Abstract
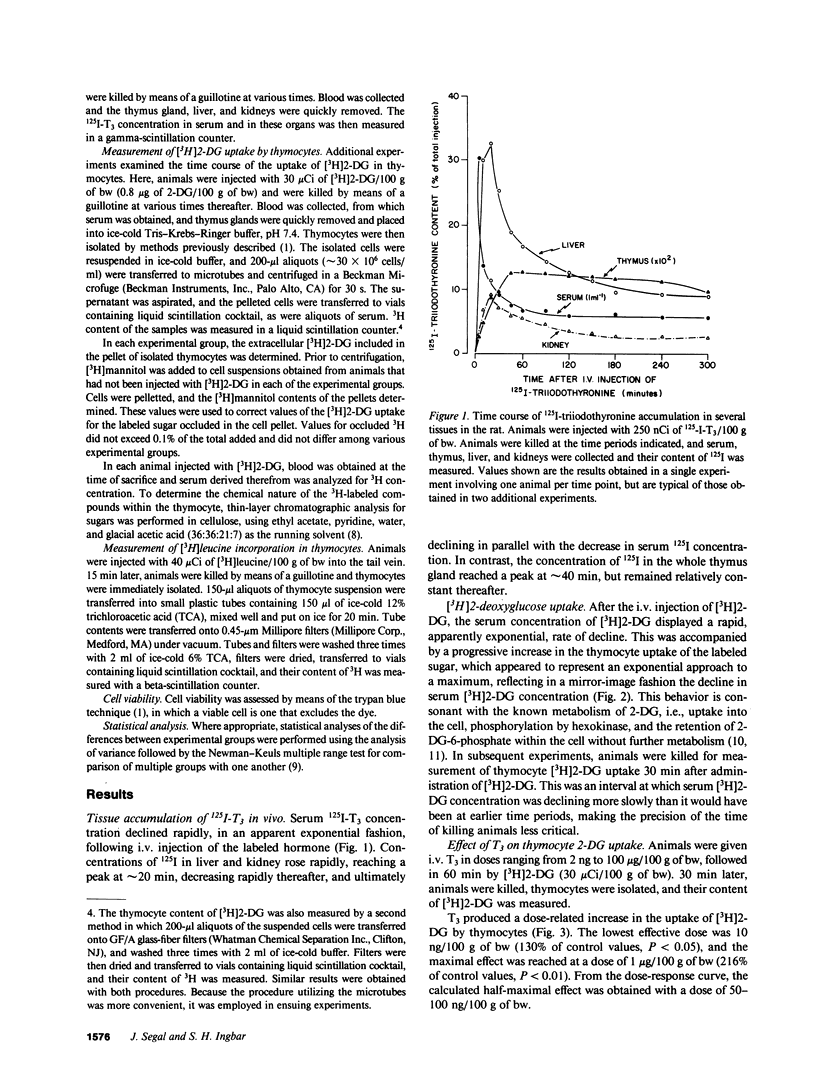
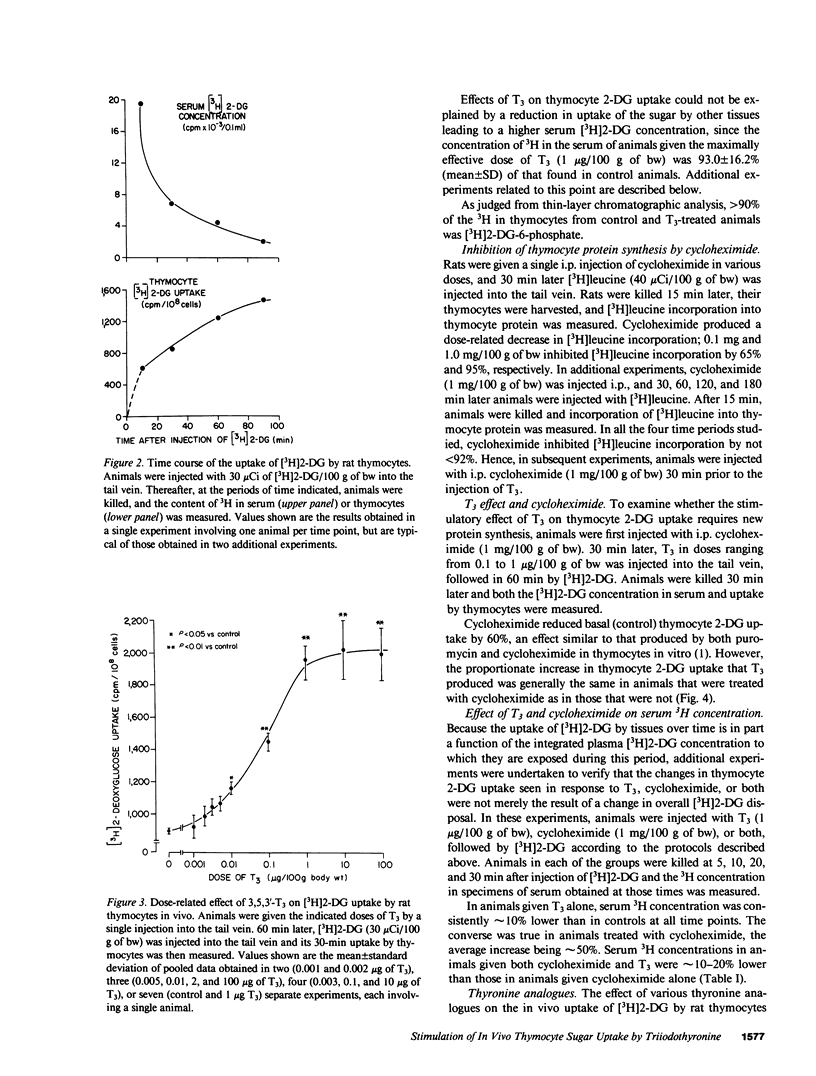
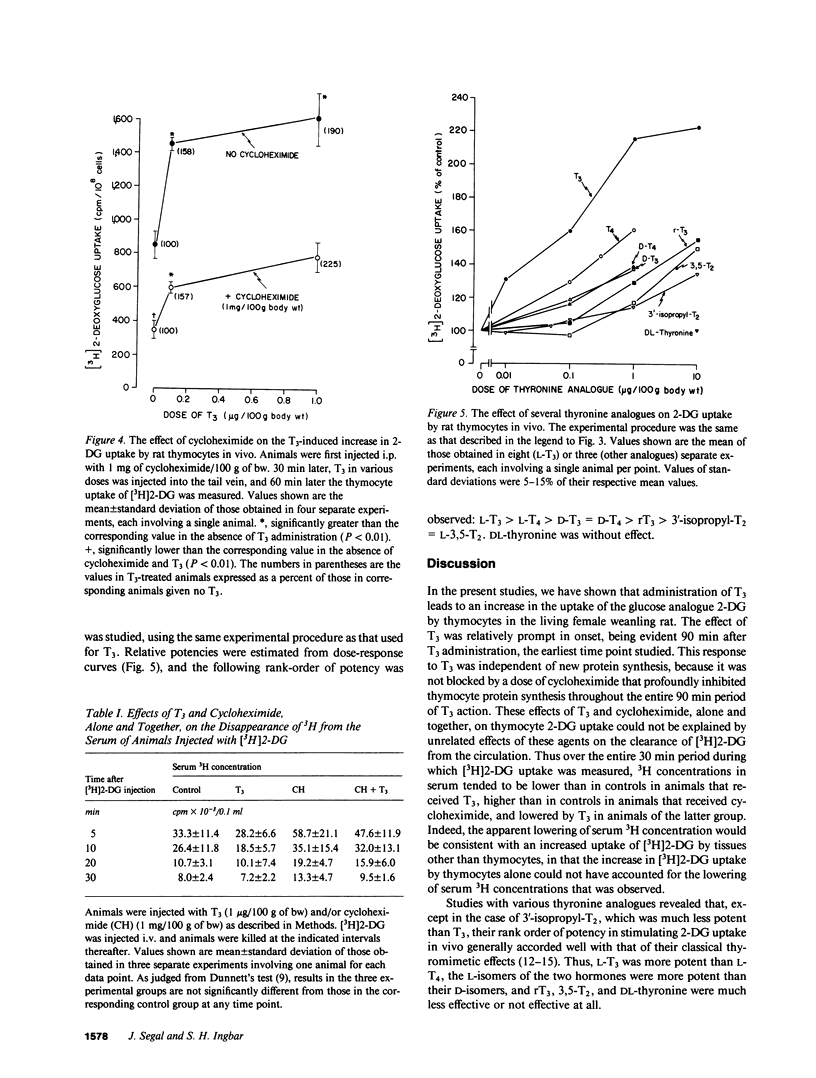
In previous studies we have demonstrated that 3,5,3'-triiodothyronine (T3) in vitro produces a prompt increase in the uptake of the sugar analogue 2-deoxyglucose (2-DG) by freshly isolated rat thymocytes. This effect is prompt, being evident at 20 min after addition of T3, is independent of new protein synthesis, and can be elicited by physiologic concentrations of the hormone. In the present studies, we have sought to determine whether physiologic doses of T3 are capable of inducing an increase in 2-DG uptake in the thymocytes of the living animal. Therefore, 26-28-d-old female rats were injected with increasing doses of i.v. T3, followed 60 min later by 3H-labeled 2-DG. 30 min later, animals were killed, thymocytes were isolated, and their 3H content determined. Uptake of [3H]2-DG was increased by T3 in a dose-dependent manner. The lowest effective dose was 10 ng/100 g of body weight (30% above control) and the maximally effective dose 1 microgram/100 g of body weight (116% above control). The effect of T3 was independent of new protein synthesis in that it was not blocked by a dose of cycloheximide that inhibited the incorporation of [3H]leucine into thymocyte protein by 92-95%. Comparable studies with various thyronine analogues revealed the following rank order of potency: L-T3 greater than L-3,5,3'5'-tetraiodothyronine (L-T4) greater than D-T3 greater than or equal to D-T4 greater than L-3,3'5'-triiodothyronine greater than 3'-isopropyl-3,5-L-diiodothyronine (T2) = 3,5-L-T2. DL-thyronine was without effect. These studies indicate that T3 in physiologic doses acts in vivo to increase the uptake of sugar by rat thymocytes by a mechanism that is extranuclear in origin, in that it is independent of new protein synthesis. The findings support the conclusion that the previously demonstrated effects of T3 on thymocyte sugar uptake in vitro, which seem clearly to be mediated at the level of the plasma membrane, have physiologic relevance.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Borges M., Eisenstein Z., Burger A. G., Ingbar S. H. Immunosequestration: a new technique for studying peripheral iodothyronine metabolism in vitro. Endocrinology. 1981 May;108(5):1665–1671. doi: 10.1210/endo-108-5-1665. [DOI] [PubMed] [Google Scholar]
- Brodde O. E., Schümann H. J., Wagner J. Decreased responsiveness of the adenylate cyclase system on left atria from hypothyroid rats. Mol Pharmacol. 1980 Mar;17(2):180–186. [PubMed] [Google Scholar]
- Challoner D. R., Allen D. O. An in vitro effect of triiodothyronine on lipolysis, cyclic AMP-C14 accumulation and oxygen consumption in isolated fat cells. Metabolism. 1970 Jul;19(7):480–487. doi: 10.1016/0026-0495(70)90002-8. [DOI] [PubMed] [Google Scholar]
- Davis P. J., Blas S. D. In vitro stimulation of human red blood cell Ca2+-ATPase by thyroid hormone. Biochem Biophys Res Commun. 1981 Apr 30;99(4):1073–1080. doi: 10.1016/0006-291x(81)90728-2. [DOI] [PubMed] [Google Scholar]
- Duran-Garcia S., Gomez-Nieto J., Fouchereau-Peron M., Padron V. F., Obregon M. J., Morreale de Escobar G., Escobar del Rey F. Effects of thyroid hormones on liver binding sites for human growth hormone, as studied in the rat. Clin Endocrinol (Oxf) 1979 Sep;11(3):275–290. doi: 10.1111/j.1365-2265.1979.tb03075.x. [DOI] [PubMed] [Google Scholar]
- Golde D. W., Bersch N., Chopra I. J., Cline M. J. Thyroid hormones stimulate erythropoiesis in vitro. Br J Haematol. 1977 Oct;37(2):173–177. doi: 10.1111/j.1365-2141.1977.tb06833.x. [DOI] [PubMed] [Google Scholar]
- Goldfine I. D., Smith G. J., Simons C. G., Ingbar S. H., Jorgensen E. C. Activities of thyroid hormones and related compounds in an in vitro thymocyte assay. J Biol Chem. 1976 Jul 25;251(14):4233–4238. [PubMed] [Google Scholar]
- Ishac E. J., Pennefather J. N., Handberg G. M. Effect of changes in thyroid state on atrial alpha- and beta-adrenoceptors, adenylate cyclase activity, and catecholamine levels in the rat. J Cardiovasc Pharmacol. 1983 May-Jun;5(3):396–405. doi: 10.1097/00005344-198305000-00009. [DOI] [PubMed] [Google Scholar]
- KIPNIS D. M., CORI C. F. Studies of tissue permeability. V. The penetration and phosphorylation of 2-deoxyglucose in the rat diaphragm. J Biol Chem. 1959 Jan;234(1):171–177. [PubMed] [Google Scholar]
- Koerner D., Schwartz H. L., Surks M. I., Oppenheimer J. H. Binding of selected iodothyronine analogues to receptor sites of isolated rat hepatic nuclei. High correlation between structural requirements for nuclear binding and biological activity. J Biol Chem. 1975 Aug 25;250(16):6417–6423. [PubMed] [Google Scholar]
- Lawrence W. D., Davis P. J., Blas S. D., Schoenl M. Interaction of thyroid hormone and sex steroids at the rabbit reticulocyte membrane in vitro: control by 17 beta-estradiol and testosterone of thyroid hormone-responsive Ca2+-ATPase activity. Arch Biochem Biophys. 1984 Nov 15;235(1):78–85. doi: 10.1016/0003-9861(84)90256-x. [DOI] [PubMed] [Google Scholar]
- Malbon C. C., Graziano M. P., Johnson G. L. Fat cell beta-adrenergic receptor in the hypothyroid rat. Impaired interaction with the stimulatory regulatory component of adenylate cyclase. J Biol Chem. 1984 Mar 10;259(5):3254–3260. [PubMed] [Google Scholar]
- Malbon C. C., Li S., Fain J. N. Hormonal activation of glycogen phosphorylase in hepatocytes from hypothyroid rats. J Biol Chem. 1978 Dec 25;253(24):8820–8825. [PubMed] [Google Scholar]
- Malbon C. C. Liver cell adenylate cyclase and beta-adrenergic receptors. Increased beta-adrenergic receptor number and responsiveness in the hypothyroid rat. J Biol Chem. 1980 Sep 25;255(18):8692–8699. [PubMed] [Google Scholar]
- Malbon C. C., Moreno F. J., Cabelli R. J., Fain J. N. Fat cell adenylate cyclase and beta-adrenergic receptors in altered thyroid states. J Biol Chem. 1978 Feb 10;253(3):671–678. [PubMed] [Google Scholar]
- McNeill J. H., Brody T. M. The effect of triiodothyronine pretreatment on amine-induced rat cardiac phosphorylase activation. J Pharmacol Exp Ther. 1968 May;161(1):40–46. [PubMed] [Google Scholar]
- Popovic W. J., Brown J. E., Adamson J. W. The influence of thyroid hormones on in vitro erythropoiesis. Mediation by a receptor with beta adrenergic properties. J Clin Invest. 1977 Oct;60(4):907–913. doi: 10.1172/JCI108845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal J., Buckley C., Ingbar S. H. Stimulation of adenylate cyclase activity in rat thymocytes in vitro by 3,5,3'-triiodothyronine. Endocrinology. 1985 May;116(5):2036–2043. doi: 10.1210/endo-116-5-2036. [DOI] [PubMed] [Google Scholar]
- Segal J., Coppens A., Ingbar S. H. The effect of thyroid status on the calmodulin content of several tissues in the rat. Endocrinology. 1985 May;116(5):1707–1711. doi: 10.1210/endo-116-5-1707. [DOI] [PubMed] [Google Scholar]
- Segal J., Gordon A. The effect 3,5,3'-triiodo-L-thyronine on the kinetic parameters of sugar transport in cultured chick embryo heart cells. Endocrinology. 1977 Nov;101(5):1468–1474. doi: 10.1210/endo-101-5-1468. [DOI] [PubMed] [Google Scholar]
- Segal J., Gordon A. The effects of actinomycin D, puromycin, cycloheximide and hydroxyurea on 3',5,3-triiodo-L-thyronine stimulated 2-deoxy-D-glucose uptake in chick embryo heart cells in vitro. Endocrinology. 1977 Jul;101(1):150–156. doi: 10.1210/endo-101-1-150. [DOI] [PubMed] [Google Scholar]
- Segal J., Ingbar S. H. An immediate increase in calcium accumulation by rat thymocytes induced by triiodothyronine: its role in the subsequent metabolic responses. Endocrinology. 1984 Jul;115(1):160–166. doi: 10.1210/endo-115-1-160. [DOI] [PubMed] [Google Scholar]
- Segal J., Ingbar S. H. Direct and synergistic interactions of 3,5,3'-triiodothyronine and the adrenergic system in stimulating sugar transport by rat thymocytes. J Clin Invest. 1980 May;65(5):958–966. doi: 10.1172/JCI109782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal J., Ingbar S. H. Specific binding sites for the triiodothyronine in the plasma membrane of rat thymocytes. Correlation with biochemical responses. J Clin Invest. 1982 Nov;70(5):919–926. doi: 10.1172/JCI110703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal J., Ingbar S. H. Stimulation by triiodothyronine of the in vitro uptake of sugars by rat thymocytes. J Clin Invest. 1979 Mar;63(3):507–515. doi: 10.1172/JCI109329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal J., Ingbar S. H. Stimulation of 2-deoxy-D-glucose uptake in rat thymocytes in vitro by physiological concentrations of triiodothyronine, insulin, or epinephrine. Endocrinology. 1980 Nov;107(5):1354–1358. doi: 10.1210/endo-107-5-1354. [DOI] [PubMed] [Google Scholar]
- Segal J., Ingbar S. H. Studies of the mechanism by which 3,5,3'- triiodothyronine stimulates 2-deoxyglucose uptake in rat thymocytes in vitro. Role of calcium and adenosine 3':5'-monophosphate. J Clin Invest. 1981 Jul;68(1):103–110. doi: 10.1172/JCI110224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal J., Schwartz H., Gordon A. The effect of triiodothyronine on 2-deoxy-D-(1-3H)glucose uptake in cultured chick embryo heart cells. Endocrinology. 1977 Jul;101(1):143–149. doi: 10.1210/endo-101-1-143. [DOI] [PubMed] [Google Scholar]
- Smith D. E., Gorski J. Extrogen control of uterine glucose metabolism. An analysis based on the transport and phosphorylation of 2-deoxyglucose. J Biol Chem. 1968 Aug 25;243(16):4169–4174. [PubMed] [Google Scholar]
- Tsai J. S., Chen A. Effect of L-triiodothyronine on (--)3H-dihydroalprenolol binding and cyclic AMP response to (--)adrenaline in cultured heart cells. Nature. 1978 Sep 14;275(5676):138–140. doi: 10.1038/275138a0. [DOI] [PubMed] [Google Scholar]
- Vaughan M. An in vitro effect of triiodothyronine on rat adipose tissue. J Clin Invest. 1967 Sep;46(9):1482–1491. doi: 10.1172/JCI105640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Will-Shahab L., Wollenberger A., Küttner I. Stimulation of rat and cat heart adenylate cyclase by triiodothyronine in the presence of 5'-guanylylimidodiphosphate. Acta Biol Med Ger. 1976;35(7):829–835. [PubMed] [Google Scholar]



