Abstract
There is evidence that subjective responses to psychoactive drugs are related to personality traits. Here, we extend previous findings by examining personality measures in relation to acute responses to d-amphetamine (AMPH) in a large sample of healthy volunteers. Healthy adults (n=286) completed the Multidimensional Personality Questionnaire Brief Form (MPQ-BF) and participated in four sessions during which they received oral AMPH (0, 5,10, 20 mg), under double-blind conditions. Subjective responses to the drug were measured using the Profile of Mood States, Addiction Research Center Inventory, and Drug Effects Questionnaire. Drug responses were reduced via principal components analysis to three higher-order factors (‘Euphoria’, ‘Arousal’, ‘Dysphoria’). Participants were rank ordered on selected MPQ-BF scales; the top and bottom third on each trait were compared on the drug response factors. High trait physical fearlessness was significantly associated with greater amphetamine-related Arousal, and high trait reward sensitivity was significantly associated with greater Euphoria. In addition, high trait impulsivity was significantly associated with greater Arousal and Euphoria. These results provide further evidence that individual differences in the subjective effects of AMPH are partially explained by differences in personality, and are consistent with the idea that both personality and responses to stimulants depend upon shared neurochemical systems.
Keywords: Amphetamine, subjective effects, personality, impulsivity, humans
Introduction
The acute effects of d-amphetamine (AMPH) differ across individuals (de Wit et al, 1986; Hart et al., 2012; Nurnberger et al., 1982; Silberman et al., 1981; Veenstra-VanderWeele et al, 2006). People differ in the intensity of response to AMPH on various measures, including physiological (Stoops et al., 2007), choice to self-administer the drug (de Wit et al., 1986; Uhlenhuth et al., 1981), and subjective feelings of euphoria, friendliness, arousal, and elation (Crabbe et al., 1983; Silberman et al., 1981; White et al., 2006). Individuals also differ in the quality of effects; that is, whether they experience anxiety or euphoria, or improvements or impairments in performance (Crabbe et al., 1983; Mattay et al., 2003; Nurnberger et al, 1982; Silberman et al, 1981). To the extent that initially pleasant effects favor repeated use of a drug, individual differences in AMPH response may contribute to risk for excessive use or abuse (see Comer et al., 2010; de Wit and Phillips, 2012; Fischman & Foltin, 1991; Jasinski, 1991 for discussion). Therefore, it is important to identify potential sources of variability in acute AMPH responses.
One factor that has been linked to acute responses to AMPH is variation in personality (Kelly et al., 2006; Stoops et al., 2007; White et al., 2002, 2006). Personality traits are stable characteristics of an individual, which appear to be biologically based (Cloninger, 1986; Eysenck and Eysenck, 1967; Tellegen, 1985). Several personality traits have been found to be associated with acute responses to stimulant drugs. For example, high scores on a sensation-seeking trait predicted greater positive AMPH-related subjective effects and choice to self-administer the drug (Kelly et al., 2006; Stoops et al., 2007). In addition, participants with high trait impulsivity scores reported greater positive subjective effects from AMPH (Oswald et al., 2007). Finally, other personality measures such as novelty seeking, reward sensitivity, and low harm avoidance have been found to be associated with greater positive AMPH-related subjective feelings of euphoria, elevated mood, and stimulation (Hutchison et al., 1999; Sax and Strakowski, 1998; White et al, 2002; 2006). Although some studies have failed to detect relationships between personality and drug responses (Alessi et al, 2003; Chait 1993; Corr and Kumari, 2000; de Wit et al., 1986), there is an accumulation of suggestive evidence.
Interestingly, certain personality traits are thought to be mediated by the same neurochemical systems that may underlie the effects of AMPH (Cloninger, 1986; Depue and Collins, 1999; Reif and Lesch, 2003). AMPH is a potent releaser of dopamine (DA), norepinephrine (NE) and, to a lesser extent, serotonin (5HT). Both DA and 5HT have been linked to individual differences in trait impulsivity (Bergh et al., 1997; Laine et al, 2001; Mann et al., 2001; Reist et al., 1996; Swann, 2003; Wade et al., 2000). In addition, reward sensitivity has been linked to DA function: high scores on reward sensitivity predict greater physiological responses to bromocriptine, a dopamine D2 agonist (Depue et al., 1994). NE has also been associated with the trait of harm avoidance: harm avoidance is negatively correlated with physiological responses to phenylephrine, a noradrenergic agonist (White and Depue, 1999). These findings suggest that personality traits may be related to acute responses to AMPH because both share common neurochemical mechanisms.
One limitation of the vast majority of existing studies of personality and acute responses to AMPH is the relatively small size of the samples. Studies of personality typically require large subject samples, whereas studies of acute responses to stimulant drugs are labor-intensive and expensive, and thus sample sizes are usually smaller. For the most part, the studies described above included from 11 to 40 participants, and we conducted one of the larger studies with 128 participants. Here we report the findings from an even larger study (n=286) examining responses to AMPH (0, 5, 10 and 20 mg) in relation to a standardized personality questionnaire, the Multidimensional Personality Questionnaire Brief Form (MPQ-BF). In our previous study (White et al, 2006) AMPH-induced positive mood was related to a measure of reward sensitivity (Social Potency), and a measure of physical fearlessness (Harm Avoidance). Here we extended these findings to an independent, larger sample and with an additional, low dose of AMPH. We also examined the relationship between AMPH response and a measure of impulsivity (MPQ-BF scale Control), and a second measure of reward sensitivity, Agentic Positive Emotionality. We hypothesized that the positive mood effects of AMPH would be (1) positively associated with a measure of impulsivity (i.e., a negative relation with Control); 2) positively associated with the measures of reward sensitivity; and (3) negatively associated with a measure of harm avoidance.
Methods
Participants
Healthy adult volunteers were recruited via newspaper, community bulletin board, and online advertisements. All potential participants completed an initial telephone and an in-person psychiatric evaluation and medical examination (including an electrocardiogram and physical examination). During this screening visit, participants completed the MPQ-BF (Patrick et al., 2002; Tellegen, 1982). Details of the scales selected for analysis are provided below. Participants also provided DNA for analyses to be reported separately. Inclusion criteria were: age between 18 and 35, at least a high school education, fluency in English, and BMI between 18 and 30. Further, because this was part of a larger study designed to investigate the genetic basis of drug responses, only Caucasians (confirmed by self-report and ancestry-informative markers in DNA) were included. Exclusion criteria included night shift work, any significant medical or psychiatric condition (e.g. cardiovascular, neurological, or major psychiatric illness including all Axis I disorders) or any other condition that would increase risk for study participation. In addition, participants were also excluded if they smoked more than three cigarettes per day, consumed more than three cups of coffee per day (i.e. approximately 300 mg caffeine per day), or tested positive for amphetamine, cocaine, opiates, phencyclidine (PCP), and marijuana (as measured by urine toxicology: Ontrak TesTstik, Roche Diagnostic Systems Inc., Somerville, NJ).
Participants provided written informed consent prior to participation. They were told that the purpose of the study was to evaluate the effects of drugs on behavior and that they could receive a stimulant, a sedative, an antihistamine, a hormone, or placebo. Participants agreed not to take any drugs including alcohol, caffeine and nicotine for 24 h prior to and following a session. Women who used hormonal contraceptives were tested regardless of menstrual cycle phase, but women not using hormonal contraceptives were tested only during the follicular phase (days 2–14; White et al, 2002).
The study was reviewed and approved by the Institutional Review Board at the University of Chicago in accordance with the Code of Federal Regulations (Title 45, Part 46) adopted by the National Institutes of Health and the Office for Protection from Research Risks of the US Federal Government. The study was conducted ethically in accordance with the Helsinki Declaration of 1964 (revised 1989) and the National Advisory Council on Drug Abuse Recommended Guidelines for the Administration of Drugs to Human Subjects.
Design
After an initial orientation session, participants completed individually four outpatient sessions. The study used a within-subjects design with three doses of AMPH (5, 10 and 20 mg) and placebo. Participants’ mood states and physiological measures were monitored at baseline and for 4 h after each drug administration. Sessions were separated by at least 48 h. After completing all sessions participants were debriefed to explain the study.
Procedure
Participants fasted from midnight and ate a light breakfast 1 h prior to the session to help standardize drug absorption. Sessions were conducted between 9:00 am and 1:00 pm. Upon reporting to the laboratory, participants provided urine and breath samples (using an Alco-Sensor III Breathalyzer, Intoximeters Inc., St Louis, MO) to confirm abstinence from alcohol and drugs, and women were tested for pregnancy.
At 9:15 am, baseline (pre-capsule) measures of heart rate and blood pressure were obtained, and participants completed the battery of self-report mood and drug effects questionnaires (see below). At 9:30 am, participants ingested capsules containing either AMPH or placebo. They completed post-capsule subjective-effects measures (see below) and cardiovascular measures at 10:00, 10:30, 11:00 am, noon, and 12:30 pm. During times when no measures were scheduled the participants were allowed to relax and watch neutral movies or read. Participants were discharged at approximately 1:00 pm provided that their heart rate and blood pressure had returned to baseline levels.
Measures of subjective effects
Three instruments were used to assess subjective effects at each time point: the Addiction Research Center Inventory (ARCI), the Drug Effects Questionnaire (DEQ) and a modified version of the Profile of Mood States (POMS) (Johanson and Uhlenhuth, 1980; McNair et al, 1971). The ARCI is a 49-item true-false questionnaire that has five empirically derived scales sensitive to specific drugs: A (amphetamine-like, stimulant effects), BG (Benzedrine Group, energy and intellectual efficiency), MBG (Morphine-Benzedrine Group, euphoric effects), LSD (Lysergic Acid Diethylamide, dysphoric effects, somatic complaints) and PCAG (Pentobarbital-Chlorpromazine-Alcohol Group, sedative effects: Martin et al., 1971). The DEQ is a visual analogue questionnaire (0–100 mm) that assesses the extent to which participants experience four subjective states: “Feel Drug”, “Feel High”, “Like Drug”, and “Want More” (Fischman and Foltin, 1991; Justice and de Wit, 2000). The POMS consists of 72 adjectives commonly used to describe momentary mood states and has been factor analyzed into eight scales and two additional composite scales. Participants indicate how they feel at the moment in relation to each of the adjectives on a 5-point scale ranging from “not at all” (0) to “extremely” (4).
Personality
The MPQ-BF is a 155-item trait personality questionnaire that consists of 11 primary trait scales (Wellbeing, Social Potency, Achievement, Social Closeness, Stress Reaction, Alienation, Aggression, Control, Harm Avoidance, Traditionalism, Absorption). These scales are grouped into three superfactors (Positive Emotionality, Negative Emotionality, and Constraint). In the current analysis, one higher-order factor and six primary trait scales were examined. The higher-order factor Agentic Positive Emotionality (APE: a combination of Achievement and Social Potency) and three scales (Social Potency, Harm Avoidance, Control) were chosen a priori based upon the previous study (White et al., 2006). APE and the Social Potency scale are both considered measures of reward sensitivity. The Harm Avoidance scale is considered a measure of physical fear versus fearlessness and the Control scale is considered a measure of planfulness versus impulsivity. Three additional scales were included because of their relevance to previous reports and to test discriminant validity of the above scales (Patrick et al., 2002; Tellegen, 1982; White et al, 2006). These included Social Closeness, which measures affiliation rather than reward sensitivity; Stress Reaction, which measures anxiety proneness rather than physical fear; and Absorption, which is considered a measure of the tendency to experience mental states vividly and quickly, providing an index of the spontaneity of mental state rather than behavior (for a discussion of MPQ-BF higher-order factors and primary trait scales, see Patrick et al., 2002).
Drug
Tablets of d-amphetamine sulfate (AMPH: 5 mg; Barr Pharmaceuticals) were encapsulated in 00 opaque capsules with lactose filler. Placebo capsules contained only lactose. The drug conditions (0, 5, 10, and 20 mg) were administered in randomized order, under double-blind conditions.
Data analysis
Overall data analysis strategy
In order to investigate the relationship between the subjective effects of AMPH and trait personality measures, we (1) characterized acute AMPH-related subjective effects for the entire sample; (2) reduced the subjective-effects measures to a smaller set of factors using principal components analysis; and (3) compared the resulting AMPH-related factor scores between individuals who scored in the highest and lowest third for each personality trait. We also examined the correlations between the different personality scales. The details of each analysis are below.
Acute AMPH-related subjective effects
To characterize the acute effects of AMPH on mood, the dependent variables from the subjective-effects questionnaires (i.e. ARCI, DEQ, and POMS) were first standardized on a 0–100 scale. Then, for each outcome measure, area-under-the-curve (AUC) was calculated using the trapezoidal method and data were analyzed with single-factor repeated measures analyses of variance (ANOVA); the factor was AMPH dose level (0, 5, 10, 20 mg). For all analyses, ANOVA provided the error terms needed to calculate the following planned comparisons: placebo versus all active AMPH doses, 5 mg versus 10 and 20 mg, and 10 mg versus 20 mg.
Reduction of subjective-effects outcome measures
To reduce the data into factors appropriate for analysis with personality, the outcome measures significantly altered by AMPH were entered into a principal components analysis (promax rotation, eigenvalue=1). Separate analyses were conducted for each active AMPH dose. Outcome measures that either: (1) did not load greater than 0.5 on any single factor; or (2) cross-loaded on multiple factors (i.e. measures that loaded greater than 0.5 on more than one factor) were removed and the remaining outcome measures were subjected to further principal component analyses. For each dose, this process was repeated three times, until all remaining outcome measures loaded greater than 0.5 on a single factor. This process resulted in three distinct factors, which we named Euphoria, Arousal, and Dysphoria (see below). Following principal components analyses, we calculated final factor scores using the average AUC values for outcome measures which loaded on the same factor at each active dose.
Relationship between personality and AMPH-related subjective effects
We used an extreme groups approach to identify potentially vulnerable subsamples in a healthy population. Participants were rank ordered based on their scores for each MPQ-BF personality trait measure, and the lowest and highest third of the sample on each trait measure were placed into two separate groups (n=95 for each group). Then, these groups were compared on the three derived drug effect factor scores (i.e. Euphoria, Dysphoria, and Arousal) using two-way repeated measures ANOVA with a within-subjects factor (i.e. AMPH dose level: 0, 5, 10, and 20 mg) and a between-subjects factor (i.e. MPQ-BF personality scale rank: low versus high). To further characterize the potential differences between personality groups, significant differences between the groups’ dose-response curves were followed with post hoc comparisons at each dose level. Using the same data analysis strategy, we also examined the relationship between personality and AMPH-related cardiovascular effects; no significant relationships were observed.
Additional analyses of AMPH-related subjective effects
We also conducted a correlational analysis using all participants to verify the relations between AMPH response and other variables including personality. The results were generally consistent with the extreme groups ANOVA and will not be discussed further. Using the entire sample we conducted linear regressions at each active AMPH dose, using the subjective-effect factors as the dependent variables; independent variables were personality scores and sex, drug-use history, age, and education. There were no significant relationships between acute drug effects and these other demographic variables.
For all analyses and comparisons, p values were considered statistically significant at less than 0.05.
Results
Sample characteristics
Valid data were obtained from 286 participants (48% female). All participants were Caucasian and 4.5% identified themselves as Hispanic. The mean age was 23 (range 18–35). Sixty percent of the sample had a college degree or higher. The average weight was 69 kg (range 48–102 kg), average height was 174 cm (range 150–203 cm) and average BMI was 22.6 (range 18–30). Overall, participants reported light-to-moderate current and past recreational drug use. That is, 95% of the sample reported current use of alcohol (mean 5.7 drinks/week; range 1–28), 31% currently smoked cigarettes (mean 3.1 cigarettes/week; range 1–21), 92% currently drank caffeine-containing beverages (mean 8.1 cups/week; range 1–21), and 28% currently smoked marijuana (mean 5.3 days/month; range 1–30). In addition, 77% of participants reported using marijuana at least once in their lifetime. Self-reported lifetime use (ever using) for other drugs was 9% for sedatives, 28% for stimulants, 21% for opioids, 33% for hallucinogens and 10% for inhalants.
Drug effects
AMPH produced the expected, dose-dependent subjective effects. The drug increased all scales of the DEQ, ARCI, and all but one scale of the POMS (i.e. Anger: data not shown).
Personality
Table 1 shows mean scores on the seven personality scales and the correlations among the scales. Overall, mean scores on the personality scales were within the range previously published (Patrick et al., 2002; White et al., 2006). The two measures of reward sensitivity, APE and Social Potency were significantly and strongly positively correlated (r=0.55). Additionally, Harm Avoidance was moderately correlated with both Control (positive relationship: r=0.37) and Absorption (negative relationship: r=−0.44). All other significant correlations between personality scales were relatively weak (Table 1).
Table 1.
Correlations between MPQ-BF trait personality measures.
| APE | SP | HA | CL | SC | SR | AB | Mean | SD | |
|---|---|---|---|---|---|---|---|---|---|
| (APE) Agentic Positive Emotionality | 1.00 | 66.2 | 12.0 | ||||||
| (SP) Social Potency | .55** | 1.00 | 7.2 | 3.0 | |||||
| (HA) Harm Avoidance | −.16** | −.01 | 1.00 | 6.3 | 2.8 | ||||
| (CL) Control | .07 | −.05 | .37** | 1.00 | 8.1 | 3.0 | |||
| (SC) Social Closeness | .05 | .28** | .18** | .03 | 1.00 | 9.2 | 2.7 | ||
| (SR) Stress Reaction | −.17** | −.11 | −.04 | −.12* | −.23** | 1.00 | 2.6 | 2.8 | |
| (AB) Absorption | .18** | .08 | −.44** | −.27** | −.15* | .18** | 1.00 | 6.2 | 2.9 |
<0.01;
<0.05
Table 2 depicts mean personality scores by group on APE, Social Potency, Harm Avoidance, Control, Stress Reaction, Social Closeness, and Absorption. The high- and low-scoring groups differed on all scales (p<0.001 for all comparisons).
Table 2.
Mean personality scores by high- and low-scoring MPQ-BF groups.
| Personality group |
||||
|---|---|---|---|---|
| Low MPQ |
High MPQ |
|||
| Mean | (SEM) | Mean | (SEM) | |
| (APE) Agentic Positive Emotionality | 46.8 | (0.5) | 65.7 | (0.4)* |
| (SP) Social Potency | 47.4 | (0.6) | 63.2 | (0.3)* |
| (HA) Harm Avoidance | 32.8 | (0.4) | 50.9 | (0.6)* |
| (CL) Control | 36.6 | (0.6) | 60.6 | (0.5)* |
| (SC) Social Closeness | 43.9 | (0.5) | 63.5 | (0.3)* |
| (SR) Stress Reaction | 31.4 | (0.2) | 51.1 | (0.5)* |
| (AB) Absorption | 33.6 | (0.5) | 53.1 | (0.5)* |
significantly different from Low MPQ p<0.001
Note: Separate high- and Low-scoring MPQ groups were formed for each personality trait (n=95 for each group).
Reduction of subjective-effects outcome measures
Principal components analysis of the subjective effects (Table 3) indicated that the factor structures at the three doses were similar. Based on the common pattern of factor loadings across doses, Factor 1 was labeled “Euphoria”, Factor 2 was labeled “Arousal”, and Factor 3 was labeled “Dysphoria”. The bold items in Table 3 indicate the outcome measures that were used to calculate the factor scores used in subsequent analyses. Overall, “Euphoria” was significantly correlated with both “Dysphoria” and “Arousal” (r=0.1 9 and 0.23 respectively; p<0.005 for both correlations). AMPH produced a dose-dependent response on all three factors (data not shown), suggesting that these factor structures are valid descriptions of AMPH-related mood effects.
Table 3.
Principal components analysis (PCA) of subjective-effect measures.
| AMPH dose conditions |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| 5 mg |
10 mg |
20 mg |
|||||||
| Factor1 Euphoria |
Factor2 Arousal |
Factor3 Dysphoria |
Factor1 Euphoria |
Factor2 Arousal |
Factor3 Dysphoria |
Factor1 Euphoria |
Factor2 Arousal |
Factor3 Dysphoria |
|
| DEQ | |||||||||
| Feel | .896 | −.100 | .028 | .890 | −.201 | .046 | .917 | −.128 | .374 |
| Like | .888 | −.041 | −.057 | .913 | −.074 | −.115 | .922 | −.104 | .065 |
| High | .873 | −.056 | .074 | .875 | −.161 | .122 | .902 | −.131 | .302 |
| More | .697 | .039 | −.002 | .769 | −.006 | −.122 | .775 | −.180 | −.088 |
| ARCI | |||||||||
| A | *** | *** | *** | .624 | .321 | .091 | .700 | .208 | .039 |
| MBG | .541 | .446 | −.061 | .671 | .337 | −.052 | .724 | .120 | −.157 |
| Benzedrine | .079 | .746 | −.095 | .266 | .741 | .041 | .263 | .706 | .063 |
| LSD | .177 | −.072 | .643 | .042 | −.118 | .720 | .391 | −.056 | .704 |
| PCAG | .130 | −.841 | −.115 | .230 | −.887 | −.096 | .223 | −.984 | −.153 |
| POMS | |||||||||
| Vigor | .185 | .781 | .001 | .221 | .698 | .030 | .333 | .682 | .049 |
| Arousal | .000 | .988 | .149 | .006 | .956 | .121 | .060 | 1.008 | .224 |
| Elation | *** | *** | *** | *** | *** | *** | .544 | .160 | −.473 |
| Friendliness | *** | *** | *** | *** | *** | *** | .414 | .022 | −.581 |
| Positive Mood | *** | *** | *** | *** | *** | *** | .470 | .209 | −.514 |
| Anxiety | −.032 | .199 | .913 | −.097 | .144 | .875 | .097 | .331 | .957 |
| Fatigue | .167 | −.802 | −.025 | .171 | −.808 | .080 | .211 | −.860 | −.023 |
| Confusion | .071 | −.613 | .389 | .066 | −.652 | .358 | .236 | −.619 | .342 |
| Depression | −.101 | −.062 | .672 | *** | *** | *** | *** | *** | *** |
Subjective-effect scales that were not significantly altered by the AMPH dose were not included in the PCA. Bold items indicate the outcome measures that were used to calculate factor scores used in subsequent analyses.
Relationship between personality and AMPH-related subjective effects
Several MPQ scales were associated with the effects of AMPH. Associations between personality and drug factors are described below.
Relationship between drug response and trait impulsivity
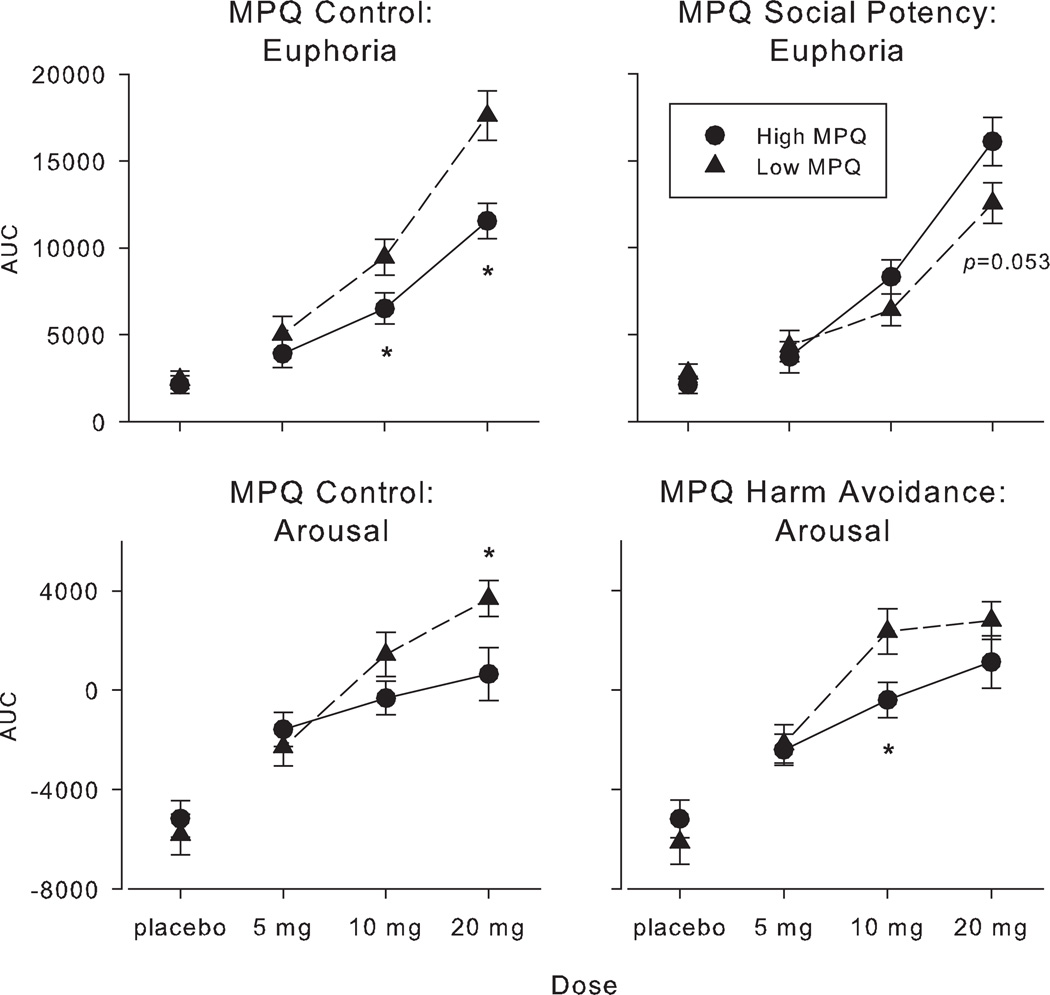
Participants who scored highly on a measure of impulsivity reported greater AMPH-related positive subjective effects (Figure 1, left panels). That is, participants who scored low on MPQ Control (i.e. who were more impulsive) reported greater increases after AMPH on both Euphoria and Arousal (F[1,188] = 7.86–10.99; p<0.01 for both analyses). Post hoc comparisons revealed that the Euphoria response was greater at the 10 mg and 20 mg dose levels (p<0.05 for both comparisons) and the Arousal response was greater at the 20 mg dose level (p=0.019).
Figure 1.
Left panels: AMPH-related ratings of Euphoria and Arousal as a function of dose and trait impulsivity (i.e. Control). Right panel (top): AMPH-related ratings of Euphoria as a function of dose and trait reward sensitivity (i.e. Social Potency). Right panel (bottom): AMPH-related ratings of Arousal as a function of dose and trait physical fearlessness (i.e. Harm Avoidance). Error bars represent one SEM. An * indicates low trait personality scores (Low MPQ) significantly different from high trait scores (High MPQ: p<0.05).
Note: Separate high- and Low-scoring MPQ groups were formed for each personality trait (n=95 for each group).
Relationship between drug response and trait reward sensitivity
Participants who scored high on Social Potency (i.e. who were more sensitive to reward) reported greater AMPH-related Euphoria (Figure 1 top right panel). That is, the two groups had significantly different dose-response curves (F[1,188]=6.39; p=0.012). Post hoc comparisons revealed that although the groups did not significantly differ at any individual dose level, comparisons at the 20 mg AMPH dose approached statistical significance (p=0.053). The subjects who scored high and low on APE did not differ in their responses to AMPH.
Relationship between drug response and trait physical fearlessness
Participants who scored high on Harm Avoidance (i.e. who reported less physical fearlessness) reported smaller increases in AMPH-induced Arousal compared with those who scored low on Harm Avoidance (Figure 1 bottom right panel; F[1,188]=3.98; p=0.048); this difference was significant at the 10 mg dose (p=0.019).
Relationship between drug response and discriminant personality measures
AMPH did not have differential effects in the groups who scored low or high on Social Closeness, Stress Reaction, and Absorption.
Discussion
We found that personality traits in healthy young adults were systematically related to their subjectively reported drug effects after low, acute doses of AMPH. Specifically, personality traits of impulsivity (MPF-BF Control), reward sensitivity (MPQ-BF Social Potency), and physical fearlessness (MPQ-BF Harm Avoidance) predicted subjective response to oral AMPH on measures of Euphoria and Arousal. These results are consistent with earlier findings indicating that individual differences in AMPH response are related to pre-existing differences in personality (Kelly et al., 2006; Oswald et al, 2007; Stoops et al, 2007; White et al., 2006), and extend previous findings to a larger sample, using several doses of AMPH and a robust principal components analysis structure to summarize the subjective effects of the drug.
One interesting finding from this study was that participants who scored low on the Control scale (i.e. those with higher levels of impulsivity) self-reported greater AMPH-related Euphoria and Arousal. This is consistent with data from previous reports, which suggest that higher trait impulsivity was associated with a greater AMPH mood response (Kelly et al., 2006; Oswald et al., 2007; Stoops et al., 2007). Interestingly, Oswald and colleagues (2007) reported that increased impulsivity, as measured by the NEO Personality Inventory impulsivity subscale, was associated not only with greater subjective effects of AMPH but, paradoxically, also with a blunted dopaminergic response to AMPH in the right ventral striatum. The authors suggested that the more impulsive individuals were more sensitive to changes in dopaminergic release. Overall, these data are consistent with the idea that there are commonalities between the neurological mechanisms of the trait of impulsivity and the acute effects of AMPH. The current results are also consistent with data suggesting that impulsivity is related to greater subjective effects and use of a number of drugs in humans (cocaine: Moeller et al., 2001; nicotine: Perkins et al., 2000) and increased self-administration in rats (cocaine: Belin et al., 2008; Dalley et al., 2007; Perry et al., 2005, 2008; methylphenidate: Marusich and Bardo, 2009; ethanol: Poulos et al., 1995; nicotine: Diergaarde et al., 2008). Thus, it is possible that individuals high on the trait of impulsivity are predisposed to develop problematic drug use or abuse.
Acute subjective response to AMPH was also associated with a trait measure of reward sensitivity. That is, individuals who scored higher on Social Potency reported greater AMPH-induced Euphoria. These data are consistent with results from our previous study (White et al., 2006), and with the idea that trait reward sensitivity and subjective responses to the stimulant have a common neural mechanism (Depue and Collins, 1999). Interestingly, this finding may be related to findings from preclinical studies indicating that animals that exhibit greater novelty seeking also exhibit a more pronounced locomotor response to AMPH, and are more likely to self-administer AMPH (Piazza et al., 1989). Thus, reward sensitivity in humans may be related to novelty seeking in rodents. The individual differences in laboratory animals have been attributed to differences in dopaminergic function (Marinelli and White, 2000). In other human studies, markers of dopaminergic function appear to be related to trait measures of reward sensitivity. For instance, in a comparison of individuals with ADHD and healthy normal volunteers, Volkow and colleagues (2011) reported that increased availability of dopamine receptors and transporters in the midbrain were positively associated with scores on MPQ-BF Positive Emotionality in both populations. Thus, individuals who are temperamentally more sensitive to reward may have a more pronounced response to AMPH and/or other stimulants. There are other possible explanations for the current findings. For example, individuals’ prior life experiences might affect both their acute self-reported responses to amphetamine and their self-reported responses on personality questionnaires. In general, the generalizability and the biological basis of this association between personality and acute subjective response to a drug remain to be determined using different experimental approaches, including brain imaging.
We also observed that participants who reported greater levels of physical fearlessness (i.e. those who scored low on the Harm Avoidance scale) self-reported greater AMPH-related Arousal but not Euphoria. This finding is largely consistent with our previous results using a smaller sample (White et al., 2006). Why fearlessness should be related to drug-induced Arousal and not Euphoria is not clear. However, it is consistent with the fact that AMPH is a potent releaser of norepinephrine, a neurotransmitter associated with alertness and arousal, and, to a lesser extent, serotonin, both of which have been implicated in individual differences in trait harm avoidance (Carver and Miller, 2006 for review; Kim et al., 2006; Moresco et al, 2002; Peirson et al., 1999). On the other hand, Arousal and Euphoria were correlated in the present study, suggesting that the two psychological constructs, or the measures, may have substantial overlap.
The current data are largely consistent with our earlier report (White et al., 2006), using a similar approach in a smaller sample. In both studies, lower Harm Avoidance and higher Social Potency were associated with greater positive subjective effects of AMPH. In the present study but not the earlier study, AMPH response was also related to impulsivity. This difference may be related to the larger sample size (i.e. n=286 versus n=128) and corresponding increase in power. Alternatively, the difference may be related to the ethnic composition of the sample (i.e. exclusively Caucasian in the current study versus 54% Caucasian in the earlier study), although there is no obvious reason why impulsivity would be less strongly related to AMPH responses in non-Caucasians. Finally, the two studies differed slightly in the methods for principal components analysis (PCA) which yielded three factors in the current study compared with two factors in the original study. Notably, the PCA analysis produced a stable structure across the three doses of AMPH dose. This similarity supports the validity and reliability of the derived factors, and afforded us the opportunity to not only directly compare the doses but also to analyze the dose-related response to AMPH.
The present findings could be expanded in several directions. First, we used relatively low doses of AMPH (i.e. 5–20 mg), which are within the therapeutic dose range but may be lower than the doses typically used by recreational drug users (Griffiths et al., 2003). The drugs are also abused via routes such as smoking, insufflation, or intravenous, which produce higher brain concentrations and more pronounced subjective effects (Hatsukami and Fischman, 1996; Simon et al., 2002). Thus, responses to higher doses of the drug may bear different relationships to personality. In the present study, the influence of personality on the subjective drug responses increased as the dose of AMPH increased, suggesting that these differences may increase even more at higher doses. Another question raised by this study is whether personality also affects responses to the drug in stimulant users. It may be that personality influences early use, but that it has less influence as drug taking continues (de Wit and Phillips, 2012). Thus, the generalizability of our observed personality-drug response correlations remains to be determined in future studies.
In conclusion, we observed that acute mood-related responses to oral AMPH were related to trait personality measures of impulsivity, reward sensitivity, and physical fearlessness. Individuals who scored higher on impulsivity reported greater AMPH-induced Euphoria and Arousal. Thus, pre-existing differences in personality appear to contribute to the subjective effects of the drug, perhaps because of shared neurochemical systems. Individuals with certain personality traits may be more susceptible to the rewarding properties of AMPH and thus may be at greater risk for using the drug repeatedly, and ultimately developing an amphetamine use disorder. Future laboratory studies might investigate the relationship between trait personality measures, the specific neurobiological underpinnings of these measures, and drug self-administration in order to better understand whether these personality traits mediate drug-taking behavior.
Acknowledgements
The assistance of Drs. Royce Lee, Margaret Wardle, and Abraham Palmer are gratefully acknowledged.
Funding
Supported by DA002812 (Harriet de Wit PI) and DA021336 (Abraham Palmer PI). Dr de Wit has received support from Unilever for a research project unrelated to this manuscript.
Footnotes
Conflict of interest
The authors declare that there are no conflict of interest.
References
- Alessi SM, Greenwald M, Johanson CE. The prediction of individual differences in response to D-amphetamine in healthy adults. Behav Pharmacol. 2003;14:19–32. doi: 10.1097/00008877-200302000-00002. [DOI] [PubMed] [Google Scholar]
- Belin D, Mar AC, Dalley JW, et al. High impulsivity predicts the switch to compulsive cocaine-taking. Science. 2008;320:1352–1355. doi: 10.1126/science.1158136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergh C, Eklund T, Sodersten P, et al. Altered dopamine function in pathological gambling. Psychol Med. 1997;21:473–475. doi: 10.1017/s0033291796003789. [DOI] [PubMed] [Google Scholar]
- Carver CS, Miller CJ. Relations of serotonin function to personality: Current views and a key methodological issue. Psychiatry Res. 2006;144:1–15. doi: 10.1016/j.psychres.2006.03.013. [DOI] [PubMed] [Google Scholar]
- Chait LD. Factors influencing the reinforcing and subjective effects of d-amphetamine in humans. Behav Pharmacol. 1993;4:191–199. [PubMed] [Google Scholar]
- Cloninger CR. A unified biosocial theory of personality and its role in the development of anxiety states. Psychiatr Dev. 1986;4:167–226. [PubMed] [Google Scholar]
- Comer SD, Bickel WK, Yi R, et al. Human behavioral pharmacology, past, present, and future: Symposium presented at the 50th annual meeting of the Behavioral Pharmacology Society. Behav Pharmacol. 2010;21:251–277. doi: 10.1097/FBP.0b013e32833bb9f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corr PJ, Kumari V. Individual differences in mood reactions to d-amphetamine: A test of three personality factors. J Psychopharma-col. 2000;14:371–377. doi: 10.1177/026988110001400406. [DOI] [PubMed] [Google Scholar]
- Crabbe JC, Jarvik LF, Liston EH, et al. Behavioral responses to amphetamines in identical twins. Acta Genet Med Gemellol (Roma) 1983;32:139–149. doi: 10.1017/s0001566000006425. [DOI] [PubMed] [Google Scholar]
- Dalley JW, Fryer TD, Brichard L, et al. Nucleus accumbens D2/3 receptors predict trait impulsivity and cocaine reinforcement. Science. 2007;315:1267–1270. doi: 10.1126/science.1137073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit H, Phillips TJ. Do initial responses to drugs predict future use or abuse? Neurosci Biobehav Rev. 2012;36:1565–1576. doi: 10.1016/j.neubiorev.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit H, Uhlenhuth EH, Johanson CE. Individual differences in the reinforcing and subjective effects of amphetamine and diazepam. Drug Alcohol Depend. 1986;16:341–360. doi: 10.1016/0376-8716(86)90068-2. [DOI] [PubMed] [Google Scholar]
- Depue RA, Collins PF. Neurobiology of the structure of personality: Dopamine, facilitation of incentive motivation, and extraversion. Behav Brain Sci. 1999;22:491–517. doi: 10.1017/s0140525x99002046. discussion 518-469. [DOI] [PubMed] [Google Scholar]
- Depue RA, Luciana M, Arbisi P, et al. Dopamine and the structure of personality: Relation of agonist-induced dopamine activity to positive emotionality. J Pers Soc Psychol. 1994;67:485–498. doi: 10.1037//0022-3514.67.3.485. [DOI] [PubMed] [Google Scholar]
- Diergaarde L, Pattij T, Poortvliet I, et al. Impulsive choice and impulsive action predict vulnerability to distinct stages of nicotine seeking in rats. Biol Psychiatry. 2008;63:301–308. doi: 10.1016/j.biopsych.2007.07.011. [DOI] [PubMed] [Google Scholar]
- Eysenck SB, Eysenck HJ. Physiological reactivity to sensory stimulation as a measure of personality. Psychol Rep. 1967;20:45–46. doi: 10.2466/pr0.1967.20.1.45. [DOI] [PubMed] [Google Scholar]
- Fischman MW, Foltin RW. Utility of subjective-effects measurements in assessing abuse liability of drugs in humans. Br J Addict. 1991;86:1563–1570. doi: 10.1111/j.1360-0443.1991.tb01749.x. [DOI] [PubMed] [Google Scholar]
- Griffiths RR, Bigelow GE, Ator NA. Principles of initial experimental drug abuse liability assessment in humans. Drug Alcohol Depend. 2003;70(3 Suppl):S41–S54. doi: 10.1016/s0376-8716(03)00098-x. [DOI] [PubMed] [Google Scholar]
- Hart AB, de Wit H, Palmer AA. Genetic factors modulating the response to stimulant drugs in humans. Curr Top Behav Neurosci. 2012 doi: 10.1007/7854_2011_187. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatsukami DK, Fischman MW. Crack cocaine and cocaine hydrochloride Are the differences myth orreality? JAMA. 1996;276:1580–1588. [PubMed] [Google Scholar]
- Hutchison KE, Wood MD, Swift R. Personality factors moderate subjective and psychophysiological responses to d-amphetamine in humans. Exp Clin Psychopharmacol. 1999;7:493–501. doi: 10.1037//1064-1297.7.4.493. [DOI] [PubMed] [Google Scholar]
- Jasinski DR. History of abuse liability testing in humans. Br J Addict. 1991;86:1559–1562. doi: 10.1111/j.1360-0443.1991.tb01748.x. [DOI] [PubMed] [Google Scholar]
- Johanson CE, Uhlenhuth EH. Drug preference and mood in humans: d-amphetamine. Psychopharmacology (Berl) 1980;71:275–279. doi: 10.1007/BF00433062. [DOI] [PubMed] [Google Scholar]
- Justice AJ, De Wit H. Acute effects of d-amphetamine during the early and late follicular phases of the menstrual cycle in women. Pharmacol Biochem Behav. 2000;66:509–515. doi: 10.1016/s0091-3057(00)00218-5. [DOI] [PubMed] [Google Scholar]
- Kelly TH, Robbins G, Martin CA, et al. Individual differences in drug abuse vulnerability: d-amphetamine and sensation-seeking status. Psychopharmacology (Berl) 2006;189:17–25. doi: 10.1007/s00213-006-0487-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SJ, Kim YS, Lee HS, et al. An interaction between the serotonin transporter promoter region and dopamine transporter polymorphisms contributes to harm avoidance and reward dependence traits in normal healthy subjects. J Neural Transm. 2006;113:877–886. doi: 10.1007/s00702-006-0444-3. [DOI] [PubMed] [Google Scholar]
- Laine TP, Ahonen A, Rasanen P, et al. Dopamine transporter density and novelty seeking among alcoholics. J Addict Dis. 2001;20:91–96. doi: 10.1300/j069v20n04_08. [DOI] [PubMed] [Google Scholar]
- Mann JJ, Brent DA, Arango V. The neurobiology and genetics of suicide and attempted suicide: A focus on the serotonergic system. Neuropsychopharmacology. 2001;24:467–477. doi: 10.1016/S0893-133X(00)00228-1. [DOI] [PubMed] [Google Scholar]
- Marinelli M, White FJ. Enhanced vulnerability to cocaine self-administration is associated with elevated impulse activity of midbrain dopamine neurons. J Neurosci. 2000;20:8876–8885. doi: 10.1523/JNEUROSCI.20-23-08876.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin WR, Sloan JW, Sapira JD, et al. Physiologic, subjective, and behavioral effects of amphetamine, methamphetamine, ephed-rine, phenmetrazine, and methylphenidate in man. Clin Pharmacol Ther. 1971;12:245–258. doi: 10.1002/cpt1971122part1245. [DOI] [PubMed] [Google Scholar]
- Marusich JA, Bardo MT. Differences in impulsivity on a delay-discounting task predict self-administration of a low unit dose of methylphenidate in rats. Behav Pharmacol. 2009;20:447–454. doi: 10.1097/FBP.0b013e328330ad6d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattay VS, Goldberg TE, Fera F, et al. Catechol O-methyltransferase vall58-met genotype and individual variation in the brain response to amphetamine. Proc Natl Acad Sci USA. 2003;100:6186–6191. doi: 10.1073/pnas.0931309100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNair D, Lorr M, Droppleman L. Profile of Mood States. San Diego: Educational and Industrial Testing Service; 1971. [Google Scholar]
- Moeller FG, Dougherty DM, Barratt ES, et al. The impact of impulsivity on cocaine use and retention in treatment. J Subst Abuse Treat. 2001;21:193–198. doi: 10.1016/s0740-5472(01)00202-1. [DOI] [PubMed] [Google Scholar]
- Moresco FM, Dieci M, Vita A, et al. In vivo serotonin 5HT(2A) receptor binding and personality traits in healthy subjects: A positron emission tomography study. Neuroimage. 2002;17:1470–1478. doi: 10.1006/nimg.2002.1239. [DOI] [PubMed] [Google Scholar]
- Nurnberger JI, Jr., Gershon ES, Simmons S, et al. Behavioral, biochemical and neuroendocrine responses to amphetamine in normal twins and 'well-state' bipolar patients. Psychoneuroendocrinology. 1982;7:163–176. doi: 10.1016/0306-4530(82)90009-9. [DOI] [PubMed] [Google Scholar]
- Oswald LM, Wong DF, Zhou Y, et al. Impulsivity and chronic stress are associated with amphetamine-induced striatal dopamine release. Neuroimage. 2007;36:153–166. doi: 10.1016/j.neuroimage.2007.01.055. [DOI] [PubMed] [Google Scholar]
- Patrick CJ, Curtin JJ, Tellegen A. Development and validation of a brief form of the Multidimensional Personality Questionnaire. Psychol Assess. 2002;14:150–163. doi: 10.1037//1040-3590.14.2.150. [DOI] [PubMed] [Google Scholar]
- Peirson AR, Heuchert JW, Thomala L, et al. Relationship between serotonin and the temperament and character inventory. Psychiatry Res. 1999;89:29–37. doi: 10.1016/s0165-1781(99)00079-7. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Gerlach D, Broge M, et al. Greater sensitivity to subjective effects of nicotine in nonsmokers high in sensation seeking. Exp Clin Psychopharmacol. 2000;8:462–471. doi: 10.1037//1064-1297.8.4.462. [DOI] [PubMed] [Google Scholar]
- Perry JL, Larson EB, German JP, et al. Impulsivity (delay discounting) as a predictor of acquisition of IV cocaine self-administration in female rats. Psychopharmacology (Berl) 2005;178:193–201. doi: 10.1007/s00213-004-1994-4. [DOI] [PubMed] [Google Scholar]
- Perry JL, Nelson SE, Carroll ME. Impulsive choice as a predictor of acquisition of IV cocaine self- administration and reinstatement of cocaine-seeking behavior in male and female rats. Exp Clin Psychopharmacol. 2008;16:165–177. doi: 10.1037/1064-1297.16.2.165. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Deminiere JM, Le Moal M, et al. Factors that predict individual vulnerability to amphetamine self-administration. Science. 1989;245:1511–1513. doi: 10.1126/science.2781295. [DOI] [PubMed] [Google Scholar]
- Poulos CX, Le AD, Parker JL. Impulsivity predicts individual susceptibility to high levels of alcohol self-administration. Behav Pharmacol. 1995;6:810–814. [PubMed] [Google Scholar]
- Reif A, Lesch KP. Toward a molecular architecture of personality. Behav Brain Res. 2003;139:1–20. doi: 10.1016/s0166-4328(02)00267-x. [DOI] [PubMed] [Google Scholar]
- Reist C, Helmeste D, Albers L, et al. Serotonin indices and impulsivity in normal volunteers. Psychiatry Res. 1996;60:177–184. doi: 10.1016/0165-1781(95)02830-7. [DOI] [PubMed] [Google Scholar]
- Sax KW, Strakowski SM. Enhanced behavioral response to repeated d-amphetamine and personality traits in humans. Biol Psychiatry AA: 1998:1192–1195. doi: 10.1016/s0006-3223(98)00168-1. [DOI] [PubMed] [Google Scholar]
- Silberman EK, Reus VI, Jimerson DC, et al. Heterogeneity of amphetamine response in depressed patients. Am J Psychiatry. 1981;138:1302–1307. doi: 10.1176/ajp.138.10.1302. [DOI] [PubMed] [Google Scholar]
- Simon SL, Richardson K, Dacey J, et al. A comparison of patterns of methamphetamine and cocaine use. J Addict Dis. 2002;21:35–44. doi: 10.1300/j069v21n01_04. [DOI] [PubMed] [Google Scholar]
- Stoops WW, Lile JA, Robbins CG, et al. The reinforcing, subject-rated, performance, and cardiovascular effects of d-amphetamine: Influence of sensation-seeking status. Addict Behav. 2007;32:1177–1188. doi: 10.1016/j.addbeh.2006.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swann AC. Neuroreceptor mechanisms of aggression and its treatment. J Clin Psychiatry. 2003;64(Suppl 4):26–35. [PubMed] [Google Scholar]
- Tellegen A. Brief Manual for the Multidimensional Personality Questionnaire. Unpublished manuscript, University of Minnesota, Minneapolis; 1982. [Google Scholar]
- Tellegen A. Structures of mood and personality and their relevance to assessing anxiety, with an emphasis on self-report. In: Tuma AH, Maser JD, editors. Anxiety and the Anxiety Disorders. Hillsdale, NJ: Erlbaum; 1985. pp. 681–706. [Google Scholar]
- Uhlenhuth EH, Johanson CE, Kilgore K, et al. Drug preference and mood in humans: Preference for d-amphetamine and subject characteristics. Psychopharmacology (Berl) 1981;74:191–194. doi: 10.1007/BF00432692. [DOI] [PubMed] [Google Scholar]
- Veenstra-VanderWeele J, Qaadir A, et al. Association between the casein kinase 1 epsilon gene region and subjective response to D-amphetamine. Neuropsychopharmacology. 2006;31:1056–1063. doi: 10.1038/sj.npp.1300936. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Newcorn JH, et al. Motivation deficit in ADHD is associated with dysfunction of the dopamine reward pathway. Mol Psychiatry. 2011;16:1147–1154. doi: 10.1038/mp.2010.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade TR, de Wit H, Richards JB. Effects of dopaminergic drugs on delayed reward as a measure of impulsive behavior in rats. Psychopharmacology (Berl) 2000;150:90–101. doi: 10.1007/s002130000402. [DOI] [PubMed] [Google Scholar]
- White TL, Depue RA. Differential association of traits of fear and anxiety with norepinephrine- and dark-induced pupil reactivity. J Pers Soc Psychol. 1999;77:863–877. doi: 10.1037//0022-3514.77.4.863. [DOI] [PubMed] [Google Scholar]
- White TL, Justice AJ, de Wit H. Differential subjective effects of D-amphetamine by gender, hormone levels and menstrual cycle phase. Pharmacol Biochem Behav. 2002;73:729–741. doi: 10.1016/s0091-3057(02)00818-3. [DOI] [PubMed] [Google Scholar]
- White TL, Lott DC, de Wit H. Personality and the subjective effects of acute amphetamine in healthy volunteers. Neuropsychopharmacology. 2006;31:1064–1074. doi: 10.1038/sj.npp.1300939. [DOI] [PubMed] [Google Scholar]