Abstract
Background
In previous studies, we showed that gene activated matrices (GAMs) containing nonviral vectors successfully deliver genes to neurons after optic nerve and spinal cord injury. In the present study, we evaluated whether adenoviral vectors delivered within a GAM increase the efficiency of local gene delivery to injured CNS neurons. Lyophilized GAMs containing collagen and adenoviral vectors were assessed in vitro and in vivo.
Methods
We evaluated viral vector stability, release kinetics and efficiency of transduction for this GAM formulation in vitro using the quantitative polymerase chain reaction (qPCR), flow cytometry and fluorescence microscopy. Using PCR, reverse transcriptase-PCR and confocal microscopy, we assessed viral DNA retrograde axonal transport, green fluorescent protein (GFP) expression in retinal ganglion cells (RGCs) after GAM implantation into the wound of the rat transected optic nerve.
Results
qPCR analyses demonstrated that 100% of viral particles were retained within the collagen after lyophilization. In vitro studies demonstrated that 60% of the particles within the GAM were infective and not released from the collagen matrix when placed in water. By 24 h, GFP expression was detected within cells that have invaded the GAM. In vivo studies demonstrated that adenoviral particles were retrogradely transported in axons from the GAM implanted at the lesion site to the RGC in the retina where the corresponding mRNA was expressed. Analysis of the efficiency of cell transduction indicated that 69% of RGC express GFP.
Conclusions
These studies demonstrate that lyophilized GAMs containing adenoviral particles within collagen are stable, retain a significant proportion of their infectivity and successfully and efficiently deliver genes to neurons after central nervous system injury.
Keywords: adenoviral vectors, CNS, collagen, gene therapy, matrix, optic nerve injury, retinal ganglion cells, retrograde axonal transport
Introduction
There is growing evidence that successful functional regeneration after central nervous system (CNS) trauma requires a combination of therapies that aim to promote neuronal survival and axonal growth, inhibit the deposition of a scar and formation of cystic cavities and block inhibitors of axonal regeneration [1–4].
There is now ample evidence that local delivery of genes within a matrix is a successful approach for delivery of therapeutic genes to promote tissue regeneration in the CNS and peripheral tissues. The matrix component not only acts as the carrier and reservoir for the gene, but also provides a local microenvironment encouraging cell invasion leading to tissue formation [5–10] and, in the CNS, provides the local environment necessary to promote axonal growth.
Previous studies from our laboratories have demonstrated that when a ‘gene activated matrix’ (GAM), a biocompatible matrix containing either plasmid DNA(s) encoding reporter genes or neurotrophic factors, was implanted into a CNS lesion, the GAM was invaded by repair cells and growing axon terminals, which internalized the plasmids and transported them retrogradely to neuronal somata [11,12].When the lyophilized GAM contained plasmids encoding a combination of neurotrophic factors, increased neuron survival and sustained neurotrophic factor expression was observed 3 months after injury and GAM implantation [11]. In follow-up studies, we showed that, when a GAM was implanted into the injured spinal cord, not only was there retrograde transport of the plasmid DNA to the perikarya of dorsal root ganglion neurons, but also we observed a significant reduction in secondary cavitation and scar deposition at the injury site [12]. Unfortunately, however, the transduction levels with condensed plasmid DNA were low and it was thus important to evaluate more efficient vectors for gene delivery.
Recombinant adenoviral vectors have been used extensively for gene delivery of therapeutic genes in diverse models of tissue regeneration because they possess many characteristics that make them ideal to deliver genes in vivo, including: (i) transduction of dividing and nondividing cells; (ii) non-integration into the cell genome and transient expression of foreign genes; (iii) production of high levels of gene expression; (iv) accommodation of large inserts; and (v) ease of production of large quantities [13]. Unfortunately, the lack of specificity, the possibility of systemic toxicity and immunogenic reactions have limited their use for systemic gene delivery approaches, although they are still a vector of choice for local gene delivery.
Previous studies from our laboratories have demonstrated that adenoviral particles encoding platelet-derived growth factor B (PDGF-B) immobilized within a collagen matrix can successfully promote tissue regeneration after implantation in various models of wound healing [6,8,9]. Moreover, with this approach, there is no reported local or systemic toxicity [9] and phase 1/2 clinical studies using GAMs containing adenovirus encoding PDGF-B to treat chronic diabetic ulcers are in progress. Because these gene delivery strategies are increasingly used in the field of tissue engineering for tooth and bone repair [14,15], we predicted that a similar strategy could be adapted for gene transfer in neuronal regeneration.
One of the practical problems with gene delivery is the question of formulation. For example, it is well recognized that the bioactivity of viral particles is often compromised during long-term storage and the freeze–thaw process. However, lyophilization of bioactive compounds and vaccines has been used extensively in pharmacy practice to ensure that biological activity is preserved over time. Because the bioactivity of adenoviral vectors is preserved by lyophilizing the vectors in the presence of sugars [16], we reasoned that a similar approach could be used for deployment as a GAM.
Our previous studies demonstrated that lyophilized GAMs containing plasmid DNA immobilized in a collagen matrix successfully deliver genes to injured neurons when implanted at the lesion site in established models of CNS injury [11,12]. In the present study, we hypothesized that the preparation of GAMs by lyophilization of adenoviral particles in the presence of collagen protects the bioactivity of the adenoviral particles within the GAM, increases the efficiency of gene delivery and provides the scaffold necessary for tissue regeneration in the injured CNS. We therefore evaluated the properties of these lyophilized GAMs in vitro and assessed their efficiency to deliver genes to the injured neurons after implantation at the lesion site of the optic nerve crush model. The results obtained from these studies show that collagen preserves the infectivity of the adenoviral vectors during the process of lyophilization. In vivo, the genes immobilized within these GAMs are internalized by the injured axons, transported retrogradely to the neuronal somata and the reporter gene is expressed. These results suggest that lyophilized GAMs containing adenoviral vectors represent an ideal approach to efficiently deliver genes to neurons after injury.
Materials and methods
Preparation of lyophylized viruses and GAMs
Adenoviral (Ad) particles (AdGFP)
Ad5.CMV-GFP (1 × 109 AdGFP particles/µl) was obtained from Q-BIOgene (Carlsbad, CA, USA). This viral preparation was free of replication competent adenovirus.
Lyophilized AdGFP (FD-AdGFP)
AdGFP resuspended in 20 mm Tris pH 8.0, 10% glycerol, 25 mm NaCl was lyophilized overnight and stored at −80 °C.
GAMs
These samples were prepared by adding 1 × 109 AdGFP particles to neutralized bovine collagen I (2.8 mg/ml; Cohesion, Palo Alto, CA, USA) as described previously [11,12]. For control experiments neutralized bovine collagen I was diluted with 1 µl of virus buffer. Samples were lyophilized overnight and stored at −80 °C.
Wet GAMs (wGAM)
These GAMs were prepared by resuspending an aliquot of lyophilized bovine collagen I (2.8 mg/ml; Cohesion) with 1 µl of AdGFP.
Collagenase treatment
GAMs, wGAMs, collagen I matrix alone and lyophilized virus alone were resuspended in 25 µl of Iscoves’s modified Dulbecco’s media (Fisher Scientific, Loughborough, UK) containing 0.2 mg/ml collagenase XI (Sigma, Poole, UK) and incubated at 37 °C for 30 min [8]. After incubation, samples were mixed thoroughly to ensure digestion of the collagen matrix. These samples were used for infection, analysis of green fluorescent protein (GFP) expression by flow cytometry and reverse transcriptase-polymerase chain reaction (RT-PCR) experiments.
In vitro studies
Human A549 cells
Cells were grown in Dulbecco’s modified Eagle’s medium medium containing 10% fetal bovine serum, glutamine and antibiotics in an incubator at 37 °C with 5% CO2.
Primary retinal cultures
Retinas from adult (200–250 g) Wistar rats were dissected as described by Lorber et al. [17]. Isolated primary retinal cells were resuspended in supplemented Neurobasal media (Invitrogen, Paisley, UK), seeded on 24-well plates at a density of 500 000 cells/well and placed for 24 h in an incubator at 37 °C with 5% CO2 [17].
GAM treatment and viral infections
To test viral infectivity, GAMs, collagen or Ad preparations were added to the A549, or primary retinal culture cells seeded on 24-well plates and centrifuged at 400 g for 1 min at room temperature to place the GAM on the bottom of the well and in contact with the cells. Cells were returned immediately to the incubator and GFP expression was analyzed after 2–3 days using fluorescence microscopy and flow cytometry.
Quantitative analysis of GFP expression by flow cytometry
GAMs and collagen were first treated with collagenase as described above. Cells were trypsinized, resuspended in phosphate-buffered saline (PBS) and analyzed immediately with a FACScalibur (BD Biosciences, Oxford, UK). Live cells were analysed for GFP expression after exclusion of dead cells using propidium iodide staining. Data were recorded and analyzed using CellQuest software (BD Biosciences). Relative cellular GFP expression was determined by comparison with control cells infected with 1 µl of viral preparation.
Viral DNA cleaning procedure
In order to use viral DNA for quantitative PCR (qPCR), the DNA was first ‘cleaned’ from viral capsid proteins and collagenase used in previous steps. Viral particles and collagenase-treated matrices were subjected to DNA purification using DNAeasy Blood and Tissue kit (Qiagen, Crawley, UK), and DNA was eluted with BPC grade water (Sigma) and used for subsequent qPCR reactions.
Quantitative PCR
qPCR was performed using SYBR Green (Abgene, Epsom, UK) and nested GFP primers. Primers were selected to generate products in the range 100–300 bp and no unspecific products such as primer dimers. Aliquots (1 µl) of a 1 : 16 dilution from the collagenase-treated matrices were used in a 15-µl reaction with Absolute QPCR SYBR Green Mix (Abgene) and 70–300 nm of each primer. The reactions were performed and analyzed on a RotorGene 3000 system (Corbett Research, Chai Wan, Hong Kong). Following the initial denaturation step (95 °C for 15 min), the cycling steps included 95 °C for 15 s, 60 °C for 20 s and 72 °C for 30 s (40 cycles). The amplification efficiency and the take-off point (Ct value) for each reaction were calculated using RotorGene (version 6.0) software (Corbett Research). Sample concentration was compared against a standard curve obtained by amplification of known viral-DNA concentrations. At least three replicates per sample were run.
In vivo studies
Optic nerve injury
Groups of four male adult Wistar (200–250 g) rats were anaesthetized with fluorothane. The optic nerve was accessed intraorbitally by a dorsal approach. After opening the dural sheath, the nerve was transected 2 mm distal to the lamina cribosa without damaging the central retinal artery. Both optic nerves were injured and either a GAM containing AdGFP or a collagen pellet was implanted between the cut optic nerve ends. After GAM implantation, the lesion site was closed by standard surgical procedures. Animals recovered from surgery with little or no morbidity and were maintained in accordance with British Home Office guidelines.
Tissue extraction and nucleic acid purification
Animals were killed and each of the retinas (R), the proximal optic nerve end including the implant (G) and the distal optic nerve (D) were dissected, snap frozen in liquid nitrogen and stored at −80 °C. RNA and DNA were extracted from tissues with Trizol (Invitrogen) in accordance with the manufacturer’s instructions and resuspended in 30 µl of water. DNA and RNA concentrations were measured using a spectrophotometer.
Reverse transcription
RNA samples (3 µg) were reverse transcribed using M-MLV (Invitrogen) reverse transcriptase in accordance with the manufacturer’s instructions. Five µl of the reaction were used for RT-PCR.
PCR analysis
DNA or cDNA were subjected to 30 cycles of PCR amplification using ReddyMix PCR master mix containing Taq polymerase (ABgene). For GFP amplification, a standard PCR reaction was followed by nested PCR using 2 µl of the first reaction. Amplification products were electrophoresed on 1% agarose gel and visualised by ethidium bromide staining using bioimaging (Syngene, Cambridge, UK). Primers used for PCR reactions were: GFP-F, 5′-ACTCTTCACTGGAGTTGT-3′; GFP-R, 5′-AAGAAGGACCATGTGGTCT-3′; nested GFPF, 5′-AGTTCTCTGTCAGTGGAGAG-3′; nested GFP-R, 5′-CCAGAATGTTGCCATCTTCC-3′; ribosome18S-F, 5′-TGGTTCCTTTGGTCGCTC-3′; and ribosome18S-R, 5′-GAGGTTATCTAGAGTCACC-3′.
Immunohistochemical analysis
Rats that received a GAM or collagen implant were killed under a CO2 chamber and perfused transcardially with 4% paraformaldehyde in 0.1m phosphate buffer, pH 7.2. After perfusion, both optic nerves and retinas were dissected (four to six tissue samples), rinsed in PBS containing 30% sucrose, embedded in OCT (Raymond Lamb, Peterborough, UK) and stored at −80 °C. Longitudinal sections (15 µm thick) of the optic nerve were cut with a cryostat and mounted on positive charged slides. Immunohistochemical staining of optic nerve sections was performed according to established techniques [12]. Primary antibodies comprised rabbit anti-bovine glial fibrillary acidic protein (GFAP; astrocytic marker, 1 : 1000; Sigma), rabbit anti-laminin (1 : 100 Sigma), mouse antirat monocyte marker ED1 (1 : 200; Serotec Ltd, Oxford, UK), mouse anti-GFP (1 : 100; Invitrogen); and mouse anti-RT97 (neurofilament marker, 1 : 50; Serotec). All antibodies were diluted in PBS containing 0.3% Tween 20 (Sigma) and 2% bovine serum albumin (BSA; Sigma). Briefly, sections were blocked with 10% normal goat serum (Vector Labs, Peterborough, UK) in PBS containing 2% BSA and 0.3% Tween 20 for 20 min. Sections were then incubated at 4 °C overnight with the primary antibodies, rinsed and incubated for 45 min in Alexa 488 goat anti-mouse and/or Alexa 594 goat anti-rabbit antibodies (Invitrogen). Finally, sections were washed and mounted with mounting medium with DAPI (Vectashield; Vector Labs). Slides were evaluated using immunofluorescence confocal microscopy (LSM510; Zeiss, Welwyn Garden City, UK).
Tracing studies
Retrograde tracing was performed by injection of 2 µl of 20% lysinated rhodamine dextran (LRD; Invitrogen) into the proximal optic nerve segment at 5 days post-lesion according to the method of Berry et al. [18]. After 48 h, the retinas were collected and whole-mounted. The total number of LRD filled and GFP positive retinal ganglion cells (RGCs) in each retina was determined using the image analysis software (ImagePro; Media Cybernetics, Marlow, UK). Fifty to 60 random fields were counted per retina.
Statistical analysis
Total counts of LRD-labelled RGC were statistically analysed using single factor analysis of variance (ANOVA).
Results
In vitro characterization of GAMs
Immobilization and retention of adenoviral particles within the GAM
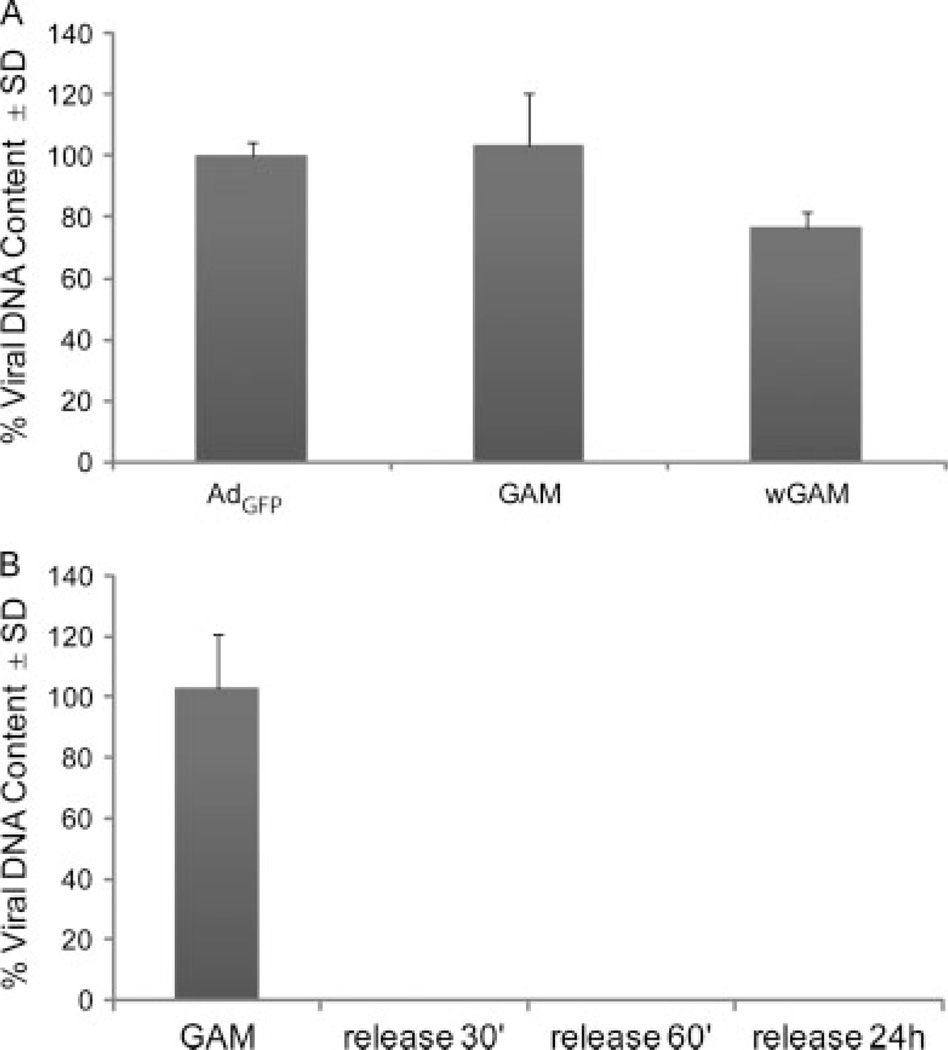
As an initial characterization of GAMs made by lyophilizing the adenoviral virus in the presence of collagen, we first determined the percentage of viral particles retained either within the GAM after the lyophilization process or within a wGAM, in which 1 µl of AdGFP was added to lyophilized collagen. GAMs were prepared as described in the Materials and Methods using the same formulation as that for in vitro and in vivo studies. The size of the GAMs after lyophilization was approximately 2 mm2. To quantify the number of viral particles immobilized within the matrix, GAMs were treated with collagenase and processed for qPCR. The results from these studies show that GAMs prepared by lyophilizing the viral particles in the presence of collagen retained 100% of the viral particles, whereas the wGAM retained only 76–78% (Figure 1A). We then determined the effect of the matrix on vector release. GAMs were resuspended in 50 µl of water and, after incubation at 37 °C for 30 min, 60 min and 24 h, water was collected and processed for qPCR. A measuring curve using serial dilutions of AdGFP was used as a reference of DNA content against which our samples were compared. When GAMs were placed in water, no viral DNA was detected, even after 24 h, indicating that the GAM acts to efficiently retain the immobilized viral vectors (Figure 1B). This is in marked contrast to Ad added directly onto a bed of lyophilized collagen matrix (wGAM), whereby 25–30% is immediately released (not shown).
Figure 1.
Viral DNA stability and retention within the GAM. (A) qPCR analysis of AdGFP, GAM and wGAM shows that GAMs retain 100% of viral DNA after lyophilization. (B) qPCR analysis of GAMs after resuspension in water demonstrates the lack of release of viral DNA into the water even after incubation for 24 h. The values on the y-axis are expressed as the mean ± SD percentage of viral DNA
Immobilized adenoviral particles retain their infectivity
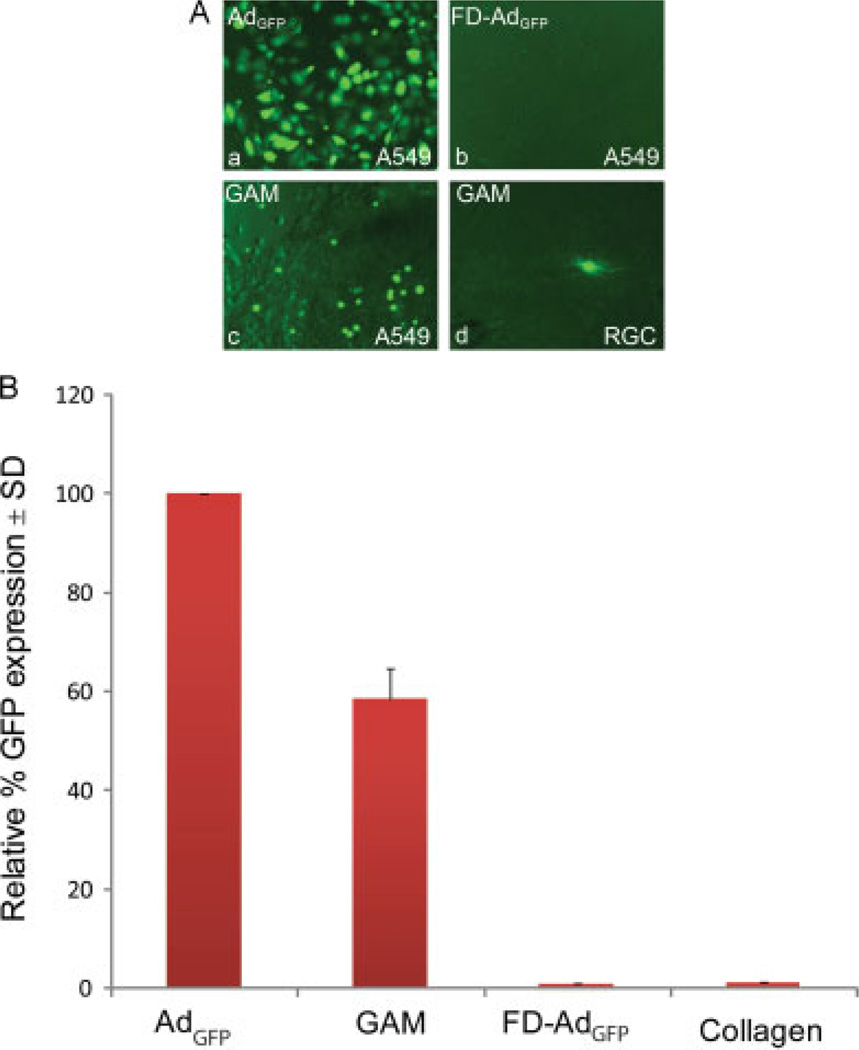
To assess whether the lyophilization of Ad in the presence of collagen also influences viral activity, A549 cells (Figure 2A, a–c) or RGCs (Figure 2A, d) were plated on 12-well plates and, 24 h later, a GAM or a collagen implant was positioned at the bottom of the well in close contact with the cells. For control studies, 1 µl of AdGFP (Figure 2A, a) or an inactive FD-AdGFP (Figure 2A, b) were added to control wells. Within the first 24 h, we observed cells migrating and invading the collagen matrix of control and GAM groups with no apparent deleterious effect on the cells. In the GAM group (Figure 2A, c), but not in the control, cells within the matrix showed GFP fluorescence after 24 h, which peaked at 48 h, suggesting that they had been transduced by the immobilized virus in the GAM. Accordingly, few GFP positive cells were observed surrounding the GAM and are assumed to represent cells that migrated out of the matrix. As expected, almost all the cells that had been infected with soluble AdGFP were GFP positive after 48 h from infection. Flow cytometry analyses showed that GAMs transduced 60% of the cells compared to soluble Ad alone. Similar studies using primary retinal cultures showed GFP positive RGCs after treatment with GAM (Figure 2A, d). Cells treated with FDAdGFP or collagen showed no GFP expression (Figure 2B).
Figure 2.
In vitro GFP expression after GAM treatment. (A) A549 cells (a–c) were plated on 12-well plates and, 24 h later, treated with either AdGFP (a), FD-AdGFP (b) or GAM (c). As expected, after AdGFP treatment, almost all cells were GFP positive, whereas no positive cells were observed after FD-AdGFP. After GAM treatment, almost all positive GFP cells were observed within the GAM. GFP positive cells were also observed after treatment of primary cultures of RGC with GAMs (d). (B) Flow cytometry analyses of in vitro A549 cells show that 60% of the cells express GFP after GAM treatment. No expression was observed in cells treated with either collagen or FD-AdGFP. The values on the y-axis are expressed as the mean ± SD relative percentage of GFP expression
In vivo characterization of GAMs
Retrograde axonal transport of viral DNA and mRNA expression after optic nerve injury
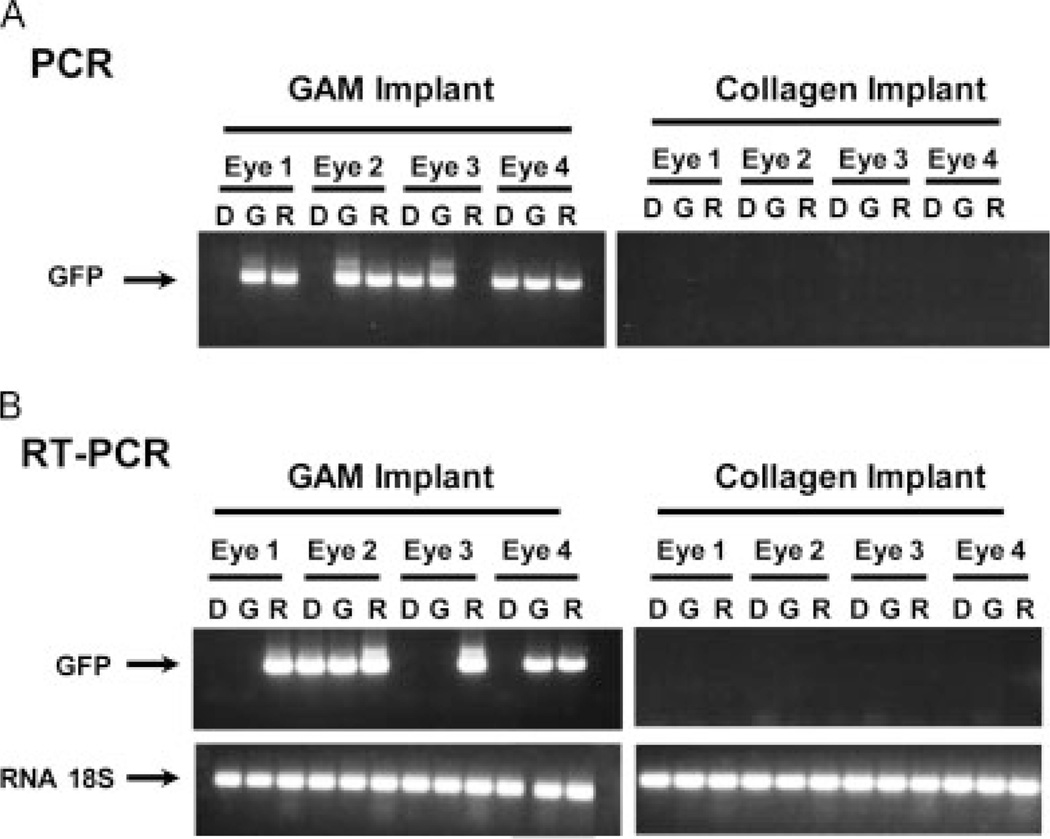
To evaluate the efficacy of GAM immobilization for injured CNS neuron uptake in vivo, we used an established optic nerve injury model as described previously [11]. The optic nerve was transected 2 mm distal of the retina and GAMs implanted at the site of the lesion. At 7 days post-injury, the retina (R), the proximal optic nerve end including the GAM implant (G) and the distal nerve end (D) were collected, processed, RNA and DNA extracted and subjected to PCR amplification. As shown in Figure 3, no viral DNA was detected in the tissues from animals treated with the collagen matrix implants (Figure 3A). By contrast, viral DNA was readily detectable in retinas and GAM/proximal optic nerve end of animals that received a GAM implant (Figure 3A). In two of four animals, we detected viral DNA in the distal end of the optic nerve presumably due to cross contamination at the time of dissection.
Figure 3.
Retrograde transport of DNA and mRNA expression after CNS injury and GAM implantation. The optic nerve was transected and GAM was implanted in the lesion site. Animals were killed 1 week post-injury and the retinae (R), GAM implant (G) and distal portion of the nerve (D) were processed for PCR (A) and RT-PCR (B) analyses to reveal DNA and RNA, respectively. Control animals received an implant of collagen I. The results of the PCR analyses demonstrate that viral DNA was detectable in all GAMs and almost all retinas of animals treated with GAM. No viral DNA was detected in animals that received collagen implants (A). The results of the RT-PCR analyses demonstrate the presence of mRNA encoding GFP in retinas treated with GAM and in almost all GAM implants. No mRNA was detected in tissues from animals that received collagen implants (B)
RT-PCR analyses failed to detect GFP cDNA in tissues from animals treated with collagen matrix implants alone. By contrast, this was detected in the retinas of animals that received the GAM implant. In two out of four animals, the GAM/proximal optic nerve end showed a strong signal, whereas only one animal had detectable levels of mRNA in the distal optic nerve end (Figure 3B).
Gene expression after GAM implantation
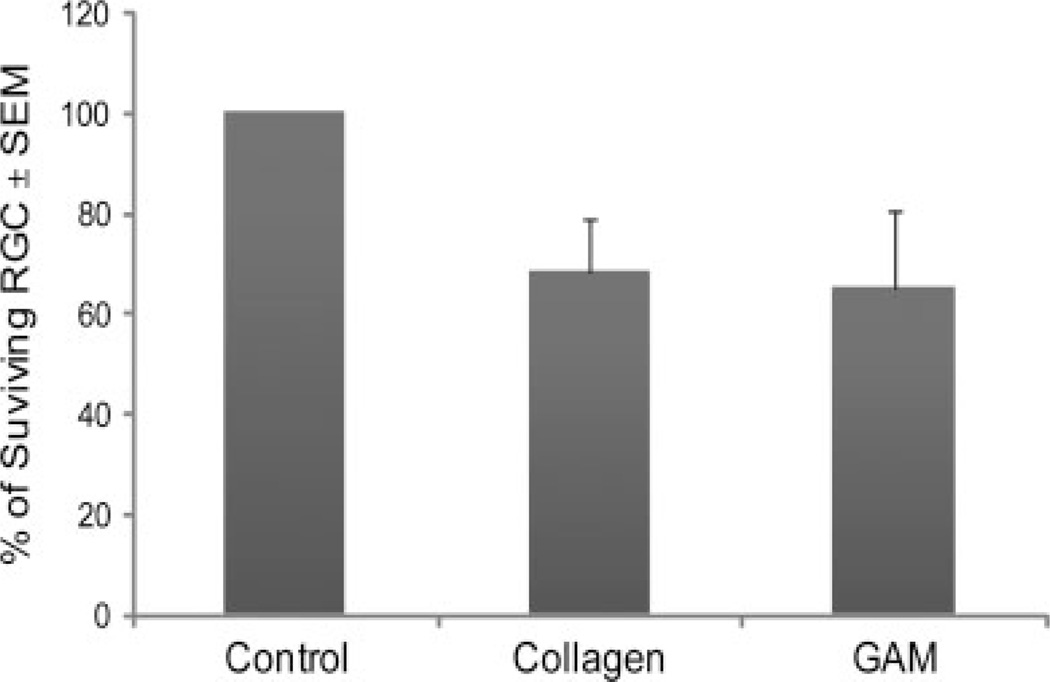
To evaluate the efficiency of gene expression in the retina, a GAM was implanted at the site of optic nerve transection and 5 days later LRD was injected into the proximal nerve end for retrograde labelling of surviving RGC. Two days later (i.e. 7 days post-injury), animals were killed and retinal whole mounts prepared. The number of RGC expressing GFP was determined by counting 50–60 fields using fluorescence microscopy and image analysis software. Data were normalized using the retinas from a control animal that did not have optic nerve injury but in which nerves were similarly injected with LRD 48 h before sacrifice to give total RGC counts. As expected, there was approximately 65–68% RGC survival in both treatments at the 7-day time point examined (Figure 4). Because there was no significative difference in the number of surviving RGC between the animal that received collagen or adenoviral GAM implants (P = 0.44), we concluded that none of the components of the lyophilized GAM were toxic to the RGC.
Figure 4.
RGC survival after optic nerve injury and GAM treatment. Rats received an injection of LRD in the proximal end of the optic nerve 48 h prior to termination of the experiment in order to label the surviving RGC in the retina. The number of rhodamine positive RGC was determined by fluorescence microscopy using the image analysis software ImagePro. The results from this experiment demonstrate that the rate of RGC survival (65–68%) is similar in animals treated with GAM or collagen implants. The values on the y-axis are expressed as the mean ± SEM percentage of surviving RGC. Single factor ANOVA statistical analysis showed no significative difference in the number of LRD-back labelled RGC in the GAM and collagen-treated groups
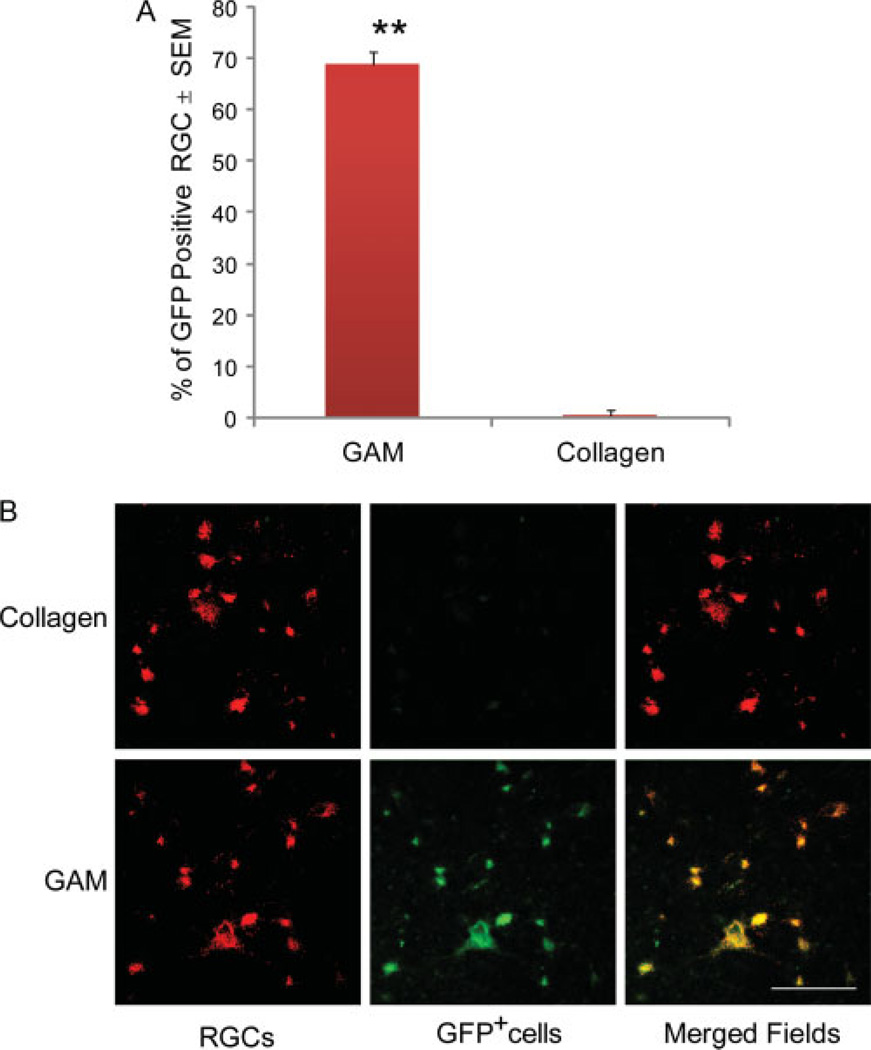
Microscopic analyses of GFP expression in retinal whole mounts demonstrated that 69% (±5%) of RGC expressed GFP after GAM treatment (Figure 5A). Confocal analyses demonstrated that the cells expressing GFP were LRD-back-labelled RGC (Figure 5B). No GFP expressing cells were detected in retinae treated with collagen matrix implants.
Figure 5.
GFP Expression in retinal ganglion cells after GAM treatment. The number of GFP positive retinal ganglion cells was determined by fluorescence microscopy using the image analysis software ImagePro. The results from these experiments show that GAM transduces 70% of retinal cells. No GFP positive cells were observed in animals that received a collagen matrix implant (A). The values on the y-axis are expressed as the mean ± SEM percentage of GFP positive RGC. Single-factor ANOVA showed significant difference in the number of GFP positive cells in the GAM compared to the collagen treated group (p ≤ 0.05). Co-localization studies and confocal microscopy demonstrates that the cells expressing GFP in the retina are back-labelled retinal ganglion cells (B). Bar = 50 microns
Histological analyses of the optic nerve lesion and GAM implant optic nerve area
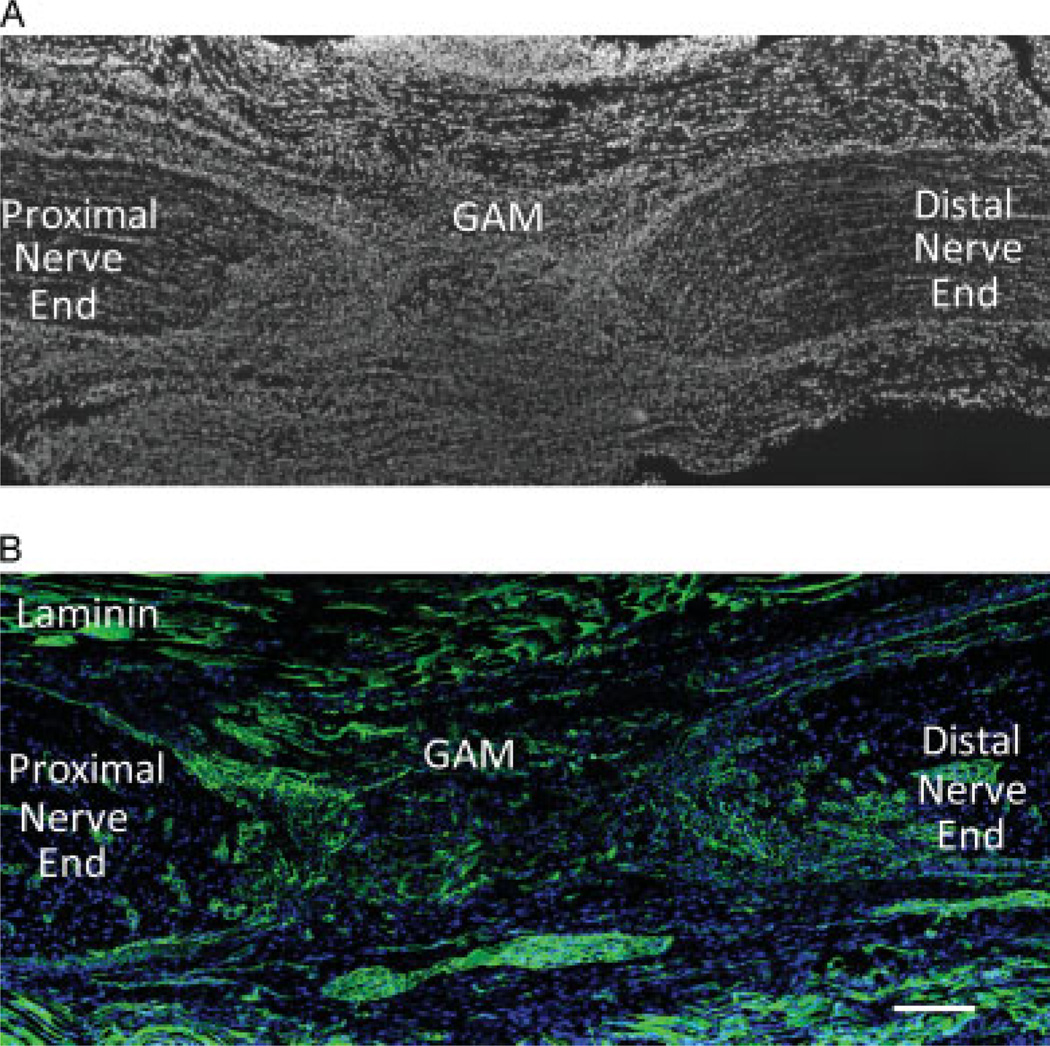
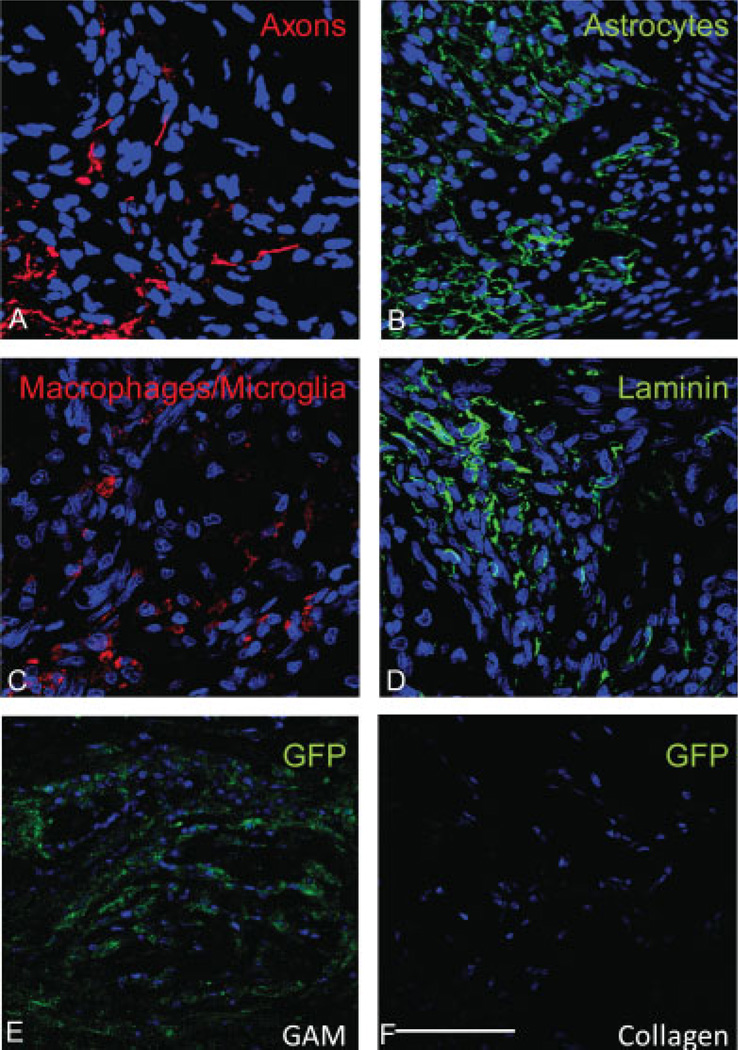
To assess the integration of the GAM implant at the optic nerve lesion site, longitudinal optic nerve sections were stained with different immunohistochemical markers to evaluate cell invasion, axon infiltration, inflammation and GFP expression. By 7 days, the GAM had completely integrated between the optic nerve stumps (Figure 6A). Using DAPI to stain cell nuclei, it was evident that the GAM had been invaded by repair cells including fibroblasts, glia, inflammatory and endothelial cells. Laminin immunoreactivity at the GAM site confirmed the integration of the GAM with the surrounding tissue and the lack of deposition of a scar at the lesion site (Figure 6B). By 7 days, very little collagen matrix from the GAM implant remained and numerous small blood vessels (visualized with laminin antibodies) were present at the optic nerve ends and within the GAM (Figure 6B). At this same time point, neurofilament positive axons were observed within and about the GAM (Figure 7A). This suggests that the collagen matrix was permissive for GAM invasion of growing axons, pinocytotic uptake and retrograde transport of the viral DNA to cell bodies, resulting in equivalent numbers of LRD-back-filled RGC expressing the transgene. GFAP positive astrocytes were detected within the GAM and in the surrounding tissue, but no glia scar was evident in the tissues analyzed (Figure 7B). As expected, ED1 positive inflammatory cells were observed within the GAM (Figure 7C). Laminin antibodies confirmed the lack of glia scar deposition and the invasion of small capillaries at the lesion site (Figure 7D). Antibodies to detect GFP showed the presence of transduced GFP positive cells within cells invading the GAM and the closely surrounding tissue (Figure 7E). No GFP positive cells were detected within the collagen implant (Figure 7F).
Figure 6.
Histological analysis of the optic nerve lesion and GAM implant area. Sagital sections of the lesioned optic nerve were labelled with DAPI (A) or immunostained with anti-laminin antibodies (B) to assess the integration of the GAM into the lesion area. By 7 days post-injury, there is very little collagen matrix left within the GAM and this has been replaced by multiple cell types and other extracellular matrix proteins. Immunohistochemical analyses with antibodies to laminin demonstrated that there is no glia scar deposition at the lesion site and laminin is observed within the GAM surrounding small capillaries. Bar = 200 microns
Figure 7.
Characterization of the cells invading the GAM: Sagittal sections of the lesion optic nerve were immunostained with antibodies to detect axons (RT97), astrocytes (GFAP), macrophages and microglia (ED1), laminin and GFP positive cells. The results from these studies show that the GAM is invaded by growing axons (A). Although there is significant staining with GFAP, no scar deposition is observed (B). Some macrophages and microglia are also present within the GAM (C). Laminin antibodies confirmed the absence of a glial scar and the invasion of the GAM by small capillaries (D). Many GFP positive cells are observed within the GAM (E). No GFP staining was observed in control collagen matrix implants (F). Bar = 50 microns
Discussion
The goal of these studies was to assess the in vitro and in vivo characteristics of GAMs in which adenoviral particles were lyophilized in the presence of collagen and to determine whether such GAMs successfully deliver genes to neurones after CNS injury. GFP was used as the reporter gene to assess GAM activity in vitro and in vivo.
The in vitro analyses demonstrate that lyophilized collagen GAMs containing adenovirus are stable, retain a significant proportion of their infectivity and can successfully and efficiently transduce dividing and nondividing cells, such as retinal ganglion cells. One of the engineered properties of the collagen GAMs is the stability of virus and their retention within the matrix. First, the collagen matrix protects the adenoviral particle from both inactivation and loss during the process of lyophilization. By contrast, adenovirus lyophilized in the presence of 10% glycerol loses all its activity. These results are in agreement with a number of recent studies showing that the lyophilization of adenoviral particles in the presence of bulking agents such as sucrose and mannitol preserves the infectivity of the viral particles [15,16,19]. Collagen can clearly play the same role and has the additional advantage that, once lyophilized, it can be deployed as a biodegradable matrix scaffold for invading repair cells. Further formulation of the collagen matrix by increasing protein : virus ratios, including bulking agents and using conduits, could provide even more temporal and spatial regulation of gene activity. Although it is known that many excipients, bulking agents and preservatives can protect viral particles from inactivation, it is also critical to take into consideration the secondary off-target effects that these chemicals may have in vivo.
The local immobilization of genes for deployment in the CNS requires a very careful formulation of the GAM, particularly when used in the optic nerve injury model. For example, the implant has to be very small (1–2 mm2) and needs to be carefully implanted at the lesion site where the nerve has been transected. A lyophilized GAM is ideal for this purpose because it is easily physically manipulated when implanted at the lesion site and, when it becomes hydrated by the wound fluid, acts as a physical bridge between the two transected nerve ends. The adenoviral particles are not released from the collagen; instead, it is the repair cells and injured axons that migrate into the collagen matrix as part of the natural remodelling and consolidation response to injury, where they can internalize the viral particles and express the gene of interest.
Our in vitro studies demonstrate that cells invading the GAM are the first to express GFP. Very few cells outside the GAM are GFP positive, and presumably comprise those that have migrated outside of the matrix after transduction, although we cannot exclude the possibility of transduction by particle leakage from the GAM. If this is the case, it must be very low levels and represent less than 1% of transduced cells. This finding is in agreement with our observation that, under the lyophilization conditions used in the present study, there is no release of the viral vector into conditioned media. Whereas other studies have observed release of the vector over time, their lyophilization process and the matrix used was significantly different from that employed in the present study [19].
The results obtained from the in vivo studies strongly support the hypothesis that injured axons in the proximal stump of the optic nerve invade the GAM, internalize the adenoviral particles and retrogradely transport them to the perykarion of the RGC, where the transgene is transcribed and translated. First, there is axonal staining in the matrix. Second, viral DNA is detected in the retina. Finally, neurons in the retina are transduced by vectors that are placed distally from their perikaryon.
Adenovirus was originally selected for these studies to determine whether our original observations of the low, albeit sustained, transduction after gene delivery with plasmid DNA, could be resolved using a more efficient vector. Adenovirus is an attractive candidate vector because it has been extensively used to deliver genes in the CNS [20–23]. It has high infectivity for dividing and nondividing cells, leading to high levels of transgene expression. Moreover, it is attractive because adenoviral vectors can be retrogradely transported by axons in the CNS and the transport of DNA is an essential feature of the GAM strategy in CNS injury as a result of its implantation at the lesion site, distal from the neuronal cell bodies. Finally, two features are required when treating CNS injury and, indeed, are present in GAMs containing adenoviral vectors: (i) the lack of integration into the host genome and (ii) the transient gene expression.
In our previous studies, the efficiency of gene delivery as defined by transfection might have been high but transduction was low. Adenovirus addresses this weakness and provides significant cell transduction, reaching almost 70% of RGC expressing GFP. By comparison, no GFP was detected with plasmid DNA, although DNA was efficiently transported to the retina. It is particularly important to note that there was no difference in the number of RGC survival in collagen or GAM implant-treated animals, indicating that the adenoviral vector within the GAM is not toxic. To our knowledge, these studies are the first to demonstrate successful cell transduction of neurons in vivo from adenoviral particles lyophilized in the presence of collagen.
As also shown in our previous studies [12], collagen provides a scaffold for tissue repair and reduces the formation of cavities at the lesion site. Cavitation is a sequela of CNS injury that is observed in rats and humans after spinal cord injury. The histological analyses from the optic nerve at the lesion site indicate that, as in spinal cord injury, the GAM is invaded by repair cells. By 7 days, the collagen as formulated here is completely integrated within the tissue. This formulation is an essential component of the approach because it acts as a depot for gene immobilization and delivery, depending on the migration of repair cells and axons. Accordingly, formulation of the matrix can have profound effects on the bioefficacy of the GAM. Depending on crosslinking, permeability, susceptibility to proteases and the kinetics of remodelling, the matrix will define and ultimately control the efficacy of gene delivery.
The importance of formulating the matrix is illustrated by the observation that there was no deposition of a glia scar at the lesion site where the GAM was implanted. Because, in this instance, we only delivered a reporter gene, we were not expecting to observe significant numbers of axons invading the GAM, although an abortive regenerative response is well described in this model [24] and presumably accounts for the detection of GFP transgene expression in the retina, distal from gene placement. The GAM was mainly invaded by repair cells, endothelial cells, glia, fibroblasts and, in the peri-GAM area, macrophages. As predicted, repair cells that invade the GAM show GFP expression and, similar to the findings of in vitro studies, a few positive cells outside the GAM were also GFP positive. Taken together, these results support our hypothesis that the efficacy of the adenoviral particles in GAMs is a result of them not being released from the matrix, but being immobilized in the formulated GAM.
In summary, these studies demonstrate that when GAMs are implanted into the CNS after injury, the matrices are invaded by the injured axons and repair cells that both internalize the viral vector. The viral DNA and, presumably, but not necessarily, the whole particle, is transported retrogradely to the distal cell bodies of RGC where the reporter gene is translated into the appropriate protein. These studies also indicate that the formulation of lyophilized GAMs containing adenoviral particles within collagen are stable, retain a significant proportion of their infectivity and can be used to retrogradely deliver genes to neuron cell bodies after CNS injury. The deployment of GAMs formulated to deliver optimal combinations of neurotrophic factors, proteases, soluble receptors and small interfering RNAs in a time-dependent fashion is currently under investigation aiming to define the optimal combination of therapeutic genes that can provide temporal–spatial control for functional neuronal regeneration.
Acknowledgements
These studies were supported by BBSRC grant (29/E18035).
References
- 1.Ahmed Z, Mazibrada G, Seabright RJ, Dent RG, Berry M, Logan A. TACE-induced cleavage of NgR and p. 75NTR in dorsal root ganglion cultures disinhibits outgrowth and promotes branching of neurites in the presence of inhibitory CNS myelin. FASEB J. 2006;20:1939–1941. doi: 10.1096/fj.05-5339fje. [DOI] [PubMed] [Google Scholar]
- 2.Ellis-Behnke RG, Liang YX, You SW, et al. Nano neuro knitting: peptide nanofiber scaffold for brain repair and axon regeneration with functional return of vision. Proc Natl Acad Sci USA. 2006;103:5054–5059. doi: 10.1073/pnas.0600559103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Geller HM, Fawcett JW. Building a bridge: engineering spinal cord repair. Exp Neurol. 2002;174:125–136. doi: 10.1006/exnr.2002.7865. [DOI] [PubMed] [Google Scholar]
- 4.Lu P, Tuszynski MH. Growth factors and combinatorial therapies for CNS regeneration. Exp Neurol. 2008;209:313–320. doi: 10.1016/j.expneurol.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Agarwal A, Mallapragada SK. Synthetic sustained gene delivery systems. Curr Topics Med Chem. 2008;8:311–310. [PubMed] [Google Scholar]
- 6.Chandler LA, Gu DL, Ma C, et al. Matrix-enabled gene transfer for cutaneous wound repair. Wound Repair Regen. 2000;8:473–479. doi: 10.1046/j.1524-475x.2000.00473.x. [DOI] [PubMed] [Google Scholar]
- 7.De Laporte L, Shea LD. Matrices and scaffolds for DNA delivery in tissue engineering. Adv Drug Deliv Rev. 2007;59:292–307. doi: 10.1016/j.addr.2007.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Doukas J, Chandler LA, Gonzalez AM, et al. Matrix immobilization enhances the tissue repair activity of growth factor gene therapy vectors. Hum Gene Ther. 2001;12:783–798. doi: 10.1089/104303401750148720. [DOI] [PubMed] [Google Scholar]
- 9.Gu DL, Nguyen T, Gonzalez AM, et al. Adenovirus encoding human platelet-derived growth factor-B delivered in collagen exhibits safety, biodistribution, and immunogenicity profiles favorable for clinical use. Mol Ther. 2004;9:699–711. doi: 10.1016/j.ymthe.2004.02.018. [DOI] [PubMed] [Google Scholar]
- 10.Heyde M, Partridge KA, Oreffo RO, Howdle SM, Shakesheff KM, Garnett MC. Gene therapy used for tissue engineering applications. J Pharm Pharmacol. 2007;59:329–350. doi: 10.1211/jpp.59.3.0002. [DOI] [PubMed] [Google Scholar]
- 11.Berry M, Gonzalez AM, Clarke W, et al. Sustained effects of gene-activated matrices after CNS injury. Mol Cell Neurosci. 2001;17:706–716. doi: 10.1006/mcne.2001.0975. [DOI] [PubMed] [Google Scholar]
- 12.Gonzalez AM, Berry M, Greenlees L, Logan A, Baird A. Matrix-mediated gene transfer to brain cortex and dorsal root ganglion neurones by retrograde axonal transport after dorsal column lesion. J Gene Med. 2006;8:901–909. doi: 10.1002/jgm.919. [DOI] [PubMed] [Google Scholar]
- 13.Printz MA, Gonzalez AM, Cunningham M, et al. Fibroblast growth factor 2-retargeted adenoviral vectors exhibit a modified biolocalization pattern and display reduced toxicity relative to native adenoviral vectors. Hum Gene Ther. 2000;11:191–204. doi: 10.1089/10430340050016265. [DOI] [PubMed] [Google Scholar]
- 14.Schek RM, Hollister SJ, Krebsbach PH. Delivery and protection of adenoviruses using biocompatible hydrogels for localized gene therapy. Mol Ther. 2004;9:130–138. doi: 10.1016/j.ymthe.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 15.Zhang Y, Song J, Shi B, et al. Combination of scaffold and adenovirus vectors expressing bone morphogenetic protein-7 for alveolar bone regeneration at dental implant defects. Biomaterials. 2007;28:4635–4642. doi: 10.1016/j.biomaterials.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 16.Croyle MA, Cheng X, Wilson JM. Development of formulations that enhance physical stability of viral vectors for gene therapy. Gene Ther. 2001;8:1281–1290. doi: 10.1038/sj.gt.3301527. [DOI] [PubMed] [Google Scholar]
- 17.Lorber B, Berry M, Logan A, Tonge D. Effect of lens lesion on neurite outgrowth of retinal ganglion cells in vitro. Mol Cell Neurosci. 2002;21:301–311. doi: 10.1006/mcne.2002.1175. [DOI] [PubMed] [Google Scholar]
- 18.Berry M, Carlile J, Hunter A. Peripheral nerve explants grafted into the vitreous body of the eye promote the regeneration of retinal ganglion cell axons severed in the optic nerve. J Neurocytol. 1996;25:147–170. doi: 10.1007/BF02284793. [DOI] [PubMed] [Google Scholar]
- 19.Hu WW, Wang Z, Hollister SJ, Krebsbach PH. Localized viral vector delivery to enhance in situ regenerative gene therapy. Gene Ther. 2007;14:891–901. doi: 10.1038/sj.gt.3302940. [DOI] [PubMed] [Google Scholar]
- 20.Barkats M, Horellou P, Colin P, Millecamps S, Faucon-Biguet N, Mallet J. 1-methyl-4-phenylpyridinium neurotoxicity is attenuated by adenoviral gene transfer of human Cu/Zn superoxide dismutase. J Neurosci Res. 2006;83:233–242. doi: 10.1002/jnr.20696. [DOI] [PubMed] [Google Scholar]
- 21.Hurtado-Lorenzo A, David A, Thomas C, Castro MG, Lowenstein PR. Use of recombinant adenovirus for gene transfer into the rat brain. Evaluation of gene transfer efficiency, toxicity, and inflammatory and immune reactions. Methods Mol Med. 2003;76:113–133. doi: 10.1385/1-59259-304-6:113. [DOI] [PubMed] [Google Scholar]
- 22.Le Gal La Salle G, Robert JJ, Berrard S, et al. An adenovirus vector for gene transfer into neurons and glia in the brain. Science (New York) 1993;259:988–990. doi: 10.1126/science.8382374. [DOI] [PubMed] [Google Scholar]
- 23.Zhou L, Baumgartner BJ, Hill-Felberg SJ, McGowen LR, Shine HD. Neurotrophin-3 expressed in situ induces axonal plasticity in the adult injured spinal cord. J Neurosci. 2003;23:1424–1431. doi: 10.1523/JNEUROSCI.23-04-01424.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lorber B, Berry M, Logan A. Different factors promote axonal regeneration of adult rat retinal ganglion cells after lens injury and intravitreal peripheral nerve grafting. J Neurosci Res. 2008;86:894–903. doi: 10.1002/jnr.21545. [DOI] [PubMed] [Google Scholar]