Abstract
Glycogenosis type III (GSD III) is an autosomal recessive disorder due to amylo-1,6-glucosidase deficiency. This disease causes limit dextrin storage in affected tissues: liver, skeletal muscles, and heart in GSD IIIa and only liver in GSD IIIb. Cardiomyopathy is quite frequent in GSD IIIa with variable severity and progression of manifestations. It is not clear if diet manipulation may interfere with cardiomyopathy’s progression. Recent case reports showed improvement of cardiomyopathy following a ketogenic diet.
Two siblings (girl and boy), 7- and 5-year-old, both affected with GSD IIIa, developed severe and rapidly worsening left ventricular hypertrophy in the first years of life, while treated with frequent diurnal and nocturnal hyperproteic meals followed by orally administered uncooked cornstarch. Subsequently they were treated with high-fat (60%) and high-protein (25%), low-carbohydrate (15%) diet. After 12 months exertion dyspnea disappeared in the girl and biochemical blood tests, cardiac enzymes, and congestive heart failure markers improved in both (CK 3439→324, 1304→581 U/L; NT-proBNP 2084→206, 782→135 pg/mL, respectively); ultrasound assessment in both patients showed a relevant reduction of the thickness of interventricular septum (30→16, 16→11 mm, respectively) and left ventricle posterior wall (18→7, 13→8 mm, respectively) and an improvement of the outflow obstruction. A diet rich in fats as well as proteins and poor in carbohydrates could be a beneficial therapeutic choice for GSD III with cardiomyopathy. Future research is needed to confirm the beneficial effect of this treatment and to design treatment strategies with the aim to provide alternative source of energy and prevent glycogen accumulation.
Keywords: Amylo-1,6-glucosidase; Cardiac hypertrophy; GSD III; High-protein diet; Ketogenic diet; Low-carbohydrate diet
Glycogen storage disease type III (GSD III) is an inherited recessive disease, due to a defect of amylo-1,6-glucosidase or glycogen debranching enzyme (MIM 232400). This disorder causes limit dextrin storage in affected tissues: liver, skeletal muscles, and heart in GSD IIIa and only liver in the less frequent GSD IIIb. Clinically patients show fasting hypoglycemia, hyperlipidemia, growth delay, enlargement of liver and both skeletal muscles, and heart involvement. Cardiomyopathy with left ventricular hypertrophy is a relatively common finding although with variable severity and progression. It may be associated with potential risk of serious arrhythmia and symptomatic heart failure (Austin et al. 2012). Functionally these patients have only partial glycogenolysis, while glycolysis and gluconeogenesis are preserved. Standard treatment consists of frequent diurnal and nocturnal hyperproteic meals followed by orally administered uncooked cornstarch, with the aim to maintain normal blood glucose (Kishnani et al. 2010). These measures are effective in maintaining the metabolic control although growth retardation, liver, and cardiac and muscular complications may still occur in the long-term follow-up even in well-controlled patients (Kishnani et al. 2010). Recent reports regarding one infant and one adult patient showed an improvement of cardiomyopathy following a ketogenic diet (Valayannopoulos et al. 2011; Sentner et al. 2012). This result was obtained with addition of synthetic ketone bodies as d,l-3-hydroxybutyrate in one case (Valayannopoulos et al. 2011) and with a high protein very-low-calorie diet in the other case (Sentner et al. 2012). Another adult patient (Dagli et al. 2009) improved increasing proteins and reducing carbohydrates intake.
We present two siblings (girl and boy), 7- and 5-year-old, both affected with GSD IIIa who had rapid deterioration of left ventricular hypertrophy. Twelve months after beginning a high-fat and high-protein diet, clinical symptoms ameliorated and biochemical tests as well as echocardiograms showed a clear improvement of cardiomyopathy.
Case Reports
Two siblings from consanguineous Tunisian parents were affected by GSD III and carried the homozygous mutation p.G1087R. The girl was diagnosed at 1.4 year on a clinical basis (hepatomegaly with hyperechogenic tissue at ultrasound, fasting hypoglycemia, hypertriglyceridemia), while the brother at birth. Both were treated with frequent hyperproteic feedings, followed by row cornstarch, because of fasting hypoglycemia. Cardiac hypertrophy was diagnosed at 2.7 years of age in the girl and at 9 months in the boy. The girl started propranolol (1 mg/kg/day) at the age of 2.7 years and the boy at 1.6 years.
Biochemical and clinical data at baseline and 1 year after starting high-fat diet are shown in Table 1.
Table 1.
Biochemical and clinical data of the two siblings before and after 1 year of high-fat diet
| Girl | Boy | |||
|---|---|---|---|---|
| Before | 12 months later | Before | 12 months later | |
| Clinical data | ||||
| Age (years) | 7 | 8 | 5 | 6 |
| Weight (kg), centile | 24.4, 75–90th | 25.3, 50–75th | 18.5, 50th | 18.3, 25th |
| Height (cm), centile | 115.8, 50th | 119, 25th | 101, 10th | 107, 10th |
| Hepatomegaly (cm from rib cage) | 6 | 6 | 8 | 8 |
| Diet | ||||
| Row cornstarch (g/kg ideal body weight/day) | 6 | 0 | 5 | 0 |
| Glucose (mg/kg ideal body weight/min) | 6.5 | 1.9 | 4.8 | 1.9 |
| Proteins (% total energy; g/kg ideal body weight) | 24; 3.5 | 26; 3.3 | 33; 5 | 25; 4.2 |
| Lipids (% total energy) | 12 | 59 | 16 | 60 |
| Carbohydrates (% total energy) | 64 | 15 | 52 | 15 |
| Kcal/day (kcal/kg ideal body weight) | 1,100; 59 | 1,120; 51 | 1,025; 60 | 1,050; 70 |
| Biochemical data | ||||
| Preprandial glucose levels (mg/dL) | 90–100 | 80–100 | 80–90 | 87–94 |
| 1 h postprandial glucose levels (mg/dL) | 70–90 | 70–90 | 120–125 | 70–90 |
| Lactate (normal range 0.44–2.22 mmol/L) | 1.9–2.1 | 0.8–2.2 | 0.6–1.1 | 0.4–0.8 |
| Triglycerides (normal range 50–200 mg/dL) | 117–179 | 127–161 | 155–224 | 246–273 |
| Cholesterol (normal range 130–200 mg/dL) | 114–176 | 170–171 | 170–204 | 164–175 |
| Creatine kinase (normal range 20–180 U/L) | 3,439–4,473 | 324–698 | 1,304–1,868 | 581–1,006 |
| Aspartate transaminase (normal range <32 U/L) | 303–413 | 264–303 | 557–1,583 | 286–324 |
| Alanine transaminase (normal range <33 U/L) | 444–544 | 280–653 | 521–1,298 | 447–693 |
| NT–pro-brain natriuretic peptide (normal range <125 pg/mL) | 1,907–2,262 | 206 | 649–917 | 135 |
| Troponin T (normal range 0–14 ng/L) | 25 | 21 | 49 | 21 |
| Myoglobin (normal range 28–72 ng/mL) | 172 | 39 | 153 | 61 |
| Echocardiogram | ||||
| Maximum thickness of the septum (mm) | 30 | 16 | 16 | 11 |
| Maximum thickness of the posterior wall (mm) | 18 | 7 | 13 | 8 |
| Outflow tract obstruction (max/medium Doppler gradient at rest, mmHg) | 90–70/30–25 | 50/21 | 32–23/10–7 | No gradient |
At 5.8 years the girl showed a heart systolic ejection murmur. Electrocardiogram recording revealed increase of hypertrophy. Two-dimensional echocardiogram showed a marked thickening of the interventricular septum (IVS) and left ventricular posterior wall (PW) (Fig. 1), with dynamic left ventricular outflow tract obstruction and mitral regurgitation (Table 1 and Fig. 1). Cardiomyopathy deteriorated rapidly with the girl complaining of weakness and exertion dyspnea. As propranolol was increased (3 mg/kg/day) without success, at 6.6 years bisoprolol was started (0.3 mg/kg/day). We observed a slight decrease of left ventricular outflow obstruction without any change of IVS and left ventricular PW thickness (Table 1). At this time heart transplantation was considered as a possible choice for the girl. A heart MRI was performed which showed a left ventricular mass index of 232 g/m2 (normal values: mean 58 g/m2, 95th centile confidence interval 42–84 g/m2).
Fig. 1.
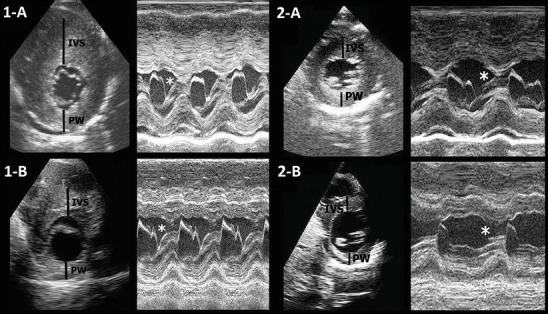
2D and M-mode echocardiography of girl (1-A and 2-A) and boy (1-B and 2-B) before and after the diet manipulation. IVS interventricular septum, PW posterior wall of the left ventricle. Note the typical systolic anterior motion of the mitral valve (SAM), responsible of outflow obstruction (asterisk) and the reduction of the thickness and the amplitude of SAM after the diet
At two years old, also the boy showed reduced physical activity associated to a rapid worsening of cardiomyopathy, with mild left ventricular obstruction, despite increase of propranolol therapy (3 mg/kg/day). At 2.8 years bisoprolol was started (0.3 mg/kg/day) and later ultrasound examination showed a slight increase of myocardial thickness (Table 1 and Fig. 1).
A high-fat (60%), low-carbohydrate (15%), high (unchanged)-protein (25%) diet was started at age seven for the girl and five for the boy (Table 1). Cornstarch was progressively reduced and eventually stopped. Fats rich in polyunsaturated fatty acids were preferred over saturated fats (Fuehrlein et al. 2004) and only extra-virgin olive oil as relish was recommended. Additional protein powders were used to increase protein intake without high saturated fat addition.
After 12 months a clinical, biochemical and echocardiographic improvement was observed in both siblings: clinical symptoms disappeared in the girl and both children became more active than before; alanine transaminase, aspartate transaminase, creatine kinase, and NT-probrain natriuretic peptide decreased substantially (Table 1); and a relevant reduction of the thickness of IVS and p of left ventricle PW was seen as well as a reduction of the outflow obstruction in both patients (Table 1 and Fig. 1). During withdrawal of cornstarch the pre- and postprandial glucose levels were maintained in the normal ranges and the patients showed good metabolic balance.
Discussion
The two patients had a severe progressive cardiomyopathy with ventricular hypertrophy and ventricular outflow tract obstruction, notwithstanding a high-protein diet as recommended by Kishnani et al. (2010). The pharmacological treatment with beta-blockers was only partially effective and also not free of risk in these patients because it might mask symptoms of hypoglycemia (Kishnani et al. 2010).
It was much impressive to observe that with the new diet they had a dramatic improvement of their clinical conditions and biochemical and echocardiographic findings.
The dietetic management of GSD III is controversial (Dagli et al. 2009): few cases showed an improvement of cardiac hypertrophy after high-protein diet (Dagli et al. 2009; Sentner et al. 2012). The rationale is that high-protein diet might reduce limit dextrin accumulation in myocardial cells and increase the use of proteins through gluconeogenesis (Sentner et al. 2012). Higher dietary protein intake might also enhance muscle protein synthesis (Kishnani et al. 2010). Another patient, 2 months old, had an improvement of cardiomyopathy after a ketogenic diet (Valayannopoulos et al. 2011). This treatment not only forced the activity of gluconeogenesis with a high protein diet but also facilitated ATP generation from fatty acid oxidation and ketolysis as alternative source of energy.
Our patients started a high-protein diet from diagnosis, but as it was not enough to maintain normoglycemia, addition of cornstarch was needed to reach a good homeostasis. With the increase of fats this addition was no more needed. Fats were increased in the diet of our children in a softer way than suggested by Valayannopoulos et al. (2011), without reaching a formal ketogenic diet, with the aim of providing them with a more comfortable diet, which could be followed for a long period of time. In our clinical experience, the complete ketogenic diet is hampered by bad compliance, being much difficult to follow in practice for a long time.
The clinical evolution of these two siblings shows that a high-fat and high-protein diet with limited carbohydrate intake could be a beneficial therapeutic choice for GSD III pediatric patients with cardiomyopathy. The patients reported in the literature (Dagli et al. 2009; Sentner et al. 2012; Valayannopoulos et al. 2011) and our present cases have in common an increased use of caloric sources different from carbohydrates. In fact the patient reported by Valayannopoulos et al. (2011) had a reduction of carbohydrates sources with a ketogenic diet, the adult reported by Dagli et al. (2009) increased protein intake to 30% and was able to halve cornstarch assumption, and the other adult reported by Sentner et al. (2012) improved on a hyperproteic severely hypocaloric diet which, although containing 61% carbohydrates, was ketogenic “per se” due to the calories limitation (900 kcal/day).
Why a high carbohydrate diet may sustain the development of cardiomyopathy in GSD III is not clear. Hyperinsulinism might play a role: glycogen storage disease patients who are excessively fed with carbohydrates might manifest lipolysis inhibition due to high insulin secretion leading to reduction of energy availability for the heart (Valayannopoulos et al. 2011).
According to these findings, it might be possible to say that the row cornstarch should not be administered to GSD III patients, whose diet should be manipulated introducing enough proteins and fats to maintain normoglycemia.
The main limitation of this clinical report is that it is not a case–control study; however each child served as its own control. Further studies are needed to confirm these results. These two siblings should also be followed longitudinally in the next years, in order to assess if the current improvement is sustained and if they do not develop side effects. Future research is needed to design alternative treatment strategies to prevent glycogen accumulation in GSD III.
Acknowledgments
We gratefully acknowledge the family and the wonderful children; the head of the Department of Pediatrics, Prof. Andrea Biondi; the nurses of the Pediatric Dept in San Gerardo Hospital; and the secretary of the Rare Metabolic Diseases Unit, Mrs. Vera Marchetti. We also acknowledge Prof. Giuseppe Vallar for his useful suggestions and Fondazione Pierfranco e Luisa Mariani for their generous financial support to our clinical activity.
Compliance with Ethics Guidelines
Conflict of Interest
Alessandra Brambilla, Savina Mannarino, Roberta Pretese, Serena Gasperini, Cinzia Galimberti and Rossella Parini declare that they have no conflict of interest.
Informed Consent
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000. Informed consent was obtained from patients’ parents for including their children in the study.
Details of the Contributions of Individual Authors
All authors are involved in the planning of the different treatment and conception and design of the paper; Alessandra Brambilla analyzed the data and prepared a draft of the article; Savina Mannarino analyzed and interpreted the cardiac data and revised the draft critically; Serena Gasperini, Roberta Pretese, and Cinzia Galimberti revised the draft critically; and Rossella Parini revised the draft critically, accepts full responsibility for the work and/or the conduct of the study, had access to the data, and controlled the decision to publish.
Footnotes
Competing interests: None declared
Contributor Information
Rossella Parini, Email: rossella.parini@unimib.it.
Collaborators: Johannes Zschocke and K Michael Gibson
References
- Austin SL, Proia AD, Spencer-Manzon MJ, Butany J, Wechsler SB, Kishnani PS. Cardiac pathology in glycogen storage disease type III. JIMD Rep. 2012;6:65–72. doi: 10.1007/8904_2011_118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagli AI, Zori RT, McCune H, Ivsic T, Maisenbacher MK, Weinstein DA. Reversal of glycogen storage disease type IIIa-related cardiomyopathy with modification of diet. J Inherit Metab Dis Suppl. 2009;32(Suppl 1):S103–106. doi: 10.1007/s10545-009-1088-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuehrlein BS, Rutemberg MS, Silver JN, et al. Differential metabolic effects of saturated versus polyunsaturated fats in ketogenic diets. J Clin Endocrinol Metab. 2004;89:1641–1645. doi: 10.1210/jc.2003-031796. [DOI] [PubMed] [Google Scholar]
- Kishnani PS, Austin SL, Arn P, et al. Glycogen storage disease type III diagnosis and management guidelines. Genet Med. 2010;12:446–463. doi: 10.1097/GIM.0b013e3181e655b6. [DOI] [PubMed] [Google Scholar]
- Sentner CP, Calistan K, Vletter WB, Smit GPA. Heart failure due to severe hypertrophic cardiomyopathy reversed by low calorie, high protein dietary adjustments in a Glycogen Storage Disease type IIIa patient. JIMD Rep. 2012;5:13–16. doi: 10.1007/8904_2011_111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valayannopoulos V, Bajolle F, Arnoux JB, et al. Successful treatment of severe cardiomyopathy in glycogen storage disease type III with d,l-3-hydroxybutyrate, ketogenic and high-protein diet. Pediatr Res. 2011;70(6):638–641. doi: 10.1203/PDR.0b013e318232154f. [DOI] [PubMed] [Google Scholar]


