Abstract
Exercise and subsequent catabolism is a potential trigger for creatine kinase (CK) concentration increase (rhabdomyolysis) in patients with LCHADD, therefore we evaluated the clinical and biochemical stability under physical exertion conditions at the age of 13 years in a currently 14-year-old LCHADD patient treated with heptanoate.
LCHADD was diagnosed during first decompensation at age 20 months. In the following 2 years, the patient had several episodes of rhabdomyolysis. Heptanoate 0.5–1 g/kg/day was started at 4 years, with no further CK elevations since. He is clinically stable, has retinopathy without vision impairment or polyneuropathy. Maximal incremental and endurance exercise tests were performed to evaluate both clinical and metabolic stability during and after exertion.
Physical fitness was adequate for age (maximum blood lactate 7.0 mmol/L, appropriate lactate performance curve, maximum heart rate of 196 bpm, maximum power 139 Watt = 2.68 Watt/kg body weight). There were no signs of clinical (muscle pain, dark urine) or metabolic derangement (stable CK, acyl carnitine profiles, blood gas analyses) – neither after maximal incremental nor endurance exertion.
This case illustrates that both under maximal incremental and endurance exertion, clinical and biochemical parameters remained stable in this currently 14-year-old LCHADD patient receiving heptanoate treatment.
Introduction
LCHAD deficiency is an inherited metabolic disorder, involving the degradation of long-chain fatty acids. The acute complication is a metabolic decompensation with hypoketotic hypoglycemia, rhabdomyolysis, hepatopathy, cardiomyopathy, and loss of consciousness until coma (Arnold et al. 2009; Spiekerkoetter et al. 2009a). Biochemical diagnosis is established through acyl carnitine profile in dried blood spot/plasma (accumulation of long-chain acyl carnitines) and excretion of organic acids in urine during the metabolic crisis (accumulation of dicarbonic acids) (Lindner et al. 2010). Diagnosis is confirmed through enzymatic analysis showing enzyme deficiency in lymphocytes/fibroblasts and molecular analysis. Close to 85% of patients are homozygous for the common mutation c.1578G>C (Tyni et al. 2012), which also occurs in compound heterozygous state. Long-term complications include retinopathy and polyneuropathy. Both clinical manifestations are seen in the teenage years and can proceed into substantial morbidity (Gillingham et al. 2005). However, first signs of retinopathy may occur as early as 2 years of life, as was the case in our patient. These complications are solely present in LCHAD and MTP deficiency, and not in the other long-chain fatty acid oxidation disorders. Therapy consists of a fat-defined (fat-reduced) diet and supplementation of medium-chain triglycerides (MCT) (Spiekerkoetter et al. 2009b; Roe and Mochel 2006; Roe et al. 2002, 2008). Therapy targeting long-term complications in patients with LCHAD deficiency includes approaches with a high supplementation of essential fatty acids (docohexaenic acid (DHA)) or anaplerotic substances, i.e. with heptanoate (Gillingham et al. 2005, 2009; Roe and Mochel 2006; Roe et al. 2002, 2008; Kinman et al. 2006). Both these therapeutic approaches lack a good parameter for follow-up and judgement of effectiveness. Follow-up on retinal background and nerve conduction velocity should be done routinely about once a year (Spiekerkoetter et al. 2009a).
We evaluated the clinical and biochemical stability under both incremental and endurance physical exertion conditions in a 13-year-old patient with LCHADD receiving heptanoate treatment for the last 9 years and a high caloric drink before exercise.
Case Report
The boy is the second child of healthy, non-consanguineous Caucasian parents, he has one older healthy sister. Until the age of 20 months his development was uneventful. At this point he suffered from a metabolic decompensation following an upper airway infection, showing coma, hepatopathy, and cardiomyopathy, leading to an intensive care unit admission and diagnosis. On eye examination, changes in retina were already visible, they have remained stable over the past 12 years since diagnosis. He does not suffer from polyneuropathy. Therapy consisting of a fat-defined diet (40% total energy intake, 50% MCT based) was started immediately. Up to the age of 4 years he repeatedly showed episodes with substantial elevation of creatine kinase (CK) concentrations and was metabolically instable (metabolic acidosis) during infections. Thus, in 2004 a therapy with heptanoate was started (currently: 2 x 15 mL in the morning and evening, equaling 0.6 g/kg/day). Heptanoate at 0.6 g/kg/day equals 15% of total energy. Total fat intake including heptanoate is 40% of total energy. Long-chain intake with 15 g/day is 0.3 g/kg/day and equals 8% of total energy. Protein intake is 1.25 g/kg/day, and kcal/kg/day are 30–35.
CK concentrations have been normal since then (see Fig. 1). It is unknown if this is the anaplerotic effect of heptanoate or if the stabilization came with increasing age (less infections). However, the patient, parents, and caretakers share the impression the boy is metabolically more stable since starting triheptanoate. At present, he is 14 years old and clinically asymptomatic. Besides following a fat-defined diet with heptanoate supplementation, he has no other medications or supplements. The boy is active, before exercise he consumes a high calorie drink (200 mL ProvideXtra® = 300 kcal, being a high energy food supplement with hydrolyzed protein and fiber, but fat, lactose and gluten free). On a regular basis, he performs karate training once a week and swimming once a week as well as being active in the local fire prevention department and the water-watch lifeguard organization. However, he has not undergone a pulse-controlled endurance training programme so far.
Fig. 1.

Creatine kinase concentrations and start of heptanoate in the patient
As exercise and subsequent catabolism is a potential trigger for metabolic derangement with CK concentration increase (=rhabdomyolysis) in LCHADD patients, we evaluated the clinical and biochemical stability under controlled physical exertion (both incremental and endurance) in this patient.
Applied Tests (Methods)
The proband first underwent a standard incremental cycle test (starting with 25 Watt, increasing by 25 Watt every 2 min) until exhaustion (Paridon et al. 2006). At the testing he was 13 years old, weighed 52 kg, and measured 167 cm (both values 90th percentile), BMI 18.6 kg/m2, heart rate 78/min, temperature 36.6°C, oxygen saturation 100%.
Samples for blood glucose, lactate, and acyl carnitine profiles in dried blood spots were taken out of the hyperemic earlobe at the end of each increment, i.e. every two minutes; as well as a sample for analysis of urinary organic acids at 0, 4, 8, 16, and 24 h after the exercise test.
As the incremental exercise test showed normal results, 3 months later we performed an endurance test (Paridon et al. 2006; Beneke et al. 1996a, b, 2000a). To establish the maximal lactate steady state (anaerobic threshold), the patient underwent three endurance exercise sessions of 30 min each with a constant Watt performance of 80, 90, and 100 Watt, respectively (Beneke et al. 1996a, b, 2000b). Each test was done at the same daytime (afternoon) followed by a 48-hour resting period between each session. The heptanoate morning dose was given as usual, and thus, 6 h before the exertion test. Blood lactate and glucose were determined every 5 min during the 30 min of the endurance tests. Samples for blood glucose, lactate, creatine kinase, and acyl carnitine profiles in dried blood spots were taken regularly during each test and 1, 2, 4, 8, 16, 24, and 48 h after each test. Dietary intake remained unchanged during the whole testing period.
The anaerobic threshold, maximal lactate steady state (MLSS) refers to the exercise intensity where there is a dynamic equilibrium between lactate formation and elimination, i.e. there is no further net lactate production (Beneke et al. 1996a, b, 2000a, b). As it is known that the MLSS corresponds to about 70% of the maximal performance during the incremental exercise test, the patient’s endurance exercise test series was started with 80 Watt, and then increased by 10 Watt each session.
Results
Standard Incremental Cycle Test
For this incremental exercise test lasting 11 min and 8 s, exhaustion was reached at 139 Watt, corresponding to a performance of 71% of the age-matched norm and equal to 2.68 Watt/kg body weight. During exercise he had adequate blood pressure and heart rate modulation (maximal heart rate 196/min at exhaustion) (Fig. 2). The ECG showed no signs of ischemia or arrhythmias. Subjectively, the patient had no symptoms.
Fig. 2.
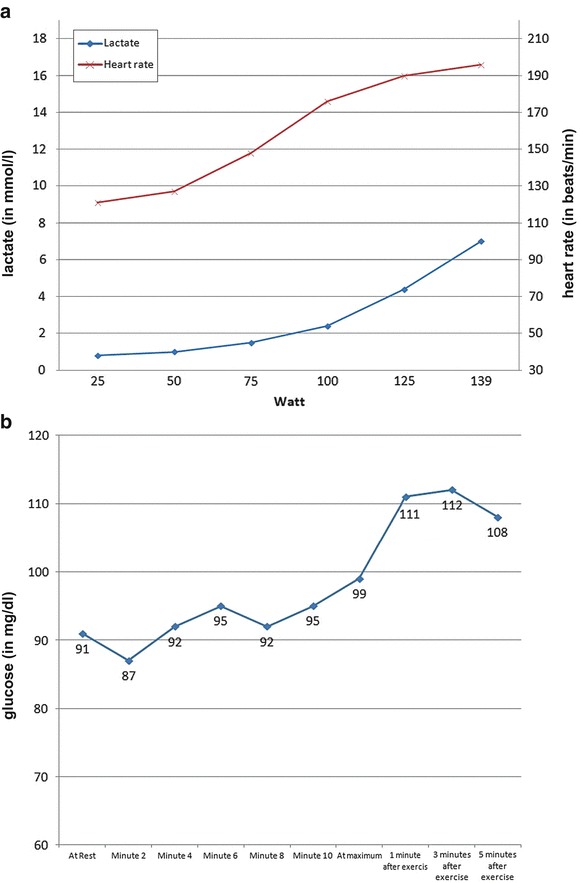
(a) Blood lactate concentration and heart rate during the standard incremental cycle test – samples taken from earlobe. Lactate in mmol/L, heart rate in beats/minute. (b) Blood glucose concentration during the standard incremental cycle test – samples taken from earlobe (in mg/dL). Y-axis is blood glucose concentration in mg/dL
Standard Endurance Cycle Test
The patient reached the maximal lactate steady state at 90 Watt (Fig. 3). Mean heart rate was 165/min.
Fig. 3.

Determination of maximal lactate steady state (MLSS) for the patient is at 90 Watt. MLSS is defined as the endurance performance where increase of blood lactate between 10 and 30 min of the endurance test is below or equal to 1 mmol/L
Anaerobic Threshold, Maximal Lactate Steady State (MLSS)
The anaerobic threshold of the patient was tested to be at 90 Watt, his mean heart rate at the anaerobic threshold was 165/min. The MLSS, calculated as the mean from the last four samples of the 90 Watt endurance exertion test, was 2.2 mmol/L. Blood glucose concentrations remained stable (Table 1).
Table 1.
Blood glucose concentrations in the patient during the endurance test (in mg/dL)
| Resting | Minute 5 | Minute 10 | Minute 15 | Minute 20 | Minute 25 | Minute 30 | |
|---|---|---|---|---|---|---|---|
| 80 Watt | 111 | 107 | 109 | 109 | 101 | 106 | 106 |
| 90 Watt | 109 | 100 | 94 | 102 | 103 | 104 | 105 |
| 100 Watt | 109 | 102 | 101 | 102 | 108 | 111 | 105 |
Metabolic Laboratory Tests
Neither during the incremental nor the endurance test, blood glucose, blood lactate, acyl carnitine profiles, or excretion of urinary organic acids showed any relevant fluctuation (data not shown). Acylcarnitine profiles as well as urinary organic acids did not show changes under the exercise tests, i.e. no increase in the amount of long-chain acylcarnitines or dicarboxylic acids.
In conclusion, the patient’s physical fitness was adequate for age (maximum blood lactate 7.0 mmol/L, appropriate lactate performance curve, maximum heart rate of 196 bpm, maximum power 139 Watt = 2.68 Watt/kg body weight). There were no signs of clinical (muscle pain, dark urine) or metabolic derangement (stable CK, acyl carnitine profiles, blood gas analyses) – neither after maximal incremental nor endurance exertion.
Discussion
LCHAD deficiency is an inherited metabolic disorder, involving the degradation of long-chain fatty acids. Exercise and subsequent catabolism is a potential trigger for a metabolic decompensation with rhabdomyolysis or increase of creatine kinase (CK) concentration. Other studies looking at exercise in long-chain fatty acid oxidation disorders have shown that (1) MCT given immediately prior to exercise improved exercise tolerance and decreased the risk of rhabdomyolysis in six LCHADD out of nine FAOD patients (Gillingham et al. 2006); (2) exercise significantly increased plasma 3-hydroxyacylcarnitines concentrations in six LCHADD out of eight FAOD patients; (3) MCT supplementation prior to exercise increased the oxidation of medium chain fats, decreased the oxidation of glucose, and lowered cardiac workload during exercise when compared with carbohydrate supplementation in eight LCHADD out of 11 FAOD patients (Behrend et al. 2012). However, these reports did not focus on the anaerobic threshold during incremental or endurance physical exertion tests in these patients.
We evaluated the clinical and biochemical stability under both incremental and endurance physical exertion conditions in a 13-year-old LCHADD patient treated with heptanoate for now nine years.
One goal was to determine the anaerobic threshold of the 13-year-old patient, i.e. exercise him at the highest lactate concentration at a steady state (maximal lactate steady state = MLSS). After he had uneventfully performed the incremental exercise test, for this purpose an endurance exercise test was added.
Both the incremental and the endurance exercise tests gave normal and stable results. Thus, they give a basis to develop a training programme for this LCHADD patient. Firstly, they are important results to evaluate the patients’ physical fitness and metabolic stability under exertion, and secondly, they can be used to develop an individual training programme for the patient. That is, as a concrete suggestion for the patient, the measurement of on-site CK does not make sense as CK concentrations remained stable at all times during the exercise tests. A more useful recommendation could be a lactate monitoring during exercise (avoid an increase above 2.2 mmol/L lactate). As a conclusion, the best practicable and concrete recommendation for this patient is to monitor his pulse during endurance exercise and generally keep heart rate below 165 beats per minute.
Heptanoate has two effects: firstly, in LCHADD it can be used as an alternative substrate and secondly, it has anaplerotic qualities. The boy has been on heptanoate treatment for 9 years. Heptanoate as an alternative substrate or its anaplerotic properties in this case seem to have had an impact on clinical stability and creatine kinase activity over the years.
In conclusion, this case illustrates that both under maximal incremental and endurance exertion, clinical and biochemical parameters remained stable in this 14-year-old LCHADD patient receiving heptanoate treatment.
Compliance with Ethical Standards
Conflict of Interest
Daniela Karall, Gerald Mair, Ursula Albrecht, Katharina Niedermayr, Thomas Karall, Wolfgang Schobersberger, and Sabine Scholl-Bürgi declare that they have no conflict of interest.
Informed Consent
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000 (5). Informed consent was obtained from the patient for being included in the study.
Footnotes
Competing interests: None declared
Contributor Information
D. Karall, Email: daniela.karall@i-med.ac.at
Collaborators: Johannes Zschocke and K Michael Gibson
References
- Arnold GL, VanHove J, Freedenberg D, et al. A Delphi clinical practice protocol for the management of very long chain acyl-CoA dehydrogenase deficiency. Mol Genet Metab. 2009;96:85–90. doi: 10.1016/j.ymgme.2008.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrend AM, Harding CO, Shoemaker JD, Matern D, Sahn DJ, Elliot DL, Gillingham MB. Substrate oxidation and cardiac performance during exercise in disorders of long chain fatty acid oxidation. Mol Genet Metab. 2012;105:110–115. doi: 10.1016/j.ymgme.2011.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beneke R, Schwarz V, Leithäuser R, et al. Maximal lactate steady-state in children. Ped Exer Sci. 1996;8:328–336. doi: 10.1097/00005768-199612000-00006. [DOI] [PubMed] [Google Scholar]
- Beneke R, Heck H, Schwarz V, et al. Maximal lactate steady state during the second decade of age. Med Sci Sports Exerc. 1996;28:1474–1478. doi: 10.1097/00005768-199612000-00006. [DOI] [PubMed] [Google Scholar]
- Beneke R, Hütler M, Leithäuser RM. Maximal lactate-steady-state independent of performance. Med Sci Sports Exerc. 2000;32:1135–1139. doi: 10.1097/00005768-200006000-00016. [DOI] [PubMed] [Google Scholar]
- Beneke R, Leithäuser R, Schwarz V, et al. Maximales Laktat-Steady-State bei Kindern und Erwachsenen. Deutsche Zeitschrift für Sportmedizin. 2000;51:100–104. [Google Scholar]
- Gillingham MB, Weleber RG, Neuringer M, et al. Effect of optimal dietary therapy upon visual function in children with long-chain 3-hydroxyacyl CoA dehydrogenase and trifunctional protein deficiency. Mol Genet Metab. 2005;86:124–133. doi: 10.1016/j.ymgme.2005.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillingham MB, Scott B, Elliott D, Harding CO. Metabolic control during exercise with and without medium-chain triglycerides (MCT) in children with long-chain 3-hydroxy acyl-CoA dehydrogenase (LCHAD) or trifunctional protein (TFP) deficiency. Mol Genet Metab. 2006;89:58–63. doi: 10.1016/j.ymgme.2006.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillingham MB, Matern D, Harding CO. Effect of feeding, exercise and genotype on plasma 3-hydroxyacylcarnitines in children with LCHAD deficiency. Top Clin Nutr. 2009;24:359–365. doi: 10.1097/TIN.0b013e3181c62182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinman RP, Kasumov T, Jobbins KA, et al. Parenteral and enteral metabolism of anaplerotic triheptanoin in normal rats. Am J Physiol Endocrinol Metab. 2006;291:E860–E866. doi: 10.1152/ajpendo.00366.2005. [DOI] [PubMed] [Google Scholar]
- Lindner M, Hoffmann GF, Matern D (2010) Newborn screening for disorders of fatty-acid oxidation: experience and recommendations from an expert meeting. J Inherit Metab Dis. doi:10.1007/s10545-010-9076-8 [DOI] [PubMed]
- Paridon SM, Alpert BS, Boas SR, et al. Clinical stress testing in the pediatric age group. Circulation. 2006;113:1905–1920. doi: 10.1161/CIRCULATIONAHA.106.174375. [DOI] [PubMed] [Google Scholar]
- Roe C, Mochel F. Anaplerotic diet therapy in inherited metabolic disease: therapeutical potential. J Inher Metab Dis. 2006;29:332–340. doi: 10.1007/s10545-006-0290-3. [DOI] [PubMed] [Google Scholar]
- Roe CR, Sweetman L, Roe DS, et al. Treatment of cardiomyopathy and rhabdomyolysis in long-chain fat oxidation disorders using an anaplerotic odd-chain triglyceride. J Clin Invest. 2002;110:259–269. doi: 10.1172/JCI0215311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roe CR, Yang BZ, Brunengraber H, et al. Carnitine palmitoyltransferase II deficiency: successful anaplerotic diet therapy. Neurology. 2008;71:260–264. doi: 10.1212/01.wnl.0000318283.42961.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiekerkoetter U, Lindner M, Santer R, et al. Management and outcome in 75 individuals with long-chain fatty acid oxidation defects: results from a workshop. J Inherit Metab Dis. 2009;32:488–497. doi: 10.1007/s10545-009-1125-9. [DOI] [PubMed] [Google Scholar]
- Spiekerkoetter U, Lindner M, Santer R, et al. Treatment recommendations in long-chain fatty acid oxidation defects: consensus from a workshop. J Inher Metab Dis. 2009;32:498–505. doi: 10.1007/s10545-009-1126-8. [DOI] [PubMed] [Google Scholar]
- Tyni T, Immonen T, Lindahl P, et al. Refined staging for chorioretinopathy in long-chain 3-hydroxyacyl coenzyme A dehydrogenase deficiency. Ophthalmic Res. 2012;48:75–81. doi: 10.1159/000334874. [DOI] [PubMed] [Google Scholar]


