Abstract
Uniquely gated by intracellular adenine nucleotides, sarcolemmal ATP-sensitive K+ (KATP) channels have been typically assigned to protective cellular responses under severe energy insults. More recently, KATP channels have been instituted in the continuous control of muscle energy expenditure under non-stressed, physiological states. These advances raised the question of how KATP channels can process trends in cellular energetics within a milieu where each metabolic system is set to buffer nucleotide pools. Unveiling the mechanistic basis of the KATP channel-driven thermogenic response in muscles thus invites the concepts of intracellular compartmentalization of energy and proteins, along with nucleotide signaling over diffusion barriers. Furthermore, it requires gaining insight into the properties of reversibility of intrinsic ATPase activity associated with KATP channel complexes. Notwithstanding the operational paradigm, the homeostatic role of sarcolemmal KATP channels can be now broadened to a wider range of environmental cues affecting metabolic well-being. In this way, under conditions of energy deficit such as ischemic insult or adrenergic stress, the operation of KATP channel complexes would result in protective energy saving, safeguarding muscle performance and integrity. Under energy surplus, downregulation of KATP channel function may find potential implications in conditions of energy imbalance linked to obesity, cold intolerance and associated metabolic disorders.
Keywords: Kir6.2, SUR, ATPase, energy expenditure, diffusion, metabolism, phosphotransfer, structure modeling
Introduction
Modern human genotypes have persisted from the prehistoric era of a hunting/gathering lifestyle, in which episodes of feasting followed by rapid energy storage were succeeded by episodes of endurance exercise to obtain more food (Booth et al., 2002; Himms-Hagen, 2004). Hence, evolutionary pressures, in an environment with ample demand for physical activity and restricted food supply, favored biological systems that conserve energy and optimize energy use (Celi, 2009). The central metabolic principle is the combustion of nutrients (mainly carbohydrates and lipids) to carbon dioxide and water transforming consumed energy to a readily usable form, primarily ATP, which is then utilized by active cellular processes to maintain the whole spectrum of cellular and core body functions concomitantly producing heat, that is, in thermogenesis (Else et al., 2004; Himms-Hagen, 2004; Levine, 2007). The main thermogenic contributors, skeletal and cardiac muscles, account for 10–20% of sedentary daily energy use, and during physical activity increase their energy consumption 20–100 times over basal levels (McArdle et al., 1996). Of note, the relatively high affinity of cellular ATPases for ATP (Läuger, 1991a), compared to the intracellular ATP levels (~6–10 mM; Bittl et al., 1987; Elliott et al., 1989; Weiss et al., 1992), causes these energy consuming systems to operate within a wide range of cellular energy states, virtually independent of intracellular ATP levels. Thus, in active muscles, fuel economy would require an intrinsic controlling mechanism capable of adjusting energy expenses in accord with ATP availability (Zingman et al., 2003). Realization of such mechanism implies the existence of an evolutionarily conserved energy sensor capable of processing changes in the intracellular adenine nucleotide pool. Uniquely gated by intracellular adenine nucleotides, ATP-sensitive K+ (KATP) channel complexes have been traditionally recognized as a suitable candidate for this role.
KATP channels and nucleotide sensing
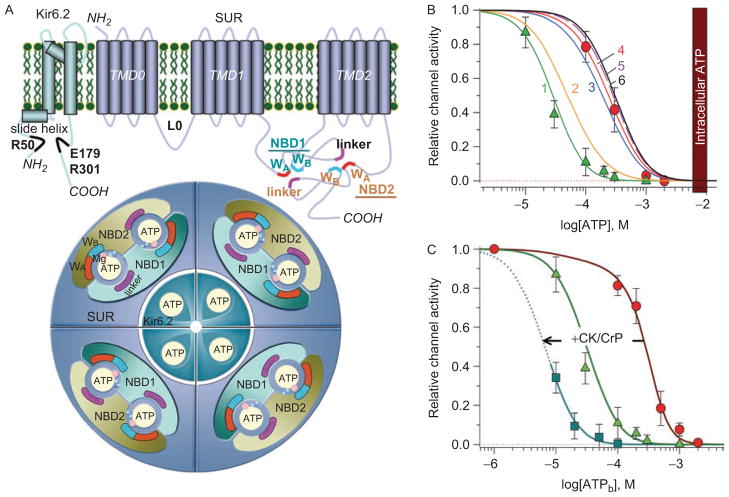
Discovered in the cardiac sarcolemma (Noma, 1983), KATP channels have been found in high density in all metabolically active tissues, where they are formed by tissue-specific multimerization of four pore-forming Kir6.1 or Kir6.2 with four regulatory SUR1 or SUR2 subunits; the latter existing in two isoforms, SUR2A and SUR2B, as a result of alternative splicing (Inagaki et al., 1995; 1996; Yamada et al., 1997; Clement et al., 1997). The progress in understanding KATP channel stoichiometry and structure–function relationships is widely discussed in a number of excellent reviews (e.g. Babenko et al., 1998; Seino, 1999; Moreau et al., 2005; Nichols, 2006; Aittoniemi et al., 2009). Briefly, in cardiac and skeletal myocytes, KATP channel complexes primarily combine Kir6.2 and SUR2A subunits (Inagaki et al., 1996; Lorenz and Terzic, 1999; Lefer et al., 2009). Numerous nucleotide-binding sites within the channel subunits confer a sophisticated nucleotide-dependent gating to KATP channel complexes (Figure 1A). While intracellular ATP keeps KATP channels closed by binding to Kir6.2 (Tucker et al., 1997; Drain et al., 1998; Craig et al., 2008), the effectiveness of this binding can be modulated through MgATP/MgADP interactions with nucleotide-binding domains (NBD1 and NBD2) of the SUR2A subunit (Shyng et al., 1997; Hosy et al., 2007) that harbor an intrinsic hydrolytic activity (Matsuo et al., 1999, 2000; Bienengraeber et al., 2000). Stabilization of MgADP at these binding pockets reduces the pore sensitivity to ATP (Zingman et al., 2001; 2007) favoring K+ efflux.
Figure 1.
Structure and nucleotide-dependent gating of KATP channel complexes. (A) Kir6.2 possesses two transmembrane (TM) pore-forming α-helices and a slide helix, which by lateral movement contributes to the gating mechanism. The putative ATP binding residues are indicated in black. SUR2A is comprised of three bundles of transmembrane-spanning domains and two cytosolic ATP-binding cassettes (ABC), i.e. nucleotide binding domains (NBD1 and NBD2). WA and WB denote the Walker A and Walker B motifs conserved within ABC proteins that, along with the linker regions, are critical for coordination of nucleotides within binding pockets. Each ATP molecule is sandwiched between the Walker motifs of one NBD and the linker region from another NBD. The whole KATP channel complex is formed by four SUR co-assembled with four Kir6.2 subunits, harboring 12 nucleotide binding sites in total. Cofactors of the hydrolytic reaction, Mg2+ (pink) and water molecules (blue), are also indicated. (B) MgADP antagonizes ATP-induced inhibition of cardiac KATP channels. In excised patches from isolated cardiomyocytes, the ATP sensitivity of KATP channels is defined by an IC50 of 27 ± 5 μM in the absence (triangles) versus 270 ± 19 μM in the presence (circles) of 100 μM ADP. At increasing ADP concentrations (curve 1: 0; 2: 10; 3: 50; 4: 100; 5: 500; 6: 1000 μM), following suturation of all binding sites no further reduction in ATP sensitivity of KATP channels can be achieved (adapted from Selivanov et al., 2004). The vertical bar corresponds to 6–10 mM intracellular ATP levels. (C) Concentration–response curves defining ATP-induced KATP channel inhibition in open cell-attached patches, where KATP channels operate in a relatively intact cellular environment, in the absence (circles) and presence (squares) of 1 mM creatine phosphate (CrP). Intracellular ATPase activities define the right-shift of the concentration-response curve (IC50 ~300 μM) in the open-cell attached configuration, relative to the values measured in excised patches (triangles), and can be effectively left-shifted to an IC50 ~10 μM by activation of the ATP-regenerating endogenous creatine kinase (CK) when the reaction substrate (CrP) is applied. By itself, CrP has no significant effect on KATP channel activity (Abraham et al., 2002).
There is a common belief that the nucleotide-gated K+ flux is the central energy controlling mechanism associated with sarcolemmal KATP channels. Indeed, due to the properties of weak inward rectification, K+ flux through KATP channels can accelerate repolarization of action potentials (AP) by driving membrane potential towards K+ equilibrium, opposing thereby associated energy expenses. The operation of KATP channels in response to reduced ATP and increased ADP levels would thus limit processes occurring during the AP propagation, including not only the operational time of L-type Ca2+ channels and associated Ca2+ release from the sarcoplasmic reticulum, but also the concomitant activity of the Na+/Ca2+ exchanger, myosin-, Ca2+- and Na+/K+-ATPases (Nichols and Lederer, 1991; Gong et al., 2000; Zingman et al., 2002; 2007; Cifelli et al., 2007; 2008). This conventional view, although apparently the most rational, still requires reconciliation of the established IC50 for channel inhibition by ATP (in the range of 20–50 μM) with millimolar intracellular ATP levels (Elliot et al., 1989; Nichols and Lederer, 1991; Alekseev et al., 2005). The MgADP-bound state of SUR2A expands the range for ATP inhibition (IC50 ~300 μM), which however still remains far below actual intracellular ATP levels (Figure 1B). On saturating all ADP-binding sites, at ADP >150–300 μM, no further reduction in ATP sensitivity can be achieved (Lederer and Nichols, 1989; Weiss et al., 1992; Abraham et al., 2002; Selivanov et al., 2004). Thus, at 6–10 mM of cytosolic ATP, MgADP-dependent KATP channel regulation would not be translated into channel opening, questioning the homeostatic role of KATP channels.
It is possible that the sensitivity of KATP channels towards ATP can be reduced in the intact cell by additional regulators. In fact, modulation of KATP channels by acidic pH has been proposed and, although found to be very inconsistent among tissues (skeletal versus cardiac muscles) as well as animal species (mammalian versus frog skeletal muscles), could contribute to channel activation, provided that significant changes in intracellular nucleotide levels occur under vigorous metabolic stress (Cook and Hales, 1984; Lederer and Nichols, 1989; Davies et al., 1992; Koyano et al., 1993; Vivaudou and Forestier, 1995; Allard et al., 1995). In addition, membrane negatively charged phosphatidylinositol phosphates (i.e. PIP2, PIP3), when applied exogenously to membrane patches, render KATP channels insensitive to ATP, suggesting phosphorylation/dephosphorylation-induced changes of membrane phospholipid composition as a mechanism for physiological regulation (Baukrowitz et al., 1998; Shyng and Nichols, 1998; Song and Ashcroft, 2001). The variability in membrane phospholipid content may, in particular, explain the inconsistency in the response to ATP of native KATP channels identified in excised patches (Findlay and Faivre, 1991; Shyng and Nichols, 1998), indicating a contribution of membrane charges brought by phospholipids in setting nucleotide-sensitivity of the channel pore (Nichols, 2006). However, activation of the creatine kinase/creatine phosphate (CK/CrP) system, that by regenerating ATP should facilitate the phosphorylation of phospholipids, effectively enhances ATP-induced channel inhibition with IC50 ~10 μM (Figure 1C; Nichols and Lederer, 1990; Bienegraeber et al., 2000; Abraham et al., 2002), which is even below the minimal IC50 values that could be achieved in the presence of lipid kinase inhibitors (Tarasov et al., 2006). Conversely, under decreased ATP/ADP ratio, reduction of lipid kinases phosphorylation rates and the resulting drop of KATP channel activity would be inconsistent with stress-induced activation of KATP channels, indicating that coupling of KATP channels with cellular energetics is mediated by adenine nucleotide signaling (Tarasov et al., 2006).
While adenine nucleotides have been recognized as the principal regulators of KATP channels, there is still an obvious lack of solid biophysical and biochemical foundations of how energy signals could be generated and transmitted within the intracellular milieu where metabolic systems are set to buffer nucleotide levels. Hence, the quest to resolve a simple, at first glance, question regarding the incongruity between the nucleotide sensitivity of KATP channel sensors and the actual, well-maintained intracellular nucleotide levels, involves several important concepts that will be discussed herein.
Nucleotide signaling in a compartmentalized cellular environment
Lack of correlation between significant changes in bulk nucleotide levels and KATP channel openings during metabolic challenge (Elliot et al., 1989; Decking et al., 1995; Zingman et al., 2002) has given rise to the suggestion that sarcolemma-associated ATPases could depress local ATP concentration, setting a nucleotide ratio at the channel site distinct from cytosolic levels (Nichols and Lederer, 1991; Weiss et al., 1992; Abraham et al., 2002; Selivanov et al., 2004). This is in accord with the recent finding that KATP channels complement specific membrane complexes combining Na+/K+ ATPase, voltage-gated Na+, K+ channels and the Na+/Ca2+ exchanger, and are anchored through ankyrins to the spectrin-actin based membrane cytoskeleton (Terzic and Kurachi, 1996; Hashemi et al., 2009; Kline et al., 2009). Such compartmentalized complexes appear to function in a microenvironment where local ion and nucleotide contents may be distinct from average cytosolic levels.
Intracellular energy consumption has traditionally been viewed as a process whereby freely diffusing high-energy phosphates are consumed where needed throughout the cytoplasm. However, this concept underwent an essential reevaluation (Saks et al., 2009) since, for instance in the heart, selective inhibition of anaerobic glycolysis versus mitochondrial oxidative phosphorylation has revealed a differential efficacy of these systems in supporting specific membrane versus contractile functions, which cannot be explained by changes in total transcellular high-energy phosphate levels (Doorey and Barry, 1983; Weiss and Hiltbrand, 1985; Kaasik et al., 2001). As the cytoplasm is now considered to be far from a “well-mixed bag”, in which high-energy phosphates diffuse readily to various cellular ATPases (Weiss and Korge, 2001), intracellular compartmentalization could in principle offer a rational basis for nucleotide-dependent KATP channel gating, provided that significant diffusional limitations exist between cellular microdomains.
The value of the diffusion coefficient for ATP in the cytosol, obtained using 31P assisted NMR (e.g. Kinsey and Moerland, 2002), implies a rather regular value of nucleotide diffusion in free space (~10−6 cm2 s−1). However, within the intracellular microenvironment where the cytoskeleton tethers proteins into macro-complexes (Hashemi et al., 2009) the free diffusional space is extremely limited, restricting metabolite mobility (Weiss and Lamp, 1987; Weiss and Korge, 2001; Kaasik et al., 2001). The existence of significant diffusion limitations has been suggested for different subcellular compartments (Saks et al., 2001) including the submembrane “fuzzy space” with a highly restricted diffusion of molecules as small as Na+ ions (Lederer et al., 1990). This “fuzzy space” would further restrict the mobility of bigger molecules to an even higher extent, and, in the presence of local ATPases, could set submembrane nucleotide concentrations distinct from bulk levels. In fact, assuming that bulk ATP is 7 mM and sarcolemmal ATPase flux is 4.7 × 10−6 μmol cm−2 s−1, as was estimated in working hearts based on 18O-assisted 31P NMR (Weiss et al., 1992; Pucar et al., 2001; Abraham et al., 2002), even a minimal contribution of sarcolemmal KATP channels to cardiomyocyte excitability, 1% of the total membrane population (Lederer and Nichols, 1989), would require a ~4 mM drop of submembrane ATP levels. Such ATP gradient can be only achieved at an apparent diffusion coefficient of 2.3 × 10−11 cm2 s−1 (Abraham et al., 2002), which is already 100,000 times lower than nucleotide diffusion in the cytosol. Notably, this is likely an under-estimation of the diffusion barrier, which could be even stronger in myocytes where components of the glycolytic system and adenylate kinase (AK) have been identified in the vicinity of KATP channels (Weiss and Lamp, 1987; Elvir-Mairena et al., 1996; Carrasco et al., 2001; Janssen et al., 2004). By regenerating local ATP, these systems could further diminish the nucleotide gradients between cellular compartments. Considering operation of 1% of the KATP channel population in the presence of AK alone, a 4 mM ATP gradient between the bulk and the submembrane space, at the same ATPase activity, would require an apparent diffusion coefficient as low as 1.6 × 10−11 cm2 s−1 (Selivanov et al., 2004). Although such extremely high diffusion restrictions have been obtained with certain assumptions, the five orders-of-magnitude difference of this apparent value from the coefficient for free diffusion of nucleotides in the cytosol cannot be compensated by varying several times the diffusion distance, i.e. the thickness of the submembrane space (0.2 μm), ATPase activity, bulk ATP or other parameters. Furthermore, these values for apparent diffusion coefficient are in agreement with estimates (~10−9–10−13 cm2 s−1) performed for cAMP and ATP in the subsarcolemmal space implementing alternative approaches (Rich et al., 2000; Kabakov, 1998). A number of observations thus indicate the existence of diffusional hindrances at the submembrane space sufficient to seclude KATP channels from bulk metabolic pathways, questioning a mechanism that is capable of shunting the existing barrier and secure communication between membrane complexes and cellular energy pools.
Nucleotide signal transmission and processing
Coupling of KATP channel proteins with cellular energetics is underscored by spatial association of the channel subunits with phosphotransfer (AK and CK) and glycolytic enzymes (Weiss and Lamp, 1987; Jovanović et al., 2007) that are capable of facilitating energy transfer (Nichols and Lederer, 1990; Elvir-Mairena et al., 1996; Carrasco et al., 2001; Abraham et al., 2002; Crawford et al., 2002; Dzeja and Terzic, 2009), and in association with cytoskeleton-bound complexes (Hashemi et al., 2009; Kline et al., 2009), could form a complete cellular metabolic unit.
The role of these reactions in the transmission of nucleotide signals may not be so evident. In particular, the phosphotransfer reaction, ADP + CrP ↔ Cr + ATP catalyzed by CK, phosphorylates ADP to ATP by transferring the phosphate group from creatine phosphate (CrP) producing creatine (Cr). The high value of the equilibrium constant for this reaction, KCK = 160 (Lawson and Veech, 1979), indicates that augmentation of total ATP turnover would deplete the intracellular pool of CrP to a greater extent than the pool of ATP. Hence, CK buffers changes in ATP and ADP levels throughout the cell, which, at first glance, contradicts the concept of nucleotide signaling. On the other hand, such buffering could be interpreted as “shuttling” of high-energy phosphates between sites of energy production and consumption (Dzeja and Terzic, 1998) or as a mechanism of nucleotide carrying or facilitated ATP diffusion flux (Meyer et al., 1984), which implies that delivery of ATP from one cellular compartment to another is accompanied by diffusion of high-energy phosphate equivalents (CrP), which then are locally involved in CK-catalyzed ATP synthesis.
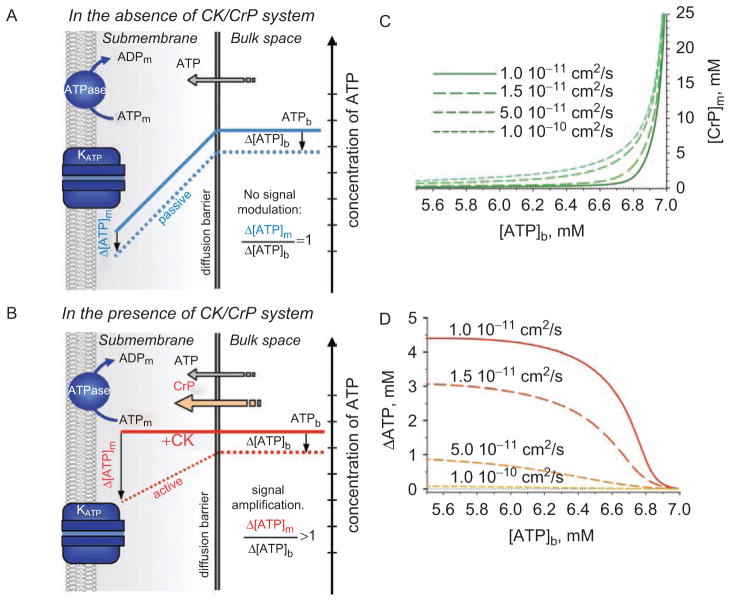
Theoretical analysis of the relationships between metabolite concentrations and fluxes in the presence of strong diffusion hindrances revealed that the CK and AK reactions, in contrast to their role to void changes in cytosolic ATP levels, may acquire properties of amplification and attenuation of nucleotide signals, respectively (Selivanov et al., 2004). Such modulation of nucleotide gradients at a diffusion barrier can be readily understood from the basic properties of phosphotransfer reactions. Assuming absence of the CK system, one may expect that a difference between bulk and submembrane ATP levels imposed by membrane ATPases is independent of bulk ATP, i.e. a drop in bulk ATP (Δ[ATP]b) would be passively followed by the same drop of submembrane ATP levels (Δ[ATP]m), with no signal modulation: Δ[ATP]m = Δ[ATP]b (Figure 2A, blue dashed line). In the presence of CK, the substantial levels of intracellular CrP maintain nucleotide content, voiding the concentration gradient between compartments. However, due to the high value of the equilibrium constant of the CK reaction, even a small reduction of bulk ATP would result in a significant drop of bulk CrP and, thereby, a reduction of CrP flux over the diffusion barrier (Figures 2B–2D). A fall of CrP in the submembrane space would result in efficient amplification of an ATP drop in this compartment: Δ[ATP]m > Δ[ATP]b (Figure 2B).
Figure 2.
Nucleotide signals at a strong diffusion barrier secluding submembrane space. (A) In the absence of the creatine kinase/creatine phosphate (CK/CrP) phosphotransfer system, local ATPase activities set a nucleotide gradient between cellular compartments (blue solid line). A drop in bulk ATP levels (Δ[ATP]b) is passively followed by changes in submembrane concentrations (Δ[ATP]m), i.e. the gradient remains unchanged (blue dotted line) with no signal modulation. (B) In the presence of CK/CrP system, the facilitated diffusion catalyzed by CK virtually voids the concentration gradient (red solid line), but actively modulates (i.e. amplifies) changes in submembrane nucleotide content (Δ[ATP]m, red dotted line) that exceed the deviation of bulk ATP. (C and D) Submembrane concentrations of creatine phosphate ([CrP]m) and ATP gradients (ΔATP) between bulk and submembrane space, respectively, calculated at the different values of apparent diffusion coefficients (system of equations 13, Selivanov et al., 2004) following a drop of bulk ATP ([ATP]b). Calculations were performed with 7 mM total nucleotide content; 40 mM total Cr/CrP level; sarcolemmal ATPase flux 4.7·10−6 μmol/cm2/s; Δx = 0.2 μm and KCK=160.
This analysis also has predicted that as long as CK effectively supports [ATP]m the contribution of AK in setting nucleotide gradients is negligible. Only when CK fails to maintain [ATP]m, switching on the AK reaction will support [ATP]m attenuating submembrane ATP changes (Selivanov et al., 2004; Alekseev et al., 2005). Such a profile of interplay between phosphotransfer systems is in agreement with experimental observations that reduction of CK flux is accompanied by up-regulation of AK-mediated phosphotransfer (Janssen et al., 2000; Pucar et al., 2001).
While these new properties for phosphotransfer reactions were drawn based on a submembrane diffusion restriction that was theoretically derived considering minimal contribution of KATP channels to membrane excitability, in a real intact cell nucleotide signal modulation would depend on the actual value of the subsarcolemmal diffusion hindrance (Figures 2C–2D), which remains undefined. In general, the concept of energy signaling in a compartmentalized environment suggests that, at diffusion barriers secluding cellular compartments, bioenergetic trends can be amplified and then processed by membrane nucleotide sensors. Therefore, by virtue of processing even minor changes in cellular nucleotide content (Abraham et al., 2002; Selivanov et al., 2004), KATP channels could decode cellular metabolic dynamics and control muscle energy expenditure not only under stress conditions but also at any level of activity, continuously promoting body energy conservation.
Membrane nucleotide sensing and optimal muscle activity thermogenesis
This homeostatic role of KATP channels has been recently demonstrated by implementing indirect calorimetry measurements in normal and KATP channel deficient mice obtained by targeted disruption of the KCNJ11 gene encoding the pore-forming Kir6.2 subunit (Kir6.2-KO) and in Tg[MyoD-Kir6.1AAA] mice with skeletal muscle specific ablation of KATP channel activity (Alekseev et al., 2010). Indeed, mice lacking KATP channels demonstrate a higher rate of oxygen consumption under sedentary conditions, reflecting increased energy expenditure and thereby heat production. In spite of the established role of KATP channels in glucose homeostasis (Miki et al., 1998; Aguilar-Bryan et al., 2001; Ashcroft, 2005) and possibly in appetite control (Spanswick et al., 1997), while unchallenged, mice lacking KATP channels did not exhibit a deficit in caloric intake or blood energy substrates (Alekseev et al., 2010). The analysis of calorimetric data at a low exercise regimen further revealed that the KATP channel deficit raises energy use through an extra energy cost of physical activity, which is primarily determined by skeletal and cardiac muscle performance (Ghanassia et al., 2007). The accelerated metabolism profile of the KATP channel-deficient animal models may, in certain aspects, resemble metabolic states associated with hyperthyroidism (Sugden et al., 1992; Chen-Zion et al., 1995) or overexpression of mitochondria uncoupling proteins in skeletal muscles (Li et al., 2000). Although sharing with hypermetabolic phenotypes the properties of enhanced insulin sensitivity and glucose uptake (Miki et al., 2002; Minami et al., 2003), lack of sarcolemmal KATP channel function does not affect mitochondrial oxidative phosphorylation capacity or nucleotide levels in myocytes (Zingman et al., 2002; Alekseev et al., 2010). Maintenance of phosphocreatine and nucleotide pools thus imposed mobilization of glycogen and fat stores resulting in the elevation of both muscle and whole body thermogenesis. The enhanced energy use in KATP channel-deficient cardiac and skeletal muscles resulted in limited body weight gain and restrained physical endurance (Alekseev et al., 2010). In this way, KATP channels emerge as an important evolutionary preserved energy-sparing system that defines muscle thermogenic response through optimization of energy use.
This finding postulates that the operational principle of this system is the feedback processing of bioenergetic cues into constraints of membrane excitability. Indeed, measurement of oxygen consumption in isolated hearts revealed that increasing pacing rates produces a moderate elevation of energy expenditure in line with KATP channel-dependent shortening of action potential duration (Alekseev et al., 2010). Lack of compensatory KATP channel-driven shortening of action potentials in KATP channel-deficient hearts was associated with significant elevation of oxygen consumption. However, paradoxically, even at the lowest workload rate (460 bits per min), when wild-type and knockout groups demonstrated indistinguishable durations of action potentials, hearts lacking KATP channels still consumed more oxygen compared to normal hearts (Alekseev et al., 2010). One possible explanation is that microscopic changes in individual action potential duration, while undetectable by MAP (monophasic action potential) recording, as implemented in the study, when integratively accumulated during thousands of cardiac cycles may contribute to energy saving. In this way, one may anticipate an advantage of progressive membrane potential shortening that may be achieved by a more robust activation of KATP channels. However, transgenic mice expressing KATP channels with a 40–100-fold reduced ATP sensitivity revealed no benefit from ischemic preconditioning, no improvement of functional recovery following metabolic inhibition along with no protection against intracellular Ca2+-induced rigor contracture (Koster et al., 2001; Rajashree et al., 2002), i.e. phenomena that are also characteristic of KATP channel-deficient hearts (Zingman et al., 2002; Gumina et al., 2003; 2007). Taken together these data suggest that a molecular mechanism responsible for the KATP channel-driven feedback control of cellular energy balance remains unresolved.
Mechanisms for the energy-saving operation of KATP channels
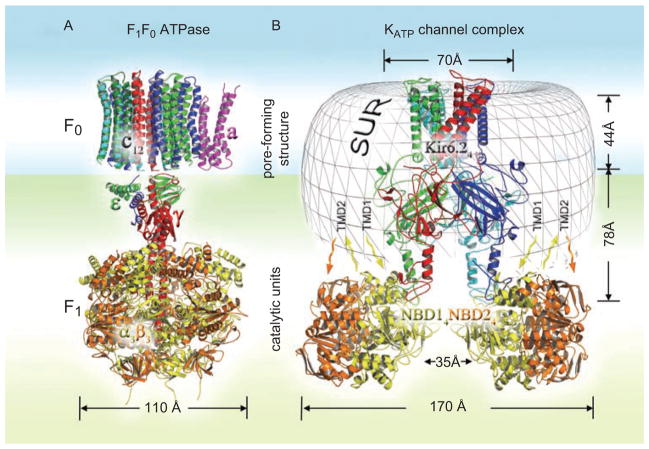
The known structure–function properties of KATP channels suggest at least two possible mechanisms by which they could affect cellular energy balance. The first, mentioned above, is the constraint of cellular energy expenses on myofibrillar contraction and re-sequestration of Ca2+ and Na+ ions by opposing membrane depolarization (Nichols and Lederer, 1991; Gong et al., 2000; Zingman et al., 2002; 2007; Cifelli et al., 2007; 2008; Alekseev et al., 2010). The second could be associated with the enzymatic properties of the regulatory SUR subunit of the channel complex and resembles the mechanism realized in a number of molecular pumps and ABC transporters. Although the molecular mechanisms of operation of different ATPases are not identical, the general principle of coupling between ion flux and associated catalytic activity is underscored by the ability of ATPase pumps to synthesize ATP using ion movement down the electrochemical gradient (Boyer et al., 1973; Läuger, 1991b; Boyer, 1997). For instance, in the F0F1 mitochondrial ATPase, due to the reversibility of this catalytic reaction, proton permeation through the F0 pore-forming subunit reduces the affinity of the F1-ATPase towards synthesized ATP (Boyer, 1997; Kagawa et al., 1997; Figure 3A). Furthermore, the reversibility of hydrolysis associated with the functioning of some ABC multidrug transporters has been demonstrated in response to downhill substrate uptake (Balakrishnan et al., 2004). In fact, even though SUR belongs to the ABCC subfamily of ABC transporters and also exhibits hydrolytic activity measured in isolated SUR proteins (Matsuo et al., 1999, 2000), in truncated NBDs fusion constructs (Zingman et al., 2001; Bienengraeber et al., 2004; Masia et al., 2005; de Wet et al., 2007; Park et al., 2008; Park and Terzic, 2010), as well as in purified recombinant Kir6.2/SUR1 complexes (Mikhailov et al., 2005), it is still unknown whether the identified intrinsic catalytic reaction possesses properties of reversibility. So far, the hydrolysis reaction rate measured in NBD fusion constructs was found to be insensitive to inorganic phosphate (Bienegraeber et al., 2004), but the catalytic properties of isolated NBDs may not necessarily correspond to properties within the whole complex. By analogy to other ATPases, the reversibility of interconversions between ATP- and ADP-Pi bound states of SUR would underscore the independence of the specific catalytic reaction upon external energy input (Boyer, 1997). Therefore, there is a theoretical possibility that K+ flux through the channel pore in response to MgADP binding to SUR could facilitate conformational transitions within SUR to release synthesized ATP. Although speculative, the latter suggestion may shed light upon some apparently unorthodox structure–function relationships, primarily associated with the unsettled role of the SUR ATPase in the regulation of K+ permeation through the channel pore, which does not require energy input, as well as upon the role of the apparently redundant nucleotide-binding sites within the constitutive complex subunits (Alekseev et al., 2005; Nichols, 2006; Aittoniemi et al., 2009).
Figure 3.
Topological features of F1F0 ATPase and KATP channel complexes. (A) Molecular architecture of the rotary motor in F1F0 ATPase (PDB: 1C17 and 1E79) comprises the pore forming F0 unit of 12 c and a subunits (magenta) as well as the catalytic nucleotide-binding F1 unit of three α (yellow) and three β (orange) subunits connected by the axle of ε and γ subunits (stator is not shown). (B) Molecular architecture of KATP channels is presented by four full-length Kir6.2 subunits (red, blue, cyan, green) embedded in the unresolved transmembrane domain (TMD0-TMD1-TMD2) structure (gray mesh). Catalytic quaternary structure of four NBD1 (yellow) and four NBD2 (orange) subunits reveals a 35 Å inner cleft (Park and Terzic, 2010) dictating the putative plane of NBDs and pore interaction at the bottom of the Kir6.2 cytoplasmic domain.
What evidence could favor the ATP-synthesis hypothesis? Although the KATP channel complex apparently cannot be associated with any known types of ATPase it shares some structural topology with A-/F-/V- or K+ translocating P-type ATPases, which are composed of distinct oligomeric segments including a catalytic unit and a membrane-embedded component, which functions in ion conduction (Müller and Grüber, 2003; Bramkamp et al., 2007; Figures 3A and 3B). Indeed, the combination of molecular simulation with single particle electron microscopy and small angle X-ray scattering (SAXS) revealed that, as in other macromolecular transporters, the ion-conducting Kir6.2 subunit is wrapped by the associated SUR unit (Mikhailov et al., 2005; Hosy et al., 2007; de Wet et al., 2007; Park and Terzic, 2010). Although the topology of cytoplasmic NBD1 and NBD2 is a matter for debate, recent SAXS analysis revealed that the inner cleft delineated by four NBDs (~35 Å) was narrower than the predicted near-to-membrane dimension of the Kir6.2 structure (~55 Å). Therefore, four pairs of NBD1–NBD2 dimers could be located at the bottom of the cytoplasmic Kir6.2 terminus (Park and Terzic, 2010), thus forming a macromolecular organization that is typical for other ATPase complexes (Figure 3B). Lack of tight physical association between NBD1/NBD2 and Kir6.2 (Schwappach et al., 2000; Mikhailov and Ashcroft 2000) provides the basis for a flexible mechano-molecular interaction between conformational transitions of Kir6.2 and nucleotide-binding TMD1-NBD1/TMD2-NBD2 cytoplasmic regions (Chan et al., 2003; Rainbow et al., 2004; Dupuis et al., 2008). Furthermore, the catalytic activity of SUR is sensitive to classical ATPase inhibitors such as ortho-vanadate or beryllium fluoride mimicking organic phosphates in binding pockets, indicating the ability of the SUR binding sites to coordinate Pi with prebound MgADP (Zingman et al., 2001). The properties of stable nucleotide binding resulting from cooperative interaction of NBDs in SUR (Ueda et al., 1999; Matsuo et al., 2000; Karger et al., 2008) may also favor the reversibility of the catalytic reaction. There is evidence that allosteric coupling between constitutive channel subunits confers not only SUR-mediated Kir6.2 channel gating but also a reversed effect of Kir6.2 on the catalytic rate of the SUR subunit (Aittoniemi et al., 2009). Of note, incidents of anoxia-induced nucleotide oscillations in whole cardiomyocytes were detected only at the outward KATP current set by a positive (+40 mV) holding potential but not at a negative holding potential (−80 mV), which may indicate a possible contribution of K+ flux-dependent ATP synthesis by KATP channels to the cellular nucleotide pool (Ganitkevich et al., 2010).
The main argument against this hypothesis appears to be the proximity of membrane potentials in resting myocytes to the potassium equilibrium, where no net K+ fluxes are expected, questioning the proposed mechanism for ATP synthesis. However, during propagation of action potentials, where major cellular energy expenses occur, K+ efflux may contribute to the process. The proton-motive force implemented in ATP synthesis can be estimated as ΔμH+ = 2.3RTΔpH + FΔΨ, where ΔpH and ΔΨ denote proton gradient (~0.8) and mitochondria membrane potential (~160 mV), respectively. Analogously, a sarcolemmal potassium-motive force can be defined as: ΔμK+ = 2.3RT·log[K]i/[K]o + FΔE, where logarithm of the ratio between intracellular and extracellular K+ denotes potassium gradient (ΔpK ~1.4) and ΔE represents a shift of membrane potential from K+ equilibrium during action potentials (60–80 mV at the phase of plateau). Regardless of the particular synthetic mechanism, direct comparison of ΔpH and ΔΨ with ΔpK and ΔE, respectively, without specific constant values, reveals close estimations for ΔμH+ and ΔμK+ indicating the feasibility of a K+ electromotive force for the suggested KATP channel ATP-synthase activity. This plain energy analysis also indicates that the direct measure of sarcolemmal ATP production by KATP channel complexes could be performed only at a shift of the membrane potential away from the K+ equilibrium, typical for resting excitable cells, and would require the combination of a reliable nucleotide reporter with the maintenance of constant electromotive force to secure a substantial K+ efflux through the channel pore, a challenging experimental task.
A synthetic function of the KATP channel complex in general does not contradict the established nucleotide-dependent channel gating and may provide an additional basis for understanding specific nucleotide interactions with constitutive channel subunits. Indeed, the proposed synthetic mechanism implies that binding of MgADP to NBD2 in SUR allosterically antagonizes inhibition of the channel pore by ATP conferring K+ efflux to switch SUR conformation to a low-affinity ATP bound state. Thus, the micromolar range of pore inhibition by ATP, at millimolar intracellular levels, becomes evidently a prerequisite to prevent futile K+ efflux and explains why a significant KATP channel current has not been detected at physiological stimuli. In this way, the question regarding channel opening at millimolar intracellular ATP levels may seem immaterial, because at basal physiological states the proposed synthetic function mandates an exclusive KATP channel complex response to ADP regardless of ATP levels, in accord with the traditional signaling mechanism based on receptor–ligand interaction. From this point of view, the amplification and tuning of nucleotide signals in the submembrane space may be considered as an ADP signaling process that switches on KATP channel complexes to compensate a deficit of local ATP in order to support the operation of ATPases in sarcolemmal metabolic units. Finally, the proposed intrinsic synthetic activity of KATP channel complexes does not deny the energy saving imparted by KATP channel-induced shortening of action potentials, because K+ permeation coupled to catalytic machinery would manifestly restrain membrane excitability at significant K+ efflux under severe energy deficit.
A generalized homeostatic role for KATP channels
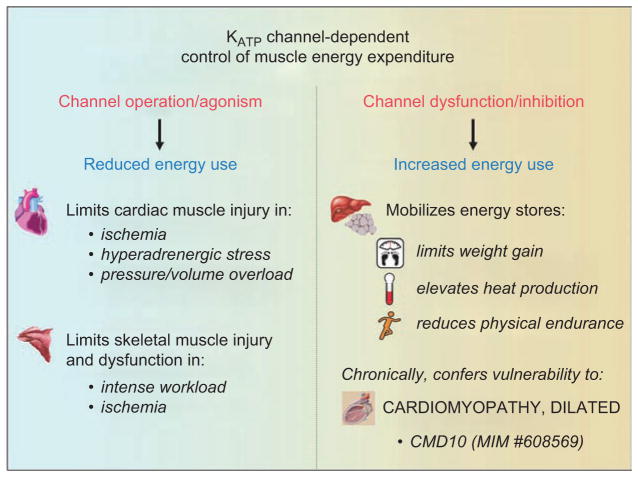
The recently documented KATP channel-driven control of energy-consuming processes under physiological states (Alekseev et al., 2010) extrapolates the homeostatic role of channel complexes to a wider range of environmental cues affecting physiological well-being. While activation of KATP channels can be implemented in limiting energy consumption by cellular ATPases during metabolic insult, interruption of KATP channel-dependent control of ATPase activities could be useful in elevating muscle energy expenditure and thereby total body thermogenesis under excessive energy input and impaired physical performance. Hence, intercommunication between cellular energetics and KATP channels allows the suggestion of a paradigm shift from the protective channel-driven shortening of action potentials towards the bidirectional (up/down) KATP channel-dependent control of muscle thermogenesis. Metabolic adaptation in the absence of KATP channels was accompanied by elevated rates of carbohydrate and lipid utilization compared to WT (Alekseev et al., 2010), which, in line with proteomic data, alters nearly 9% of all detected protein species, predominantly bioenergetic complexes including several dehydrogenases and transferases involved in the TCA (tricarboxylic acid) cycle or fatty acid β-oxidation pathway (Arrell et al., 2009). In this way, inefficient cellular energetics induced by KATP channel malfunction seems to be a common denominator for a spectrum of pathological remodeling and metabolic disorders (Figure 4).
Figure 4.
Bi-directional control of muscle energy expenditure by KATP channel complexes. Active sarcolemmal KATP channels constrain energy use and protect striated muscles during excessive energy demand. Downregulation of channel function results in increased mobilization of energy resources limiting body weight gain and increasing heat production.
Energy saving has been typically assigned to a protective response of sarcolemmal KATP channels under energy deficits, such as acute ischemia (Gumina et al., 2003; Moses et al., 2005), sympathetic distress (Zingman et al., 2002; Kane et al., 2004; Liu et al., 2004; Reyes et al., 2007; 2009a) or muscle fatigue (Renaud et al., 2002). It has been also demonstrated that KATP channels play a crucial role in other syndromes of energy overload. In particular, KATP channels influence outcome in sympathetic overstimulation such as in endurance exercise or cocaine intoxication (Zingman et al., 2002; Kane et al., 2004; Reyes et al., 2007; 2009a) as well as pressure and volume overload (Kane et al., 2006; Yamada et al., 2006). Defects in nucleotide-dependent channel gating and/or disturbed communication between KATP channels and intracellular energy routes could all be envisioned as molecular mechanisms contributing to cardiac remodeling and impaired performance induced by energy imbalance (Hodgson et al., 2003; Kane et al., 2005; Zingman et al., 2007). Indeed, mutations in the regulatory SUR2A subunit predispose to dilated cardiomyopathy and adrenergic atrial fibrillation (Bienengraeber et al., 2004; Olson et al. 2007; Olson and Terzic, 2010), while a common single nucleotide polymorphism in the pore-forming Kir6.2 subunit has been associated with increased left ventricular size in the setting of hypertension (Reyes et al., 2008) and with reduced heart rate response to exercise in patients with heart failure (Reyes et al., 2009b).
The previously unrecognized role for sarcolemmal KATP channels in the continuous regulation of cellular energy use under non-stressed physiological states suggests a potentially advantageous KATP channel downregulation under conditions of energy surplus, such as in obesity (Alekseev et al., 2010). The protective function of KATP channels against body weight gain at the expense of physical endurance would be in accord to inefficient energy use by cardiac and skeletal muscles lacking the KATP channel energy-controlling mechanism. Downregulation of KATP channel function specifically in skeletal muscle, despite exposing this tissue to risk of injury, under certain circumstances may have a net benefit. Indeed, the consequences imposed on health by morbid obesity are so severe that current strategies are associated with risks that are deemed acceptable in order to achieve weight loss or control. Moreover, the consequences of KATP channel downregulation on muscle fiber integrity were found as a result of intense exercise and were not identified in control sedentary animals (Thabet et al., 2005), i.e. under conditions consistent with the population at greatest risk for obesity.
An area of investigation unveiled by an energy-management role of KATP channels is that of the involvement of the channel complex in temperature control. By setting exercise and non-exercise activity thermogenesis (Alekseev et al., 2010), sarcolemmal KATP channels could also contribute to heat production during acute cold exposure. As part of a peripheral system aimed at controlling muscle energy economy, it is conceivable that KATP channels, downregulated at reduced ambient temperature, could increase thermogenesis during muscle shivering, thus elevating heat to maintain core body temperature. While this hypothesis remains to be tested, recent data suggests that increasing muscle-specific thermogenesis confers protection against acute cold exposure (Brand and Esteves 2005; Wijers et al., 2008), setting the basis for possible KATP channel involvement in thermoregulation.
Concluding remarks
Sarcolemmal KATP channel complexes have been recognized as important components of an evolutionarily conserved biological system aimed at energy conservation and optimization of energy use (Alekseev et al., 2010). This recently acquired physiological function of KATP channels in striated muscles, beyond the commonly accepted response to severe energy deficit, invites the reevaluation of the conventional view on the role, regulation and therapeutic implication of sarcolemmal KATP channels. Although the central feature underlying this homeostatic role is the established sensitivity of KATP channels toward changes in intracellular adenine nucleotides, nucleotide-dependent channel operation cannot be exclusively resolved based on the homogeneity of intracellular energy pool and could rather rely upon compartmentalization of protein complexes and nucleotide fluxes between intracellular microdomains. The vast population of sarcolemmal KATP channels is thus a prerequisite to support energy homeostasis through the maintenance of local ATPase activities within local metabolic units, secluded at sarcolemma by strong diffusion barriers, contributing to muscle thermogenesis. The operational paradigm of KATP channel-driven thermogenic response provides at least two possible mechanisms of the control of energy use that may not be mutually exclusive. First, KATP channels reduce energy consumption, operating as a “safety valve” against action potential prolongation, which, however, is not readily applicable under physiological stimuli in the absence of detectable changes in action potentials duration. Second, KATP channels may support energy resources in the submembrane compartment, acting as ATP synthases utilizing K+ ion flow down the electrochemical gradient, which at present remains hypothetical and requires gaining insight into the properties of reversibility of SUR-associated ATPase activity. Alternatively, a distinct yet unrecognized function of KATP channel complexes that revolves around SUR hydrolytic activity may be further suggested. Recent advances in the KATP channel field suggest that these sarcolemmal energy sensors may serve as a peripheral target for bi-directional regulation of muscle energy utilization, which may find implication in a broad range of energy imbalance conditions linked, for instance, to heart disease, muscle myopathy, obesity and cold intolerance. In this way, the KATP channel-dependent muscle thermogenic response could be evaluated for rational diagnosis, prognosis and selection of preventive personalized therapy supported by the newest developments in proteomics, metabolomics and pharmacogenomics (Waldman et al., 2009).
Acknowledgments
The authors are thankful to Dr. A. Aleksandrov (University of North Carolina) for valuable discussion.
Footnotes
Declaration of interest
This work was supported by Gerstner Family Career Development Award in Individualized Medicine to AEA and NIH (HL64822) to AT.
References
- Abraham MR, Selivanov VA, Hodgson D, Pucar D, Zingman LV, Wieringa B, Dzeja PP, Alekseev AE, Terzic A. Coupling of cell energetics with membrane metabolic sensing: Integrative signaling through creatine kinase phosphotransfer disrupted by M-CK gene knockout. J Biol Chem. 2002;277:24427–24434. doi: 10.1074/jbc.M201777200. [DOI] [PubMed] [Google Scholar]
- Aguilar-Bryan L, Bryan J, Nakazaki M. Of mice and men: KATP channels and insulin secretion. Recent Prog Horm Res. 2001;56:47–68. doi: 10.1210/rp.56.1.47. [DOI] [PubMed] [Google Scholar]
- Aittoniemi J, Fotinou C, Craig TJ, de Wet H, Proks P, Ashcroft FM. SUR1: a unique ATP-binding cassette protein that functions as an ion channel regulator. Philos Trans R Soc Lond B Biol Sci. 2009;364:257–267. doi: 10.1098/rstb.2008.0142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allard B, Lazdunski M, Rougier O. Activation of ATP-dependent K+ channels by metabolic poisoning in adult mouse skeletal muscle: role of intracellular Mg2+ and pH. J Physiol. 1995;485:283–296. doi: 10.1113/jphysiol.1995.sp020730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alekseev AE, Hodgson DM, Karger AB, Park S, Zingman LV, Terzic A. ATP-sensitive K+ channel channel/enzyme multimer: metabolic gating in the heart. J Mol Cell Cardiol. 2005;38:895–905. doi: 10.1016/j.yjmcc.2005.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alekseev AE, Reyes S, Yamada S, Hodgson-Zingman DM, Sattiraju S, Zhu Z, Sierra A, Gerbin M, Coetzee WA, Goldhamer DJ, Terzic A, Zingman LV. Sarcolemmal ATP-sensitive K+ channels control energy expenditure determining body weight. Cell Metab. 2010;11:58–69. doi: 10.1016/j.cmet.2009.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrell DK, Zlatkovic J, Kane GC, Yamada S, Terzic A. ATP-sensitive K+ channel knockout induces cardiac proteome remodeling predictive of heart disease susceptibility. J Proteome Res. 2009;8:4823–4834. doi: 10.1021/pr900561g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashcroft FM. ATP-sensitive potassium channelopathies: focus on insulin secretion. J Clin Invest. 2005;115:2047–2058. doi: 10.1172/JCI25495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babenko AP, Aguilar-Bryan L, Bryan J. A view of SUR/KIR6.X, KATP channels. Annu Rev Physiol. 1998;60:667–687. doi: 10.1146/annurev.physiol.60.1.667. [DOI] [PubMed] [Google Scholar]
- Balakrishnan L, Venter H, Shilling RA, van Veen HW. Reversible transport by the ATP-binding cassette multidrug export pump LmrA: ATP synthesis at the expense of downhill ethidium uptake. J Biol Chem. 2004;279:11273–11280. doi: 10.1074/jbc.M308494200. [DOI] [PubMed] [Google Scholar]
- Baukrowitz T, Schulte U, Oliver D, Herlitze S, Krauter T, Tucker SJ, Ruppersberg JP, Fakler B. PIP2 and PIP as determinants for ATP inhibition of KATP channels. Science. 1998;282:1141–1144. doi: 10.1126/science.282.5391.1141. [DOI] [PubMed] [Google Scholar]
- Bienengraeber M, Alekseev AE, Abraham MR, Carrasco AJ, Moreau C, Vivaudou M, Dzeja PP, Terzic A. ATPase activity of the sulfonylurea receptor: a catalytic function for the KATP channel complex. FASEB J. 2000;14:1943–1952. doi: 10.1096/fj.00-0027com. [DOI] [PubMed] [Google Scholar]
- Bienengraeber M, Olson TM, Selivanov VA, Kathmann EC, O’Cochlain F, Gao F, Karger AB, Ballew JD, Hodgson DM, Zingman LV, Pang YP, Alekseev AE, Terzic A. ABCC9 mutations identified in human dilated cardiomyopathy disrupt catalytic KATP channel gating. Nat Genet. 2004;36:382–387. doi: 10.1038/ng1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittl JA, DeLayre J, Ingwall JS. Rate equation for creatine kinase predicts the in vivo reaction velocity: 31P NMR surface coil studies in brain, heart, and skeletal muscle of the living rat. Biochemistry. 1987;26:6083–6090. doi: 10.1021/bi00393a021. [DOI] [PubMed] [Google Scholar]
- Booth FW, Chakravarthy MV, Gordon SE, Spangenburg EE. Waging war on physical inactivity: using modern molecular ammunition against an ancient enemy. J Appl Physiol. 2002;93:3–30. doi: 10.1152/japplphysiol.00073.2002. [DOI] [PubMed] [Google Scholar]
- Boyer PD. The ATP synthase – a splendid molecular machine. Ann Rev Biochem. 1997;66:717–749. doi: 10.1146/annurev.biochem.66.1.717. [DOI] [PubMed] [Google Scholar]
- Boyer PD, Cross RL, Momsen W. A new concept for energy coupling in oxidative phosphorylation based on a molecular explanation of oxygen exchange reaction. Proc Nat Acad Sci USA. 1973;70:2837–2839. doi: 10.1073/pnas.70.10.2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramkamp M, Altendorf K, Greie J-C. Common patterns and unique features of P-type ATPases: a comparative view on the KdpFABC complex from Escherichia coli. Mol Membr Biol. 2007;24:375–386. doi: 10.1080/09687680701418931. [DOI] [PubMed] [Google Scholar]
- Brand MD, Esteves TC. Physiological functions of the mitochondrial uncoupling proteins UCP2 and UCP3. Cell Metab. 2005;2:85–93. doi: 10.1016/j.cmet.2005.06.002. [DOI] [PubMed] [Google Scholar]
- Carrasco A, Dzeja P, Alekseev A, Pucar D, Zingman L, Abraham M, Hodgson D, Bienengraeber M, Puceat M, Janssen E, Wieringa B, Terzic A. Adenylate kinase phosphotransfer communicates cellular energetic signals to ATP-sensitive potassium channels. Proc Natl Acad Sci USA. 2001;98:7623–7628. doi: 10.1073/pnas.121038198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celi FS. Brown adipose tissue – when it pays to be inefficient. N Engl J Med. 2009;360:1553–1556. doi: 10.1056/NEJMe0900466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan KW, Zhang H, Logothetis DE. N-terminal transmembrane domain of the SUR controls trafficking and gating of Kir6 channel subunits. EMBO J. 2003;22:3833–3843. doi: 10.1093/emboj/cdg376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen-Zion M, Bassukevitz Y, Beitner R. Rapid changes in carbohydrate metabolism in muscle induced by triiodothyronine; the role of glucose 1,6-bisphosphate. Biochem Mol Med. 1995;56:19–25. doi: 10.1006/bmme.1995.1051. [DOI] [PubMed] [Google Scholar]
- Cifelli C, Bourassa F, Gariépy L, Banas K, Benkhalti M, Renaud J-M. KATP channel deficiency in mouse flexor digitorum brevis causes fibre damage and impairs Ca2+ release and force development during fatigue in vitro. J Physiol. 2007;582:843–857. doi: 10.1113/jphysiol.2007.130955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cifelli C, Boudreault L, Gong B, Bercier JP, Renaud J-M. Contractile dysfunctions in ATP-dependent K+ channel-deficient mouse muscle during fatigue involve excessive depolarization and Ca2+ influx through L-type Ca2+ channels. Exp Physiol. 2008;93:1126–1138. doi: 10.1113/expphysiol.2008.042572. [DOI] [PubMed] [Google Scholar]
- Clement JP, Kunjilwar K, Gonzalez G, Schwanstecher M, Panten U, Aguilar-Bryan L, Bryan J. Association and stoichiometry of KATP channel subunits. Neuron. 1997;18:827–838. doi: 10.1016/s0896-6273(00)80321-9. [DOI] [PubMed] [Google Scholar]
- Cook DL, Hales CN. Intracellular ATP directly blocks K+ channels in pancreatic β-cells. Nature. 1984;311:271–273. doi: 10.1038/311271a0. [DOI] [PubMed] [Google Scholar]
- Craig TJ, Ashcroft FM, Proks P. How ATP inhibits the open KATP channel. J Gen Physiol. 2008;132:131–44. doi: 10.1085/jgp.200709874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford RM, Ranki JH, Botting CH, Budas GR, Jovanovic A. Creatine kinase is physically associated with the cardiac ATP-sensitive K+ channel in vivo. FASEB J. 2002;16:102–104. doi: 10.1096/fj.01-0466fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies NW, Standen NB, Stanfield PR. The effect of intracellular pH on ATP-dependent potassium channels of frog skeletal muscle. J Physiol. 1992;445:549–568. doi: 10.1113/jphysiol.1992.sp018939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decking UK, Reffelmann T, Schrader J, Kammermeier H. Hypoxia-induced activation of KATP channels limits energy depletion in the guinea pig heart. Am J Physiol. 1995;269:H734–742. doi: 10.1152/ajpheart.1995.269.2.H734. [DOI] [PubMed] [Google Scholar]
- de Wet H, Mikhailov MV, Fotinou C, Dreger M, Craig TJ, Vénien-Bryan C, Ashcroft FM. Studies of the ATPase activity of the ABC protein SUR1. FEBS J. 2007;274:3532–3544. doi: 10.1111/j.1742-4658.2007.05879.x. [DOI] [PubMed] [Google Scholar]
- Doorey AJ, Barry WH. The effects of inhibition of oxidative phosphorylation and glycolysis on contractility and high-energy phosphate content in cultured chick heart cells. Circ Res. 1983;53:192–201. doi: 10.1161/01.res.53.2.192. [DOI] [PubMed] [Google Scholar]
- Drain P, Li L, Wang J. KATP channel inhibition by ATP requires distinct functional domains of the cytoplasmic C terminus of the pore-forming subunit. Proc Natl Acad Sci USA. 1998;95:13953–13958. doi: 10.1073/pnas.95.23.13953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupuis JP, Revilloud J, Moreau CJ, Vivaudou M. Three C-terminal residues from the sulphonylurea receptor contribute to the functional coupling between the KATP channel subunits SUR2A and Kir6.2. J Physiol. 2008;586:3075–3085. doi: 10.1113/jphysiol.2008.152744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzeja PP, Terzic A. Phosphotransfer reactions in the regulation of ATP-sensitive K+ channels. FASEB J. 1998;12:523–529. doi: 10.1096/fasebj.12.7.523. [DOI] [PubMed] [Google Scholar]
- Dzeja P, Terzic A. Adenylate kinase and AMP signaling networks: Metabolic monitoring, signal communication and body energy sensing. Int J Mol Sci. 2009;10:1729–1772. doi: 10.3390/ijms10041729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott AC, Smith GL, Allen DG. Simultaneous measurements of action potential duration and intracellular ATP in isolated ferret hearts exposed to cyanide. Circ Res. 1989;64:583–591. doi: 10.1161/01.res.64.3.583. [DOI] [PubMed] [Google Scholar]
- Else PL, Turner N, Hulbert AJ. The evolution of endothermy: role for membranes and molecular activity. Physiol Biochem Zool. 2004;77:950–958. doi: 10.1086/422767. [DOI] [PubMed] [Google Scholar]
- Elvir-Mairena JR, Jovanovic A, Gomez LA, Alekseev AE, Terzic A. Reversal of the ATP-liganded state of ATP-sensitive K+ channels by adenylate kinase activity. J Biol Chem. 1996;271:31903–31908. doi: 10.1074/jbc.271.50.31903. [DOI] [PubMed] [Google Scholar]
- Findlay I, Faivre JF. ATP-sensitive K channels in heart muscle. Spare channels. FEBS Lett. 1991;279:95–97. doi: 10.1016/0014-5793(91)80259-6. [DOI] [PubMed] [Google Scholar]
- Ganitkevich V, Mattea V, Benndorf K. Glycolytic oscillations in single ischemic cardiomyocytes at near anoxia. J Gen Physiol. 2010;135:307–319. doi: 10.1085/jgp.200910332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghanassia E, Brun J-F, Mercier J, Raynaud E. Oxidative mechanisms at rest and during exercise. Clinica Chimica Acta. 2007;383:1–20. doi: 10.1016/j.cca.2007.04.006. [DOI] [PubMed] [Google Scholar]
- Gong B, Miki T, Seino S, Renaud J-M. A KATP channel deficiency affects resting tension, not contractile force, during fatigue in skeletal muscle. Am J Physiol. 2000;279:C1351–1358. doi: 10.1152/ajpcell.2000.279.5.C1351. [DOI] [PubMed] [Google Scholar]
- Gumina RJ, Pucar D, Bast P, Hodgson DM, Kurtz CE, Dzeja PP, Miki T, Seino S, Terzic A. Knockout of Kir6.2 negates ischemic preconditioning-induced protection of myocardial energetics. Am J Physiol. 2003;284:H2106–2139. doi: 10.1152/ajpheart.00057.2003. [DOI] [PubMed] [Google Scholar]
- Gumina RJ, O’Cochlain DF, Kurtz CE, Bast P, Pucar D, Mishra P, Miki T, Seino S, Macura S, Terzic A. KATP channel knockout worsens myocardial calcium stress load in vivo and impairs recovery in stunned heart. Am J Physiol. 2007;292:H1706–1713. doi: 10.1152/ajpheart.01305.2006. [DOI] [PubMed] [Google Scholar]
- Hashemi SM, Hund TJ, Mohler PJ. Cardiac ankyrins in health and disease. J Mol Cell Cardiol. 2009;47:203–209. doi: 10.1016/j.yjmcc.2009.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himms-Hagen J. Exercise in a pill: feasibility of energy expenditure targets. Curr Drug Targets CNS Neurol Disord. 2004;3:389–409. doi: 10.2174/1568007043337076. [DOI] [PubMed] [Google Scholar]
- Hodgson DM, Zingman LV, Kane GC, Perez-Terzic C, Bienengraeber M, Ozcan C, Gumina RJ, Pucar D, O’Coclain F, Mann DL, Alekseev AE, Terzic A. Cellular remodeling in heart failure disrupts KATP channel-dependent stress tolerance. EMBO J. 2003;22:1732–1742. doi: 10.1093/emboj/cdg192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosy E, Dérand R, Revilloud J, Vivaudou M. Remodelling of the SUR-Kir6.2 interface of the KATP channel upon ATP binding revealed by the conformational blocker rhodamine 123. J Physiol. 2007;582:27–39. doi: 10.1113/jphysiol.2007.134288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki N, Gonoi T, Clement JP, 4th, Namba N, Inazawa J, Gonzalez G, Aguilar-Bryan L, Seino S, Bryan J. Reconstitution of IKATP: an inward rectifier subunit plus the sulfonylurea receptor. Science. 1995;270:1166–1170. doi: 10.1126/science.270.5239.1166. [DOI] [PubMed] [Google Scholar]
- Inagaki N, Gonoi T, Clement J, Wang CZ, Aguilar-Bryan L, Bryan J, Seino S. A family of sulfonylurea receptors determines the pharmacological properties of ATP-sensitive K+ channels. Neuron. 1996;16:1011–1017. doi: 10.1016/s0896-6273(00)80124-5. [DOI] [PubMed] [Google Scholar]
- Janssen E, Dzeja PP, Oerlemans F, Simonetti A, Heerschap A, de Haan A, Rush PS, Terjung RR, Wieringa B, Terzic A. Adenylate kinase 1 gene deletion disrupts muscle energetic economy despite metabolic rearrangement. EMBO J. 2000;19:6371–6381. doi: 10.1093/emboj/19.23.6371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen E, Kuiper J, Hodgson D, Zingman LV, Alekseev AE, Terzic A, Wieringa B. Two structurally distinct and spatially compartmentalized adenylate kinases are expressed from the AK1 gene in mouse brain. Mol Cell Biochem. 2004;256–257:59–72. doi: 10.1023/b:mcbi.0000009859.15267.db. [DOI] [PubMed] [Google Scholar]
- Jovanović S, Jovanović A, Crawford RM. M-LDH serves as a regulatory subunit of the cytosolic substrate-channelling complex in vivo. J Mol Biol. 2007;371:349–361. doi: 10.1016/j.jmb.2007.05.081. [DOI] [PubMed] [Google Scholar]
- Kaasik A, Veksler V, Boehm E, Novotova M, Minajeva A, Ventura-Clapier R. Energetic crosstalk between organelles: architectural integration of energy production and utilization. Circ Res. 2001;89:153–159. doi: 10.1161/hh1401.093440. [DOI] [PubMed] [Google Scholar]
- Kabakov AY. Activation of KATP channels by Na/K pump in isolated cardiac myocytes and giant membrane patches. Biophys J. 1998;75:2858–2867. doi: 10.1016/S0006-3495(98)77728-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagawa Y, Hamamoto T. Intramolecular rotation in ATP synthase: dynamic and crystallographic studies on thermophilic F1. Biochem Biophys Res Commun. 1997;240:247–256. doi: 10.1006/bbrc.1997.7574. [DOI] [PubMed] [Google Scholar]
- Kane GC, Behfar A, Yamada S, Perez-Terzic C, O’Cochlain F, Reyes S, Dzeja PP, Miki T, Seino S, Terzic A. ATP-sensitive K+ channel knockout compromises the metabolic benefit of exercise training, resulting in cardiac deficits. Diabetes. 2004;53:S169–175. doi: 10.2337/diabetes.53.suppl_3.s169. [DOI] [PubMed] [Google Scholar]
- Kane GC, Liu XK, Yamada S, Olson TM, Terzic A. Cardiac KATP channels in health and disease. J Mol Cell Cardiol. 2005;38:937–943. doi: 10.1016/j.yjmcc.2005.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane GC, Behfar A, Dyer RB, O’Cochlain DF, Liu XK, Hodgson DM, Reyes S, Miki T, Seino S, Terzic A. KCNJ11 gene knockout of the Kir6.2 KATP channel causes maladaptive remodeling and heart failure in hypertension. Hum Mol Genet. 2006;15:2285–2297. doi: 10.1093/hmg/ddl154. [DOI] [PubMed] [Google Scholar]
- Karger AB, Park S, Reyes S, Bienengraeber M, Dyer RB, Terzic A, Alekseev AE. Role for SUR2A ED domain in allosteric coupling within the KATP channel complex. J Gen Physiol. 2008;131:185–196. doi: 10.1085/jgp.200709852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsey ST, Moerland TS. Metabolite diffusion in giant muscle fibers of the spiny lobster Panulirus argus. J Exp Biol. 2002;205:3377–3386. doi: 10.1242/jeb.205.21.3377. [DOI] [PubMed] [Google Scholar]
- Kline CF, Kurata HT, Hund TJ, Cunha SR, Koval OM, Wright PJ, Christensen M, Anderson ME, Nichols CG, Mohler PJ. Dual role of KATP channel C-terminal motif in membrane targeting and metabolic regulation. Proc Natl Acad Sci USA. 2009;106:16669–16674. doi: 10.1073/pnas.0907138106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koster JC, Knopp A, Flagg TP, Markova KP, Sha Q, Enkvetchakul D, Betsuyaku T, Yamada KA, Nichols CG. Tolerance for ATP-insensitive KATP channels in transgenic mice. Circ Res. 2001;89:1022–1029. doi: 10.1161/hh2301.100342. [DOI] [PubMed] [Google Scholar]
- Koyano T, Kakei M, Nakashima H, Yoshinaga M, Matsuoka T, Tanaka H. ATP-regulated K+ channels are modulated by intracellular H+ in guinea-pig ventricular cells. J Physiol. 1993;463:747–766. doi: 10.1113/jphysiol.1993.sp019620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Läuger P. Distinguished Lecture Series of the Society of General Physiologists. Vol. 5. Sunderland, MA: Sinauer Associates, Inc., Publishers; 1991a. Electrogenic ion pumps; pp. 168–249. [Google Scholar]
- Läuger P. Distinguished Lecture Series of the Society of General Physiologists. Vol. 5. Sunderland, MA: Sinauer Associates, Inc., Publishers; 1991b. Electrogenic ion pumps; pp. 191–192. [Google Scholar]
- Lawson JW, Veech RL. Effects of pH and free Mg2+ on the Keq of the creatine kinase reaction and other phosphate hydrolyses and phosphate transfer reactions. J Biol Chem. 1979;254:6528–6537. [PubMed] [Google Scholar]
- Lederer WJ, Nichols CG. Nucleotide modulation of the activity of rat heart ATP-sensitive K+ channels in isolated membrane patches. J Physiol. 1989;419:193–211. doi: 10.1113/jphysiol.1989.sp017869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lederer WJ, Niggli E, Hadley RW. Sodium-calcium exchange in excitable cells: fuzzy space. Science. 1990;248:283. doi: 10.1126/science.2326638. [DOI] [PubMed] [Google Scholar]
- Lefer DJ, Nichols CG, Coetzee WA. Sulfonylurea receptor 1 subunits of ATP-sensitive potassium channels and myocardial ischemia/reperfusion injury. Trends Cardiovasc Med. 2009;19:61–67. doi: 10.1016/j.tcm.2009.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine JA. Nonexercise activity thermogenesis – liberating the life-force. J Intern Med. 2007;262:273–287. doi: 10.1111/j.1365-2796.2007.01842.x. [DOI] [PubMed] [Google Scholar]
- Li B, Nolte LA, Ju J-S, Han DH, Coleman T, Holloszy JO, Semenkovich CF. Skeletal muscle respiratory uncoupling prevents diet-induced obesity and insulin resistance in mice. Nat Med. 2000;6:1115–1120. doi: 10.1038/80450. [DOI] [PubMed] [Google Scholar]
- Liu XK, Yamada S, Kane GC, Alekseev AE, Hodgson DM, O’Cochlain F, Jahangir A, Miki T, Seino S, Terzic A. Genetic disruption of Kir6.2, the pore-forming subunit of ATP-sensitive K+ channel, predisposes to catecholamine-induced ventricular dysrhythmia. Diabetes. 2004;53(Suppl 3):S165–168. doi: 10.2337/diabetes.53.suppl_3.s165. [DOI] [PubMed] [Google Scholar]
- Lorenz E, Terzic A. Physical association between recombinant cardiac ATP-sensitive K+ channel subunits Kir6.2 and SUR2A. J Mol Cell Cardiol. 1999;31:425–434. doi: 10.1006/jmcc.1998.0876. [DOI] [PubMed] [Google Scholar]
- Masia R, Enkvetchakul D, Nichols CG. Differential nucleotide regulation of KATP channels by SUR1 and SUR2A. J Mol Cell Cardiol. 2005;39:491–501. doi: 10.1016/j.yjmcc.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Matsuo M, Kioka N, Amachi T, Ueda K. ATP binding properties of the nucleotide-binding folds of SUR1. J Biol Chem. 1999;274:37479–37482. doi: 10.1074/jbc.274.52.37479. [DOI] [PubMed] [Google Scholar]
- Matsuo M, Tanabe K, Kioka N, Amachi T, Ueda K. Different binding properties and affinities for ATP and ADP among sulfonylurea receptor subtypes, SUR1, SUR2A, and SUR2B. J Biol Chem. 2000;275:28757–28763. doi: 10.1074/jbc.M004818200. [DOI] [PubMed] [Google Scholar]
- McArdle WD, Katch FI, Katch VL. Human energy expenditure during rest and physical activity. In: Balado D, editor. Exercise Physiology. 4. Baltimore, MD: Williams and Wilkins; 1996. pp. 151–164. [Google Scholar]
- Meyer RA, Sweeney HL, Kushmerick MJ. A simple analysis of the “phosphocreatine shuttle”. Am J Physiol. 1984;246:C365–377. doi: 10.1152/ajpcell.1984.246.5.C365. [DOI] [PubMed] [Google Scholar]
- Mikhailov MV, Ashcroft SJ. Interactions of the sulfonylurea receptor 1 subunit in the molecular assembly of beta-cell KATP channels. J Biol Chem. 2000;275:3360–3364. doi: 10.1074/jbc.275.5.3360. [DOI] [PubMed] [Google Scholar]
- Mikhailov MV, Campbell JD, de Wet H, Shimomura K, Zadek B, Collins RF, Sansom MS, Ford RC, Ashcroft FM. 3-D structural and functional characterization of the purified KATP channel complex Kir6-2-Sur1. EMBO J. 2005;24:4166–4175. doi: 10.1038/sj.emboj.7600877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miki T, Nagashima K, Tashiro F, Kotake K, Yoshitomi H, Tamamoto A, Gonoi T, Iwanaga T, Miyazaki J, Seino S. Defective insulin secretion and enhanced insulin action in KATP channel-deficient mice. Proc Natl Acad Sci USA. 1998;95:10402–10406. doi: 10.1073/pnas.95.18.10402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miki T, Minami K, Zhang L, Morita M, Gonoi T, Shiuchi T, Minokoshi Y, Renaud J-M, Seino S. ATP-sensitive potassium channels participate in glucose uptake in skeletal muscle and adipose tissue. Am J Physiol. 2002;283:E1178–1184. doi: 10.1152/ajpendo.00313.2002. [DOI] [PubMed] [Google Scholar]
- Minami K, Morita M, Saraya A, Yano H, Terauchi Y, Miki T, Kuriyama T, Kadowaki T, Seino S. ATP-sensitive K+ channel- mediated glucose uptake is independent of IRS-1/phosphatidylinositol 3-kinase signaling. Am J Physiol. 2003;285:E1289–1296. doi: 10.1152/ajpendo.00278.2003. [DOI] [PubMed] [Google Scholar]
- Moreau C, Prost AL, Dérand R, Vivaudou M. SUR, ABC proteins targeted by KATP channel openers. J Mol Cell Cardiol. 2005;38:951–963. doi: 10.1016/j.yjmcc.2004.11.030. [DOI] [PubMed] [Google Scholar]
- Moses MA, Addison PD, Neligan PC, Ashrafpour H, Huang N, McAllister SE, Lipa JE, Forrest CR, Pang CY. Inducing late phase of infarct protection in skeletal muscle by remote preconditioning: efficacy and mechanism. Am J Physiol Regul Integr Comp Physiol. 2005;289:R1609–1617. doi: 10.1152/ajpregu.00395.2005. [DOI] [PubMed] [Google Scholar]
- Müller V, Grüber G. ATP synthases: structure, function and evolution of unique energy converters. Cell Mol Life Sci. 2003;60:474–494. doi: 10.1007/s000180300040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols CG. KATP channels as molecular sensors of cellular metabolism. Nature. 2006;440:470–476. doi: 10.1038/nature04711. [DOI] [PubMed] [Google Scholar]
- Nichols CG, Lederer WJ. The regulation of ATP-sensitive K+ channel activity in intact and permeabilized rat ventricular myocytes. J Physiol. 1990;423:91–110. doi: 10.1113/jphysiol.1990.sp018013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols CG, Lederer WJ. Adenosine triphosphate-sensitive potassium channels in the cardiovascular system. Am J Physiol. 1991;261:H1675–1686. doi: 10.1152/ajpheart.1991.261.6.H1675. [DOI] [PubMed] [Google Scholar]
- Noma A. ATP-regulated K+ channels in cardiac muscle. Nature. 1983;305:147–148. doi: 10.1038/305147a0. [DOI] [PubMed] [Google Scholar]
- Olson TM, Terzic A. Human KATP channelopathies: diseases of metabolic homeostasis. Pflugers Arch. 2010;460:295–306. doi: 10.1007/s00424-009-0771-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson TM, Alekseev AE, Moreau C, Liu XK, Zingman LV, Miki T, Seino S, Asirvatham SJ, Jahangir A, Terzic A. KATP channel mutation confers risk for vein of Marshall adrenergic atrial fibrillation. Nat Clin Pract Cardiovasc Med. 2007;4:110–116. doi: 10.1038/ncpcardio0792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S, Terzic A. Quaternary Structure of KATP channel SUR2A nucleotide binding domains resolved by synchrotron radiation X-ray scattering. J Struct Biol. 2010;169:243–251. doi: 10.1016/j.jsb.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S, Lim BB, Perez-Terzic C, Mer G, Terzic A. Interaction of asymmetric ABCC9-encoded nucleotide binding domains determines KATP channel SUR2A catalytic activity. J Proteome Res. 2008;7:1721–1728. doi: 10.1021/pr7007847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pucar D, Dzeja PP, Bast P, Juranic N, Macura S, Terzic A. Cellular energetics in the preconditioned state: Protective role for phosphotransfer reactions captured by 18O-assisted 31P NMR. J Biol Chem. 2001;276:44812–44819. doi: 10.1074/jbc.M104425200. [DOI] [PubMed] [Google Scholar]
- Rainbow RD, James M, Hudman D, Al Johi M, Singh H, Watson PJ, Ashmole I, Davies NW, Lodwick D, Norman RI. Proximal C-terminal domain of sulphonylurea receptor 2A interacts with pore-forming Kir6 subunits in KATP channels. Biochem J. 2004;379:173–181. doi: 10.1042/BJ20031087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajashree R, Koster JC, Markova KP, Nichols CG, Hofmann PA. Contractility and ischemic response of hearts from transgenic mice with altered sarcolemmal KATP channels. Am J Physiol. 2002;283:H584–590. doi: 10.1152/ajpheart.00107.2002. [DOI] [PubMed] [Google Scholar]
- Renaud JM. Modulation of force development by Na+, K+, Na+/K+ pump and KATP channel during muscular activity. Can J Appl Physiol. 2002;27:296–315. doi: 10.1139/h02-017. [DOI] [PubMed] [Google Scholar]
- Reyes S, Kane GC, Miki T, Seino S, Terzic A. KATP channels confer survival advantage in cocaine overdose. Mol Psychiatry. 2007;12:1060–1061. doi: 10.1038/sj.mp.4002083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes S, Terzic A, Mahoney DW, Redfield MM, Rodeheffer RJ, Olson TM. KATP channel polymorphism is associated with left ventricular size in hypertensive individuals: a large-scale community-based study. Hum Genet. 2008;123:665–667. doi: 10.1007/s00439-008-0519-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes S, Kane GC, Zingman LV, Yamada S, Terzic A. Targeted disruption of KATP channels aggravates cardiac toxicity in cocaine abuse. Clin Transl Sci. 2009a;2:361–365. doi: 10.1111/j.1752-8062.2009.00145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes S, Park S, Johnson BD, Terzic A, Olson TM. KATP channel Kir6.2 E23K variant overrepresented in human heart failure is associated with impaired exercise stress response. Hum Genet. 2009b;126:779–789. doi: 10.1007/s00439-009-0731-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich TC, Fagan KA, Nakata H, Schaack J, Cooper DM, Karpen JW. Cyclic nucleotide-gated channels colocalize with adenylyl cyclase in regions of restricted cAMP diffusion. J Gen Physiol. 2000;116:147–161. doi: 10.1085/jgp.116.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saks VA, Kaambre T, Sikk P, Eimre M, Orlova E, Paju K, Piirsoo A, Appaix F, Kay L, Regitz-Zagrosek V, Fleck E, Seppet E. Intracellular energetic units in red muscle cells. Biochem J. 2001;356:643–657. doi: 10.1042/0264-6021:3560643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saks V, Monge C, Guzun R. Philosophical basis and some historical aspects of system biology: from Hegel to Noble – applications for bioenergetic research. Int J Mol Sci. 2009;10:1161–1192. doi: 10.3390/ijms10031161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwappach B, Zerangue N, Jan YN, Jan LY. Molecular basis for KATP assembly: transmembrane interactions mediate association of a K+ channel with an ABC transporter. Neuron. 2000;26:155–167. doi: 10.1016/s0896-6273(00)81146-0. [DOI] [PubMed] [Google Scholar]
- Seino S. ATP-sensitive potassium channels: A model of heteromultimeric potassium channel/receptor assemblies. Annu Rev Physiol. 1999;61:337–362. doi: 10.1146/annurev.physiol.61.1.337. [DOI] [PubMed] [Google Scholar]
- Selivanov VA, Alekseev AE, Hodgson DM, Dzeja PP, Terzic A. Nucleotide-gated KATP channels integrated with creatine and adenylate kinases: amplification, tuning and sensing of energetic signals in the compartmentalized cellular environment. Mol Cell Biochem. 2004;256–257:243–256. doi: 10.1023/b:mcbi.0000009872.35940.7d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shyng SL, Nichols CG. Membrane phospholipid control of nucleotide sensitivity of KATP channels. Science. 1998;282:1138–1141. doi: 10.1126/science.282.5391.1138. [DOI] [PubMed] [Google Scholar]
- Shyng SL, Ferrigni T, Nichols CG. Regulation of KATP channel activity by diazoxide and MgADP: Distinct functions of the two nucleotide binding folds of the sulfonylurea receptor. J Gen Physiol. 1997;110:643–654. doi: 10.1085/jgp.110.6.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song DK, Ashcroft FM. ATP modulation of ATP-sensitive potassium channel ATP sensitivity varies with the type of SUR subunit. J Biol Chem. 2001;276:7143–7149. doi: 10.1074/jbc.M009959200. [DOI] [PubMed] [Google Scholar]
- Spanswick D, Smith MA, Groppi VE, Logan SD, Ashford MLJ. Leptin inhibits hypothalamic neurons by activation of ATP-sensitive potassium channels. Nature. 1997;390:521–525. doi: 10.1038/37379. [DOI] [PubMed] [Google Scholar]
- Sugden MC, Holness MJ, Liu YL, Smith DM, Fryer LG, Kruszynska YT. Mechanisms regulating cardiac fuel selection in hyperthyroidism. Biochem J. 1992;286:513–517. doi: 10.1042/bj2860513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarasov AI, Girard CAJ, Ashcroft FM. ATP sensitivity of the ATP-sensitive K+ channel in intact and permeabilized pancreatic β-cells. Diabetes. 2006;55:2446–2454. doi: 10.2337/db06-0360. [DOI] [PubMed] [Google Scholar]
- Terzic A, Kurachi Y. Actin microfilament disrupters enhance KATP channel opening in patches from guinea-pig cardiomyocytes. J Physiol. 1996;492:395–404. doi: 10.1113/jphysiol.1996.sp021316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thabet M, Miki T, Seino S, Renaud J-M. Treadmill running causes significant fiber damage in skeletal muscle of KATP channel-deficient mice. Physiol Genomics. 2005;22:204–212. doi: 10.1152/physiolgenomics.00064.2005. [DOI] [PubMed] [Google Scholar]
- Tucker SJ, Gribble FM, Zhao C, Trapp S, Ashcroft FM. Truncation of Kir6.2 produces ATP-sensitive K+ channels in the absence of the sulphonylurea receptor. Nature. 1997;387:179–183. doi: 10.1038/387179a0. [DOI] [PubMed] [Google Scholar]
- Ueda K, Komine J, Matsuo M, Seino S, Amachi T. Cooperative binding of ATP and MgADP in the sulfonylurea receptor is modulated by glibenclamide. Proc Natl Acad Sci USA. 1999;96:1268– 1272. doi: 10.1073/pnas.96.4.1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivaudou M, Forestier C. Modification by protons of frog skeletal muscle KATP channels: effects on ion conduction and nucleotide inhibition. J Physiol. 1995;486:629–645. doi: 10.1113/jphysiol.1995.sp020840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldman SA, Kraft WK, Nelson TJ, Terzic A. Clinical pharmacology: a paradigm for individualized medicine. Biomark Med. 2009;3:679–684. doi: 10.2217/bmm.09.76. [DOI] [PubMed] [Google Scholar]
- Weiss J, Hiltbrand B. Functional compartmentation of glycolytic versus oxidative metabolism in isolated rabbit heart. J Clin Invest. 1985;75:436–447. doi: 10.1172/JCI111718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss JN, Korge P. The cytoplasm: no longer a well-mixed bag. Circ Res. 2001;89:108–110. [PubMed] [Google Scholar]
- Weiss JN, Lamp ST. Glycolysis preferentially inhibits ATP-sensitive K+ channels in isolated guinea pig cardiac myocytes. Science. 1987;238:67–69. doi: 10.1126/science.2443972. [DOI] [PubMed] [Google Scholar]
- Weiss JN, Venkatesh N, Lamp ST. ATP-sensitive K+ channels and cellular K+ loss in hypoxic and ischaemic mammalian ventricle. J Physiol. 1992;447:649–673. doi: 10.1113/jphysiol.1992.sp019022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijers SL, Schrauwen P, Saris WH, van Marken Lichtenbelt WD. Human skeletal muscle mitochondrial uncoupling is associated with cold induced adaptive thermogenesis. PLoS One. 2008;3:e1777. doi: 10.1371/journal.pone.0001777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada M, Isomoto S, Matsumoto S, Kondo C, Shindo T, Horio Y, Kurachi Y. Sulphonylurea receptor 2B and Kir6.1 form a sulphonylurea-sensitive but ATP-insensitive K+ channel. J Physiol. 1997;499:715–720. doi: 10.1113/jphysiol.1997.sp021963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada S, Kane GC, Behfar A, Liu XK, Dyer RB, Faustino RS, Miki T, Seino S, Terzic A. Protection conferred by myocardial ATP-sensitive K+ channels in pressure overload-induced congestive heart failure revealed in KCNJ11 Kir6.2-null mutant. J Physiol. 2006;577:1053–1065. doi: 10.1113/jphysiol.2006.119511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zingman LV, Alekseev AE, Bienengraeber M, Hodgson D, Karger AB, Dzeja PP, Terzic A. Signaling in channel/enzyme multimers: ATPase transitions in SUR module gate ATP-sensitive K+ conductance. Neuron. 2001;31:233–45. doi: 10.1016/s0896-6273(01)00356-7. [DOI] [PubMed] [Google Scholar]
- Zingman LV, Hodgson DM, Bast PH, Kane GC, Perez-Terzic C, Gumina RJ, Pucar D, Bienengraeber M, Dzeja PP, Miki T, Seino S, Alekseev AE, Terzic A. Kir6.2 is required for adaptation to stress. Proc Natl Acad Sci USA. 2002;99:13278–13283. doi: 10.1073/pnas.212315199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zingman LV, Hodgson DM, Alekseev AE, Terzic A. Stress without distress: homeostatic role for KATP channels. Mol Psychiatry. 2003;8:253–254. doi: 10.1038/sj.mp.4001323. [DOI] [PubMed] [Google Scholar]
- Zingman LV, Alekseev AE, Hodgson-Zingman DM, Terzic A. ATP-sensitive potassium channels: metabolic sensing and cardioprotection. J Appl Physiol. 2007;103:1888–1893. doi: 10.1152/japplphysiol.00747.2007. [DOI] [PubMed] [Google Scholar]