Abstract
Background
Psychological stress is often associated with poor health-related outcomes. One potential biomarker for chronic stress, hair cortisol, is minimally invasive compared to other cortisol collection techniques. This pilot study examined the relationships between hair cortisol and self-reported perceived stress, stressful life events, depressive symptoms, and dispositional optimism among adolescents.
Methods
This cross-sectional study comprised of a convenience sample of 27 adolescents (age: M=14.96, SD=1.63) recruited from a Southern California after-school program. Along with demographic and hair characteristics (e.g., hair color, type, etc.), participants completed the Perceived Stress Scale, Stressful Life Events checklist, CES-D (depressive symptoms), and Life Orientation Test (optimism). Hair cortisol was measured by analyzing hair samples approximately 1 cm from the scalp representing one month of cortisol exposure.
Results
Hair cortisol had a significant inverse association with dispositional optimism (r=−0.44, p<0.05). Hair cortisol was not significantly associated with self-reported perceived stress, stressful life events, or depressive symptoms.
Conclusion
Assessment of hair cortisol may prove beneficial as an objective measure in research examining chronic stress-related outcomes among adolescents. Resiliency or protective dispositions, such as optimism, merit attention in relation to this biomarker.
Keywords: Hair cortisol, Stress, Stressful life events, Dispositional optimism, Adolescents
Introduction
Psychological stress is a common risk factor for poor mental, behavioral, and physical health. Stress leads to the release of various pituitary and adrenal hormones that influence the immune system [1]. The release of these hormones are generally adaptive in response to acute stressors (e.g., short-term difficulties, laboratory mental stress), but over time, stress that is chronic (e.g., uncontrollable and occurring over a prolonged period) can lead to a dysregulated neuroendocrine or neuroimmune response, including cortisol secretion in response to stress. Cortisol levels increase at stressor onset and reduce as time passes. A meta-analyses of chronic stress and cortisol secretion finds chronic stress and uncontrollable stressors to elicit elevated levels of circulating cortisol and slow recoveries to basal levels [2]. However, findings among persons with posttraumatic stress disorder (PTSD) finds lower levels of cortisol secretion, suggesting that reaction to chronic stress can include both hyper- and hypo-cortisol responses.
An important limitation to the field of stress research is that there has been no objective biomarker for chronic (vs. acute) stress. Although repeated and pooled measures of stress biomarkers (e.g., saliva/urinary cortisol levels) have been used to assess chronic stress, these approaches are expensive and are logistically burdensome within the context of field studies of large populations. Further, the interpretation of the pattern of diurnal variation in salivary cortisol is also not well validated as an indicator of chronic stress, partially because of high levels of variance reflecting multiple diurnal patterns [3]. (For example, consider two individuals, one with a high, vs. one with a blunted, cortisol awakening response. The total cortisol exposure over the course of a day could be equivalent between these individuals if the former showed a quick return to basal levels and the latter showed a delayed, but sustained increase following awakening.)
Hair cortisol is a promising biomarker for assessing chronic stress [4]. Whereas salivary and plasma cortisol are indicators of acute stress (over hours), and urine collected appropriately may be an indicator for stress during the prior day, hair cortisol is an indicator of exposure during the period of growth of hair and therefore fills an important methodological gap [5,6]. Hair grows approximately 1cm per month and the laboratory methodologies for assessing this hormone in hair have recently been developed and validated. In the amounts needed, hair can be collected quickly and is acceptable to participants in culturally diverse settings [7].
Hair cortisol levels have been positively associated with self-reported levels of perceived stress among adults [8] and children [9] and have been responsive to specific major life stressors. For example, among rhesus monkeys, hair cortisol levels increased following moving them to a new location [5]. Among neonates, days on a ventilator (a chronic stressor) was associated with elevated hair cortisol levels [10]. Among young children, hair cortisol levels increased following school entry [11]; and among adults, those with long term unemployment (12+ months) had higher hair cortisol levels than those currently employed [12]. Moreover, adults with chronic pain had higher hair cortisol levels than those without chronic pain [13]. However, other studies among adults found weak or no associations between the hair cortisol and the perceived stress scale (PSS), depressive symptoms, or chronic stress [12–14]. Further, one study among young adults found an inverse association between hair cortisol and perceived stress and depressive symptoms [15]. Thus, more research on hair cortisol is needed to further elucidate these relationships, particularly among younger populations where stress may disproportionately contribute towards the development of inflammatory chronic diseases [16–19].
No studies have examined the relationship between hair cortisol and stress-buffering/protective factors. Dispositional optimism has well-established protective relationships with both perceived stress and stressful life events (e.g., through greater use of adaptive strategies for coping with stressors) [20]. In addition, stress is one potential pathway through which optimism positively influences various physical health outcomes [21]. For example, dispositional optimism is associated with lower salivary cortisol awakening response [22,23]. Further, optimism has been shown to mitigate the negative inflammatory effects of laboratory stressors [24], although other studies have not found this relationship [22]. Thus, dispositional optimism is an important contributor to consider in relation to biomarkers of chronic stress.
The primary aim of the current study was to examine the relationships between hair cortisol and self-reported perceived stress, stressful life events, depressive symptoms, and dispositional optimism among adolescents. We hypothesized that hair cortisol would be positively associated with perceived stress and stressful life events scales and inversely associated with dispositional optimism.
Method
Participants
Participants included a convenience sample of 28, primarily Hispanic (93%), adolescents (mean age=14.96, years, SD=1.63, min=12, max=18; 70% female) recruited from a local after school program. One participant was excluded due to unrealistic survey responses (i.e., responded positively to all 65 stressful life events). Personal assent and parental informed consent were obtained from all participants, and hair samples and self-report surveys were collected using a protocol approved by Institutional Review Board of the University of Southern California. The total procedure required approximately 20 minutes and participants received a $10 gift card for their time.
Measures
Demographics and hair characteristics
We assessed age, gender, hair color, hair type, hair treatment/dying, hair blow drying, and use of hand creams (which may contain hydrocortisone).
Perceived Stress
The 10 item Perceived Stress Scale was used to assess stress over the prior month (PSS) [25]. The PSS assesses the extent respondents felt their lives were unpredictable, uncontrollable, or overwhelming over the prior month (e.g., “In the last month, how often have you felt that you were unable to control the important things in your life?). Each item has a 5-point response format ranging from “never” to “very often.” The Cronbach’s alpha for this sample is 0.78.
Stressful Life Events
We used a well-validated and age appropriate 65-item check list of stressful life events experienced over the prior 3 months [26]. This scale was developed and validated among a sample of 1,074 multi-ethnic youth (mean age=12.75) from urban Los Angeles middle schools and includes a range of commonly experienced events spanning from temporary (e.g., test taking, arguments with friends) to more severe (e.g., injury, family death) stressors. For each endorsed event, the stressfulness of the event was rated on a 1 (not at all stressful) to 4 (very stressful) scale. The overall sum of endorsed events was used to form a total stressful life event score for each participant. A weighted sum was used to form a total life event stressfulness score for each participant. The Cronbach’s alpha for this sample is 0.89.
Depressive symptoms was ascertained with the widely used Center for Epidemiological Studies Depression Scale (CES-D) [27]. The CES-D is a 20-item measure of current (i.e., past week) depressive symptoms (e.g., “I felt depressed,” “I felt lonely,” and “I felt that everything I did was an effort”). Responses are made on a 4-point scale, ranging from 0 (rarely or none of the time, less than one day) to 3 (most or all of the time, 5–7 days). The Cronbach’s alpha for this sample is 0.76.
Optimism was assessed with the revised Life Orientation Test (LOT-R) [28]. This widely used 6 item scale assesses future life expectancies using a 5-point response format ranging from “strongly agree” to “strongly disagree.” Examples of items include “In uncertain times I usually expect the best” and “I rarely count on good things happening to me” (reverse scored). The Cronbach’s alpha for this sample is 0.68.
Hair samples
At least 10mg (~50 strands of hair) was collected from the vertex posterior, where there is the most uniform growth pattern between individuals, using procedures previously developed [5]. Fine tipped surgical scissors were used to obtain hair strands as close to the scalp as possible. Hair samples were placed in foil, indicated with the direction of the scalp end, labeled with participant ID, and placed in a plastic bag for transportation and storage.
Hair cortisol assessment
Between 10–20 mg of hair from the 1cm closest to the scalp end was utilized to assess cortisol using a protocol developed by Davenport et al. (2006) [5]. Each hair segment is placed into a falcon tube, isopropanol is added, and the tube mixed using an overhead rotator for 3 minutes. After decanting, the wash cycle is repeated and the sample is dried, powdered, and weighed (50 mg) into a cryo vial. Methanol is added (with vials rotated for 24 hours) for extraction of the steroid. Samples are spun (microcentrifuge at 10 rpm for 2 min) and 1 ml of the clear supernatant is transferred into a new cryo vial. The alcohol is evaporated and twenty microliters are used for cortisol determination using a commercially available immunoassay with chemiluminescence detection (CLIA, IBL-Hamburg, Germany). Intra- and interassay coefficient of variance of this assay is below 8% and validation work (among adults) using liquid chromatography mass spectrometry/MS has shown a strong correlation (r-square=.96); CLIA results were linearly inflated over the LCMS/MS results by 15%, an acceptable deviation [6].
Statistical analyses
Although inconsistent results have been found for the associations between hair cortisol levels and hair characteristics, recent reviews have recommended that these characteristics be assessed as potential confounding factors [29,30]. Thus, we first examined descriptive statistics of all study variables and tested whether hair cortisol varied by potential covariates (e.g., hair color/type, frequency of hair washing). Spearman correlations were then performed to examine the relationships between study variables, using pairwise deletion of missing data. Multiple regression analyses were performed to examine the strength of underlying univariate relationships. In a series of exploratory analyses, we examined whether hair cortisol had a curvilinear relationship (i.e., U-shaped, representing both hyper-and hypo-cortisol responses) with perceived stress, life events, or optimism. Here squared stress, depressive symptoms, and optimism terms (i.e., quadratic terms sensitive to detecting curvilinear trends) were added to separate regression equations of hair cortisol after statistically controlling for the linear effects of the stress, depressive symptoms, and optimism terms, respectively. Due to skewness, hair cortisol levels (Mean pg/mg=16.78, SD=1.44, min=7.61, max=42.52) were log transformed for all analyses. We present results for the first segment of hair to retain the largest sample size.
Results
Descriptive Statistics
Independent sample T-test comparisons of cortisol levels did not differ between participants who: had treated (bleached/dyed/colored) their hair in the past 3 months, had their hair permed or straightened in the past 3 months, had black vs. brown hair, or had straight vs. wavy/curly hair, male vs. female gender (all p’s<0.28; Table 1). Hair cortisol was also not associated with number of days per week hair was shampooed (P <0.21).
Table 1.
Demographic statistics of adolescent sample (N=27).
| Descriptive Statistics of Sample | |
|---|---|
| Mean Age (Std Dev) | 14.96 (1.63) |
| Female Gender (vs. male) | 70% |
| Hispanic Ethnicity (vs non-Hispanic) | 93% |
| Hair treated with bleached, dye or colored in past 3 months (vs. none) | 30.80% |
| Hair chemically permed or straightened in past 3 months (vs none) | 48.10% |
| Black Hair Color (vs brown) | 51.90% |
| Straight Hair (vs curly/wavy) | 48.10% |
| Number of days per week hair was shampooed | 5.72 (1.57) |
Univariate Associations
Descriptive data and correlations of the main study variables are located in Table 2 and Table 3, respectively. Mean levels of hair cortisol were similar to those previously reported in populations of different ages [31]. Hair cortisol levels were not significantly related to self-reported perceived stress, life event checklist summary scores, life event stressfulness scores, or reports of depressive symptoms. There was a significant inverse relationship between hair cortisol and dispositional optimism (Figure 1). Optimism also had a significant inverse relationship with perceived stress and depressive symptoms.
Table 2.
Descriptive statistics of study variables.
| Descriptive Statistics of Study Variables | ||||
|---|---|---|---|---|
| Min | Max | Mean | (SD) | |
| Hair Cortisol [log(pg/mg)] | ||||
| (N=27) | 2.03 | 3.75 | 2.82 | (0.37) |
| Perceived Stress | ||||
| (N=27) | 3.00 | 30.00 | 16.51 | (6.76) |
| Life events sum | ||||
| (N=26) | 5.00 | 47.00 | 18.23 | (9.50) |
| Life events stressfulness | ||||
| (N=26) | 5.00 | 115.00 | 42.62 | (26.83) |
| Depressive symptoms | ||||
| (N=25) | 4.00 | 29.00 | 15.70 | (6.66) |
| Optimism (OPT) | ||||
| (N=24) | 13.00 | 27.00 | 19.13 | (3.34) |
Table 3.
Spearman Correlation Matrix of study variables.
| Spearman Correlation Matrix | |||||
|---|---|---|---|---|---|
| 1. HC | 2. PSS | 3. LE | 4. LES | 5. DEP | |
| 1. Hair Cortisol [HC; Log (pg/mg)] (N=27) |
|||||
| 2. Perceived Stress (PSS) (N=27) |
.14 | ||||
| 3. Life events sum (LE) (N=26) |
.32 | .31 | |||
| 4. Life events stressfulness(LES) (N=26) |
.22 | .49** | .89*** | ||
| 5. Depressive symptoms(DEP) (N=25) |
.15 | .54** | .43* | .59** | |
| 6. Optimism (OPT) (N=24) |
−.44* | −.40* | −.11 | −.10 | −.40+ |
p-values: +<.10,
<=0.05;
<=0.01;
<0.001; pairwise deletion of missing data
Figure 1.
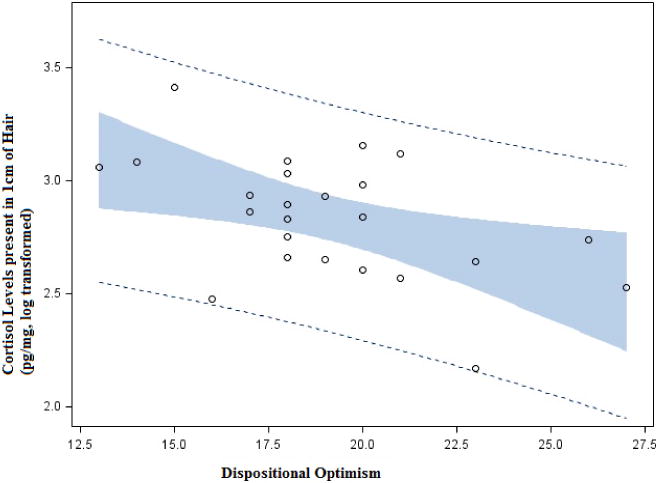
Scatterplot depicting the inverse relationship between dispositional optimism and hair cortisol (log transformed).
Multivariable Associations
Hair cortisol was regressed against optimism scores in a separate multivariable models controlling for perceived stress, and depressive symptoms, respectively. The relationship between optimism and hair cortisol remained significant when controlling for perceived stress (Model F(2,21)=3.81, p=0.04; R2=.27; Optimism B=−0.53, t=−2.71, p=0.01) or depressive symptoms (Model F(2,22)=3.47, p=0.05; R2=0.27; Optimism B=−0.52, t=−2.44, p=0.02). (Neither stress nor depressive symptoms were related to cortisol in these models.) None of the exploratory analyses testing whether hair cortisol had a curvilinear relationship with perceived stress, life events, or optimism, were significant (data not shown; all p’s <0.21).
Discussion
These preliminary data support an inverse association between dispositional optimism and hair cortisol (as a potential biomarker of chronic cortisol exposure) among younger populations. Dispositional optimism is linked with beneficial physical responses to stress e.g.,[32,33], including lower levels of salivary cortisol awakening response [22,23], and to our knowledge this is the first study to find a similar relationship with a biomarker for chronic stress. Although not measured in this study, two possible pathways that might explain this relationship is that optimists (vs. pessimists) are more likely to have higher levels of social support and use adaptive coping strategies in response to stress [20]. Adaptive coping strategies include health promoting behaviors, such as diet, exercise, and avoidance of substance use [20]. Thus, future work concerning optimism and hair cortisol should consider potential pathways that may explain this relationship.
Given the result noted above, more stable trait-like measures may prove useful in future work examining hair cortisol. For example, personality characteristics, such as neuroticism and hostility, and chronic stressful neighborhood characteristics, such as crime or noise [34], might have a greater effect on chronic cortisol exposure (vs. acute cortisol assessments) than temporal characteristics (such as affective states/moods) because they are more resistant to change.
There are numerous potential reasons that there were no significant relationships between hair cortisol and self-reported perceived stress or stressful life events. First, our sample size was limited, reducing our statistical power, and there may have been unknown factors related to unique characteristics of the participants (e.g., pubertal status). Nevertheless, the relatively small to medium relationships (r’s=0.14–0.32) are consistent with prior work [13,14] and suggests low to modest overlap between the self-reported stress measures and hair cortisol.
Second, although our assessments of stress were well-validated and temporally aligned to the collected samples, they may not have adequately reflected ongoing chronic stress. For example, the prior 3 month life event checklist (and perceived stress for the prior 30 days) may have captured events that influenced cortisol levels temporarily, but not long enough to impact levels detected in hair. Repeated assessments of life events and both serum/urinary and hair cortisol levels (to reflect more acute and chronic cortisol exposure, respectively) may be able to determine whether chronic stressor exposure has a greater long term impact on HPA activity. Prior work with chronic unemployment [12] and chronic pain [13] further support this possibility.
Third, there may be a “lack of psychoendocrine covariance” (e.g., [12]). That is, psychological stress is a dynamic process and people may report psychological stress when they are not stressed physiologically and alternatively may be physiologically stressed, but not report any psychological stress. Thus, future work should include more objective (e.g., independent observers/assessments) in addition to utilizing self-report stress assessments to further explore this possibility.
Hair cortisol is a potential indicator for assessing chronic stress exposure, particularly in larger studies among younger populations where response burden to in-depth interviews of psychological stress can be difficult. Because hair cortisol is relatively inexpensive to obtain and transport, significantly less invasive than serum sampling, not reactive to/biased from the assessment method/procedure, and is more feasible than extensive standardized psychological surveys or interviews within the context of large cohort studies, it has great potential for use in research among diverse and urban populations. This is potentially useful in studies of more vulnerable populations (e.g., low socioeconomic status, immunocompromised, children) where chronic stress is posited to disproportionately contribute towards the development of inflammatory chronic diseases (e.g., [16–19]) and increase the deleterious impact of physical risk factors for inflammatory outcomes (e.g., air pollution; [35]). Thus, hair cortisol may serve as an important indicator to consider in research examining resiliency factors that are posited to buffer the impact of stress on various health outcomes [36,37].
Acknowledgments
We thank Dr. Clemens Kirschbaum and his lab for performing the hair cortisol assays.
Source of Funding:
The second author was supported by the National Cancer Institute of the National Institutes of Health under award number T32CA009492.
References
- 1.Sapolsky RM. Why zebras don’t get ulcers: a guide to stress, stress related diseases, and coping. New York: 1994. [Google Scholar]
- 2.Miller GE, Chen E, Zhou ES. If it goes up, must it come down? Chronic stress and the hypothalamic-pituitary-adrenocortical axis in humans. Psychol Bull. 2007;133:25–45. doi: 10.1037/0033-2909.133.1.25. [DOI] [PubMed] [Google Scholar]
- 3.Fries E, Dettenborn L, Kirschbaum C. The The cortisol awakening response (CAR): facts and future directions. Int J Psychophysiol. 2009;72:67–73. doi: 10.1016/j.ijpsycho.2008.03.014. [DOI] [PubMed] [Google Scholar]
- 4.Gow R, Thomson S, Rieder M, Van Uum S, Koren G. An assessment of cortisol analysis in hair and its clinical applications. Forensic Sci Int. 2010;196:32–37. doi: 10.1016/j.forsciint.2009.12.040. [DOI] [PubMed] [Google Scholar]
- 5.Davenport MD, Tiefenbacher S, Lutz CK, Novak MA, Meyer JS. Analysis of endogenous cortisol concentrations in the hair of rhesus macaques. Gen Comp Endocrinol. 2006;147:255–261. doi: 10.1016/j.ygcen.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 6.Kirschbaum C, Tietze A, Skoluda N, Dettenborn L. Hair as a retrospective calendar of cortisol production-Increased cortisol incorporation into hair in the third trimester of pregnancy. Psychoneuroendocrinology. 2009;34:32–37. doi: 10.1016/j.psyneuen.2008.08.024. [DOI] [PubMed] [Google Scholar]
- 7.Kim SR, Wipfli H, Avila-Tang E, Samet JM, Breysse PN. Method validation for measurement of hair nicotine level in nonsmokers. Biomed Chromatogr. 2009;23:273–279. doi: 10.1002/bmc.1110. [DOI] [PubMed] [Google Scholar]
- 8.Kalra S, Einarson A, Karaskov T, Van Uum S, Koren G. The relationship between stress and hair cortisol in healthy pregnant women. Clinical and investigative medicine. 2007;30:E103–E107. doi: 10.25011/cim.v30i2.986. [DOI] [PubMed] [Google Scholar]
- 9.Vanaelst B, Michels N, De Vriendt T, Huybrechts I, Vyncke K, et al. Cortisone in hair of elementary school girls and its relationship with childhood stress. Eur J Pediatr. 2013;172:843–846. doi: 10.1007/s00431-013-1955-1. [DOI] [PubMed] [Google Scholar]
- 10.Yamada J, Stevens B, de Silva N, Gibbins S, Beyene J, et al. Hair cortisol as a potential biologic marker of chronic stress in hospitalized neonates. Neonatology. 2007;92:42–49. doi: 10.1159/000100085. [DOI] [PubMed] [Google Scholar]
- 11.Groeneveld MG, Vermeer HJ, Linting M, Noppe G, van Rossum EF, et al. Children’s hair cortisol as a biomarker of stress at school entry. Stress. 2013;16:711–715. doi: 10.3109/10253890.2013.817553. [DOI] [PubMed] [Google Scholar]
- 12.Dettenborn L, Tietze A, Bruckner F, Kirschbaum C. Higher cortisol content in hair among long-term unemployed individuals compared to controls. Psychoneuroendocrinology. 2010;35:1404–1409. doi: 10.1016/j.psyneuen.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 13.Van Uum SH, Sauvé B, Fraser LA, Morley-Forster P, Paul TL, et al. Elevated content of cortisol in hair of patients with severe chronic pain: a novel biomarker for stress. Stress. 2008;11:483–488. doi: 10.1080/10253890801887388. [DOI] [PubMed] [Google Scholar]
- 14.Dowlati Y, Herrmann N, Swardfager W, Thomson S, Oh PI, et al. Relationship between hair cortisol concentrations and depressive symptoms in patients with coronary artery disease. Neuropsychiatric disease and treatment. 2010;6:393–400. [PMC free article] [PubMed] [Google Scholar]
- 15.Gerber M, Kalak N, Elliot C, Holsboer-Trachsler E, Pühse U. Both hair cortisol levels and perceived stress predict increased symptoms of depression: an exploratory study in young adults. Neuropsychobiology. 2013;68:100–109. doi: 10.1159/000351735. [DOI] [PubMed] [Google Scholar]
- 16.Taylor SE, Lerner JS, Sage RM, Lehman BJ, Seeman TE. Early environment, emotions, responses to stress, and health. J Pers. 2004;72:1365–1393. doi: 10.1111/j.1467-6494.2004.00300.x. [DOI] [PubMed] [Google Scholar]
- 17.Baum A, Garofalo JP, Yali AM. Socioeconomic status and chronic stress. Does stress account for SES effects on health? Ann N Y Acad Sci. 1999;896:131–144. doi: 10.1111/j.1749-6632.1999.tb08111.x. [DOI] [PubMed] [Google Scholar]
- 18.Milam J, McConnell R, Yao L, Berhane K, Jerrett M, et al. Parental stress and childhood wheeze in a prospective cohort study. J Asthma. 2008;45:319–323. doi: 10.1080/02770900801930277. [DOI] [PubMed] [Google Scholar]
- 19.Leserman J. Role of depression, stress, and trauma in HIV disease progression. Psychosom Med. 2008;70:539–545. doi: 10.1097/PSY.0b013e3181777a5f. [DOI] [PubMed] [Google Scholar]
- 20.Carver CS, Scheier MF, Segerstrom SC. Optimism. Clin Psychol Rev. 2010;30:879–889. doi: 10.1016/j.cpr.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rasmussen HN, Scheier MF, Greenhouse JB. Optimism and physical health: a meta-analytic review. Ann Behav Med. 2009;37:239–256. doi: 10.1007/s12160-009-9111-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Endrighi R, Hamer M, Steptoe A. Associations of trait optimism with diurnal neuroendocrine activity, cortisol responses to mental stress, and subjective stress measures in healthy men and women. Psychosom Med. 2011;73:672–678. doi: 10.1097/PSY.0b013e31822f9cd7. [DOI] [PubMed] [Google Scholar]
- 23.Lai JC, Evans PD, Ng SH, Chong AM, Siu OT, et al. Optimism, positive affectivity, and salivary cortisol. Br J Health Psychol. 2005;10:467–484. doi: 10.1348/135910705X26083. [DOI] [PubMed] [Google Scholar]
- 24.Brydon L, Walker C, Wawrzyniak AJ, Chart H, Steptoe A. Dispositional optimism and stress-induced changes in immunity and negative mood. Brain Behav Immun. 2009;23:810–816. doi: 10.1016/j.bbi.2009.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cohen S. Perceived stress in a probability sample of the United States The Social Psychology of Health. Sage; Newbury Park, CA: 1988. [Google Scholar]
- 26.Booker CL, Gallaher P, Unger JB, Ritt-Olson A, Johnson CA. Stressful life events, smoking behavior, and intentions to smoke among and multiethnic sample of sixth graders. Ethn Health. 2004;9:369–397. doi: 10.1080/1355785042000285384. [DOI] [PubMed] [Google Scholar]
- 27.Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- 28.Scheier MF, Carver CS, Bridges MW. Distinguishing optimism from neuroticism (and trait anxiety, self-mastery, and self-esteem): a reevaluation of the Life Orientation Test. J Pers Soc Psychol. 1994;67:1063–1078. doi: 10.1037//0022-3514.67.6.1063. [DOI] [PubMed] [Google Scholar]
- 29.Stalder T, Kirschbaum C. Analysis of cortisol in hair–state of the art and future directions. Brain Behav Immun. 2012;26:1019–1029. doi: 10.1016/j.bbi.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 30.Staufenbiel SM, Penninx BW, Spijker AT, Elzinga BM, van Rossum EF. Hair cortisol, stress exposure, and mental health in humans: a systematic review. Psychoneuroendocrinology. 2013;38:1220–1235. doi: 10.1016/j.psyneuen.2012.11.015. [DOI] [PubMed] [Google Scholar]
- 31.Steudte S, Stalder T, Dettenborn L, Klumbies E, Foley P, et al. Decreased hair cortisol concentrations in generalised anxiety disorder. Psychiatry Res. 2011;186:310–314. doi: 10.1016/j.psychres.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 32.Thomas JL, Britt TW, Odle-Dusseau H, Bliese PD. Dispositional optimism buffers combat veterans from the negative effects of warzone stress on mental health symptoms and work impairment. J Clin Psychol. 2011;67:866–880. doi: 10.1002/jclp.20809. [DOI] [PubMed] [Google Scholar]
- 33.Terrill AL, Ruiz JM, Garofalo JP. Look on the bright side: do the benefits of optimism depend on the social nature of the stressor? J Behav Med. 2010;33:399–414. doi: 10.1007/s10865-010-9268-6. [DOI] [PubMed] [Google Scholar]
- 34.Steptoe A, Feldman PJ. Neighborhood problems as sources of chronic stress: development of a measure of neighborhood problems, and associations with socioeconomic status and health. Ann Behav Med. 2001;23:177–185. doi: 10.1207/S15324796ABM2303_5. [DOI] [PubMed] [Google Scholar]
- 35.Shankardass K, McConnell R, Jerrett M, Milam J, Richardson J, et al. Parental stress increases the effect of traffic-related air pollution on childhood asthma incidence. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:12406–12411. doi: 10.1073/pnas.0812910106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pressman SD, Cohen S. Does positive affect influence health? Psychol Bull. 2005;131:925–971. doi: 10.1037/0033-2909.131.6.925. [DOI] [PubMed] [Google Scholar]
- 37.Steptoe A, Dockray S, Wardle J. Positive affect and psychobiological processes relevant to health. J Pers. 2009;77:1747–1776. doi: 10.1111/j.1467-6494.2009.00599.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


