Abstract
Background
In a murine anthracycline-related cardiotoxicity model, increases in cardiovascular magnetic resonance (CMR) myocardial contrast-enhanced T1-weighted signal intensity are associated with myocellular injury and decreases in left ventricular ejection fraction (LVEF). We sought to determine if T1- and T2-weighted measures of signal intensity associate with decreases in LVEF in human subjects receiving potentially cardiotoxic chemotherapy.
Methods and Results
In 65 individuals with breast cancer (n=51) or a hematologic malignancy (n=14), we measured left ventricular volumes, EF, and contrast-enhanced T1-weighted and T2-weighted signal intensity prior to and 3 months after initiating potentially cardiotoxic chemotherapy using blinded, unpaired analysis of CMR images. Participants were aged 51±12 years of whom 55% received an anthracycline, 38% received a monoclonal antibody, and 6% received an antimicrotubule agent. Overall, LVEF decreased from 57±6% to 54±7% (p<0.001) due to an increase in end-systolic volume (p<0.05). T1-weighted signal intensities also increased from 14.1±5.1 to 15.9±6.8 (p<0.05) with baseline values trending higher among individuals who received chemotherapy prior to study enrollment (p=0.06). Changes in T1-weighted signal intensity did not differ within the 17 LV myocardial segments (p=0.97). Myocardial edema quantified from T2-weighted images did not change significantly after 3 months (p=0.70).
Conclusions
Concordant with previous animal studies, CMR measures of contrast-enhanced T1-weighted signal intensity occur commensurate with small but significant LVEF declines 3 months after receipt of potentially cardiotoxic chemotherapy. These data indicate that changes in T1-weighted signal intensity may serve as an early marker of subclinical injury related to the administration of potentially cardiotoxic chemotherapy in human subjects.
Keywords: cardiotoxicity, cardiovascular magnetic resonance imaging, left ventricular ejection fraction, T1-weighted and T2-weighted imaging
Commonly administered chemotherapeutic regimens are associated with acute and chronic cardiac injury and future cardiovascular (CV) events.1 Methods to detect early evidence of subclinical cardiac injury could be useful for selecting individuals who might benefit from therapeutic interventions to prevent deterioration in left ventricular (LV) performance or subsequent CV events.1,2 Cardiovascular magnetic resonance (CMR) assessed changes in myocardial T1 and T2 magnetic relaxation from ex-vivo samples, small animal studies, and human case series have been observed after the administration of anthracycline-based chemotherapy (Anth-bC).3-6 Moreover, in one study, regional changes in LV lateral wall myocardial signal intensity occurred after receipt of trastuzumab for breast cancer.6
Previously in a murine model of Anth-bC cardiotoxicity, we demonstrated that increases in global T1-weighted late gadolinium contrast-enhanced signal intensity (LGE-SI) CMR a) identified histopathologic evidence of myocardial injury consistent with anthracycline administration, and b) predicted future decrements in LV ejection fraction (LVEF) during the longitudinal receipt of doxorubicin, an anthracycline.7 To date, however, it is unknown whether similar changes in contrast-enhanced T1-weighted and T2-weighted CMR measures of signal intensity are associated with LVEF changes among patients receiving Anth-bC or other potentially cardiotoxic chemotherapy for breast cancer, leukemia, or lymphoma. In addition, it is uncertain whether regional (e.g., lateral or septal wall) changes in of these measures are associated with LVEF changes above and beyond global measures.
Accordingly, we performed a prospective longitudinal cohort study to determine if either regional or global changes in T1-weighted and T2-weighted CMR signal intensity were associated with coincident changes in LV performance early after receipt of potentially cardiotoxic chemotherapy.
Methods
Study Population
This study was approved by the Institutional Review Board at the Wake Forest University School of Medicine and all study participants provided written, witnessed informed consent. The study was funded by the National Cancer Institute of the National Institutes of Health, R33CA12196, and by the Susan G. Komen Foundation, BCTR07007769. Eligible participants, recruited through the Comprehensive Cancer Center of Wake Forest University School of Medicine, included those scheduled to receive Anth-bC and other known cardiotoxic agents used in the treatment of breast cancer, leukemia, or lymphoma. Participants were ineligible for enrollment if they exhibited a contraindication for CMR, history of myocardial infarction, or an estimated glomerular filtration rate (GFR) < 60 ml/min/1.73m2 calculated using serum creatinine obtained from blood draw prior to each CMR examination.8
Study Design
CMR examinations were performed on a Magnetom Avanto 1.5 Tesla scanner (Siemens Medical Solutions USA, Malvern, Pennsylvania) according to previously published techniques at baseline (before chemotherapy initiation) and three months after chemotherapy initiation.9-11 All studies were analyzed by analysts blinded to participant identifiers, study group, and the date or results of other CMR examinations (a blinded, unpaired read). Prior to each CMR examination, blood samples were taken via venipuncture for serum troponin-I (Beckman Coulter Inc., Brea, California) and B-type natriuretic peptide (Phoenix Pharmaceuticals, Inc., Burlingame, California) assessments.12,13
LV Volumes and Ejection Fraction
LV volumes (end diastolic [LVEDV], end systolic [LVESV], and stroke volume [LVSV]), were determined from cine white-blood steady-state free precession images encompassing the left ventricle in 8 mm short-axis planes separated by 2 mm.10,11 Image acquisition parameters included a 40 cm field of view (FOV),192×109 matrix, 10-ms repetition time (TR), 1.12-ms echo time (TE), 20-degree flip angle (FA), 930 Hz/pixel bandwidth, and 40 ms temporal resolution. Endocardial contours were drawn for LV volume calculations at end-diastole and end-systole on offline workstations and summed by Simpson’s rule for calculation of LVEF.14
Contrast-enhanced T1-weighted Imaging
Study participants received 0.2 mmol/kg of intravenous gadoteridol contrast (ProHance; Bracco Diagnostics, Princeton, New Jersey) during each CMR examination. Gradient-echo inversion recovery late gadolinium enhancement images were acquired exactly ten minutes after contrast administration (assessed by stopwatch) in 6–12 short-axis slices encompassing the left ventricle.7 The imaging parameters included a 292×360 mm FOV, 256×192 matrix, 24 views per segment, 8 mm thick slice with no gap, 25° FA, TI of 140-360 ms, 130 Hz/pixel bandwidth, 3.3 ms TE, and 808 ms TR based on heart rate. In accordance with prior publications, the TI was selected for a uniform dark myocardium at the baseline CMR exam and held constant at the follow-up exam.7
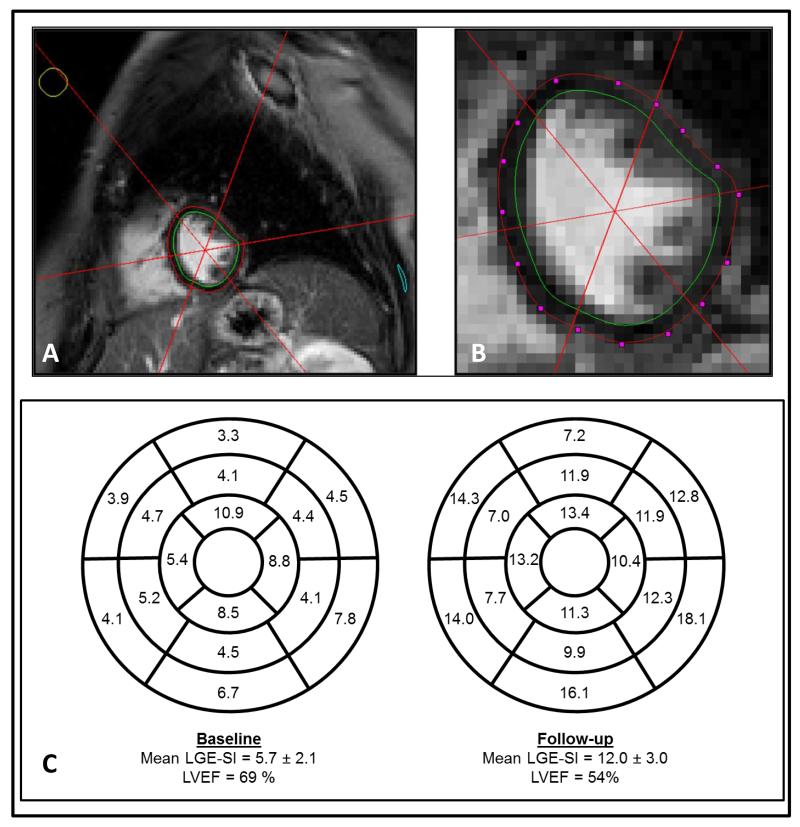
Images were transferred offline for post-processing in MATLAB (The Mathworks, Inc.; Natick, Massachusetts) software, where myocardial endocardial and epicardial boundaries were identified by carefully tracing the myocardial boundaries to avoid partial volume effects (Figures 1A and 1B).7 A spoke tool divided the LV myocardium into the 17-segment American Heart Association (AHA) model.15 Thereafter, the 3-dimensional coordinates of each voxel, corresponding segment number, and signal intensity values were exported for subsequent analyses. The mean and standard deviation of contrast-enhanced T1-weighted signal intensity (LGE-SI) was calculated for each AHA segment within the myocardium for each examination (Figure 1C).
Figure 1.
Panel A and B: Segmentation of endocardial (green) and epicardial (red) borders using splines for extraction of myocardial voxels in CMR analysis. Panel C: AHA 17-segment models for one participant at baseline and three months after chemotherapy demonstrating diffuse increases in contrast-enhanced T1-weighted signal intensity across the myocardium commensurate with a large LVEF drop.
Using this technique in our lab, we have performed repeated measures separated by two weeks in 7 asymptomatic, cancer-free individuals without a substantive change in their clinical condition. Images were analyzed in an unpaired, blinded fashion with excellent correlation between repeated measures (y=0.87×+1.2, R2=0.98). We then performed a blinded reproducibility analysis with CMR examinations separated by two months and found excellent agreement between visits (6.38±0.67 vs 6.35±0.68) with high correlation (y=1.01×−0.1, R2=0.99).
Myocardial precontrast T1 was quantified in 10 participants at baseline and follow-up using a modified Look-Locker inversion recovery sequence.16 Breath-held T1 mapping images were acquired in the mid-cavity short-axis plane using a 380×342 mm FOV, 240×151 matrix, 35° FA, 110 Hz/pixel bandwidth, 8 mm slice thickness, 1.95 ms TE, and 3.9 ms TR. Mean myocardial T1 was calculated after segmentation (Figure 1) and adjusted for heart rate.16
T2-weighted Imaging
Breath-held dark-blood T2-weighted turbo spin echo images were acquired prior to contrast administration in the mid-cavity short-axis plane using a 303×360 mm FOV, 256×216 matrix, 180° FA, 780 Hz/pixel bandwidth, 66 ms TE, and TR of twice the RR interval.17 Myocardial contours were drawn with the addition of region of interest in the serratus anterior muscle to measure T2-weighted signal intensity in the skeletal muscle. Myocardial relative enhancement was quantified as the ratio of myocardial to skeletal muscle signal intensity.17,18 Percent myocardial edema was calculated from the amount of myocardial voxels with a relative enhancement ≥2.18
Statistical Analyses
All statistical analyses were completed using SAS statistical software (v9.2; Cary, North Carolina). Statistical means and standard deviations were calculated for all continuous variables, and the frequencies and percentages for all categorical variables were tabulated. Serial measurements compared using Student’s paired t-tests. The study population was dichotomized for subgroup analyses into participants with or without prior chemotherapy exposure before current chemotherapy treatment. Two-sample Student’s t-tests were used to test difference of means in subgroup analyses and correlations of variables were analyzed by linear regression models and Pearson correlation coefficients. Categorical variables were compared between the subgroups using Fisher’s exact and chi-square tests.
After overall cohort analyses, outcomes were investigated with a series of sensitivity analyses performed with analysis of covariance. Adjustments for multiple comparisons were made for sensitivity analyses after accounting for a maximum of five baseline covariates. Outcome variables were adjusted for only for covariates determined to be significant in sensitivity analyses. Additionally, longitudinal mixed models were used to determine the association of serial measurements with segment, slice, and LV wall region location. Logistic regression modeling was used to determine the prognostic value of the baseline LGE-SI in forecasting future LVEF changes. A pre-selected composite endpoint of a clinically significant LVEF decrease was defined as a decrease in the follow-up measurement ≥10%, or an absolute value less than 50%. Prior chemotherapy and number of comorbidities were included in the modeling to account for baseline risk factors that may impact LV fibrosis and LVEF for reasons other than chemotherapy. All values are reported as mean ± standard deviation unless stated otherwise; a value of p ≤ 0.05 was considered significant.
Results
The demographic data of the study population including CV comorbidities and cancer diagnoses are displayed in Table 1. The total amount of anthracyclines in doxorubicin equivalent dosage ranged from 60-500 mg/m2 with a median dose of 240 mg/m2. Of the 23 participants previously treated with chemotherapy, 14 received an anthracycline (average dose of 240 mg/m2 11±21 months prior to enrollment). All baseline and change in CMR measurements are listed in Table 2 (see Supplemental Table for follow-up CMR measurements). No differences in baseline LVEF were identified for age (p=0.76), gender (p=0.34), cancer type (p=0.43), hypertension (p=0.95), diabetes (p=0.58), hyperlipidemia (p=0.14), or a history of tobacco use (p=0.91). Similarly, no differences in baseline contrast-enhanced T1-weighted measures (LGE-SI) were associated with age (p=0.23), cancer type (p=0.29), hypertension (0.41), diabetes (0.71), hyperlipidemia (0.29), or a history of tobacco use (0.15). The LV mass index did not statistically change at the follow-up examination (51±12 g/m2) compared with baseline values (54±11 g/m2, p=0.35).
Table 1. Study participant descriptive characteristics of baseline cardiovascular risks and cancer treatment.
| All Subjects (n=65) |
No Prior Chemo (n=42) |
Prior Chemo (n=23) |
No Prior vs Prior Chemo: p-value |
|
|---|---|---|---|---|
| Characteristics | ||||
| Age, years | 51±12 | 49±13 | 54±10 | 0.17 |
| Women:Men | 56:9 | 33:9 | 23:0 | *0.02 |
| White:Black: Asian | 53:11:1 | 34:8:0 | 19:3:1 | 0.45 |
| Weight, kg | 81.4±25.3 | 84.5±28.4 | 75.7±17.5 | 0.13 |
| Height, m | 1.68±0.08 | 1.70±0.09 | 1.64±0.06 | *0.002 |
| Body Mass Index, kg/m2 | 29±7 | 29±8 | 28±5 | 0.64 |
| Comorbidities | ||||
| Diabetes, n(%) | 10(15%) | 7 (17%) | 3(13%) | 1.00 |
| Hypertension, n(%) | 31(48%) | 19(45%) | 12(52%) | 0.61 |
| Hyperlipidemia, n(%) | 15(23%) | 10(24%) | 5(22%) | 1.00 |
| Tobacco Use, n(%) | 29(45%) | 18(43%) | 11(48%) | 0.80 |
| Total Comorbidities | ||||
| Mean±Std Dev | 1.3±1.2 | 1.3±1.3 | 1.3±1.2 | 0.85 |
| Median (25th-75th percentile) | 1(0-2) | 1(0-2) | 1(0-2) | |
| Cancer Type | ||||
| Breast Cancer, n(%) | 51(78%) | 28(67%) | 23(100%) | *<0.01 |
| Leukemia, n(%) | 3(5%) | 3(7%) | 0 | 0.55 |
| Lymphoma, n(%) | 11(17%) | 11(26%) | 0 | *<0.01 |
| Cancer-Related Treatment † | ||||
| Anthracycline Agent, n(%) | 36(55%) | 32(76%) | 4(17%) | *<0.01 |
| Anthracycline Dose∥, mg/m2 | 240±85 | 240±91 | 240±0 | 0.99 |
| Monoclonal Antibody, n(%) | 25(38%) | 7(17%) | 18(78%) | *<0.01 |
| Antimicrotubule Agent, n(%) | 4(6%) | 3(7%) | 1(4%) | 0.99 |
| Radiation Treatment, n(%) | 7(11%) | 2(5%) | 5(22%) | 0.09 |
| Radiation Dose, Gy | 31±20 | 24±14 | 36±24 | 0.38 |
Values reported as either mean±standard deviation or frequency (percentage). P-values reported from t-test of differences in means between subgroups of prior chemotherapy for continuous variables and Fisher’s exact / chi-square tests for categorical variables. p<0.05 considered statistically significant.
Anthracycline based treatments including daunorubicin, doxorubicin, or epirubicin; Monoclonal antibody treatments including bevacizumab, gemtuzumab, or trastuzumab; Antimicrotubule based treatments including docetaxel and taxol.
Anthracycline dose calculated from cumulative doxorubicin equivalent dose.
Table 2. CMR measurements of study cohorts at baseline examination and longitudinal change from baseline (3 month - baseline).
| All Subjects (n=65) |
No Prior Chemo (n=42) |
Prior Chemo (n=23) |
No Prior vs Prior Chemo: p-value |
|
|---|---|---|---|---|
| Baseline Values | ||||
| LVEDV, ml | 109±35 | 113±35 | 101±33 | 0.17 |
| LVEDV indexed, ml/m2 | 57±13 | 58±12 | 55±15 | 0.47 |
| LVESV, ml | 46±17 | 46±16 | 46±17 | 0.94 |
| LVESV indexed, ml/m2 | 24±7 | 24±6 | 25±8 | 0.40 |
| Stroke Volume, ml | 63±21 | 67±21 | 55±18 | *0.02 |
| Stroke Volume indexed, ml/m2 | 33±8 | 34±8 | 30±8 | 0.05 |
| T2w Relative Enhancement | 1.3±0.3 | 1.3±0.3 | 1.3±0.3 | 0.44 |
| Myocardial Edema, % | 4.2±12 | 4.2±14 | 4.4±7.8 | 0.95 |
| LGE-SI | 14.1±5.1 | 13.2±4.7 | 15.7±5.3 | 0.06 |
| LVEF, % | 57±6 | 59±6 | 55±6 | *0.007 |
| Change from Baseline | ||||
| LVEDV, ml | 5±28 | 2±29 | 11±25 | 0.22 |
| LVEDV indexed, ml/m2 | 2±15 | 0±16 | 6±13 | 0.16 |
| LVESV, ml | 5±14 | 4±14 | 8±13 | 0.24 |
| LVESV indexed, ml/m2 | 3±7 | 2±7 | 4±7 | 0.19 |
| Stroke Volume, ml | 0±17 | −2±18 | 2±16 | 0.31 |
| Stroke Volume indexed, ml/m2 | 0±9 | −1±10 | 2±8 | 0.22 |
| T2w Relative Enhancement | 0.11±0.47 | 0.19±0.53 | 0±0.30 | 0.10 |
| Myocardial Edema, % | 1.7±16 | 3.5±20 | −1.3±6.8 | 0.21 |
Values reported as mean ± standard deviation. p<0.05 considered statistically significant. p-values reported in last column from t-test of differences in means for continuous variables and Fisher’s exact / chi-square tests for categorical differences between subgroups of prior chemotherapy.
A small increase in GFR was observed during the study from 96±18 ml/min/1.73m2 at baseline to 100±20 ml/min/1.73m2 at follow-up CMR examination (p=0.02). Overall, we observed no statistically significant change from baseline to follow-up in serum troponin-I levels (0.031±0.09 ng/ml to 0.045±0.05 ng/ml, p=0.11), or B-type natriuretic peptide levels (46.8±17.9 pg/ml to 44.1±16.8 pg/ml, p=0.41).
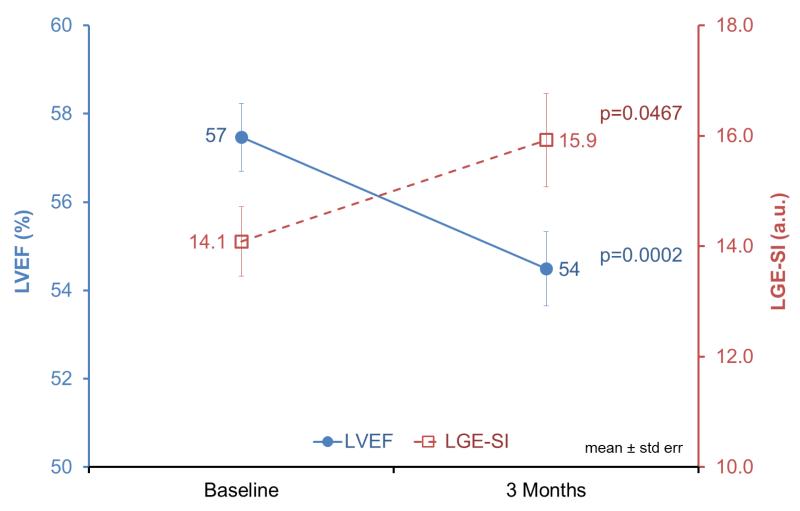
The LVEF declined among all participants from 57±6% to 54±7% three months post-chemotherapy (p=0.0002, Figure 2). After accounting for prior chemotherapy administration, LVEF changed significantly (p=0.01) and was lower at baseline in those treated previously (p=0.02), however, the LVEF change was no different between the subgroups dichotomized by prior chemotherapy administration (p=0.92). In secondary analyses, the LVEF declined due to reduced contractility as measured by an increase in LVESV (p<0.05) rather than by volume depletion (LVEDV did not change significantly [p=0.32]). The LVEF change was not affected by age (p=0.75), gender (p=0.80), cancer type (p=0.60), administration of anthracycline versus other chemotherapy drug classes (p=0.29), or CV risk factors including hypertension (p=0.94), hyperlipidemia (p=0.78), diabetes (p=0.42), or history of tobacco use (p=0.61).
Figure 2.
LVEF dropped from 57±1% to 54±1% in the three months following chemotherapy exposure (blue solid line, p<0.001). Concurrently, contrast-enhanced T1-weighted signal intensity (LGE-SI) increased from 14.1±0.6 to 15.9±0.8 in the study population (red dashed line, p<0.05).
No focal areas of increased LGE-SI consistent with an infarct or underlying fibrosis were visually appreciated in any study participant. At baseline, a reduced LVEF was not associated with increased T1-weighted signal (R2=0.02, p=0.25). LGE-SI increased from 14.1±5.1 to 15.9±6.8 at follow-up (p<0.05). The standard deviation of LGE-SI did not significantly change over the 3 months (δσ=0.17±2.1, p=0.52). Changes in LGE-SI were not associated with anthracycline dose (p=0.98) or anthracycline drug received (p=0.91). Similarly, changes in LGE-SI were not associated with demographic variables including age (p=0.77), gender (p=0.44), cancer type (p=0.46), administration of anthracycline versus other chemotherapy drug classes (p=0.81), or CV risk factors including hyperlipidemia (p=0.01), hypertension (p=0.44), diabetes (p=0.62), or history of tobacco use (p=0.27; p<0.006 considered statistically significant after correction for multiple comparisons). LGE-SI changes were not associated with a change in LV mass index (p=0.55). Additionally, LGE-SI changes were not associated with serum troponin-I and B-type natriuretic peptide levels (p=0.68 and p=0.18, respectively).
The LGE-SI increased at follow-up across all AHA segments (p<0.0001) and within each short-axis slice (p=0.009) without a greater increase in any particular slice (p=0.94) or AHA segment (p=0.97). Additionally, increase in LGE-SI was similar across wall regions of the left ventricle, specifically in the septal wall, anterior wall, and posterior-lateral walls (p=0.92).
We measured mid-cavity myocardial T1 by mapping in ten individuals, of whom six were newly diagnosed cancer patients and four were previously treated with anthracyclines. Four newly diagnosed individuals received anthracyclines and all others were treated with monoclonal antibodies. Overall, this subgroup had fewer cardiovascular risk factors than found in the rest of the study population (0.50±0.53 vs 1.53±1.3 total risk factors, p=0.0003). Over three months, precontrast T1 did not change significantly (973±36 vs 982±29 ms, p=0.53). In these same individuals, the mid-cavity contrast-enhanced T1-weighted measures remained stable between baseline and follow-up (15.2±5.6 to 15.2±7.9, p=0.99) as did the LVEF (56±6 to 56±7, p=0.93). T1 did not change significantly for participants whether newly diagnosed or previously treated (p=0.99 for both). The change in precontrast T1 values were significantly correlated with LGE-SI changes (R=−0.64, p<0.05) and trended to correlate with LVEF changes (R=−0.60, p=0.06).
In newly diagnosed cancer patients (n=6), myocardial T1 trended toward a correlation with mid-cavity T1 signal intensity (R2=0.27, p=0.08). These two measures were significantly correlated with one another in individuals previously treated with anthracyclines (R2=0.63, p=0.02). Myocardial T1 did not correlate with percent edema in either subgroup (p>0.21 for both).
Analyzable T2-weighted images were acquired at baseline and follow-up in 52 individuals. Compared to baseline, we observed no appreciable differences three months after chemotherapy initiation in relative enhancement (1.3±0.3 at baseline vs 1.4±0.3 at 3 months, p=0.17) or percent edema (4.2±12% vs 5.6±11%, p=0.70). No differences between those with or without prior chemotherapy were found for change in relative enhancement (p=0.23) or percent edema (p=0.34). Similarly, no statistically significant changes in relative enhancement (p=0.27) and percent edema (p=0.59) were observed amongst the 31 individuals treated with anthracyclines.
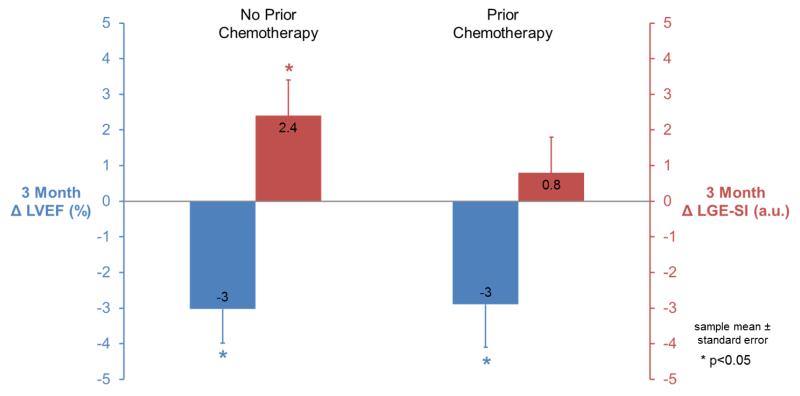
Stratified analyses were performed on individuals with and without chemotherapy treatment prior to enrollment. First, the LVSV and consequently LVEF, were reduced at baseline in participants with prior chemotherapy (p=0.02 and p=0.007, respectively). Three months after chemotherapy initiation, those receiving chemotherapy for the first time had decreases in LVEF from 59±6% to 56±6% (p=0.003) and increases in LGE-SI from 13.2±4.7 to 15.6±6.8 (p=0.04; Figure 3). In participants receiving their 2nd or 3rd course of chemotherapy, LVESV increased (p=0.006) and LVEF decreased from 55±6% to 52±7% (p=0.02) over the study. In these same participants, myocardial contrast-enhanced T1-weighted measures trended toward an elevation at baseline relative to newly diagnosed individuals (p=0.06), and a smaller increase in contrast-enhanced T1-weighted measures between baseline and follow-up was observed (15.7±5.3 to 16.5±7.0, p=0.60; Figure 3). LGE-SI was assessed in a 6 month follow-up examination in n=60 of the study participants and trended to remain elevated when compared to baseline values (15.4±6.6, p=0.10).
Figure 3.
Study participants without prior chemotherapy exposure (left) exhibited both significantly increased contrast-enhanced T1-weighted signal intensity (LGE-SI, red, right axis) and decreased LVEF (blue, left axis) three months after chemotherapy initiation. Conversely, in those with prior chemotherapy exposure (right), LGE-SI was elevated at rest and did not increase after additional chemotherapy even though LVEF decreased.
In participants with a low to moderate risk of developing CAD (≤2 CV comorbidities), we examined whether baseline LGE-SI, baseline LVEF, and prior chemotherapy exposure were predictive of a clinical LVEF decline at 3 months. Of the 52 participants, 19 experienced a primary event of clinical LVEF decline. After adjusting for prior chemotherapy administration, baseline LGE-SI signal and LVEF trended to be significant predictors of a LV decline (p=0.09 and p=0.16, respectively). For those with prior chemotherapy, a higher LGE-SI (β=0.28, p=0.07) or lower LVEF (β=−0.14, p=0.09) at baseline increased the probability of having the clinical LVEF decline three months after chemotherapy initiation.
Discussion
Three conclusions can be drawn from the results of this study. First, global measures of contrast-enhanced T1-weighted images obtained 10 minutes after the administration of 0.2 mmol/kg of gadoteridol contrast are associated with a decline in LVEF in patients receiving chemotherapy for the treatment of breast cancer or a hematologic malignancy. These findings persist regardless of age, gender, cancer treatment, or the presence of underlying CV comorbidities. The observed LVEF declines were driven by increases in LVESV (p<0.05) — a measure reduced myocardial contractility — rather than declines in LVEDV (p=0.32) that may occur in cancer patients who are in poor health and can exhibit intravascular volume depletion. Second, patients previously receiving chemotherapy trended toward higher resting measures of contrast-enhanced T1-weighted signal intensity within the LV myocardium relative to newly diagnosed patients without prior chemotherapy (p=0.06, Table 2). Finally, increases in contrast-enhanced T1-weighted signal intensity occur globally and diffusely throughout the LV myocardium without regional differences among LV myocardial segments (p=0.92) or slice locations (p=0.94). This increase in T1 signal intensity occurred without evidence of increased myocardial edema measured on T2-weighted images following chemotherapy administration (p=0.70).
Previously, we have shown LV dysfunction occurs early after cardiotoxic chemotherapy due to an increase in LVESV and diminished contractility measured by myocardial strain with CMR.19 Furthermore, increased LGE-SI forecasted clinical LVEF changes in a murine model of cardiotoxicity with histopathologic evidence of vacuolization and acute myocardial injury.7 In this study, we sought to determine if similar changes T1-weighted imaging occur commensurate with LVEF in human subjects treated for cancer. No participants with prior infarction were enrolled into the study and no participants had evidence of focal hyperenhancement due to acute infarction throughout the study. GFR remained > 60 ml/min/1.73m2 throughout both examination study points and is therefore presumed to minimally impact repeated T1-weighted measurements. Furthermore, the small increase in GFR observed in this study would, if anything, increase gadolinium clearance and bias the results towards the null.
Baseline mean contrast-enhanced T1-weighted signal intensity was increased in participants previously treated with chemotherapy versus chemotherapy-naïve participants. There are several possibilities for this finding. First, recent studies using T1 mapping strategies have shown an increase in T1 values in the setting of chronic cardiomyopathy and fibrosis.16,20-23 Since our baseline T1-weighted measures were obtained several years after initial receipt of cardiotoxic chemotherapy, chronic replacement fibrosis may have been present in our participants. Second, as we have previously shown in animals, acute, subacute, and chronic myocellular injury can be appreciated after doxorubicin with techniques that assess myocardial T1.3-5 Histopathologically, this increase in T1 signal may be related to myocytes injured by oxidative stress.5,22 Further histopathologic studies of previously treated animal models or humans could clarify this issue.
In patients with up to two CV risk factors who had previously received chemotherapy, elevated resting contrast-enhanced T1-weighted signal intensity trended to be more predictive of a clinical LVEF drop than baseline LVEF suggesting that T1 related myocardial tissue characteristics may have utility in identifying underlying risk for development of chemotherapy-induced cardiotoxicity. We present focused analyses on the 3 month follow-up examination that is chronologically closest to chemotherapy discontinuation. Baseline LGE-SI was elevated after a prolonged interval from past cancer therapy in those with chemotherapy administration prior to study entry. Likewise, LGE-SI remained elevated when assessed after a prolonged period from chemotherapy administration (6 months), albeit additional confounding cancer therapies (radiation, adjuvant therapy) were administered between the 3 and 6 month assessments for which we are underpowered to investigate further.
Blood biomarkers and T2-weighted measures of edema did not significantly change at the 3-month follow-up visit. The timing of our blood collection and image acquisition relative to the dosing of chemotherapy may have contributed to this finding. In prior CMR studies of acute ischemia or inflammation associated with T2-related changes associated with myocardial edema occur commensurate with the disease process and resolve by two weeks.24 Our study design scheduled the 3 month follow-up CMR examination to precede chemotherapy administration on the day of the study visit thereby reflecting a pre-dose rather than post-dose effect which has been evaluated in other studies.5,25 Future serial CMR studies during the subacute phase immediately following a cycle of chemotherapy are needed to determine whether T2-weighted imaging or T2 mapping may detect active myocardial water accumulation associated with acute or immediate injury in addition to T1 mapping which can appreciate both acute and chronic injury.20,23
The major therapeutic agents received by enrolled participants (Table 1 - anthracyclines, antimicrotubules, and monoclonal antibodies) are each associated with heart failure.26 Anthracyclines and antimicrotubules, however, are also associated with EKG changes and myocardial ischemia/infarction; Monoclonal antibodies are associated with LV dysfunction and cardiomyopathies.26 In this study, we observed similar findings in individuals treated with anthracycline-based or trastuzumab-based chemotherapy for breast cancer or a hematologic malignancy.
The mean contrast-enhanced T1-weighted signal intensity increased globally and diffusely across all LV myocardial segments of the 17-segment AHA model (p<0.0001). Regional changes in LV lateral wall myocardial signal intensity after receipt of trastuzumab for breast cancer have been observed previously,6 however, we did not find any regional T1 changes with respect to LV wall region (p=0.92) or short-axis slice (p=0.94). Perhaps the inclusion of only participants with confirmed trastuzumab-induced cardiomyopathy in the retrospective report by Fallah-Rad is responsible for the prior finding. At that time, more pronounced fibrosis may have been present, or participants may have experienced a secondary viral type infection after their cancer treatment.27 Our prospective study sought to identify early noninvasive markers of myocardial tissue changes using serial imaging across a somewhat diverse population that included several cancer types and treatment regimens that have been previously associated with the development of chemotherapy-induced cardiotoxicity.
Importantly, the histopathologically-validated quantitative methodology tested previously in animal models identified elevations in T1 signal commensurate with small but significant declines in LVEF related to increases in LV end-systolic volume after receipt of cardiotoxic chemotherapy. Previous studies have measured T1 and extracellular volume (ECV) in adult and pediatric survivors of cancer, showing disparate results. Both pediatric studies found T1 and ECV of survivors within normal ranges, yet showed associations with reduced functional capacity and anthracycline dose.28,29 In adult survivors, ECV was elevated compared to controls.30 We found no longitudinal change in precontrast T1, however, this healthier subgroup had fewer cardiovascular risk factors than the rest of the study population which may also explain the stable longitudinal LGE and LVEF measurements in this subgroup. Further studies are needed to investigate changes in T1 in the subacute and acute phases of cardiotoxicity.
Our study does have potential limitations. First, we did not obtain myocardial biopsy samples for correlation with CMR image results, but our methodology has been validated histopathologically in a previous animal model of chemotherapy-induced cardiotoxicity.7 Second, we were ethically unable to include cancer subjects who had chemotherapy withheld as a control group in our study. To address this, we have previously quantified the variability of the method in case-control animal model study7 and the reproducibility in human subjects free of acute or chronic disease with no change in their medical condition (R2=0.99). Third, our study population included an admixture of chemotherapy treatments and patient demographics. Larger studies could clarify the utility of this methodology and newer techniques to determine whether those receiving specific chemotherapeutic regimens were more or less susceptible to cardiac injury based on their demographics including pre-existing CV disease and LV dysfunction. Finally, the T1-weighted and T2-weighted imaging methodology requires constant imaging parameters at successive visits (such as the inversion time and time passed between contrast administration and image acquisition). We were unable to obtain direct T1, T2, or extracellular volume quantification in most of our study participants due to the limited availability of mapping sequences at the time this study was conducted and as such, are underpowered to draw conclusions regarding these results. These findings, however, demonstrate that changes in magnetic relaxation, upon which T1 and T2 mapping sequences are based, are associated with myocardial injury early after chemotherapy exposure in an admixture of cancer patients. Moreover, qualitative T1-weighted and T2-weighted imaging sequences are commercially available unlike mapping sequences which are limited to academic centers for development currently. It will be advantageous in future studies to include emerging quantitative T1 (native and postcontrast), T2, and extracellular volume mapping to discriminate acutely-injured myocardium from chronically-injured myocardium.23
In conclusion, similar to our findings observed in animals, potentially cardiotoxic chemotherapy is associated with an increase in signal intensity on T1-weighted images and a decline in LVEF in humans receiving chemotherapy. In individuals without prior exposure to chemotherapy, a further increase in signal intensity within the LV myocardium on contrast-enhanced T1-weighted images is associated with a decline in LVEF upon the receipt of potentially cardiotoxic chemotherapy. The results of this study suggest future studies should be conducted to determine if newer quantitative methods of myocardial T1 and T2 mapping identify subclinical myocardial injury in patients receiving potentially cardiotoxic chemotherapy.
Supplementary Material
Acknowledgements
Prohance contrast agent was provided for the study by Bracco Diagnostics (Princeton, New Jersey).
Sources of Funding
Research supported in part by NIH grant R33CA12196, American Heart Association Predoctoral Fellowship 09PRE2210050, and a grant from the Susan G. Komen Foundation BCTR07007769.
Footnotes
Disclosures
None.
References
- 1.Ewer MS, Yeh E. Cancer and the heart. BC Decker Inc.; Lewiston, NY: 2006. Print. [Google Scholar]
- 2.Albini A, Pennesi G, Donatelli F, Cammarota R, De Flora S, Noonan DM. Cardiotoxicity of anticancer drugs: The need for cardio-oncology and cardio-oncological prevention. J Natl Cancer Inst. 2010;102:14–25. doi: 10.1093/jnci/djp440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wassmuth R, Lentzsch S, Erdbruegger U, Schulz-Menger J, Doerken B, Dietz R, Friedrich MG. Subclinical cardiotoxic effects of anthracyclines as assessed by magnetic resonance imaging- A pilot study. Am Heart J. 2001;141:1007–1013. doi: 10.1067/mhj.2001.115436. [DOI] [PubMed] [Google Scholar]
- 4.Thompson RC, Canby RC, Lojeski EW, Ratner AV, Fallon JT, Pohost GM. Adriamycin cardiotoxicity and proton nuclear magnetic resonance relaxation properties. Am Heart J. 1987;113:1444–1449. doi: 10.1016/0002-8703(87)90660-0. [DOI] [PubMed] [Google Scholar]
- 5.Cottin Y, Ribuot C, Maupoil V, Godin D, Arnould L, Brunotte F, Rochette L. Early incidence of adriamycin treatment on cardiac parameters in the rat. Can J Physiol Pharmacol. 1994;72:140–145. doi: 10.1139/y94-022. [DOI] [PubMed] [Google Scholar]
- 6.Rad N, Lytwyn M, Fang T, Kirkpatrick I, Jassal DS. Delayed contrast enhancement cardiac magnetic resonance imaging in trastuzumab induced cardiomyopathy. J Cardiovasc Magn Reson. 2008;10:5. doi: 10.1186/1532-429X-10-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lightfoot JC, D’Agostino RB, Jr., Hamilton CA, Jordan J, Torti FM, Kock ND, Workman S, Hundley WG. Novel approach to early detection of doxorubicin cardiotoxicity by gadolinium-enhanced cardiovascular magnetic resonance imaging in an experimental model. Circ Cardiovasc Imaging. 2010;3:550–558. doi: 10.1161/CIRCIMAGING.109.918540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim RJ, Wu E, Rafael A, Chen EL, Parker MA, Simonetti O, Klocke FJ, Bonow RO, Judd RM. The use of contrast-enhanced magnetic resonance imaging to identify reversible myocardial dysfunction. N Engl J Med. 2000;343:1445–1453. doi: 10.1056/NEJM200011163432003. [DOI] [PubMed] [Google Scholar]
- 10.Rehr RB, Malloy CR, Filipchuk NG, Peshock RM. Left-ventricular volumes measured by MR imaging. Radiology. 1985;156:717–719. doi: 10.1148/radiology.156.3.4023232. [DOI] [PubMed] [Google Scholar]
- 11.Longmore DB, Klipstein RH, Underwood SR, Firmin DN, Hounsfield GN, Watanabe M, Bland C, Fox K, Poolewilson PA, Rees RSO, Denison D, McNeilly AM, Burman ED. Dimensional accuracy of magnetic-resonance in studies of the heart. Lancet. 1985;1:1360–1362. doi: 10.1016/s0140-6736(85)91786-6. [DOI] [PubMed] [Google Scholar]
- 12.John J, Woodward DB, Wang YP, Yan SB, Fisher D, Kinasewitz GT, Heiselman D. Troponin-I as a prognosticator of mortality in severe sepsis patients. J Crit Care. 2010;25:270–275. doi: 10.1016/j.jcrc.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 13.Daugaard G, Lassen U, Bie P, Pederson EB, Jensen KT, Abildgaard U, Hesse B, Kjaer A. Natriuretic peptides in the monitoring of anthracycline induced reduction in left ventricular ejection fraction. Eur J Heart Fail. 2005;7:87–93. doi: 10.1016/j.ejheart.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 14.Sechtem U, Pflugfelder PW, Gould RG, Cassidy MM, Higgins CB. Measurement of right and left-ventricular volumes in health-individuals with cine MR imaging. Radiology. 1987;163:697–702. doi: 10.1148/radiology.163.3.3575717. [DOI] [PubMed] [Google Scholar]
- 15.Cerqueira MD, Weissman NJ, Dilsizian V, Jacobs AK, Kaul S, Laskey WK, Pennell DJ, Rumberger JA, Ryan R, Verani MS. Amer Heart Assoc Writing Grp M. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart - A statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Int J Cardiovasc Imaging. 2002;18:539–542. [PubMed] [Google Scholar]
- 16.Messroghli DR, Walters K, Plein S, Sparrow P, Friedrich MG, Ridgway JP, Sivananthan MU. Myocardial T1 mapping: Application to patients with acute and chronic myocardial infarction. Magn Reson Med. 2007;58:34–40. doi: 10.1002/mrm.21272. [DOI] [PubMed] [Google Scholar]
- 17.Abdel-Aty H, Boye P, Zagrosek A, Wassmuth R, Kumar A, Messroghli D, Bock P, Dietz R, Friedrich MG, Schulz-Menger J. Diagnostic performance of cardiovascular magnetic resonance in patients with suspected acute myocarditis: comparison of different approaches. J Am Coll Cardiol. 2005;45:1815–1822. doi: 10.1016/j.jacc.2004.11.069. [DOI] [PubMed] [Google Scholar]
- 18.Schulz-Menger J, Bluemke DA, Bremerich J, Flamm SD, Fogel MA, Friedrich MG, Kim RJ, von Knobelsdorff-Brenkenhoff F, Kramer CM, Pennell DJ, Plein S, Nagel E. Standardized image interpretation and post processing in cardiovascular magnetic resonance: Society for Cardiovascular Magnetic Resonance (SCMR) Board of Trustees Task Force on Standardized Post Processing. J Cardiovasc Magn Reson. 2013;15 doi: 10.1186/1532-429X-15-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Drafts BC, Twomley KM, D’Agostino R, Jr., Lawrence J, Avis N, Ellis LR, Thohan V, Jordan J, Melin SA, Torti FM, Little WC, Hamilton CA, Hundley WG. Low to moderate dose anthracycline-based chemotherapy is associated with early noninvasive imaging evidence of subclinical cardiovascular disease. JACC Cardiovasc Imaging. 2013;6:877–885. doi: 10.1016/j.jcmg.2012.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ferreira V, Piechnik S, Dall’Armellina E, Karamitsos T, Francis J, Friedrich M, Robson M, Neubauer S. Quantification of acute myocardial injury by ShMOLLI T1-Mapping, T2-weighted and late gadolinium imaging in patients presenting with chest pain, positive troponins and non-obstructive coronary arteries. J Cardiovasc Magn Reson. 2011;13:1–2. [Google Scholar]
- 21.Messroghli DR, Radjenovic A, Kozerke S, Higgins DM, Sivananthan MU, Ridgway JP. Modified Look-Locker Inversion recovery (MOLLI) for high-resolution T1 mapping of the heart. Magn Reson Med. 2004;52:141–146. doi: 10.1002/mrm.20110. [DOI] [PubMed] [Google Scholar]
- 22.Mewton N, Liu CY, Croisille P, Bluemke D, Lima JAC. Assessment of myocardial fibrosis with cardiovascular magnetic resonance. J Am Coll Cardiol. 2011;57:891–903. doi: 10.1016/j.jacc.2010.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jiji RS, Kramer CM, Salerno M. Non-invasive imaging and monitoring cardiotoxicity of cancer therapeutic drugs. J Nucl Cardiol. 2012;19:377–388. doi: 10.1007/s12350-012-9512-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dall’Armellina E, Karia N, Lindsay AC, Karamitsos TD, Ferreira V, Robson MD, Kellman P, Francis JM, Forfar C, Prendergast BD, Banning AP, Channon KM, Kharbanda RK, Neubauer S, Choudhury RP. Dynamic changes of edema and late gadolinium enhancement after acute myocardial infarction and their relationship to functional recovery and salvage index. Circ Cardiovasc Imaging. 2011;4:228–236. doi: 10.1161/CIRCIMAGING.111.963421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cardinale D, Sandri MT, Martinoni A, Tricca A, Civelli M, Lamantia G, Cinieri S, Martinelli G, Cipolla CM, Fiorentini C. Left ventricular dysfunction predicted by early troponin I release after high-dose chemotherapy. J Am Coll Cardiol. 2000;36:517–522. doi: 10.1016/s0735-1097(00)00748-8. [DOI] [PubMed] [Google Scholar]
- 26.Vasu S, Hundley WG. Understanding cardiovascular injury after treatment for cancer: An overview of current uses and future directions of cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2013;15:66. doi: 10.1186/1532-429X-15-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mahrholdt H, Wagner A, Deluigi CC, Kispert E, Hager S, Meinhardt G, Vogelsberg H, Fritz P, Dippon J, Bock CT, Klingel K, Kandolf R, Sechtem U. Presentation, patterns of myocardial damage, and clinical course of viral myocarditis. Circulation. 2006;114:1581–1590. doi: 10.1161/CIRCULATIONAHA.105.606509. [DOI] [PubMed] [Google Scholar]
- 28.Tham EB, Haykowsky MJ, Chow K, Spavor M, Kaneko S, Khoo NS, Pagano JJ, Mackie AS, Thompson RB. Diffuse myocardial fibrosis by T1-mapping in children with subclinical anthracycline cardiotoxicity: Relationship to exercise capacity, cumulative dose and remodeling. J Cardiovasc Magn Reson. 2013;15:48. doi: 10.1186/1532-429X-15-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Toro-Salazar OH, Gillan E, O’Loughlin MT, Burke GS, Ferranti J, Stainsby J, Liang B, Mazur W, Raman SV, Hor KN. Occult cardiotoxicity in childhood cancer survivors exposed to anthracycline therapy. Circ Cardiovasc Imaging. 2013;6:873–880. doi: 10.1161/CIRCIMAGING.113.000798. [DOI] [PubMed] [Google Scholar]
- 30.Neilan TG, Coelho-Filho OR, Shah RV, Feng JH, Pena-Herrera D, Mandry D, Pierre-Mongeon F, Heydari B, Francis SA, Moslehi J, Kwong RY, Jerosch-Herold M. Myocardial extracellular volume by cardiac magnetic resonance imaging in patients treated with anthracycline-based chemotherapy. Am J Cardiol. 2013;111:717–722. doi: 10.1016/j.amjcard.2012.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.