Abstract
Objective
The purpose of this study was to determine suture-holding properties of tissue engineered neocartilage relative to native articular cartilage. To this end, suture pull-out strength was quantified for native articular cartilage and for neocartilages possessing various mechanical properties.
Methods
Suture holding properties were examined in vitro and in vivo. Neocartilage from bovine chondrocytes was engineered using two sets of exogenous stimuli resulting in neotissue of different biochemical compositions. Compressive and tensile properties and glycosaminoglycan, collagen, and pyridinoline cross-link contents were assayed (study 1). Suture pull-out strength was compared between neocartilage constructs, and bovine and leporine native cartilage. Uniaxial pull-out test until failure was performed after passing 6-0 Vicryl through each tissue (study 2). Subsequently, neocartilage was implanted into a rabbit model to examine short-term suture holding ability in vivo (study 3).
Results
Neocartilage glycosaminoglycan and collagen content per wet weight reached 4.55% ± 1.62% and 4.21 ± 0.77%, respectively. Tensile properties for neocartilage constructs reached 2.6 ± 0.77 MPa for Young’s modulus and 1.39 ± 0.63 MPa for ultimate tensile strength. Neocartilage reached ~33% of suture pull-out strength of native articular cartilage. Neocartilage cross-link content reached 50% of native values, and suture pull-out strength correlated positively with cross-link content (R2=0.74). Neocartilage sutured into rabbit osteochondral defects was successfully maintained for 3 weeks.
Conclusion
This study shows that pyridinoline cross-links in neocartilage may be vital in controlling suture pull-out strength. Neocartilage produced in vitro with one-third of native tissue pull-out strength appears sufficient for construct suturing and retention in vivo.
Keywords: self-assembling process, tissue engineering, articular cartilage, suture pull-out
Introduction
Tissue engineered replacement cartilage seeks to fill the gap between pain reducing procedures and total joint arthroplasty. To this end, scaffold-free, self-assembled neocartilage has been generated with clinically relevant properties approaching those of native articular cartilage (Hu and Athanasiou 2006). For practical purposes, any tissue engineered cartilage construct must be mechanically robust enough to be handled during surgery and maintain integrity while being secured to the surrounding native tissue (Farhadi et al. 2006). The ability of constructs to be stably fixed within the defect site allows for possible integration with the surrounding tissue, a criterion important for implant survival. Movement can result in delamination, causing the entire implant to be removed and destroyed; this is a common mode of failure in current cartilage repair modalities. At a minimum, movement can hinder interdigitation by disrupting newly formed matrices that bridge the neotissue to the native tissues. At longer time scales, tissue engineered constructs must possess mechanical properties sufficient to resist joint forces. By meeting these primary criteria, biomimetic tissue engineered constructs improve their clinical applicability.
A variety of cartilage retention techniques are in clinical or research stages. Particular focus has been placed on tissue glues or bonding agents, including fibrin glue, cyanoacrylate-based adhesives, or other biocompatible adhesives (Strehin et al. 2010). These function by forming a bridge between the neotissue and native tissue. However, such a bridge may also function as a barrier to integration. Since cartilage’s metabolic activity ceases rapidly in response to defect creation, the potential for integration drops steeply after surgery and may be minimal by the time the adhesive is fully degraded or resorbed. Implant movability is decreased as the adhesive’s surface area increases; extension of the barrier, which may deter long-term integration, is a trade-off for initial fixation of the implant.
Clinical-based approaches for cell-based cartilage repair commonly use suturing of various materials to retain the implant within the defect site. For instance, the cartilage repair technique of autologous chondrocyte implantation (ACI) requires suturing a periosteal flap or collagen membrane over the defect site to help retain the implanted cell suspension. Other treatments utilize an overlay material sutured onto the articular cartilage. Cartilage suturing is also the most commonly used technique in reshaping the cartilage of the tip of the nose during rhinoplasty (Toriumi and Tardy 1995), or for correcting defects of the external ear during otoplasty (Mustarde 1978). Suturing of cartilage is, thus, a well-established and important technique that may be translated to engineered neocartilage.
To date, the characteristics that influence native articular cartilage to hold suture have not been characterized. Implant failure can result from suture pull-out, and it is, thus, important also to characterize the suture holding ability of engineered neocartilage with respect to native tissue. It is likely that, the closer the neocartilage’s biochemical and biomechanical properties are to those of the native tissue, its ability to hold suture will also approach that of native cartilage. Toward this goal, various soluble and biomechanical stimuli have been identified (Natoli et al. 2009a; Elder and Athanasiou 2008; Huey and Athanasiou 2011b). Of these, TGF-β, hydrostatic pressure (HP), and chondroitinase-ABC (C-ABC), alone or in combination, have elicited increases in compressive, tensile, and biochemical properties of treated constructs (Natoli et al. 2009a; Elder and Athanasiou 2008). Combinations of TGF-β and HP have demonstrated synergistic increases in collagen content and tensile properties, while C-ABC treatment of self-assembled constructs increases tensile properties alone or in combination with TGF-β (Huey and Athanasiou 2011a). Neocartilage produced with and without these stimuli possesses widely different properties and are useful for assessing how suture-holding may be related to other tissue properties, particularly collagen cross-linking. Here the term suture-holding is used to denote the capability of a material to both accept suture and resist pull-out of this suture from the material.
Biochemical cross-links in articular cartilage have recently been identified to contribute to the tissue’s tensile properties by interlinking/polymerizing multiple collagen fibers into larger fibrils (Responte et al. 2007). Covalent cross-links dramatically improve the collagen-imparted tensile properties of tissue, reduce protease access, and decrease collagen solubility. Therefore, polymerizing these collagen fibers into larger fibrils through cross-linking may improve suture-holding by providing greater resistance to suture pull-out. To seek out an alternative to tissue glues, the ability of tissue engineered cartilage to be implanted using sutures was investigated in this study. Determining the suture-holding properties of tissue engineered neocartilage holds implications for their successful use as cartilage replacements. To be successful, the neocartilage must be robust enough to survive the forces involved in suturing as well as those in the joint. For this study, four materials were compared in terms of suture pull-out strength: stimulated or unstimulated neocartilage, bovine native articular cartilage, and leporine native articular cartilage. Initial pass of the suture (monotonic test) into the sample and the pull-out of the suture from the tissue(s) was standardized across all samples (Fig. 1).
Fig. 1.
Overview of studies performed, including construct production, stimulation, validation and implantation. Initial self-assembly and treatment with combinations of stimuli (study 1) were applied to produce a robust construct with sufficient sutureholding capacity (study 2) for surgical implantation in a in vivo rabbit osteochondral patellar defect (study 3).
The primary purpose of this study was to assess the suture-holding capacity of treated and unstimulated self-assembled neocartilage constructs compared to native tissue from both bovine and leporine sources. We hypothesized that neocartilage constructs treated with exogenous stimuli would have enhanced suture pull-out strength compared to unstimulated control neocartilage, and that this suture pull-out strength would be on the order of native articular cartilage.
Materials and Methods
Cartilage and chondrocyte acquisition
Articular cartilage was isolated from bovine articular cartilage stifle joints (knees) of animals 4–8 weeks old (Research 87, Boston, MA). Leporine articular cartilage tissue was acquired from six skeletally mature New Zealand White rabbits (approximately 4 kg; 6–8 months old). The rabbits were humanely euthanized for purposes unrelated to this study, under approval by the Institutional Animal Care and Use Committee, University of California, Davis (IACUC). Animals had not undergone orthopaedic or hormonal manipulation. For chondrocyte isolation, cartilage was enzymatically digested immediately, as described below. Otherwise, native cartilage was frozen in protease inhibitor until testing (Athanasiou et al. 1991).
Study 1. Tissue engineered cartilage construct production
Chondrocyte isolation and construct seeding
Cartilage was aseptically minced and digested overnight using 0.2% collagenase (Worthington, Lakewood, NJ) in culture medium containing 3% fetal bovine serum (Atlanta Biologicals, Lawrenceville, GA), as described previously (Hu and Athanasiou 2006). 4.5 million chondrocytes in 100 µl of chondrogenic media (DMEM containing 1% Penicillin-Streptomycin-Fungizone, 1% non-essential amino acids, 1% Insulin-Transferrin-Selenium, 100 nM dexamethasone, 50 µg/ml ascorbate-2-phosphate, 40 µg/ml L-proline, 100 µg/ml sodium pyruvate) were seeded into non-adherent 5 mm agarose wells and allowed to self-assemble to produce constructs as described previously (Elder and Athanasiou 2009b; Hu and Athanasiou 2006). At no time were chondrocytes embedded in the agarose. Chondrocyte constructs were then cultured in a humidified incubator at 37°C and 10% CO2. All cell culture media components were purchased from Invitrogen (Carlsbad, Ca) or Sigma-Aldrich (St. Louis, MO) unless otherwise noted. Transforming growth factor β-1 (TGF-β1) was obtained from Peprotech Inc. (Rocky Hill, NJ), and Chondroitinase-ABC (C-ABC) was obtained from Sigma-Aldrich.
Exogenous stimulus regimen (TGF-β1, hydrostatic pressure, and chondroitinase ABC)
TGF-β1 at 30 ng/ml was applied to combinatorial stimulated constructs from days 0–14, and 10 MPa of static hydrostatic pressure (HP) was applied for 1 hour during days 10–14 as described previously (Elder and Athanasiou 2009a). Stimulated constructs were further treated with C-ABC at 2 U/ml for 4 hours on days 15 and 29 (Murphy et al. 2013; Natoli et al. 2009b; Natoli et al. 2009a). Control constructs were not treated with any exogenous stimuli.
Biochemistry and biomechanical testing
After 42 days in culture, the resulting neocartilage constructs were assayed. Neocartilage pieces were weighed, lyophilized for 96 hours, and reweighed. Samples were then digested to completion using a sequential pepsin-elastase digestion. Collagen content was assayed using the chloramine-T hydroxyproline assay (Woessner 1961). Glycosaminoglycan (GAG) content was assayed using the Biocolor Biglycan GAG assay kit (Biocolor, UK) (Hu and Athanasiou 2006). For histology, neocartilage pieces were frozen in OCT cutting media, sectioned at 14 µm on a cryotome, and adhered to Superfrost Plus slides. Prior to staining sections were fixed in formalin. Sections were stained for Safranin-O/Fast green and Picosirius Red as previously described (Hu and Athanasiou 2006).
The compressive aggregate modulus values of constructs were determined as previously described (Elder and Athanasiou 2009c). Briefly, aggregate modulus was measured using a creep indentation apparatus (Athanasiou et al. 1994) with a 0.8 mm flat porous indenter tip, a tare weight of 0.2 g and a test load of 0.7 g (Elder and Athanasiou 2009b). Using the linear biphasic theory compressive aggregate modulus, Poission’s ratio, and permeability were determined (Mow et al. 1989).
The tensile mechanical properties (Young’s modulus, EY, and Ultimate Tensile Strength, UTS) of constructs were determined using a uniaxial pull-apart-test until failure (Aufderheide and Athanasiou 2007). Gauge length and sample thickness were measured using digital calipers (Hu and Athanasiou 2006). An Instron 5565 materials testing system (Instron, Norwood, MA) was used to achieve a rate of displacement of 1% of the gauge length per second until failure. The cross-sectional area and load-displacement curve was used to generate a stress-strain curve, from which the linear region of the curve was used to determine the Young’s modulus and UTS.
Study 2. Comparison of suture pull-out strength for neocartilage and native cartilage
Single pass suture pull-out
Native bovine and leporine tissues were removed from the underlying bone and cut to 0.5 mm thickness comprising the articulating surface and middle zone, which was then cut into 5 mm discs using a dermal punch. Dimensions were chosen to mimic those of the engineered neocartilage at 5 mm dia. by 0.5 mm thick. Suture consisted of 6-0 Vicryl (Polyglactin 910, Ethicon® Somerville NJ) on a tapered needle, as typically used in flap suturing during ACI.
For the single pass suture test, specimens were attached to paper test strips using cyanoacrylate glue, and suture was passed through the unglued portion of the construct at 2 mm from the edge of the specimen. The suture was then attached to one set of grips while the paper test strip was gripped in the opposite set of grips. A uniaxial pullapart test until failure (pull-out) was then performed in a manner similar to the previously described tensile test, but at a rate of 0.02 mm/s, equivalent to a rate of displacement of 1% of the 2 mm distance from the suture to the edge of the specimen per second until failure.
Cross-link detection
High performance liquid chromatography (HPLC) was used to quantify pyridinoline cross-links. Samples were weighed wet and then digested in 800µL of 6 N HCl at 100°C for 20h. Following digestion, samples were dried using a vacuum concentrator, then resuspended in 50 µL of 10 nmol pyridoxine/mL and 2.4 µmol homoarginine/mL solution, and then diluted fivefold with a solution of 0.5% HFBA acetonitrile in 10% acetonitrile. Samples were injected at 10 µL into a 25 mm C18 column (Shimadzu, Columbia, MD) and eluted using a solvent profile described previously (Eleswarapu et al. 2011). Pyridinoline standards (Quidel, San Diego, CA) were used to generate a standard curve.
Study 3. In vivo suture-holding of engineered cartilage
Rabbit surgical procedure
Three skeletally mature New Zealand White rabbits (weight range 3.5–4.5 kg; age range 6–8 months) were used in this study with IACUC approval. Rabbit surgeries were performed under general anesthesia using a mixture of ketamine (35 mg/kg), xylazine (5 mg/kg), and ace promazine (0.5 mg/kg). Following endotracheal intubation, anesthesia was maintained with Isoflurane 1–3% accompanied by mechanical ventilation. The left knee and surrounding area was shaved and the skin was aseptically prepared for surgery. Intra- and post-operative analgesia was provided with buprenorphine (0.03 mg/kg) twice per day and meloxicam (0.1 mg/kg) once a day for a period of five days.
A medial parapatellar approach to the knee was performed and the patella was subluxed laterally and everted. A 5 mm punch was used to uniformly mark a circular section at the center of the patellar articular cartilage. A dental unit mounted with a sterile burr was used to create a 5 mm-diameter osteochondral defect until bleeding subchondral bone was encountered. All procedures were performed under continuous sterile 0.9% saline irrigation to avoid thermal insult. Stimulated neocartilage was placed upon the defect and sutured to the surrounding rim of native cartilage using 6-0 vicryl with a tapered needle, equivalent to that used for flap suturing during ACI and tested in study 2. Rabbits were allowed weight bearing on the operated legs after 1 week and were euthanized at 3 weeks via intravenous pentobarbital overdose. Samples were decalcified, paraffin embedded, sectioned, and stained for hematoxylin and eosin (H&E).
Statistical Analysis
For the stimulated group n=4–7 was used for all assays, for the unstimulated group n=3–7 was used for all assays. For native cartilage n=6–9 was used for all assays. Groups per test type were analyzed by one-way ANOVA and by Tukey-Kramer post hoc test, when appropriate, using the statistical analysis software package JMP (SAS, Cary, NC). p<0.05 was defined as being statistically significant. For comparisons between stimulated or unstimulated neocartilage or between native cartilages t-test was used. Data used to generate the correlation (Fig. 4) was only from individual samples with data for both pull-out strength and pyridinoline content (paired), significance was calculated using Pearson’s correlation. All data are reported as mean ± standard deviation. In all figures, different letters between groups indicate statistical significance.
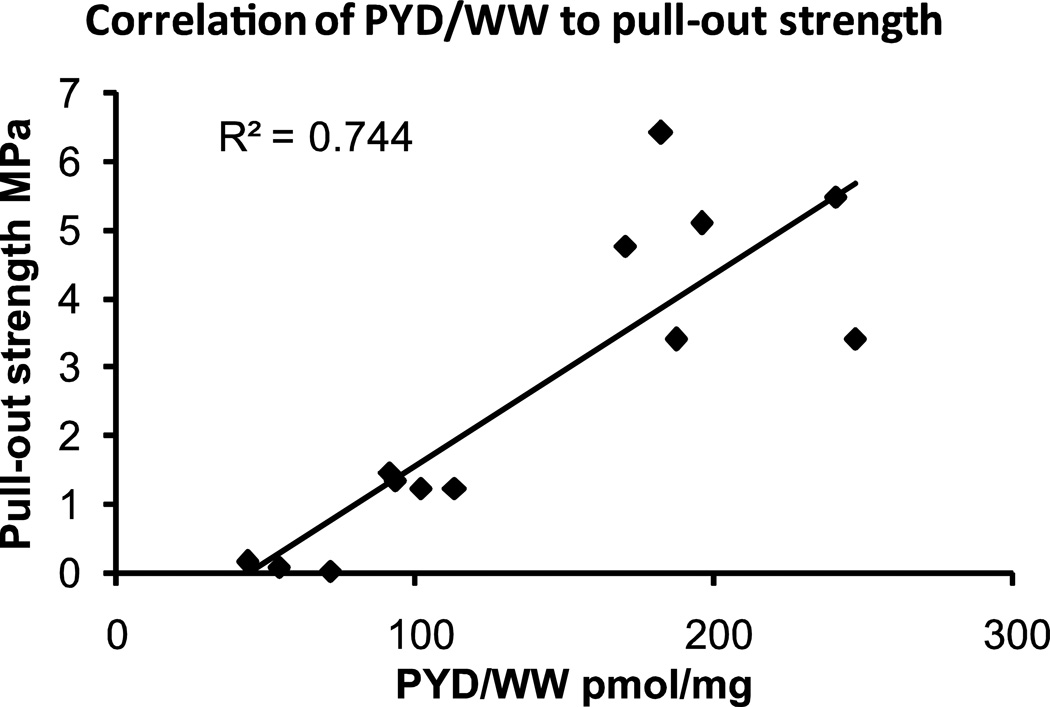
Fig. 4.
Correlation between mechanical properties, cross-linking and suture pull-through strength p<0.0001.
Results
Study 1
Construct gross appearance and histology
Engineered neocartilage constructs were white and glossy in appearance with firm consistency. Thickness of stimulated and unstimulated neocartilage constructs (control) was 0.54 ± 0.05 mm, and 1.40 ± 0.26 mm, respectively. Diameter was 4.93 ± 0.51 mm and 5.07 ± 0.10 mm for stimulated and unstimulated neocartilage constructs, respectively. Representative gross morphology and histological stains are displayed in Fig. 2b, 2c. Picrosirius Red showed intense collagen staining in stimulated constructs, with lesser staining in unstimulated control constructs. Safranin-O and Fast Green staining appeared similar between both stimulated and unstimulated constructs (Fig. 2).
Fig. 2.
(a) Tensile properties of stimulated constructs, Young’s modulus (EY) (left) and ultimate tensile strength (UTS) (right) in MPa. All data are reported as mean +/− standard deviation. Different letters between groups indicate statistical significance at p<0.05. (b) Gross morphology of stimulated and unstimulated constructs, tissue was white and glossy in appearance with a firm consistency. (c) Representative Picrosirius Red and Safranin-O/Fast green histology staining of the stimulated and unstimulated control constructs (40×).
Construct biochemical and mechanical properties
Total collagen content was 4.21 ± 0.77% and 2.50 ± 0.35% for stimulated constructs and for unstimulated controls, respectively. Glycosaminoglycan (GAG) content was 1.29 ± 0.54% and 4.55 ± 1.62% for stimulated and unstimulated control constructs, respectively. Water content for stimulated constructs and for unstimulated controls was 82.8 ± 0.6% and 86.5 ± 1.3%. All biochemical properties assayed were significantly different between stimulated and unstimulated control constructs.
Stimulated constructs exhibited a Young’s modulus (EY) of 2.64 ± 0.77 MPa and an ultimate tensile strength (UTS) of 1.39 ± 0.63 MPa and were significantly different compared to unstimulated (EY = 0.44 ± 0.12 MPa and UTS = 0.14 ± 0.06 MPa) (Fig. 2a). Aggregate modulus for stimulated and unstimulated control constructs was not significantly different at 90.4 ± 16.3 kPa and 134.0 ± 57.7 kPa, respectively.
Study 2
Suture pull-out strength
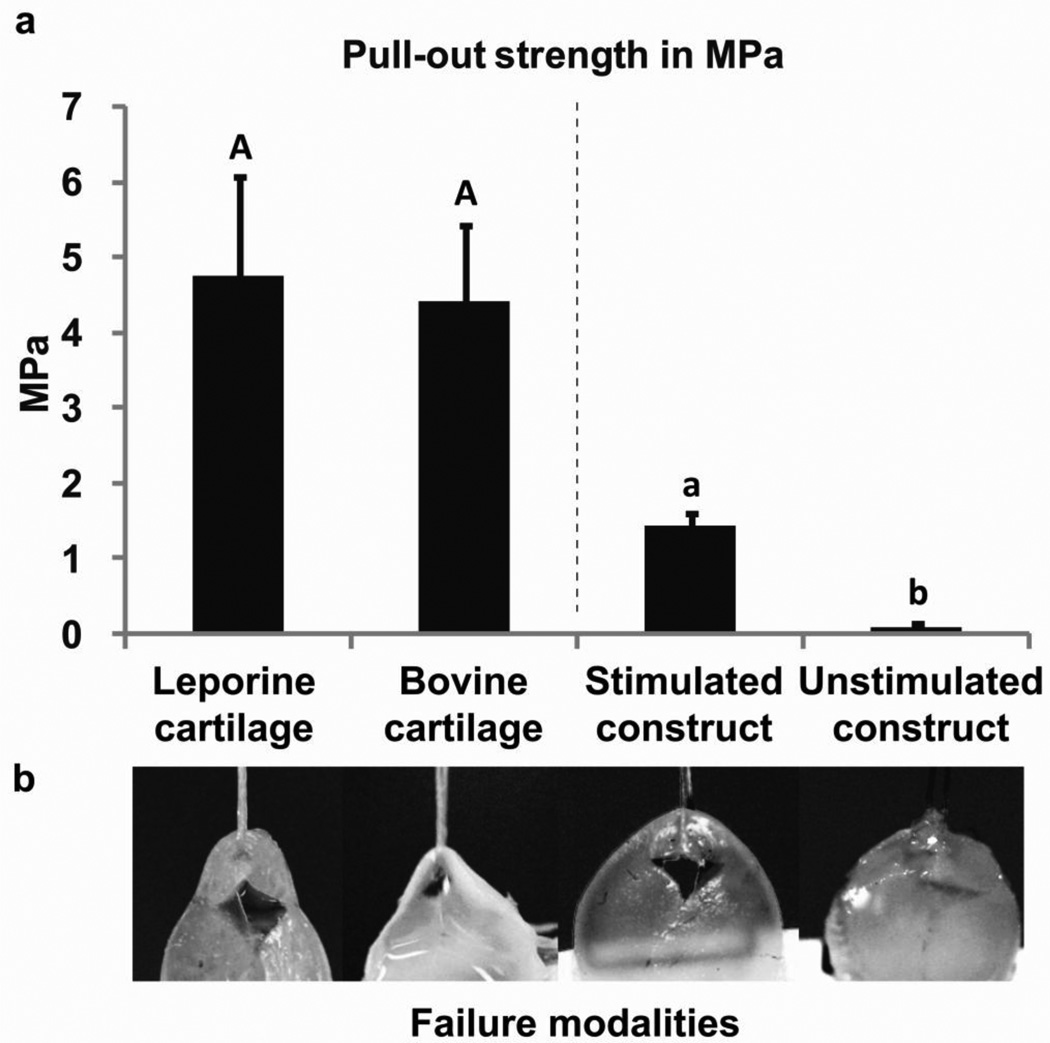
Suture pull-out strengths for bovine (4.40 ± 1.03 MPa) and rabbit native articular cartilage (4.76 ± 1.33 MPa) were similar. Stimulated neocartilage constructs had suture pull-out strength of 1.45 ± 0.18 MPa, or approximately 30–33% of native cartilage. Unstimulated control pull-out strength was much lower (0.08 ± 0.06 MPa, or 1.8–2% of native cartilage) (Fig. 3). Unstimulated constructs were also inherently more difficult to set-up for the suture pull-out test, with suture pull-out sometimes occurring during loading of the sample into the testing apparatus. Mode of failure for stimulated neocartilage constructs and native cartilages were similar; triangular/diamond-shaped tears occurred, with bunching of the sample at the suture-specimen interface, until complete suture pull-through. Unstimulated neocartilage control constructs had a single clean dissection with no apparent bunching of the sample (Fig. 3).
Fig. 3.
(a) Suture pull-out strength of native cartilages was similar. Comparison between engineered cartilages were significantly different p<0.0001. All data are reported as mean +/− standard deviation. Different letters between groups indicate statistical significance at p<0.05. (b) Comparison of failure modalities for samples during suture pull-out. From left to right leporine cartilage, bovine cartilage, stimulated tissue engineered construct and unstimulated control construct. Note the triangular-to-diamond shape tearing of the first two tissues and stimulated construct compared to the clean straight pull-through of the suture on the unstimulated control construct (far right). -Images were adjusted for brightness and contrast to improve visibility.
Cross-link (pyridinoline) content
Stimulated constructs had increased cross-links compared to unstimulated control constructs, at 50% and 25% of native rabbit tissue values, respectively. Stimulated constructs had 113 ± 19 pmol pyridinoline/mg (pyd/mg), and unstimulated constructs had 57 ± 14 pmol pyd/mg. This was compared to native cartilage, with the leporine cartilage tissue having 212 ± 38 pmol pyd/mg while juvenile bovine cartilage tissue was 167 ± 53 pmol pyd/mg; cross-link content correlated to suture pull-out strength per same sample with an R2 of 0.74 (Fig. 4).
Study 3
Rabbit implantation
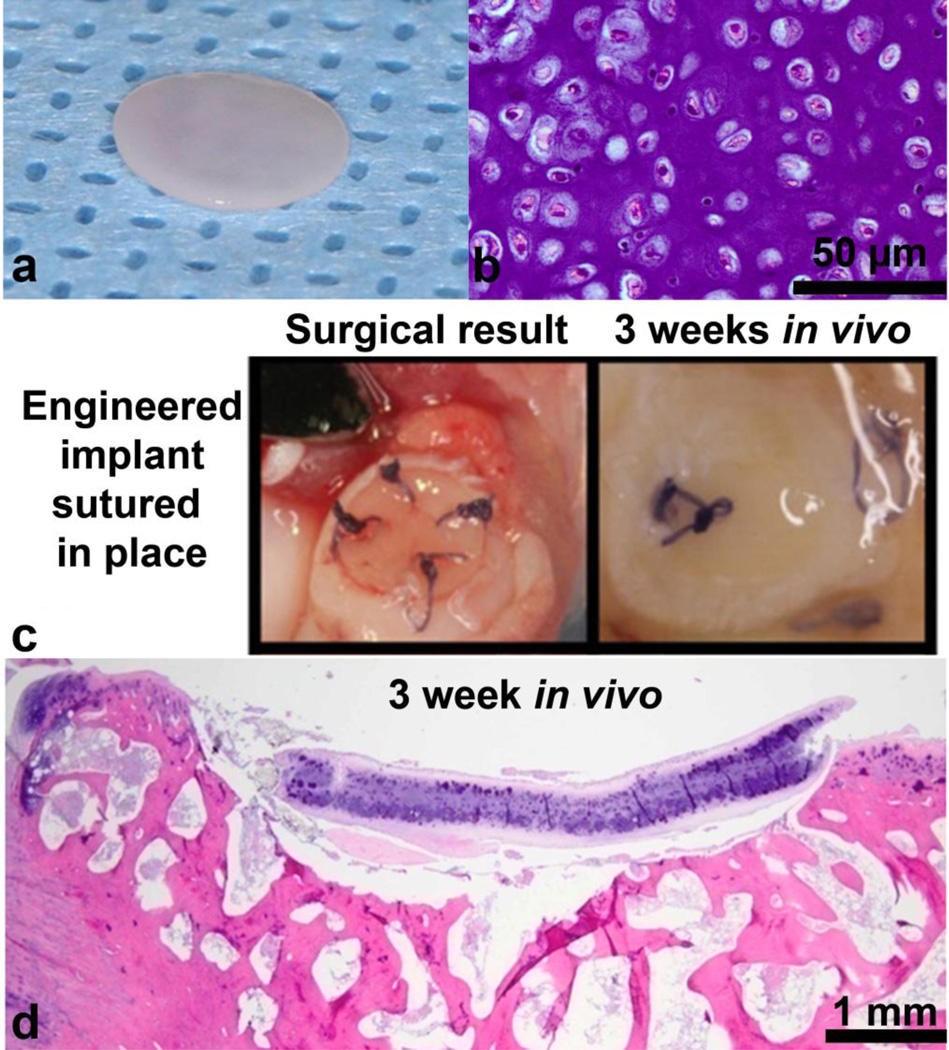
Implanted neocartilage constructs (stimulated) appeared glossy (Fig. 5a) and were capable of being sutured into the osteochondral defect (Fig. 5c left). Following surgery, rabbits were allowed normal locomotion and did not display obvious lameness of the operated legs. After sacrifice at 3 weeks, they were observed to have remained within the osteochondral patellar defects (Fig. 5c right). As expected, Vicryl sutures had begun to degrade without being fully degraded (Fig. 5c right). H&E staining of the implant was similar to pre-implantation neocartilage (Fig. 5b); intense GAG staining was present along with chondrocytes in lacunae (Fig. 5d). Integration with the surrounding native cartilage or underlying bone was not seen at this time point.
Fig. 5.
Stimulated tissue engineered neocartilage constructs were implanted into a rabbit petallar-femoral defect for 3 weeks. Constructs were capable of being sutured into place and persisted until the 3 week sacrifice time. Rabbit in vivo implantation proof-of-concept. (a) Stimulated construct pre-implantation. (b) H&E staining of stimulated construct before implantation (40×). (c) (Left) Stimulated construct sutured into rabbit patellar osteochondral defect using a restorable 6-0 Vicryl, (Right) after 3 weeks in vivo. Sutures have begun resorbing as expected, while the stimulated construct is still in place within the defect. (d)H&E staining of stimulated construct within rabbit patellar defect after 3 weeks (4×).
Discussion
This study was motivated by the practical need for any implantable tissue engineered cartilage construct to be mechanically robust enough to be handled during surgery and to maintain integrity while being sutured to the surrounding native tissue. This study examined the suture-holding capacity of engineered neocartilage possessing different biochemical and biomechanical characteristics, using the native tissue as a reference point. Here we showed that engineered neocartilage can reach suture pull-out strength of ~1/3 that of native tissue. We also showed that suture pull-out strength is correlated with the tissue’s cross-link content (R2=0.74). The feasibility of using sutures to secure neocartilage into a cartilage defect in vivo was also demonstrated.
In the native cartilage, cross-link content was higher for the rabbit cartilage (212 ± 38 pmol pyd/mg) than the bovine (167 ± 53 pmol pyd/mg) cartilage tested. This difference was likely due to the rabbit cartilage having been harvested from skeletally mature animals as compared to the bovine cartilage, which was harvested from juvenile knees. A variety of collagen cross-links have been found to increase with age, including the pyridinoline cross-link (Eyre et al. 1988). Cross-links form through the actions of the enzyme lysyl oxidase at hydroxylysyl pyridinoline residues at the n- and c-telopeptide sites of collagen. Lysyl oxidase functions by oxidizing lysine residues, resulting in several intermediate products. However, the formation of the pyridinoline cross-link is not directly an enzyme-mediated process but rather the product of a cascade; cross-linking therefore increases as a function of time, with adult cartilage having more cross-links than juvenile cartilage. This increase in cross-linking, along with a maturation-dependent increase in collagen content, directly correlates to increased tensile properties (Williamson et al. 2003b; Williamson et al. 2003a). Inasmuch as the tensile properties of articular cartilage are necessary for its function, formation of cross-links at a level consistent with that of native tissue remains a major goal in tissue engineering of cartilage replacements (Fry et al. 1962; Asanbaeva et al. 2008).
It is expected that by enhancing suture pull-out strength the construct’s clinical relevance improves as well. Neocartilage must be able to survive both surgical implantation and the challenging mechanical environment of the joint while the construct integrates with the surrounding tissue. Previous work has shown that in vivo incubation before implantation of a scaffold-based tissue engineered cartilage construct for facial reconstruction, enhanced suture-pull out and resulted in improved clinical outcomes (Farhadi et al. 2006). In conjunction with the importance of the neotissue’s mechanical properties, the anchoring technique used to secure the implant also influences the implant’s long-term success. A caveat of the suturing method is that suturing is frequently destructive to the surrounding tissue; in particular, suturing in articular cartilage may lead to early osteoarthritic changes in the adjacent tissue (Hunziker and Stahli 2008). Construct retention at the site of the defect, ideally via a modality that does not introduce barriers to integration or deleterious changes, must be considered to allow for integration of neocartilage and success in cartilage repair strategies.
Suture-pull out strength is related not only to cross-link content but likely also to collagen structural organization. The role of collagen structural organization in suture-holding is evidenced by the difference in failure modality of the constructs. As suture pulled through native cartilage and the stimulated neocartilage constructs a diamond-shaped tear was evident. This diamond-shaped tearing mode of failure for suture pull-out has previously been linked in other tissues to the collagen organization of the tissue, with collagen bundles pulling through the tissue (Trowbridge et al. 1989). In contrast, the failure modality for unstimulated control constructs demonstrated a clean dissection of the material by the suture, with minimal evidence of resistance to the suture. This difference in collagen organization may explain how, while the unstimulated constructs had the lowest cross-link content, at approximately 26.8% of the rabbit and bovine native tissue value, the pull-out strength was much lower, at only 1.8% of native tissue. Thus, while cross-link content is an important contributor to construct mechanical properties, the near native tissue levels of cross-link content generated by this study indicates that future directions in tissue engineering should focus on enhancement of the collagen structural organization to better replicate native tissue abilities.
Combinations of three stimuli, including HP, TGF-β, and C-ABC produce neocartilage constructs with clinically relevant properties with regard to suture-holding capacity. These stimuli have been previously proven successful by our group; with combinations of HP and TGF-β resulting in synergistic increases in collagen content and enhanced Young’s modulus. Additionally, treatment of constructs with C-ABC have been reported to enhance construct tensile properties (both Young’s modulus and UTS) (Natoli et al. 2009a; Natoli et al. 2009b). In addition, the combination of all three stimuli has likewise generated robust neocartilage from non-articular chondrocyte sources (Murphy et al. 2013). In this study, we evaluated this stimulated tissue engineered neocartilage for its ability to be sutured into a cartilage defect, giving the constructs clinical relevance. Toward this end, we compared stimulated neocartilage to the gold standard material—bovine articular cartilage (e.g., the native source tissue), and rabbit articular cartilage (e.g., the native recipient tissue in implantation). This combination of stimuli appears to generate constructs with clinically relevant properties suitable for future in vivo studies.
Employing a short-term study utilizing three rabbits it was shown stimulated constructs were sufficiently robust for in vivo suturing into a patellar osteochondral defect site. A rabbit patellar osteochondral defect model was employed as the dimensions of the rabbit patella (~0.5 mm cartilage thickness, diameter of ~6 mm) closely approximate that of the neocartilage produced (~0.5 mm cartilage thickness, ~5 mm diameter). The site was also amenable to suturing of the construct into the defect. Despite the neocartilage only exhibiting ~1/3 of the pull-out strength of the native tissue, neocartilage constructs were able to be sutured into rabbit patellar defects without suture pull-out. More importantly, the neocartilage and suture remained intact and in place after three weeks of implantation, two of which consisted of unrestricted movement by the animal. For this early time point, the suture had only partially resorbed (Fig. 5c right), and the cartilage has not yet integrated with the surrounding native tissue. However, cartilage histology showed viable tissue with matrix distribution and morphology similar to healthy native tissue and to neocartilage prior to implantation. This short-term study demonstrated that clinically, neocartilage with 1/3 of native tissue pull-out strength is sufficient for implant suturing and retention.
Our results show the ability to produce clinically relevant engineered cartilage via a combination of stimuli and indicate that pyridinoline cross-links in engineered cartilage may be vital to controlling its suture pull-out strength. The mechanical characteristics that influence suture pull-out strength have seldom been characterized for native or engineered cartilage. Suture holding properties of cartilage likely depend on pyridinoline content and collagen organization. Controlling pyridinoline in engineered constructs should be a design criterion, as pyridinoline correlates well with pull-out strength and thus in vivo suitability. The present study indicates that tissue engineered cartilage constructs with biomechanical properties that are within the range of native tissue may also have sufficient clinically relevant properties for implantation and defect repair.
Acknowledgements
The authors would like to acknowledge the support for this work of NIH R01AR053286, and the Arthritis Foundation Postdoctoral Fellowship of which G. DuRaine is a recipient.
No financial support or benefits from commercial sources were received.
Footnotes
The authors have no conflicts of interest.
References
- Asanbaeva A, Masuda K, Thonar EJ, Klisch SM, Sah RL. Cartilage growth and remodeling: modulation of balance between proteoglycan and collagen network in vitro with beta-aminopropionitrile. Osteoarthritis Cartilage. 2008;16(1):1–11. doi: 10.1016/j.joca.2007.05.019. doi:S1063-4584(07)00198-7 [pii] 10.1016/j.joca.2007.05.019. [DOI] [PubMed] [Google Scholar]
- Athanasiou KA, Agarwal A, Dzida FJ. Comparative study of the intrinsic mechanical properties of the human acetabular and femoral head cartilage. J Orthop Res. 1994;12(3):340–349. doi: 10.1002/jor.1100120306. [DOI] [PubMed] [Google Scholar]
- Athanasiou KA, Rosenwasser MP, Buckwalter JA, Malinin TI, Mow VC. Interspecies comparisons of in situ intrinsic mechanical properties of distal femoral cartilage. J Orthop Res. 1991;9(3):330–340. doi: 10.1002/jor.1100090304. [DOI] [PubMed] [Google Scholar]
- Aufderheide AC, Athanasiou KA. Assessment of a bovine co-culture, scaffold-free method for growing meniscus-shaped constructs. Tissue engineering. 2007;13(9):2195–2205. doi: 10.1089/ten.2006.0291. [DOI] [PubMed] [Google Scholar]
- Elder BD, Athanasiou KA. Synergistic and additive effects of hydrostatic pressure and growth factors on tissue formation. PLoS One. 2008;3(6):e2341. doi: 10.1371/journal.pone.0002341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elder BD, Athanasiou KA. Effects of temporal hydrostatic pressure on tissue-engineered bovine articular cartilage constructs. Tissue Eng Part A. 2009a;15(5):1151–1158. doi: 10.1089/ten.tea.2008.0200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elder BD, Athanasiou KA. Hydrostatic pressure in articular cartilage tissue engineering: from chondrocytes to tissue regeneration. Tissue Eng Part B Rev. 2009b;15(1):43–53. doi: 10.1089/ten.teb.2008.0435. doi:10.1089/ten.teb.2008.0435 10.1089/ten.teb.2008.0435 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elder BD, Athanasiou KA. Systematic assessment of growth factor treatment on biochemical and biomechanical properties of engineered articular cartilage constructs. Osteoarthritis Cartilage. 2009c;17(1):114–123. doi: 10.1016/j.joca.2008.05.006. doi:S1063-4584(08)00166-0 [pii] 10.1016/j.joca.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eleswarapu SV, Responte DJ, Athanasiou KA. Tensile properties, collagen content, and crosslinks in connective tissues of the immature knee joint. PLoS One. 2011;6(10):e26178. doi: 10.1371/journal.pone.0026178. doi:10.1371/journal.pone.0026178 PONE-D-11-14856 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyre DR, Dickson IR, Van Ness K. Collagen cross-linking in human bone and articular cartilage. Age-related changes in the content of mature hydroxypyridinium residues. Biochem J. 1988;252(2):495–500. doi: 10.1042/bj2520495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farhadi J, Fulco I, Miot S, Wirz D, Haug M, Dickinson SC, Hollander AP, Daniels AU, Pierer G, Heberer M, Martin I. Precultivation of engineered human nasal cartilage enhances the mechanical properties relevant for use in facial reconstructive surgery. Ann Surg. 2006;244(6):978–985. doi: 10.1097/01.sla.0000247057.16710.be. doi:10.1097/01.sla.0000247057.16710.be 00000658-200612000-00018 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry P, Harkness ML, Harkness RD, Nightingale M. Mechanical properties of tissues of lathyritic animals. J Physiol. 1962;164:77–89. doi: 10.1113/jphysiol.1962.sp007003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu JC, Athanasiou KA. A self-assembling process in articular cartilage tissue engineering. Tissue Eng. 2006;12(4):969–979. doi: 10.1089/ten.2006.12.969. [DOI] [PubMed] [Google Scholar]
- Huey DJ, Athanasiou KA. Maturational growth of self-assembled, functional menisci as a result of TGF-beta1 and enzymatic chondroitinase-ABC stimulation. Biomaterials. 2011a;32(8):2052–2058. doi: 10.1016/j.biomaterials.2010.11.041. doi:S0142-9612(10)01480-8 [pii] 10.1016/j.biomaterials.2010.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huey DJ, Athanasiou KA. Tension-compression loading with chemical stimulation results in additive increases to functional properties of anatomic meniscal constructs. PLoS One. 2011b;6(11):e27857. doi: 10.1371/journal.pone.0027857. doi:10.1371/journal.pone.0027857 PONE-D-11-10874 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunziker EB, Stahli A. Surgical suturing of articular cartilage induces osteoarthritis-like changes. Osteoarthritis Cartilage. 2008;16(9):1067–1073. doi: 10.1016/j.joca.2008.01.009. doi:S1063-4584(08)00021-6 [pii] 10.1016/j.joca.2008.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mow VC, Gibbs MC, Lai WM, Zhu WB, Athanasiou KA. Biphasic indentation of articular cartilage--II. A numerical algorithm and an experimental study. J Biomech. 1989;22(8–9):853–861. doi: 10.1016/0021-9290(89)90069-9. doi:0021-9290(89)90069-9 [pii] [DOI] [PubMed] [Google Scholar]
- Murphy MK, DuRaine GD, Reddi A, Hu JC, Athanasiou KA. Inducing articular cartilage phenotype in costochondral cells. Arthritis Res Ther. 2013;15(6):R214. doi: 10.1186/ar4409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustarde JC. Correction of prominent ears using buried mattress sutures. Clin Plast Surg. 1978;5(3):459–464. [PubMed] [Google Scholar]
- Natoli RM, Responte DJ, Lu BY, Athanasiou KA. Effects of multiple chondroitinase ABC applications on tissue engineered articular cartilage. J Orthop Res. 2009a;27(7):949–956. doi: 10.1002/jor.20821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natoli RM, Revell CM, Athanasiou KA. Chondroitinase ABC treatment results in greater tensile properties of self-assembled tissue-engineered articular cartilage. Tissue Eng Part A. 2009b;15(10):3119–3128. doi: 10.1089/ten.tea.2008.0478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Responte DJ, Natoli RM, Athanasiou KA. Collagens of articular cartilage: structure, function, and importance in tissue engineering. Crit Rev Biomed Eng. 2007;35(5):363–411. doi: 10.1615/critrevbiomedeng.v35.i5.20. doi:4d8cbde20903daf0,1fff0bc6456f7bb7 [pii] [DOI] [PubMed] [Google Scholar]
- Strehin I, Nahas Z, Arora K, Nguyen T, Elisseeff J. A versatile pH sensitive chondroitin sulfate-PEG tissue adhesive and hydrogel. Biomaterials. 2010;31(10):2788–2797. doi: 10.1016/j.biomaterials.2009.12.033. doi:S0142-9612(09)01420-3 [pii] 10.1016/j.biomaterials.2009.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toriumi DM, Tardy ME. Cartilage suturing techniques for correction of nasal tip deformities. Operative Techniques in Otolaryngology-Head and Neck Surgery. 1995;6(4):265–273. [Google Scholar]
- Trowbridge EA, Lawford PV, Crofts CE. Pericardial heterografts: a comparative study of suture pull-out and tissue strength. J Biomed Eng. 1989;11(4):311–314. doi: 10.1016/0141-5425(89)90065-4. [DOI] [PubMed] [Google Scholar]
- Williamson AK, Chen AC, Masuda K, Thonar EJ, Sah RL. Tensile mechanical properties of bovine articular cartilage: variations with growth and relationships to collagen network components. J Orthop Res. 2003a;21(5):872–880. doi: 10.1016/S0736-0266(03)00030-5. doi:S0736026603000305 [pii] 10.1016/S0736-0266(03)00030-5. [DOI] [PubMed] [Google Scholar]
- Williamson AK, Masuda K, Thonar EJ, Sah RL. Growth of immature articular cartilage in vitro: correlated variation in tensile biomechanical and collagen network properties. Tissue engineering. 2003b;9(4):625–634. doi: 10.1089/107632703768247322. [DOI] [PubMed] [Google Scholar]
- Woessner JF., Jr The determination of hydroxyproline in tissue and protein samples containing small proportions of this imino acid. Arch Biochem Biophys. 1961;93:440–447. doi: 10.1016/0003-9861(61)90291-0. [DOI] [PubMed] [Google Scholar]