Dixon JB, Weltman MD, Tilg H, Moschen AR, Purdie DM, Demetris AJ, Clouston AD. Progressive fibrosis in nonalcoholic steatohepatitis: association with altered regeneration and a ductular reaction. Gastroenterology. 2007;133(1):80–90. doi: 10.1053/j.gastro.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 29.Wallace K, Burt AD, Wright MC. Liver fibrosis. Biochem J. Apr. 2008;411(1):1–18. doi: 10.1042/BJ20071570. [DOI] [PubMed] [Google Scholar]
- 30.Omenetti A, Diehl AM. The adventures of sonic hedgehog in development and repair. II. Sonic hedgehog and liver development, inflammation, and cancer. Am J Physiol Gastrointest Liver Physiol. 2008;294(3):G595–8. doi: 10.1152/ajpgi.00543.2007. [DOI] [PubMed] [Google Scholar]
- 31.Chan IS, Guy CD, Chen Y, Lu J, Swiderska-Syn M, Michelotti GA, Karaca G, Xie G, Krüger L, Syn WK, Anderson BR, Pereira TA, Choi SS, Baldwin AS, Diehl AM. Paracrine Hedgehog signaling drives metabolic changes in hepatocellular carcinoma. Cancer Res. Dec 15. 2012;72(24):6344–50. doi: 10.1158/0008-5472.CAN-12-1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Choi SS, Omenetti A, Syn WK, Diehl AM. The role of Hedgehog signaling in fibrogenic liver repair. Int J Biochem Cell Biol. 2011;43(2):238–244. doi: 10.1016/j.biocel.2010.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hishinuma I, Nakamura T. Alpha-tocopherol and inhibition of cytolysis in glutathione-depleted hepatocytes in primary culture. J Nutr Sci Vitaminol (Tokyo) 1988;34:11–23. doi: 10.3177/jnsv.34.11. [DOI] [PubMed] [Google Scholar]
- 34.Miyazaki M, Bai L, Tsuboi S, Seshimo K, Namba M. Effects of antioxidants on survival of adult rat hepatocytes under various oxygen tensions in serum-free primary culture. Acta Med Okayama. 1991;45:441–444. doi: 10.18926/AMO/32182. [DOI] [PubMed] [Google Scholar]
- 35.Watanabe M, Sugimoto M, Ito K. The acrolein cytotoxicity and cytoprotective action of alpha-tocopherol in primary cultured rat hepatocytes. Gastroenterol Jpn. 1992;27:199–205. doi: 10.1007/BF02777723. [DOI] [PubMed] [Google Scholar]
- 36.Factor VM, Laskowska D, Jensen MR, Woitach JT, Popescu NC, Thorgeirsson SS. Vitamin E reduces chromosomal damage and inhibits hepatic tumor formation in a transgenic mouse model. Proc Natl Acad Sci U S A. 2000;97:2196–2201. doi: 10.1073/pnas.040428797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thakur PC, Stuckenholz C, Rivera MR, Davison JM, Yao JK, Amsterdam A, Sadler KC, et al. Lack of de novo phosphatidylinositol synthesis leads to endoplasmic reticulum stress and hepatic steatosis in cdipt-deficient zebrafish. Hepatology. 2011;54:452–462. doi: 10.1002/hep.24349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Witek RP, Yang L, Liu R, Jung Y, Omenetti A, Syn WK, Choi SS, Cheong Y, Fearing CM, Agboola KM, Chen W, Diehl AM. Liver cell-derived microparticles activate hedgehog signaling and alter gene expression in hepatic endothelial cells. Gastroenterology. 2009;136(1):320–330. doi: 10.1053/j.gastro.2008.09.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Syn WK, Choi SS, Liaskou E, Karaca GF, Agboola KM, Oo YH, Mi Z, Pereira TA, Zdanowicz M, Malladi P, Chen Y, Moylan C, Jung Y, Bhattacharya SD, Teaberry V, Omenetti A, Abdelmalek MF, Guy CD, Adams DH, Kuo PC, Michelotti GA, Whitington PF, Diehl AM. Osteopontin is induced by hedgehog pathway activation and promotes fibrosis progression in nonalcoholic steatohepatitis. Hepatology. 2011;53(1):106–115. doi: 10.1002/hep.23998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ge X, Leung TM, Arriazu E, Lu Y, Urtasun R, Christensen B, Fiel MI, Mochida S, Sørensen ES, Nieto N. Osteopontin binding to lipopolysaccharide lowers tumor necrosis factor-α and prevents early alcohol-induced liver injury in mice. Hepatology. 2013 Nov 9; doi: 10.1002/hep.26931. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sutti S, Jindal A, Locatelli I, Vacchiano M, Gigliotti L, Bozzola C, Albano E. Adaptive immune responses triggered by oxidative stress contribute to hepatic inflammation in NASH. Hepatology. 2013 Oct 1; doi: 10.1002/hep.26749. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 42.Xie J, Bartels CM, Barton SW, Gu D. Targeting hedgehog signaling in cancer: research and clinical developments. Onco Targets Ther. 2013;6:1425–1435. doi: 10.2147/OTT.S34678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim J, Aftab BT, Tang JY, Kim D, Lee AH, Rezaee M, Kim J, Chen B, King EM, Borodovsky A, Riggins GJ, Epstein EH, Jr, Beachy PA, Rudin CM. Itraconazole and arsenic trioxide inhibit Hedgehog pathway activation and tumor growth associated with acquired resistance to smoothened antagonists Cancer. Cell. 2013;23(1):23–34. doi: 10.1016/j.ccr.2012.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang J, Mook RA, Jr, Lu J, Gooden DM, Ribeiro A, Guo A, Barak LS, Lyerly HK, Chen W. Identification of a novel Smoothened antagonist that potently suppresses Hedgehog signaling. Bioorg Med Chem. 2012;20(22):6751–6757. doi: 10.1016/j.bmc.2012.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Iaffaldano P, Ruggieri M, Viterbo RG, Mastrapasqua M, Trojano M. The improvement of cognitive functions is associated with a decrease of plasma Osteopontin levels in Natalizumab treated relapsing multiple sclerosis. Brain Behav Immun. 2013 Aug 30; doi: 10.1016/j.bbi.2013.08.009. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 46.McGlinn E, van Bueren KL, Fiorenza S, Mo R, Poh AM, Forrest A, Soares MB, Bonaldo Mde F, Grimmond S, Hui CC, Wainwright B, Wicking C. Pax9 and Jagged1 act downstream of Gli3 in vertebrate limb development. Mech Dev. 2005;122(11):1218–33. doi: 10.1016/j.mod.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 47.Chen Y, Choi SS, Michelotti GA, Chan IS, Swiderska-Syn M, Karaca GF, Xie G, Moylan CA, Garibaldi F, Premont R, Suliman HB, Piantadosi CA, Diehl AM. Hedgehog controls hepatic stellate cell fate by regulating metabolism. Gastroenterology. 2012;143(5):1319–1329. doi: 10.1053/j.gastro.2012.07.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pajvani UB, Qiang L, Kangsamaksin T, Kitajewski J, Ginsberg HN, Accili D. Inhibition of Notch uncouples Akt activation from hepatic lipid accumulation by decreasing mTorc1 stability. Nat Med. 2013;19(8):1054–60. doi: 10.1038/nm.3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pospisilik JA, Schramek D, Schnidar H, Cronin SJ, Nehme NT, Zhang X, Knauf C, et al. Drosophila genome-wide obesity screen reveals hedgehog as a determinant of brown versus white adipose cell fate. Cell. 2010;140:148–160. doi: 10.1016/j.cell.2009.12.027. [DOI] [PubMed] [Google Scholar]
- 50.Teperino R, Amann S, Bayer M, McGee SL, Loipetzberger A, Connor T, Jaeger C, Kammerer B, Winter L, Wiche G, Dalgaard K, Selvaraj M, Gaster M, Lee-Young RS, Febbraio MA, Knauf C, Cani PD, Aberger F, Penninger JM, Pospisilik JA, Esterbauer H. Hedgehog partial agonism drives Warburg-like metabolism in muscle and brown fat. Cell. Oct 12. 2012;151(2):414–426. doi: 10.1016/j.cell.2012.09.021. [DOI] [PubMed] [Google Scholar]