Abstract
Eosinophilia is a feature of the host immune response that distinguishes parasitic worms from other pathogens, yet a discrete function for eosinophils in worm infection has been elusive. The aim of this study was to clarify the mechanism(s) underlying the striking and unexpected observation that eosinophils protect intracellular, muscle-stage Trichinella spiralis larvae against NO-mediated killing. Our findings indicate that eosinophils are specifically recruited to sites of infection at the earliest stage of muscle infection, consistent with a local response to injury. Early recruitment is essential for larval survival. By producing IL-10 at the initiation of infection, eosinophils expand IL-10+ myeloid dendritic cells and CD4+ IL-10+ T lymphocytes that inhibit iNOS expression and protect intracellular larvae. The results document a novel immunoregulatory function of eosinophils in helminth infection, in which eosinophil-derived IL-10 drives immune responses that eventually limit local nitric oxide production. In this way, the parasite co-opts an immune response in a way that enhances its own survival.
Introduction
Eosinophilia is a prominent consequence of Th2 immunity mounted in response to infections by parasitic helminths. Cytotoxic effects of eosinophils, mediated by cationic granule proteins, have been considered to be their central influence in worm infections (1, 2). Recent investigations of the roles of eosinophils in health and disease have provided new insights into the versatility of this cell population. In mouse models, eosinophils influence insulin resistance (3, 4), promote the regenerative response to toxic injury of skeletal muscle (5) and liver (6), and are required for recruitment of Th2 cells to the lung in allergy (7–9). Eosinophils constitutively express IL-4 (10) and production of IL-4 or IL-13 is key to the role of eosinophils in each of these contexts. Eosinophils also regulate adaptive immunity by producing cytokines (11), and this property has been tested in experiments that are relevant to the outcome of worm infection. In one example, eosinophils serve as an early source of IL-4, promoting Th2 cell polarization when Schistosoma mansoni eggs are injected into peritoneal cavities of mice (12). Moreover, eosinophils express MHCII and costimulatory molecules CD80 and CD86 on the cell surface and are capable of presenting allergens and helminth antigens to T cells (13–15). Perhaps equally likely is the potential for eosinophils to promote maturation of dendritic cells (DCs) in order to enhance antigen-specific Th2 immune responses (16, 17). More recently, a role for eosinophils in modulating goblet cell hyperplasia and IgE responses during microfilariae infection has been reported (18). While these studies provide evidence for immunoregulatory function of eosinophils in worm-induced Th2 immunity, the significance of antigen presentation or IL-4 secretion have yet to be confirmed in the course of infection.
Despite their prominence in the response to infection by intestine-dwelling parasitic worms, including Trichinella spiralis, findings from experiments in eosinophil ablated strains of mice consistently indicate that eosinophils do not contribute in a discernable way to intestinal immunity (19–21). During Trichinella infection, eosinophils promote the growth and survival of T. spiralis larvae as they colonize skeletal muscle (21, 22). In two strains of mice in which the eosinophil lineage has been ablated (PHIL and ΔdblGATA) (23, 24), growing larvae in muscle are killed by a nitric oxide (NO)-dependent mechanism (22). Providing mice with eosinophils during the first 10 days of muscle infection prevents killing (22) suggesting that eosinophils may directly regulate inducible nitric oxide synthase (iNOS) expression by local macrophages and neutrophils. Other findings document that expression of iNOS in leukocytes infiltrating sites of infection is regulated, in part, by CD4+ T cells that produce IL-10 (25, 26). The relationship between eosinophils and CD4+IL-10+ T cells has not yet been elucidated.
In the studies reported here, we aimed to elucidate the specific activity of eosinophils that regulates local NO production. We found that eosinophils are rapidly and specifically recruited to sites of infection and that the presence of eosinophils at the earliest stage of muscle infection is necessary for larval survival four weeks later. Neither antigen presentation nor production of IL-4 by eosinophils are essential for preserving the parasite. In contrast, by producing IL-10, eosinophils expand IL-10+ myeloid DCs and CD4+IL-10+ T cell that suppress local NO production and thus preserve larvae in muscle. Our results provide evidence that a parasitic worm co-opts the regulatory capacity of eosinophils in a way that supports its own survival.
Materials and Methods
Rats and mice
Adult Albino Oxford strain rats were produced and maintained in the Baker Institute vivarium. ΔdblGATA (eosinophil-ablated), PHIL (eosinophil-ablated), VertX (IL-10 reporter), Rag1−/−, IL-5-expressing transgenic (NJ.1638) (IL-5Tg+), IL-5Tg+ × MHCII−/−, IL-5Tg+ × IL-4−/− mice were bred at Cornell Transgenic Mouse Core Facility and offspring were transferred to the Baker Institute. IL-5Tg+ × IL-4−/− and IL-5Tg+ × MHCII−/− mice were generated by crossing and backcrossing on the deficient strains and genotype was confirmed by PCR. IL-10−/− mice were purchased from The Jackson laboratory. Rag2−/−γc−/− (innate lymphoid cell-ablated) mice were purchased from Taconic. Arg1flox/flox;Tie2cre (Arginase1 specifically ablated in myeloid cells) mice were a gift from Dr. Thomas Wynn (NIAID). PHIL mice were genotyped as described previously (23). All strains were on a C57BL/6 background. C57BL/6NHsd mice were purchased from Taconic as wild type (WT) control. Animal care was in accordance with the guidelines of the Association for Assessment and Accreditation of Laboratory Animal Care and experiments were performed with the approval of the Institutional Animal Care and Use Committee of Cornell University.
Parasite and Antigens
Trichinella spiralis first-stage larvae (L1) and newborn larvae (NBL) were recovered from rats as described previously (21). For oral infection, L1 were suspended in 2% nutrient broth (Difco), 0.6% gelatin (Fisher Scientific) and doses of 300 L1 were administered by gavage. For synchronous infection, 25,000 NBL were suspended in 0.25 ml serum-free DMEM (Mediatech, Inc.) and delivered by retro-orbital injection. Mice were euthanized by CO2 inhaltion at the times indicated in each experiment. Whole body muscle larvae burdens were assessed 28 days postinfection (dpi) as described previously (21). In some experiments, larvae were recovered from diaphragms 17 dpi by digesting minced tissue for 15 min at 37 °C in 5 mg/ml collagenase I (Sigma), a method that allows evaluation of the cellular response at a time that is relevant to larval survival. Crude somatic antigens from L1 were prepared as previously described (21).
Eosinophil transfer experiments
Eosinophils were recovered from infected IL-5Tg+, IL-5Tg+ × MHCII−/−, IL-5Tg+ × IL-4−/− or infected IL-10−/− mice 12–20 dpi as described previously (22). Cells were pooled from spleens and peritoneal lavage fluid and purified by magnetic bead selection as previously described (22). Briefly, eosinophils were labeled with PE-conjugated anti-Siglec-F antibody (BD) and anti-PE microbeads (Miltenyi Biotec). Average purity of eosinophils from this procedure was >93%. After washing twice with PBS, 5 × 106 eosinophils were resuspended in 200 μl sterile PBS and injected i.v. into ΔdblGATA mice every 48 hours for 6 days.
Similar results were obtained by transferring eosinophils into PHIL and ΔdblGATA mice as described previously (22). Because ΔdblGATA mice are more vigorous and productive, we elected to pursue the question of immune regulation, requiring complex adoptive transfer protocols (transfer of eosinophils, CD4+ T cells, or BMDCs, as described below) in that strain.
Cell isolation from lymph node for DC phenotyping
Cells from cervical lymph nodes (dLNs) for DC phenotyping were isolated as described (27). Briefly, dLNs were digested at 37 °C on a shaker for 15 min with 1.75 Wunsch Units/ml Liberase CI (Roche) and 80 Units/ml DNase I (Sigma) in Hanks buffered saline (Cellgro) containing 1mM MgCl2, 1.8mM CaCl2, 50 U/ml penicillin and 50 μg/ml streptomycin (Gibco). 100 μl 0.1M EDTA (pH 7.3) per milliliter was then added to stop the reaction, and the tube was immediately diluted to 15 ml with DMEM containing 50 U/ml penicillin and 50 μg/ml streptomycin. Undigested tissue was manually dispersed on a stainless steel tea strainer, using 12 ml syringe pestle. Cell preparations were passed through 70 μm filters into a 50 ml tube to obtain a single-cell suspension. Cells were washed with PBS containing 2% FCS and analyzed by flow cytometry for DC phenotyping as described below.
CD4+ T cell isolation and transfer
Single-cell suspensions were prepared from dLN recovered from dLNs of infected WT or IL-10−/− donor mice on 17 dpi as described previously (25). CD4+ T cells were enriched by negative selection on magnetic beads using CD4 T Cell Isolation Kit II (Miltenyi Biotec) and an AutoMACS magnetic cell separator. Average purity was 94%. 3–5 × 106 CD4+ T cells were suspended in PBS and injected i.v. into ΔdblGATA recipients that had been infected with 300 L1 4 days previously.
Culture of bone marrow-derived dendritic cells (BMDCs)
BMDCs were generated as described (28). Briefly, bone marrow cells were isolated from naïve WT or IL-10−/− mice. Cells were plated (5 × 105/ml) in complete RPMI media in the presence of recombinant murine GM-CSF (20ng/ml; Peprotech) for 3 days. Fresh medium containing GM-CSF (20ng/ml) was added to cultures on 3 and 6 days. On day 9, non-adherent cells were harvested and stained for CD11c, MHCII and CD11b to evaluate the purity (>90% CD11c+CD11b+MHCII+ cells) by flow cytometry. BMDCs were then primed with 50μg/ml L1 crude somatic antigen for 18 hours. Primed cells were washed in PBS, suspended in PBS, and 5 × 106 BMDCs were injected i.v. into ΔdblGATA mice that had been infected with 300 L1 4 days previously.
Flow cytometry
Cells from individual diaphragms were recovered following perfusion of blood from tissues, as described previously (22), and cultured ex vivo for 6 h with 250 ng/ml ionomycin (Sigma-Aldrich), 50 ng/ml PMA (Sigma-Aldrich), and 1 μg/ml brefeldin A (BD Pharmingen). After a 15 min incubation with Fc block (eBioscience) and 10% normal mouse serum, cells were incubated for 15 min with PE-Cy5-conjugated anti-CD4 (eBioscience). Samples were then treated with fixation/permeabilization buffer (eBioscience), and permeabilized cells were incubated for 1 h with PE-conjugated anti-IL-4 (eBioscience) or anti-IL-10 (eBioscience).
For dendritic cell phenotyping, cells from dLNs and diaphragms were incubated FITC-conjugated anti-CD11b, PE-conjugated anti-CD11c and Pacific blue-conjugated anti-MHCII. Data were acquired using a Gallios flow cytometer (Beckman Coulter) and analyzed with FlowJo software (Tree Star).
In vivo cell proliferation assay
BrdU was injected (i.p.) to mice on 14 dpi and dLNs were removed 24h later. dLN cells were recovered and cultured ex vivo for 6 h with 250 ng/ml ionomycin (Sigma-Aldrich), 50 ng/ml PMA (Sigma-Aldrich), and 1 μg/ml brefeldin A (BD Pharmingen). BrdU incorporation into T cells was assessed by co-labeling cells with PE-Cy5-conjugated anti-CD4 and PE-conjugated anti-IL-4 and following the manufacturer's protocol for the FITC BrdU Flow Kit (BD Pharmingen).
Parasite measurement
Area of parasites was measured as described previously (22). Briefly, developing L1 larvae were recovered from mice (17dpi) by digesting minced diaphragms for 15 min at 37°C in PBS containing 2% FCS and 5mg/ml collagenase I (Sigma). Larvae were treated with 70% ethanol (warm up at 56°C) overnight. Larvae were then centrifuged and resuspended in 5% glycerol/70% ethanol for one day before cytospin. The cytospin slides were fixed with methanol and stained with HEMA-3 (Fisher Healthcare), and measurements were performed using a BX51 microscope. The area of each larva was calculated using Microsuite Basic Olympus software. At least 25 larvae were measured per mouse, and values are expressed in micrometers squared.
Multiplexed cytokine assay
Cytokines were measured in serum using the Bio-Plex 200 multiplex system (Bio-Rad) and the Bio-Plex Pro TM mouse cytokine 23-plex Assay kit (M60-009RDPD), following manufacturer's instructions.
NO determination
Total NO end products (nitrates and nitrites) were measured in dLN culture supernatants prepared as described previously (25) using the Total Nitric Oxide Assay Kit (Thermo Scientific, Prod# EMSNOTOT), following the manufacturer's instructions.
Quantitative RT-PCR for selected chemokines and cytokines
Total RNA was isolated from masseter tissue using TRIzol reagent (Invitrogen). Reverse transcription of the RNA (1 μg) was performed using SuperScript III First-Strand Synthesis System (Invitrogen). Quantitative RT-PCR (qRT-PCR) was performed for IL-13, CCL17, CCL22 and CCL11 using the Applied Biosystems PRISM 7500 Sequence Detection System and its analysis software, SDS 2.3 and RQ Manager. The reactions were performed using the Taqman Universal PCR Master Mix. All primers were purchased from Applied Biosystems.
Statistical analysis
All experiments were performed two to four times with similar results. Means ± SD were calculated from data collected from individual mice unless otherwise indicated. Significant differences were determined using Student's t test or One-way ANOVA with Tukey's post hoc test for multiple means. Statistical analysis was performed with GraphPad Prism 5 software.
Results
Arginase 1 is required for the suppression of NO-mediated larval clearance but does not support larval growth
Infective first-stage larvae of T. spiralis colonize the intestine, develop into adult worms, and release migratory newborn larvae (NBL) that eventually enter skeletal muscle, invade myotubes, grow rapidly, and establish chronic, intracellular infection. Diaphragm and masseter are preferred muscles for infection in mice and the life cycle is complete in 28 days. At this time, first-stage larvae in muscle are fully infectious. In the absence of eosinophils, larval growth is impaired and larval survival is reduced (21, 22).
We have reported previously that Arginase 1 (Arg1) expression is decreased in skeletal muscles of eosinophil-ablated mice at 17dpi (22) and that infiltrating neutrophils and macrophages were iNOS+ in WT mice. With the knowledge that Arg1 competes with iNOS in controlling the production of NO, and that NO is toxic for growing T. spiralis larvae (22), we first aimed to reinforce the importance of production of NO in mediating the killing of muscle larvae. Therefore, we tested larval clearance using Arg1flox/flox;Tie2cre mice that lack of Arg1 in myeloid cell populations (29). Compared with WT mice, larval burdens were reduced in Arg1flox/flox;Tie2cre mice (Fig. 1A), although larvae grew normally (Fig. 1B). The reduction in muscle burden was associated with enhanced NO production in antigen-stimulated dLN cell cultures (Fig. 1C). These results, together with those obtained previously (21, 22) support a role for NO producing myeloid cells in larval clearance. Importantly, this toxicity is separable from the compromise of larval growth that also occurs in eosinophil-ablated mice.
Figure 1. Arg1 is required for larval survival but not growth.
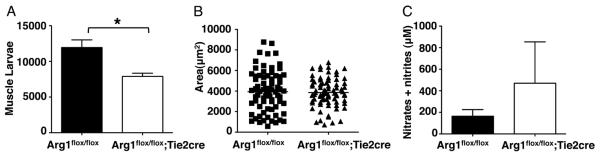
(A) Larval burdens in muscle of Arg1flox/flox;Tie2cre and Arg1flox/flox mice, 26 dpi. (B) Body size (area) of larvae recovered from infected Arg1flox/flox;Tie2cre and Arg1flox/flox mice, 17dpi. Bars represent means from at least 75 larvae pooled from masseters of 3 mice. (C) NO endproducts (nitrates and nitrites) in antigen-stimulated dLN cell cultures. Each data set was collected from two experiments with similar results. Values represent mean ± SD; n = 3 - 4 mice. Significant differences were determined by Student's t test or ANOVA and Tukey's test. *p < 0.05
IL-10 derived from CD4+ T cells compensates for eosinophil deficiency
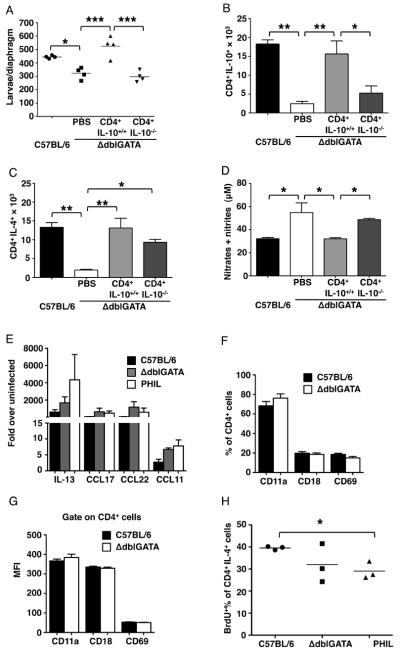
We have shown previously that iNOS in infected muscles of IL-10-deficient mice was reduced by adoptive transfer of IL-10-competent CD4+ T cells (25). By testing the impact of eosinophil-ablation on CD4+ T cells at sites of infection, we found that reduced larval survival correlated with impaired recruitment of CD4+IL-10+, as well as CD4+IL-4+ T cells, to sites of infection (compare WT to PBS treated eosinophil-ablated mice in Fig. 2A – C). When CD4+ T cells from dLN of WT infected mice were transferred to ΔdblGATA mice, they infiltrated skeletal muscle and promoted leukocyte infiltration (data not shown), documenting that the local environment in ablated mice was not deficient in chemoattractants or endothelial cell surface molecules necessary for T cell and leukocyte recruitment. Indeed, local gene expression of chemokines that recruit Th2 cells was unaltered in ablated mice (Fig. 2 E). Furthermore, expression of markers of T cell activation was similar in cells recovered from diaphragms of T. spiralis-infected WT, PHIL or ΔdblGATA mice (Fig. 2 F and G). Thus, the local environment was compatible with CD4+ T cell recruitment and CD4+ T cells were being activated, but they were compromised in their abilities to produce IL-4 and IL-10. In vivo labeling with BrdU revealed that expansion of CD4+IL-4+ T cells in dLN was diminished in ΔdblGATA mice and significantly impaired in PHIL mice (Fig. 2 H), suggesting that production rather than recruitment of effector CD4+ T cells was deficient in the absence of eosinophils. Transfer of CD4+ T cells from infected WT donors to eosinophil-ablated mice (4 dpi) improved larval burdens, promoted infiltration of CD4+IL-10+ cells and inhibited production of NO (Fig. 2 A, B and D). In contrast, transfer of CD4+ T cells from infected IL-10−/− donors did not have these effects (Fig. 2 A, B and D). CD4+IL-4+ cells were recruited independently of the IL-10 status of donor cells (Fig. 2C). Finding that CD4+IL-10+ T cells were capable of compensating for the absence of eosinophils in controlling local NO production, supports the conclusion that the direct action of eosinophils on NO producing leukocytes was not required to protect larvae from killing.
Figure 2. IL-10 derived from CD4+ T cells protects larvae in muscle.
(A) – (D), 5 × 106 CD4+ T cells from infected WT or IL-10−/− mice were transferred to ΔdblGATA recipient mice on 4 dpi. (A) Larval burdens in diaphragms, 17 dpi. (B) Number of CD4+IL-10+ cells and (C) CD4+IL-4+ cells in diaphragms of mice, 17 dpi. (D) NO endproducts in antigen-stimulated dLN cell cultures. (E) qRT-PCR analysis of IL-13, CCL11, CCL17 and CCL22 in masseter muscles of WT, PHIL, ΔdblGATA mice, 14 dpi. (F) Percentage and (G) MFI of CD11a+, CD18+ and CD69+ on gated CD4+ T cells recovered from dLNs of WT and ΔdblGATA mice, 14 dpi. A – F, Each data set was collected from two experiments with similar results. Values represent mean ± SD; n = 3 - 4 mice. Significant differences were determined by Student's t test or ANOVA and Tukey's test. *p < 0.05, **p < 0.001, ***p < 0.0001.
Eosinophils promote accumulation of IL-10+ myeloid DCs and IL-10-producing DCs compensate for eosinophil deficiency
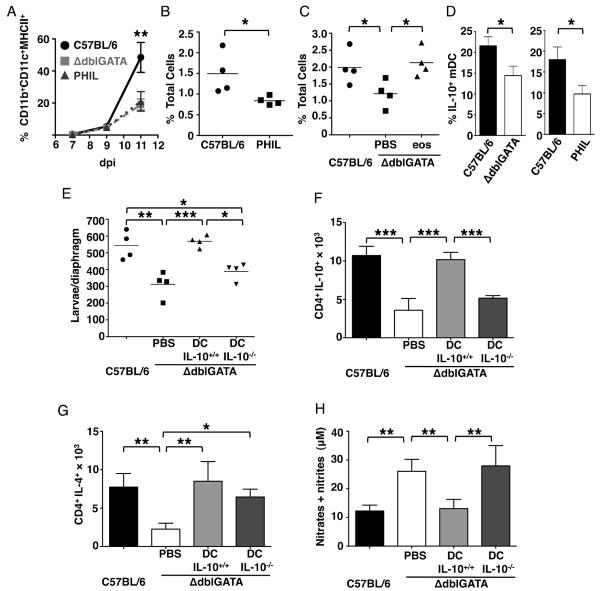
The finding that eosinophils influence CD4+IL-10+ T cells, together with the knowledge that eosinophils influence DCs in allergy (16), prompted us to test the impact of eosinophil-ablation on DCs in T. spralis infection. Quantification of CD11c+ cells in dLNs of infected WT, PHIL and ΔdblGATA mice revealed no differences on 7, 9 and 11 days of infection (data not shown); however, fewer CD11c+CD11b+MHCIIhigh cells were recovered from dLN and diaphragm on day 11 in eosinophil-ablated mice (Fig. 3 A – C). The results are consistent with earlier findings that eosinophils were required for DC trafficking and accumulation in lung dLNs following aeroallergen provocation (30). Indeed, accumulation of CD11c+CD11b+MHCIIhigh cells (myeloid DCs, mDCs) in T. spiralis-infected diaphragm significantly improved when eosinophils were restored to ΔdblGATA mice (Fig. 3C), confirming that recruitment or expansion of mDCs was dependent upon eosinophils. Moreover, the percentage of diaphragm mDCs that were IL-10+ was reduced in eosinophil-ablated mice (Fig. 3D). Wild-type BMDC (> 90% CD11c+CD11b+MHCII+) that were incubated with larval antigens prior to transfer to ΔdblGATA mice on 4 dpi increased survival of larvae (Fig. 3E) and improved recruitment of CD4+IL-4+ and CD4+IL-10+ T cells to diaphragms (Fig. 3 F and G). Production of NO was also suppressed upon transfer (Fig. 3 H). In contrast, transfer of larval antigen primed BMDC from IL-10−/− donors only improved the number of CD4+IL-4+ T cells in diaphragm without affecting CD4+IL-10+ T cells, larval survival or NO production (Fig. 3 E – H). Thus, eosinophils both recruit mDCs and support their production of IL-10. Such cells likely drive expansion of CD4+IL-10+ cells that limit iNOS expression (25) and NO-mediated larval killing.
Figure 3. Eosinophils promote accumulation of IL-10+ mDC that protect larvae.
(A) Percentage of CD11b+CD11c+MHCII+ mDCs in total CD11c+ cells in dLN 7, 9 and 11 dpi. (B) Percentage mDC of total diaphragm cells, 11 dpi. (C) Percentage mDC of total diaphragm cells from ΔdblGATA mice (11 dpi) that received 5 × 106 eosinophils or PBS on alternate days from 5 – 9 dpi. (D) Percentage of mDCs that were IL-10+, 11 dpi. (E) – (H), ΔdblGATA mice received 5 × 106 antigen-primed BMDCs from WT or IL-10−/− mice, 4 dpi. (E) Larval burdens in diaphragms, 17 dpi. (F) Number of CD4+IL-10+ cells and (G) CD4+IL-4+ cells in diaphragms, 17 dpi. (H) NO endproducts in antigen-stimulated dLN cell cultures. Each data set was collected from two experiments with similar results. Values represent mean ± SD; n = 3 - 4 mice. Significant differences were determined by Student's t test or ANOVA and Tukey's test. *p < 0.05, **p < 0.001, ***p < 0.0001.
Eosinophils arrive and exert their influence immediately following muscle infection
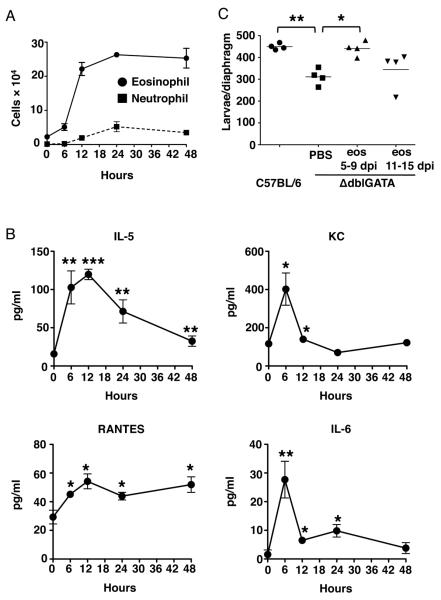
To further investigate the function of eosinophils, we sought to determine the time at which eosinophils normally extravascate at sites of skeletal muscle infection. In order to synchronize infection of the muscle, we injected NBL intravenously into WT mice. Eosinophil numbers increased dramatically in the diaphragm between 6 and 12 hours following injection (Fig. 4A) preceded by increases in serum IL-5, RANTES and KC (Fig. 4B), three important drivers of eosinophil chemotaxis. IL-6 in serum also increased upon injection (Fig. 4B). Results for other mediators in the panel were not significant. Neutrophils did not extravascate in diaphragm until 12–24 hours following injection (Fig. 4A), documenting a strong selection for eosinophils in the early recruitment of leukocytes to infected tissue (the ratio of neutrophils to eosinophils in the blood is approximately 25:1). Transfer of eosinophils collected from IL-5Tg+ mice to ΔdblGATA mice during two different intervals confirmed that larval survival was dependent upon the presence of eosinophils between day 5 and 9 following oral infection, a period during which NBL first arrive in muscle (Fig. 4C). Transfer of similar numbers of eosinophils between days 11 and 15 had a modest but not statistically significant effect on larval survival. Experimental outcomes were similar when eosinophil donors were either uninfected or infected (data not shown). Taken together, the results support the conclusion that rapid recruitment of eosinophils to sites of infection positions them to influence the initiation of the immune response.
Figure 4. Eosinophils are rapidly and specifically recruited to muscle to promote larval survival during infection.
(A) Numbers of eosinophils (Siglec-F+ cells) and neutrophils (Ly-6G+ (clone 1A8) cells) in diaphragms of naïve or WT mice, 6, 12, 24 and 48 hours post injection with 25,000 NBL. (B) Concentrations of IL-5, KC, RANTES and IL-6 in sera from mice in (A). (C) Larval burdens in diaphragms, 17 dpi, of ΔdblGATA mice that received 5×106 eosinophils or PBS every 48 hrs between 5 – 9 dpi or 11 – 15 dpi (oral infection). Each data set was collected from two experiments with similar results. Values represent mean ± SD; n = 3 - 4 mice. Significant differences were determined by Student's t test or ANOVA and Tukey's test. *p < 0.05, **p < 0.001, ***p < 0.0001.
Type 2 innate lymphoid cells are not required to promote muscle eosinophilia and larval survival
The newly discovered type 2 innate lymphoid cells (ILC2) have been demonstrated to recruit eosinophils by releasing preformed IL-5 (3, 31). In order to determine whether ILC2 influence recruitment of eosinophils to sites of infection, as well as larval survival, we injected NBL into ILC-ablated Rag2−/−γc−/− mice. (Intravenous infection controls the dose of NBL that are delivered to the muscle by circumventing the prolonged survival of fecund intestinal worms that occurs in lymphocyte-deficient mice.) Although there were fewer eosinophils in the spleens in Rag2−/−γc−/− compared to Rag1−/− mice (Fig. 5A), the recruitment of eosinophils to sites of infection was normal in the absence of ILC2 (Fig. 5B). More importantly, larval burdens were similar in the two strains of mice (Fig. 5C). Taken together, the results indicate that muscle eosinophilia and larval survival are not dependent on ILC2.
Figure 5. ILC2 are dispensable for muscle eosinophilia and larval survival.
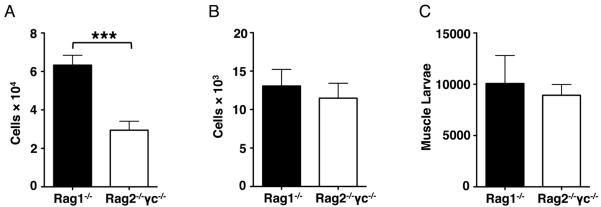
(A) – (C), Rag1−/− and Rag2−/−γc−/− mice were injected 25,000 NBL intravenously. Numbers of eosinophils in (A) spleens and (B) diaphragms, 13 dpi. (C) Total body larval burdens in muscle, 24 dpi. Each data set was collected from two experiments with similar results. Values represent mean ± SD; n = 4 mice. Significant differences were determined by Student's t test. ***p < 0.0001.
Antigen presentation by eosinophils is not required to preserve larvae
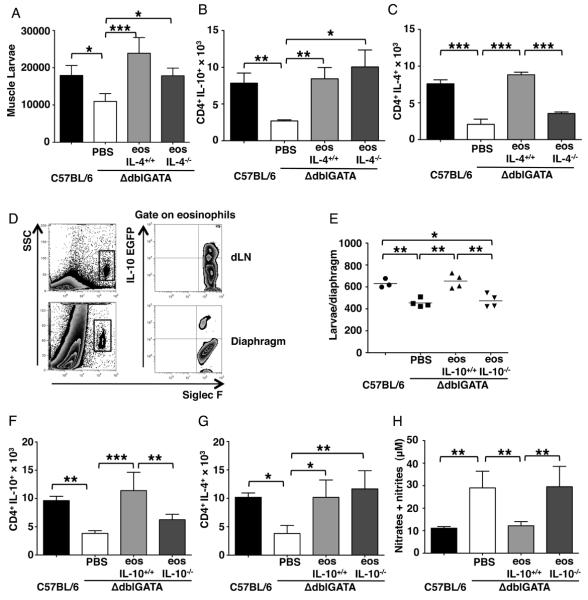
The requirement for eosinophils during the earliest phase of the IL-10-dependent immune response suggested that, in addition to promoting IL-10 production by mDCs (Fig. 3D), eosinophils may themselves present antigen. To better understand whether larval survival is a consequence of MHCII-dependent antigen presentation by eosinophils, we transferred MHCII−/− eosinophils isolated from infected IL-5Tg+ × MHCII−/− mice to infected ΔdblGATA mice (5 – 9 dpi). Both MHCII+/+ and MHCII−/− eosinophils supported larval burdens, larval growth (Fig. 6A and B), and accumulation of CD4+IL-4+ cells in diaphragm (Fig. 6 C). Thus, antigen presentation by eosinophils is not required to promote larval survival or to drive Th2 responses.
Figure 6. Antigen presentation by eosinophils is not required to preserve larvae.
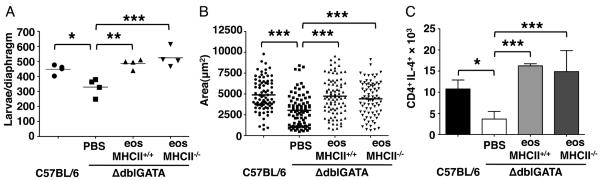
(A) – (C), ΔdblGATA mice received PBS or 5 × 106 eosinophils from infected IL-5Tg+ or IL-5Tg+MHCII−/− mice every 48 h from 5 – 9 dpi. (A) Larval burdens in diaphragms, 17 dpi. (B) Body size (area) of larvae, 17dpi. (C) Numbers of CD4+IL-4+ cells in diaphragms, 17 dpi. Each data set was collected from two experiments with similar results. Values represent mean ± SD; n = 4 mice. Significant differences were determined by ANOVA and Tukey's test. *p < 0.05, **p < 0.001, ***p < 0.0001.
Eosinophil-derived IL-10, but not IL-4 is required for larval survival
We next examined whether eosiniophil-derived IL-4 promotes larval survival in skeletal muscle. Transfer of IL-4−/− eosinophils isolated from IL-5Tg+ × IL-4−/− mice to infected ΔdblGATA mice improved larval burdens and this correlated with an increase in the number of CD4+IL-10+ cells in diaphragms (Fig. 7 A and B) but not CD4+IL-4+ T cells (Fig. 7C). In other experiments, IL-4−/−, IL-13−/− and WT mice supported similar larval burdens following infection with T. spiralis (data not shown). More importantly, production of NO in both IL-4−/− and IL-13−/− mice was comparable to WT mice (data not shown). Thus, the results support the conclusion that both IL-4 and IL-13 are dispensable in eosinophil-mediated regulation of immunity that prevents killing of T. spiralis larvae by NO.
Figure 7. Eosinophil derived IL-10 preserves larvae.
(A) – (C), ΔdblGATA mice received PBS or 5 × 106 eosinophils from infected IL-5Tg+ or IL-5Tg+IL-4−/− mice every 48 h from 5 – 9 dpi. (A) Total body larval burdens in muscle, 28 dpi. (B) Number of CD4+IL-10+ cells and (C) CD4+IL-4+ cells in diaphragms of mice, 17dpi. (D) IL-10 detected in eosinophils recovered from dLNs and diaphragms of infected Vertx mice, 13dpi. (E) – (H), ΔdblGATA mice received 5 × 106 eosinophils from infected WT or IL-10−/− mice, or PBS every 48 h from 5 – 9 dpi. (E) Larval burdens in diaphragms, 17 dpi. (F) Numbers of CD4+IL-10+ cells and (G) CD4+IL-4+ cells in diaphragms of mice, 17 dpi. (H) NO endproducts in antigen-stimulated dLN cell cultures. Each data set was collected from two experiments with similar results. Values represent mean ± SD; n = 3 - 4 mice. Significant differences were determined by Student's t test or ANOVA and Tukey's test. *p < 0.05, **p < 0.001, ***p < 0.0001.
We detected eosinophils producing IL-10 in infected reporter mice (VertX strain (32)) confirming that eosinophils in dLN and diaphragms expressed the gene (Fig. 7D). Furthermore, transfer of eosinophils from IL-10−/− mice to ΔdblGATA recipients failed to improve larval burdens, recruit CD4+IL-10+ T cells, or reduce NO production, while IL-10 competent eosinophils rescued larvae, enhanced recruitment of CD4+IL-10+ (but not IL-4+) T cells, and suppressed production of NO (Fig. 7 E – H). Thus, the mechanism of action of eosinophils in preserving larvae in muscles depended upon intrinsic production of IL-10 that directed the adaptive immune response towards IL-10+ mDCs and CD4+IL-10+ T cells, ultimately inhibiting expression of iNOS and preventing NO-mediated killing of the parasite.
Discussion
The aim of this study was to bring clarity to the striking and unexpected observation that eosinophils protect intracellular, muscle-stage T. spiralis larvae against NO-mediated killing. Testing two strains of eosinophil-ablated mice, engineered by two very different approaches, reduced the likelihood that the results are artifactual, and our previous experiments consistently replicated results in PHIL and ΔdblGATA mice (cite Fabre, Geb). In the current studies, we employed these strains in adoptive transfer experiments that are powerful tools for examining the contribution of effector cell populations. We set out to address two questions: what are the crucial regulators that prevent larval killing by NO and how do eosinophils exert their influence? We discovered a dominant role for IL-10 throughout the immune response that culminates in the protection of larvae in muscle.
Our previous findings showed that growing larvae were at greatest risk for NO mediated killing and suggested that the combination of impaired growth and local NO that was evident in eosinophil-ablated mice was responsible for the high rate of larval clearance. Although the mechanism by which eosinophils support growth of T. spiralis is not well understood, it is noteworthy that eosinophils also promote development and fecundity of filarial worms (33). Our evidence suggests that impaired growth and killing of T. spiralis are mediated by distinct mechanisms. Specifically, infection of Arg1-deficient mice showed that larval killing can occur in the absence of impaired growth. Similarly, growth is normal in IL-10−/− mice while local iNOS expression is high, and larval killing is evident (22, 34). Reduction in larval burdens at 28 days following oral infection is similar in these two strains (44% in Arg1flox/flox;Tie2cre, 50% in IL-10−/−) and in ΔdblGATA (48%). PHIL mice consistently show a higher rate of clearance (67–77%), a finding that is unexplained. We speculate that IL-10 and Arg1 deficiencies yield much higher local NO that overcomes the contribution of impaired growth to net larval clearance that may occur in eosinophil-ablated mice. The relationship between impaired growth and larval clearance requires further investigation. We have shown previously that CD4+CD25− T cells are critical sources of IL-10 that limits NO production by cells that infiltrate Trichinella-infected muscle (22, 25); however, the functions of eosinophils in supporting larval growth appear to operate independently of adaptive immunity (Huang and Appleton, unpublished data), and are being investigated separately. In this report, we describe the processes associated with eosinophil-driven adaptive immunity that give rise to control of NO production.
Taken together with earlier findings that inhibition of iNOS by drug treatment correlated with improved larval survival (21), that macrophages account for 80% of leukocytes at sites of infection, and that iNOS+ cells at sites of infection are largely macrophages and neutrophils (22), the results from Arg1flox/flox;Tie2cre mice support the conclusion that myeloid cells are important targets of eosinophil-dependent immune regulation during T. spiralis infection. Moreover, adoptive transfer of IL-10+ mDCs or CD4+ T cells to eosinophil-ablated mice compensated for eosinophils in protecting larvae from killing. We interpret these findings to indicate that eosinophils do not themselves need to interact with macrophages or neutrophils in order to limit their NO production. Rather, our results support the conclusion eosinophils promote an adaptive immune response rich in IL-10 that limits NO production.
In addition to limiting NO production, Arg1 is the key enzyme in collagen deposition by macrophages (35). A collagen capsule is a defining feature of the Trichinella nurse cell and large numbers of macrophages are apposed to the capsule (36, 37). Nevertheless, the nurse cell, rather than the macrophage, has been reported to supply collagen for the capsule (36). Although the expression of Arg1 is decreased in diaphragms of eosinophil-ablated mice, capsule formation is normal in surviving nurse cells (21), indicating myeloid cells may be dispensable for collagen deposition. Normal larval growth in Arg1flox/flox;Tie2cre mice provides further, indirect evidence that local myeloid cells are not required for collagen capsule formation.
Eosinophils induce T cell responses by distinct mechanisms in different systems (13–16, 38). Restoring eosinophils to ablated, infected mice promoted the expansion of mDCs and CD4+IL-10+ T cells. We found no evidence that eosinophils promoted CD4+ T cell activation via antigen presentation, rather our data are consistent with an eosinophil influence on DC recruitment and activation that subsequently drives T cell differentiation. Importantly, CD11b+ mDCs, a DC subset, can initiate the production of IL-10 from T cells in helminth infections by secreting IL-10 (27, 39–42). In allergic asthma, eosinophils have been shown to be required for accumulation of mDCs in the lung and dLN (30). In T. spiralis infected, eosinophil-ablated mice, the reduction in CD11b+ mDCs at sites of infection and dLN was not likely the result of a deficient chemokine response (as is observed in asthma (30)) as gene expression of several chemokines known to be essential for recruitment of monocytes was normal in eosinophil-deficient infected tissue (data not shown). Although the deficiency in total cells is not readily explained by our data, clearly the cytokine profile of mDCs was influenced by eosinophils. IL-10 induces maturation of DCs, and triggers the secretion of IL-10 from DCs (42, 43). Thus, we speculate that in addition to promoting the accumulation of mDCs, eosinophils provide IL-10 that stimulates DCs to become IL-10 producers. This, in turn, creates a suitable microenvironment for the development of CD4+IL-10+ T cells which limit iNOS expression and thereby protect larvae.
We speculate that mice mount innate and adaptive IL-10 responses in order to limit tissue injury and suppress immune-mediated inflammation caused by migrating NBL, and that such a response coincidentally supports T. spiralis in establishing intracellular infection. Eosinophils were selectively recruited to skeletal muscle within hours of intravenous injection of NBL. This response is distinct from the well-documented, T cell-dependent eosinophilia induced by intestinal worms several days after oral infection (44), a process that, in natural infections, would be superimposed on the innate response to injury caused by NBL. We are able to isolate the response to NBL by using intravenous infections; however, the relationship between that response and the ongoing intestinal immune response merits investigation. The innate response to NBL included increases in serum concentrations of three key mediators of eosinophil chemotaxis: IL-5, KC and RANTES (45). The nature of the assay performed did not reveal that source of the chemokines and it is possible that they were derived from muscle, lung or other tissues that larvae enter during migration. The timing of detection in blood is consistent with the mediators being preformed rather than induced. We speculate that the release of these mediators is triggered by injury caused by large NBL (150 mm) that deploy a stylet to aid their exit from the blood and penetration of tissue. Endothelial cells express KC and RANTES, and rapidly secrete them in response to injury (46, 47). Moreover, NBL activate the complement system (48), which may trigger tissue resident mast cells to release IL-5, KC and RANTES (49–52).
The cellular sources and significance of IL-10 varies among helminth infections (53–58). By infecting IL-10 reporter mice, we observed that eosinophils in dLN and muscle produce IL-10 during T. spiralis infection. IL-10 was not detected in eosinophils in uninfected mice, indicating that it was induced by infection. The immediate release of chemokines and recruitment of eosinophils to sites of infection are consistent with an innate response to injury caused by NBL. IL-10 is a feature of the response to injury, inhibiting inflammation and minimizing tissue damage (59). By regulating macrophage phenotype, IL-10 promotes muscle regeneration and growth (60).
Injured tissues can release mediators known for initiating the IL-10 response, including IL-6 and IL-27 (61, 62). In T. spiralis infection, serum IL-6 was dramatically increased at 6 hours after NBL injection. Exercise-associated muscle injury induces increase of muscle-derived IL-6 in plasma (61), and the transient increase of IL-6 may enhance production of IL-10 (63). Both IL-6 and IL-27 influence T cell populations to produce IL-10 (64–67). We detected increased expression of IL-27 as well as increased numbers of IL-27 receptor expressing eosinophils at sites of infection (Huang and Appleton, unpublished data), suggesting a potential role for IL-27 in triggering eosinophils to secrete IL-10; however, pilot experiments showed a only a modest trend towards decreased larval burdens in IL-27 receptor deficient mice, with a partial improvement in larval survival following adoptive transfer of normal eosinophils to receptor deficient mice. Thus, although IL-27 may contribute, it does not fully account for IL-10 induction in eosinophils at sites of infection. The influence of IL-6 on eosinophils merits further investigation.
IL-25 and IL-33 are found in endothelial cells and function as alarmins during inflammation. Both stimulate ILC2 (68) to release IL-5 and IL-13, which in turn activate T helper cells and recruit eosinophils (69). The timing of eosinophil recruitment was not consistent with the notion that ILC2 nucleate this innate eosinophil response, and indeed, infection of Rag2−/−γc−/− mice showed normal muscle eosinophilia and larval survival. Although it has been shown that ILC2 are essential for maintaining the integrity of mucosal sites, such as clearance of intestinal helminth parasites and promoting tissue repair after helminth infections (70–72), our data support a dispensable role for ILC2 in muscle infection.
Study of T. spiralis infection in mice is particularly valuable because there are few natural animal models of either tissue-dwelling helminth infection or skeletal muscle responses to injury caused by infection. By combining eosinophil ablation and restoration with this natural infection, our findings document a novel immunoregulatory function of eosinophils in helminth infection. Eosinophils enter sites of infection immediately following tissue invasion by T. spiralis larvae, which is essential for larval survival. Furthermore, by producing IL-10, eosinophils expand IL-10-secreting DCs and CD4+ T cells, thereby controlling the activation of proinflammatory macrophages and neutrophils that otherwise kill parasite larvae by releasing NO. We speculate that Trichinella has adapted to its host by exploiting a mechanism that is in place to limit tissue injury. The remarkable functional versatility of eosinophils that has become increasingly evident is an important consideration in devising strategies for new prophylactic and therapeutic approaches to reducing the burden of parasitic worm infections.
Acknowledgments
We thank Dr. Thomas Wynn for providing the Arg1flox/flox;Tie2cre mice; Dr. Andrew Macdonald for sharing the protocol for dendritic cells analysis in lymph nodes; and Dr. Cynthia Leifer and Dr. Eric Denkers for helpful advice.
Abbreviations used in this paper
- Arg1
Arginase 1
- dLN
draining lymph node
- ILC2
type 2 innate lymphoid cell
- iNOS
inducible nitric oxide synthase
- mDC
myeloid dendritic cells
- NBL
newborn larvae
Footnotes
This work was supported by NIH grants AI081043 and AI097555 (J.A.) and HL065228 (J.L.).
Disclosures: The authors have no financial conflict of interest.
References
- 1.Rosenberg HF, Dyer KD, Foster PS. Eosinophils: changing perspectives in health and disease. Nat Rev Immunol. 2013;13:9–22. doi: 10.1038/nri3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shamri R, Xenakis JJ, Spencer LA. Eosinophils in innate immunity: an evolving story. Cell Tissue Res. 2011;343:57–83. doi: 10.1007/s00441-010-1049-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Molofsky AB, Nussbaum JC, Liang HE, Van Dyken SJ, Cheng LE, Mohapatra A, Chawla A, Locksley RM. Innate lymphoid type 2 cells sustain visceral adipose tissue eosinophils and alternatively activated macrophages. J Exp Med. 2013;210:535–549. doi: 10.1084/jem.20121964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu D, Molofsky AB, Liang HE, Ricardo-Gonzalez RR, Jouihan HA, Bando JK, Chawla A, Locksley RM. Eosinophils sustain adipose alternatively activated macrophages associated with glucose homeostasis. Science. 2011;332:243–247. doi: 10.1126/science.1201475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heredia JE, Mukundan L, Chen FM, Mueller AA, Deo RC, Locksley RM, Rando TA, Chawla A. Type 2 innate signals stimulate fibro/adipogenic progenitors to facilitate muscle regeneration. Cell. 2013;153:376–546. 388. doi: 10.1016/j.cell.2013.02.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goh YP, Henderson NC, Heredia JE, Red Eagle A, Odegaard JI, Lehwald N, Nguyen KD, Sheppard D, Mukundan L, Locksley RM, Chawla A. Eosinophils secrete IL-4 to facilitate liver regeneration. Proc Natl Acad Sci U S A. 2013;110:9914–9919. doi: 10.1073/pnas.1304046110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Walsh ER, Thakar J, Stokes K, Huang F, Albert R, August A. Computational and experimental analysis reveals a requirement for eosinophil-derived IL-13 for the development of allergic airway responses in C57BL/6 mice. J Immunol. 2011;186:2936–2949. doi: 10.4049/jimmunol.1001148. [DOI] [PubMed] [Google Scholar]
- 8.Walsh ER, Sahu N, Kearley J, Benjamin E, Kang BH, Humbles A, August A. Strain-specific requirement for eosinophils in the recruitment of T cells to the lung during the development of allergic asthma. J Exp Med. 2008;205:1285–1292. doi: 10.1084/jem.20071836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jacobsen EA, Ochkur SI, Pero RS, Taranova AG, Protheroe CA, Colbert DC, Lee NA, Lee JJ. Allergic pulmonary inflammation in mice is dependent on eosinophil-induced recruitment of effector T cells. J Exp Med. 2008;205:699–710. doi: 10.1084/jem.20071840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Velazquez JR, Lacy P, Mahmudi-Azer S, Bablitz B, Milne CD, Denburg JA, Moqbel R. Interleukin-4 and RANTES expression in maturing eosinophils derived from human cord blood CD34+ progenitors. Immunology. 2000;101:419–425. doi: 10.1046/j.1365-2567.2000.00104.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spencer LA, Szela CT, Perez SA, Kirchhoffer CL, Neves JS, Radke AL, Weller PF. Human eosinophils constitutively express multiple Th1, Th2, and immunoregulatory cytokines that are secreted rapidly and differentially. J Leukoc Biol. 2009;85:117–123. doi: 10.1189/jlb.0108058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sabin EA, Kopf MA, Pearce EJ. Schistosoma mansoni egg-induced early IL-4 production is dependent upon IL-5 and eosinophils. J Exp Med. 1996;184:1871–1878. doi: 10.1084/jem.184.5.1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duez C, Dakhama A, Tomkinson A, Marquillies P, Balhorn A, Tonnel AB, Bratton DL, Gelfand EW. Migration and accumulation of eosinophils toward regional lymph nodes after airway allergen challenge. J Allergy Clin Immunol. 2004;114:820–825. doi: 10.1016/j.jaci.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 14.Mawhorter SD, Pearlman E, Kazura JW, Boom WH. Class II major histocompatibility complex molecule expression on murine eosinophils activated in vivo by Brugia malayi. Infect Immun. 1993;61:5410–5412. doi: 10.1128/iai.61.12.5410-5412.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Padigel UM, Hess JA, Lee JJ, Lok JB, Nolan TJ, Schad GA, Abraham D. Eosinophils act as antigen-presenting cells to induce immunity to Strongyloides stercoralis in mice. J Infect Dis. 2007;196:1844–1851. doi: 10.1086/522968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang D, Chen Q, Su SB, Zhang P, Kurosaka K, Caspi RR, Michalek SM, Rosenberg HF, Zhang N, Oppenheim JJ. Eosinophil-derived neurotoxin acts as an alarmin to activate the TLR2-MyD88 signal pathway in dendritic cells and enhances Th2 immune responses. J Exp Med. 2008;205:79–90. doi: 10.1084/jem.20062027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lotfi R, Lotze MT. Eosinophils induce DC maturation, regulating immunity. J Leukoc Biol. 2008;83:456–460. doi: 10.1189/jlb.0607366. [DOI] [PubMed] [Google Scholar]
- 18.Cadman ET, Thysse KA, Bearder S, Cheung AY, Johnston AC, Lee JJ, Lawrence RA. Eosinophils are important for protection, immunoregulation and pathology during infection with nematode microfilariae. PLoS Pathog. 2014;10:e1003988. doi: 10.1371/journal.ppat.1003988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Svensson M, Bell L, Little MC, DeSchoolmeester M, Locksley RM, Else KJ. Accumulation of eosinophils in intestine-draining mesenteric lymph nodes occurs after Trichuris muris infection. Parasite Immunol. 2011;33:1–11. doi: 10.1111/j.1365-3024.2010.01246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Swartz JM, Dyer KD, Cheever AW, Ramalingam T, Pesnicak L, Domachowske JB, Lee JJ, Lee NA, Foster PS, Wynn TA, Rosenberg HF. Schistosoma mansoni infection in eosinophil lineage-ablated mice. Blood. 2006;108:2420–2427. doi: 10.1182/blood-2006-04-015933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fabre V, Beiting DP, Bliss SK, Gebreselassie NG, Gagliardo LF, Lee NA, Lee JJ, Appleton JA. Eosinophil deficiency compromises parasite survival in chronic nematode infection. J Immunol. 2009;182:1577–1583. doi: 10.4049/jimmunol.182.3.1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gebreselassie NG, Moorhead AR, Fabre V, Gagliardo LF, Lee NA, Lee JJ, Appleton JA. Eosinophils preserve parasitic nematode larvae by regulating local immunity. J Immunol. 2012;188:417–425. doi: 10.4049/jimmunol.1101980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee JJ, Dimina D, Macias MP, Ochkur SI, McGarry MP, O'Neill KR, Protheroe C, Pero R, Nguyen T, Cormier SA, Lenkiewicz E, Colbert D, Rinaldi L, Ackerman SJ, Irvin CG, Lee NA. Defining a link with asthma in mice congenitally deficient in eosinophils. Science. 2004;305:1773–1776. doi: 10.1126/science.1099472. [DOI] [PubMed] [Google Scholar]
- 24.Yu C, Cantor AB, Yang H, Browne C, Wells RA, Fujiwara Y, Orkin SH. Targeted deletion of a high-affinity GATA-binding site in the GATA-1 promoter leads to selective loss of the eosinophil lineage in vivo. J Exp Med. 2002;195:1387–1395. doi: 10.1084/jem.20020656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beiting DP, Gagliardo LF, Hesse M, Bliss SK, Meskill D, Appleton JA. Coordinated control of immunity to muscle stage Trichinella spiralis by IL-10, regulatory T cells, and TGF-beta. J Immunol. 2007;178:1039–1047. doi: 10.4049/jimmunol.178.2.1039. [DOI] [PubMed] [Google Scholar]
- 26.Beiting DP, Bliss SK, Schlafer DH, Roberts VL, Appleton JA. Interleukin-10 limits local and body cavity inflammation during infection with muscle-stage Trichinella spiralis. Infect Immun. 2004;72:3129–3137. doi: 10.1128/IAI.72.6.3129-3137.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Phythian-Adams AT, Cook PC, Lundie RJ, Jones LH, Smith KA, Barr TA, Hochweller K, Anderton SM, Hammerling GJ, Maizels RM, MacDonald AS. CD11c depletion severely disrupts Th2 induction and development in vivo. J Exp Med. 2010;207:2089–2096. doi: 10.1084/jem.20100734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lutz MB, Kukutsch N, Ogilvie AL, Rossner S, Koch F, Romani N, Schuler G. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J Immunol Methods. 1999;223:77–92. doi: 10.1016/s0022-1759(98)00204-x. [DOI] [PubMed] [Google Scholar]
- 29.Pesce JT, Ramalingam TR, Mentink-Kane MM, Wilson MS, El Kasmi KC, Smith AM, Thompson RW, Cheever AW, Murray PJ, Wynn TA. Arginase-1-expressing macrophages suppress Th2 cytokine-driven inflammation and fibrosis. PLoS Pathog. 2009;5:e1000371. doi: 10.1371/journal.ppat.1000371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jacobsen EA, Zellner KR, Colbert D, Lee NA, Lee JJ. Eosinophils regulate dendritic cells and Th2 pulmonary immune responses following allergen provocation. J Immunol. 2011;187:6059–6068. doi: 10.4049/jimmunol.1102299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nussbaum JC, Van Dyken SJ, von Moltke J, Cheng LE, Mohapatra A, Molofsky AB, Thornton EE, Krummel MF, Chawla A, Liang HE, Locksley RM. Type 2 innate lymphoid cells control eosinophil homeostasis. Nature. 2013;502:245–248. doi: 10.1038/nature12526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Madan R, Demircik F, Surianarayanan S, Allen JL, Divanovic S, Trompette A, Yogev N, Gu Y, Khodoun M, Hildeman D, Boespflug N, Fogolin MB, Grobe L, Greweling M, Finkelman FD, Cardin R, Mohrs M, Muller W, Waisman A, Roers A, Karp CL. Nonredundant roles for B cell-derived IL-10 in immune counter-regulation. J Immunol. 2009;183:2312–2320. doi: 10.4049/jimmunol.0900185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Babayan SA, Read AF, Lawrence RA, Bain O, Allen JE. Filarial parasites develop faster and reproduce earlier in response to host immune effectors that determine filarial life expectancy. PLoS Biol. 2010;8:e1000525. doi: 10.1371/journal.pbio.1000525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Helmby H, Grencis RK. Contrasting roles for IL-10 in protective immunity to different life cycle stages of intestinal nematode parasites. Eur J Immunol. 2003;33:2382–2390. doi: 10.1002/eji.200324082. [DOI] [PubMed] [Google Scholar]
- 35.Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 36.Polvere RI, Kabbash CA, Capo VA, Kadan I, Despommier DD. Trichinella spiralis: synthesis of type IV and type VI collagen during nurse cell formation. Exp Parasitol. 1997;86:191–199. doi: 10.1006/expr.1997.4180. [DOI] [PubMed] [Google Scholar]
- 37.Wu Z, Sofronic-Milosavljevic L, Nagano I, Takahashi Y. Trichinella spiralis: nurse cell formation with emphasis on analogy to muscle cell repair. Parasit Vectors. 2008;1:27. doi: 10.1186/1756-3305-1-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shi HZ, Xiao CQ, Li CQ, Mo XY, Yang QL, Leng J, Chen YQ. Endobronchial eosinophils preferentially stimulate T helper cell type 2 responses. Allergy. 2004;59:428–435. doi: 10.1046/j.1398-9995.2003.00405.x. [DOI] [PubMed] [Google Scholar]
- 39.Smith KA, Harcus Y, Garbi N, Hammerling GJ, MacDonald AS, Maizels RM. Type 2 innate immunity in helminth infection is induced redundantly and acts autonomously following CD11c(+) cell depletion. Infect Immun. 2012;80:3481–3489. doi: 10.1128/IAI.00436-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Balic A, Smith KA, Harcus Y, Maizels RM. Dynamics of CD11c(+) dendritic cell subsets in lymph nodes draining the site of intestinal nematode infection. Immunol Lett. 2009;127:68–75. doi: 10.1016/j.imlet.2009.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Koyama K. Dendritic cells have a crucial role in the production of cytokines in mesenteric lymph nodes of B10.BR mice infected with Trichuris muris. Parasitol Res. 2008;102:349–356. doi: 10.1007/s00436-007-0768-4. [DOI] [PubMed] [Google Scholar]
- 42.Chen CC, Louie S, McCormick BA, Walker WA, Shi HN. Helminth-primed dendritic cells alter the host response to enteric bacterial infection. J Immunol. 2006;176:472–483. doi: 10.4049/jimmunol.176.1.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.De Smedt T, Van Mechelen M, De Becker G, Urbain J, Leo O, Moser M. Effect of interleukin-10 on dendritic cell maturation and function. Eur J Immunol. 1997;27:1229–1235. doi: 10.1002/eji.1830270526. [DOI] [PubMed] [Google Scholar]
- 44.Basten A, Boyer MH, Beeson PB. Mechanism of eosinophilia. I. Factors affecting the eosinophil response of rats to Trichinella spiralis. J Exp Med. 1970;131:1271–1287. doi: 10.1084/jem.131.6.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Borchers MT, Ansay T, DeSalle R, Daugherty BL, Shen H, Metzger M, Lee NA, Lee JJ. In vitro assessment of chemokine receptor-ligand interactions mediating mouse eosinophil migration. J Leukoc Biol. 2002;71:1033–1041. [PubMed] [Google Scholar]
- 46.Kawai T, Seki M, Hiromatsu K, Eastcott JW, Watts GF, Sugai M, Smith DJ, Porcelli SA, Taubman MA. Selective diapedesis of Th1 cells induced by endothelial cell RANTES. J Immunol. 1999;163:3269–3278. [PubMed] [Google Scholar]
- 47.Armstrong DA, Major JA, Chudyk A, Hamilton TA. Neutrophil chemoattractant genes KC and MIP-2 are expressed in different cell populations at sites of surgical injury. J Leukoc Biol. 2004;75:641–648. doi: 10.1189/jlb.0803370. [DOI] [PubMed] [Google Scholar]
- 48.Hong Y, Kim CW, Ghebrehiwet B. Trichinella spiralis: activation of complement by infective larvae, adults, and newborn larvae. Exp Parasitol. 1992;74:290–299. doi: 10.1016/0014-4894(92)90152-z. [DOI] [PubMed] [Google Scholar]
- 49.Bradding P, Roberts JA, Britten KM, Montefort S, Djukanovic R, Mueller R, Heusser CH, Howarth PH, Holgate ST. Interleukin-4, -5, and -6 and tumor necrosis factor-alpha in normal and asthmatic airways: evidence for the human mast cell as a source of these cytokines. Am J Respir Cell Mol Biol. 1994;10:471–480. doi: 10.1165/ajrcmb.10.5.8179909. [DOI] [PubMed] [Google Scholar]
- 50.Sun J, Sukhova GK, Yang M, Wolters PJ, MacFarlane LA, Libby P, Sun C, Zhang Y, Liu J, Ennis TL, Knispel R, Xiong W, Thompson RW, Baxter BT, Shi GP. Mast cells modulate the pathogenesis of elastase-induced abdominal aortic aneurysms in mice. J Clin Invest. 2007;117:3359–3368. doi: 10.1172/JCI31311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Enoksson M, Lyberg K, Moller-Westerberg C, Fallon PG, Nilsson G, Lunderius-Andersson C. Mast cells as sensors of cell injury through IL-33 recognition. J Immunol. 2011;186:2523–2528. doi: 10.4049/jimmunol.1003383. [DOI] [PubMed] [Google Scholar]
- 52.Galli SJ, Nakae S, Tsai M. Mast cells in the development of adaptive immune responses. Nat Immunol. 2005;6:135–142. doi: 10.1038/ni1158. [DOI] [PubMed] [Google Scholar]
- 53.Chen F, Liu Z, Wu W, Rozo C, Bowdridge S, Millman A, Van Rooijen N, Urban JF, Jr., Wynn TA, Gause WC. An essential role for TH2-type responses in limiting acute tissue damage during experimental helminth infection. Nat Med. 2012;18:260–266. doi: 10.1038/nm.2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mendlovic F, Flisser A. Dendritic cells in the gut: interaction with intestinal helminths. J Biomed Biotechnol. 2010;2010:250563. doi: 10.1155/2010/250563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schnoeller C, Rausch S, Pillai S, Avagyan A, Wittig BM, Loddenkemper C, Hamann A, Hamelmann E, Lucius R, Hartmann S. A helminth immunomodulator reduces allergic and inflammatory responses by induction of IL-10-producing macrophages. J Immunol. 2008;180:4265–4272. doi: 10.4049/jimmunol.180.6.4265. [DOI] [PubMed] [Google Scholar]
- 56.Mangan NE, Fallon RE, Smith P, van Rooijen N, McKenzie AN, Fallon PG. Helminth infection protects mice from anaphylaxis via IL-10- producing B cells. J Immunol. 2004;173:6346–6356. doi: 10.4049/jimmunol.173.10.6346. [DOI] [PubMed] [Google Scholar]
- 57.Hesse M, Piccirillo CA, Belkaid Y, Prufer J, Mentink-Kane M, Leusink M, Cheever AW, Shevach EM, Wynn TA. The pathogenesis of schistosomiasis is controlled by cooperating IL-10-producing innate effector and regulatory T cells. J Immunol. 2004;172:3157–3166. doi: 10.4049/jimmunol.172.5.3157. [DOI] [PubMed] [Google Scholar]
- 58.Schopf LR, Hoffmann KF, Cheever AW, Urban JF, Jr., Wynn TA. IL-10 is critical for host resistance and survival during gastrointestinal helminth infection. J Immunol. 2002;168:2383–2392. doi: 10.4049/jimmunol.168.5.2383. [DOI] [PubMed] [Google Scholar]
- 59.Saraiva M, O'Garra A. The regulation of IL-10 production by immune cells. Nat Rev Immunol. 2010;10:170–181. doi: 10.1038/nri2711. [DOI] [PubMed] [Google Scholar]
- 60.Deng B, Wehling-Henricks M, Villalta SA, Wang Y, Tidball JG. IL-10 triggers changes in macrophage phenotype that promote muscle growth and regeneration. J Immunol. 2012;189:3669–3680. doi: 10.4049/jimmunol.1103180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Febbraio MA, Pedersen BK. Muscle-derived interleukin-6: mechanisms for activation and possible biological roles. Faseb J. 2002;16:1335–1347. doi: 10.1096/fj.01-0876rev. [DOI] [PubMed] [Google Scholar]
- 62.Xu F, Liu Q, Lin S, Shen N, Yin Y, Cao J. IL-27 is elevated in acute lung injury and mediates inflammation. J Clin Immunol. 2013;33:1257–1268. doi: 10.1007/s10875-013-9923-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Steensberg A, Fischer CP, Keller C, Moller K, Pedersen BK. IL-6 enhances plasma IL-1ra, IL-10, and cortisol in humans. Am J Physiol Endocrinol Metab. 2003;285:E433–437. doi: 10.1152/ajpendo.00074.2003. [DOI] [PubMed] [Google Scholar]
- 64.Stumhofer JS, Silver JS, Laurence A, Porrett PM, Harris TH, Turka LA, Ernst M, Saris CJ, O'Shea JJ, Hunter CA. Interleukins 27 and 6 induce STAT3-mediated T cell production of interleukin 10. Nat Immunol. 2007;8:1363–1371. doi: 10.1038/ni1537. [DOI] [PubMed] [Google Scholar]
- 65.McGeachy MJ, Bak-Jensen KS, Chen Y, Tato CM, Blumenschein W, McClanahan T, Cua DJ. TGF-beta and IL-6 drive the production of IL-17 and IL-10 by T cells and restrain T(H)-17 cell-mediated pathology. Nat Immunol. 2007;8:1390–1397. doi: 10.1038/ni1539. [DOI] [PubMed] [Google Scholar]
- 66.Jin JO, Han X, Yu Q. Interleukin-6 induces the generation of IL- 10-producing Tr1 cells and suppresses autoimmune tissue inflammation. J Autoimmun. 2013;40:28–44. doi: 10.1016/j.jaut.2012.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hunter CA, Kastelein R. Interleukin-27: balancing protective and pathological immunity. Immunity. 2012;37:960–969. doi: 10.1016/j.immuni.2012.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Walker JA, Barlow JL, McKenzie AN. Innate lymphoid cells--how did we miss them. Nat Rev Immunol. 2013;13:75–87. doi: 10.1038/nri3349. [DOI] [PubMed] [Google Scholar]
- 69.Neill DR, Wong SH, Bellosi A, Flynn RJ, Daly M, Langford TK, Bucks C, Kane CM, Fallon PG, Pannell R, Jolin HE, McKenzie AN. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature. 2010;464:1367–1370. doi: 10.1038/nature08900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wong SH, Walker JA, Jolin HE, Drynan LF, Hams E, Camelo A, Barlow JL, Neill DR, Panova V, Koch U, Radtke F, Hardman CS, Hwang YY, Fallon PG, McKenzie AN. Transcription factor RORalpha is critical for nuocyte development. Nat Immunol. 2012;13:229–236. doi: 10.1038/ni.2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Turner JE, Morrison PJ, Wilhelm C, Wilson M, Ahlfors H, Renauld JC, Panzer U, Helmby H, Stockinger B. IL-9-mediated survival of type 2 innate lymphoid cells promotes damage control in helminth-induced lung inflammation. J Exp Med. 2013;210:2951–2965. doi: 10.1084/jem.20130071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Spencer SP, Wilhelm C, Yang Q, Hall JA, Bouladoux N, Boyd A, Nutman TB, Urban JF, Jr., Wang J, Ramalingam TR, Bhandoola A, Wynn TA, Belkaid Y. Adaptation of innate lymphoid cells to a micronutrient deficiency promotes type 2 barrier immunity. Science. 2014;343:432–437. doi: 10.1126/science.1247606. [DOI] [PMC free article] [PubMed] [Google Scholar]