Abstract
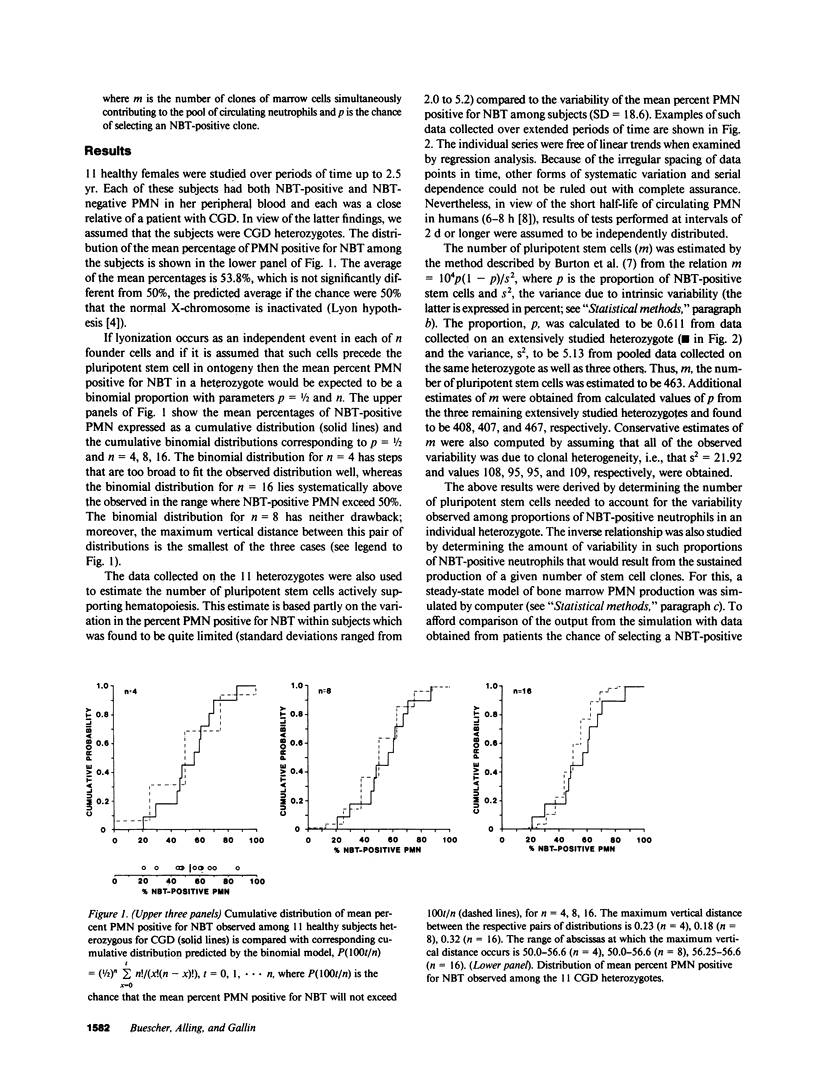
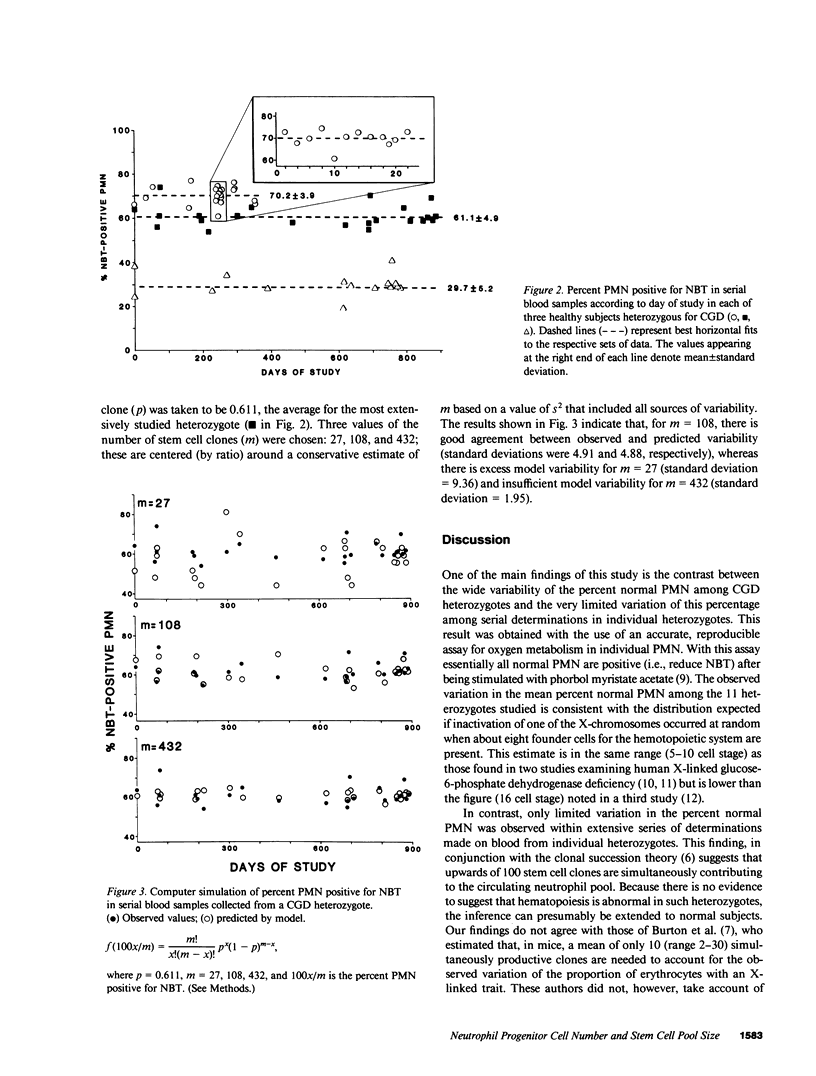
In families with X-linked chronic granulomatous disease (CGD), heterozygous females have two stable populations of polymorphonuclear leukocytes (PMN) in their blood; one normal, the other, deficient in oxygen metabolism. The two types of PMN can be distinguished by the ability or lack of ability to reduce nitroblue tetrazolium dye. The variation in the percent normal PMN among 11 CGD heterozygotes was shown to follow a binomial distribution based on eight independent trials and a chance of success of 50%. This is consistent with the occurrence of X-chromosome inactivation (lyonization) when eight embryonic founder cells for the hematopoietic system are present. Serial determinations of the percent normal PMN in individual heterozygotes showed very limited variability (standard deviations ranged from 2.0% to 5.2%) most of which could be ascribed to experimental error. An estimate of the remaining variation (residual variance) was introduced into a well-known formula to calculate the appropriate number of pluripotent stem cells necessary to support hematopoiesis and a figure exceeding 400 was obtained. Thus, the data indicate that in humans there is a highly polyclonal system of hematopoiesis.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CARTWRIGHT G. E., ATHENS J. W., WINTROBE M. M. THE KINETICS OF GRANULOPOIESIS IN NORMAL MAN. Blood. 1964 Dec;24:780–803. [PubMed] [Google Scholar]
- Fialkow P. J. Primordial cell pool size and lineage relationships of five human cell types. Ann Hum Genet. 1973 Jul;37(1):39–48. doi: 10.1111/j.1469-1809.1973.tb01813.x. [DOI] [PubMed] [Google Scholar]
- Gallin J. I., Buescher E. S., Seligmann B. E., Nath J., Gaither T., Katz P. NIH conference. Recent advances in chronic granulomatous disease. Ann Intern Med. 1983 Nov;99(5):657–674. doi: 10.7326/0003-4819-99-5-657. [DOI] [PubMed] [Google Scholar]
- Gandini E., Gartler S. M., Angioni G., Argiolas N., Dell'Acqua G. Developmental implications of multiple tissue studies in glucose-6-phosphate dehydrogenase-deficient heterozygotes. Proc Natl Acad Sci U S A. 1968 Nov;61(3):945–948. doi: 10.1073/pnas.61.3.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAY H. E. HOW MANY CELL-GENERATIONS? Lancet. 1965 Aug 28;2(7409):418–419. doi: 10.1016/s0140-6736(65)90763-4. [DOI] [PubMed] [Google Scholar]
- Mills E. L., Rholl K. S., Quie P. G. X-linked inheritance in females with chronic granulomatous disease. J Clin Invest. 1980 Aug;66(2):332–340. doi: 10.1172/JCI109861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nesbitt M. N., Gartler S. M. The applications of genetic mosaicism to developmental problems. Annu Rev Genet. 1971;5:143–162. doi: 10.1146/annurev.ge.05.120171.001043. [DOI] [PubMed] [Google Scholar]
- Repine J. E., Rasmussen B., White J. G. An improved nitroblue tetrazolium test using phorbol myristate acetate-coated coverslips. Am J Clin Pathol. 1979 May;71(5):582–585. doi: 10.1093/ajcp/71.5.582. [DOI] [PubMed] [Google Scholar]



