Abstract
Background
Small cell lung cancer (SCLC) is the most aggressive form of lung cancer with poor disease outcome. Chemotherapeutic agent paclitaxel (PA) is commonly used as a second-line treatment in SCLC, but response rates are low.
Materials and Methods
86M1 SCLC cells were treated in the presence or absence of Paclitaxel and TRAIL or the combination for 24 hours. Western blot analysis was utilized to examine protein expression, cell surface protein expression and membrane integrity were elucidated by flow cytometry, and immunofluorescence microscopy was used to demonstrate translocation of proteins to the cell nucleus.
Results
Human 86M1 SCLC cells were found to be resistant to PA killing in vitro. This resistance is mediated by up-regulation of pro-survival protein BCL-xl. However, PA also increases surface expression of death receptors 4 and 5 (DR4 and DR5, respectively). The death receptors’ ligand increased SCLC killing by PA through an apparent caspase-independent route involving activation/translocation of AIF.
Conclusion
The addition of TRAIL to PA can potentiate apoptosis in a relatively PA-resistant SCLC line (specifically 86M1 cells). More importantly, we are the first to report an active method of resistance to paclitaxel in SCLC via BCL-xl up-regulation.
Small cell lung cancer (SCLC) is an aggressive form of lung cancer. Although SCLC is a highly chemosensitive disease, outcome is generally poor and the 5-year survival rate is <10% (1). Diagnosis of extensive stage (ES) comprises approximately two-thirds of new SCLC cases, and the median survival of these patients is only 2-4 months if untreated, with survival increasing to 6-8 months with chemotherapy. This disease is very responsive to first-line chemotherapy with response rates of greater than 50% routinely observed. However, these responses are often short-lived and disease recurrence in the ES patient population is frequent. Patients with relapsed disease, or patients who fail to respond to chemotherapy generally succumb to their disease within a few months (1). Treatment of patients with relapsed SCLC is especially challenging if the disease is platinum-resistant, when disease progression occurs within 3 months of completion of a platinum containing regimen. In these patients, median survival ranges from 3.7 to 4.7 months (2-7). In SCLC, paclitaxel is primarily considered a second-line therapy after the failure of platinum-based treatment regimens (8). Several modes of action of paclitaxel have been described. The drug is most well known as a microtubule stabilizer. Specifically, paclitaxel binds to tubulin and interferes with spindle formation in mitosis, ultimately arresting cells in G1 and G2/M phases of the cell cycle, leading to cell death (9-13). In addition to stabilizing microtubules paclitaxel may act to sequester free tubulin, effectively depleting the cell’s supply of tubulin (14). Beyond these effects on microtubules, more recent research has indicated that paclitaxel also induces programmed cell death in cancer cells by binding to the pro-survival protein Bcl-2, blocking its function (15, 16). A varietyof pharmacoimmunologic effects have also been attributed to paclitaxel (17-19).
There are two major pathways of apoptotic cell death. One pathway involves changes in mitochondrial membrane potential and the translocation of proteins from the mitochondria into the cytoplasm, including translocation of cytochrome c, ultimately triggering the activation of caspases and other proteins, subsequently leading to apoptosis (20, 21). BCL family proteins play key roles in the mitochondria and are central to regulating mitochondrial membrane integrity. The family consists of both pro-survival proteins, such as the well characterized BCL-2 and BCL-xl (22), as well as pro-apoptotic proteins including BAX and BAK (23). The other major apoptotic pathway is primarily dependent on caspase-8 activation driven by the binding of death receptors on the cell surface by death ligands. Two well characterized death receptors (DR) are DR4 and DR5 which are both activated by tumor necrosis factor related apoptosis inducing ligand (TRAIL). TRAIL is present on activated cytotoxic T lymphocytes (CTL), but not on unstimulated peripheral blood T-cells (24). Binding of DR4/5 by TRAIL results in the recruitment of Fas-Associated protein with Death Domain (FADD) and FADD-like interleukin-1 beta-converting enzyme (FLICE) to the intracellular portion of the DR. The resulting complex can then cleave and activate caspase-8, leading to apoptosis through mitochondrial dependent (BID cleavage) or mitochondrial independent (caspase-3 cleavage) pathways (25-28). It has been reported that paclitaxel exerts effects on both of the aforementioned pathways (29-34). Additionally, paclitaxel has been shown to regulate the expression of DR4 and DR5 (35-37). However, much of this remains controversial and there is disagreement in the literature relating to the exact mechanism of paclitaxel induced cancer cell apoptosis. This discord is primarily due to the variation in the concentration of paclitaxel used in the studies – some studies use biologically relevant doses of paclitaxel (25-150 nM), whereas other studies describe results obtained from cells treated with 10 to 20-fold the aforementioned doses (38).
In the study described herein we sought to elucidate mechanisms by which SCLC are resistant to PA as well as assessing a potential treatment strategy to overcome these mechanisms.
Materials and Methods
Cell propagation
86M1 SCLC cells (39, 40) were maintained in complete RPMI (RPMI-1640 media, supplemented with 10% fetal bovice serum, 100 U/ml penicillin, 100 μg/ml streptomycin, and 2 mM L-glutamine and penicillin-streptomycin; Invitrogen, Carlsbad, California, USA). Cells were enumerated, and live cells counted, using a hemacytometer and trypan blue exclusion.
Reagents
Paclitaxel was obtained as a 7 mM stock from Cardinal Health (Dublin, Ohio, USA)supplied through the Moffitt Pharmacy. Recombinant human TRAIL was obtained from R&D Systems (Minneapolis, Minnesota, USA).
Cell death assay
Sensitivity of 86M1 SCLC to paclitaxel was determined by lactase dehydrogenase (LDH) release using the CytoTox 96 Non-Radioactive Cytotoxicity Assay (Promega, Madison, Wisconsin, USA). Briefly, SCLC cells were culture for 48 hours with or without different concentrations of paclitaxel ranging from 0.1 to 1000 nM. Following drug exposure, the plates were gently centrifuged and 50 μl of the culture supernatant was added to a new 96 well flat bottom plate. Kit substrate, 50 μl, was added and the plate was incubated for 30 minutes. Stop solution was then added, and the samples were gently mixed. Absorbance at 490 nm was recorded. Media background controls were also performed and subtracted from all of the recorded OD values. Specific cell lysis was quantitated using the following formula: (sample release minus spontaneous release) divided by (maximum release minus spontaneous release) ×100.
Death receptor expression
Surface expression of DR4 and DR5 was analyzed by flow cytometric analysis. Cells were treated with or without 100 nM paclitaxel for 20 hours. Cell culture suspensions were then harvested and cell washed in phosphate-buffered saline (PBS) and stained with phycoerythrin (PE)-conjugated anti-DR4 or -DR5 antibodies (EBiosciences, San Diego, California, USA) diluted 1:50 in PBS. The cells were stained at room temperature for 20 minutes. The cells were then washed twice in PBS. A viability dye (7AAD) was then added. Live cells were gated as 7AAD-egative and this population was used to evaluate DR expression.
Acridine orange/ethidium bromide staining
Apoptosis was visualized by chromatin condensation. Cells were treated with or without 100 nM paclitaxel for 20 hours. Where indicated, cells were then treated with 50 ng/ml TRAIL (R&D Systems) for the final 1-4 hours of culture, following which the cells were harvested. The cells were then incubated with 100 μg/ml acridine orange (Roche, San Francisco, California, USA), a DNA fluorochrome which is able to cross the intact plasma membrane, for 5 minutes as previously described (41). Since these were suspended cells, the cells were then placed on a slide and coverslipped. Chromatin morphology was analyzed using an Olympus inverted fluorescence microscope and Q Capture Pro Software (Olympus, Center Valley, Pennsylvania, USA).
Annexin V staining
After treatment with paclitaxel and/or TRAIL, cells were harvested and washed in cold PBS and then resuspended at a density of 1-10×106 cells per ml in 1× Annexin V binding buffer (BD Biosciences, Franklin Lakes, New Jersey, USA). 100 μl of the cells were labelled with 10 μl Annexin V-Pacific Blue conjugate. The cells were incubated for 15-20 minutes in the dark, after which 400 μl 1× Annexin binding buffer was added to each tube. Cells were immediately analyzed using an LSRII cytometer (BD Biosciences). Annexin staining was analyzed using Flow Jo software (Tree Star Inc., Ashland, Oregon, USA). Apoptosis was quantified by the percentage of positive cells falling under the defined positive curve.
Cell fractionation
Mitochondrial and cytosolic cell fractions were obtained using the Mitchondria/Cytosol Fractionation kit from BioVision (Mountain View, California, USA). Fractionation was carried out according to the manufacturer’s instructions. An antibody raised against a mitochondrial-specific protein, Cox IV (Cell Signal Technologies, Danvers, Massachusetts, USA), was detected by Western blot to validate fractionation and as a loading control for mitochondrial fraction protein loading. Proteins were subjected to Western blot analysis as described below.
Western blot analysis
Following treatment with or without 100 nM paclitaxel for 20 hours, and, where indicated, 50 ng/ml of TRAIL for the final 1-4 hours of culture, cells were harvested, washed, and resuspended in 1× CHAPS buffer (Cell Signal Technologies) supplemented with 5 mM dithiothreitol (DTT) (Cell Signal Technologies) and 1 mM phenylmethylsulfonyl fluoride (PMSF) (Sigma, St. Louis, Missouri, USA). Protein concentration was determined using the BioRad (Hercules, California, USA) protein assay and the lysates were diluted to equal concentrations. 3× or 10× sodium dodecyl sulfate (SDS)-loading buffer (BioRad) was added to a final concentration of 1× and samples were boiled for 5 minutes. The proteins were resolved on 4-20 or 8-16% gradient pre-cast SDS-Hepes polyacrilamide gels (Pierce/Fisher, Pittsburgh, Pennsylvania, USA) and then transferred to polyvinylidine fluoride (PVDF) membrane (Millipore, Billerica, Massachusetts, USA). Membranes were blocked with Tris-buffered saline containing 5% non-fat milk (Carnation, Glendale, California, USA) and 0.5% Tween (Fisher, Pittsburgh, Pennsylvania, USA). For phospho-protein blotting, the 5% non-fat milk was replaced with 5% bovine serum albumin (BSA) (Sigma). The membranes were incubated in primary antibodies over night at 4°C (antibodies indicated in figures and figure legends). The membranes were then washed and incubated for 1 hour in horseradish peroxidase-conjugated secondary goat anti-rabbit or rabbit anti-mouse antibodies (Millipore). Proteins were detected using ECL plus (Amersham) and hyperfilm (Amersham). All antibodies were purchase from Cell Signal Technologies, except anti-AKT and anti-pAKT which were purchased from Santa Cruz Biotechnology Inc. (Santa Cruz, California, USA).
Flow cytometric analysis of mitochondrial membrane potential and caspase activation
Mitochondrial membrane potential and caspase activation were determined by flow cytometry using the Dual Sensor MitoCasp kit (Cell Technology Inc., Santa Monica, California, USA). The cells were acquired using a FACSCalibur cytometer (BD Biosciences) and staining intensity was analyzed using Flow Jo software (Tree Star Inc.).
AIF fluorescence microscopy
SCLC cells were treated as indicated and then harvested. The cells were permeablized and fixed using Fix and Perm (Calbiochem, San Diego, California, USA). The cells were then incubated with Fluorescein isothiocyanate (FITC)-conjugated anti-AIF antibody (Abcam, Cambridge, Massachusetts, USA) and washed in PBS. The cells were then incubated with 4′,6-diamidino-2-phenylindole (DAPI) and allowed to settle on a slide under a coverslip. Fluorescence microscopy was performed using a Zeiss LSM 510 Laser Scanning Confocal Microscope (Zeiss, Thornwood, New York, USA). Images and co-localization were evaluated using Image Pro Plus 6.2 (Media Cybernetics, Silver Spring, Maryland, USA).
Results
86M1 SCLC cells are resistant to killing by paclitaxel
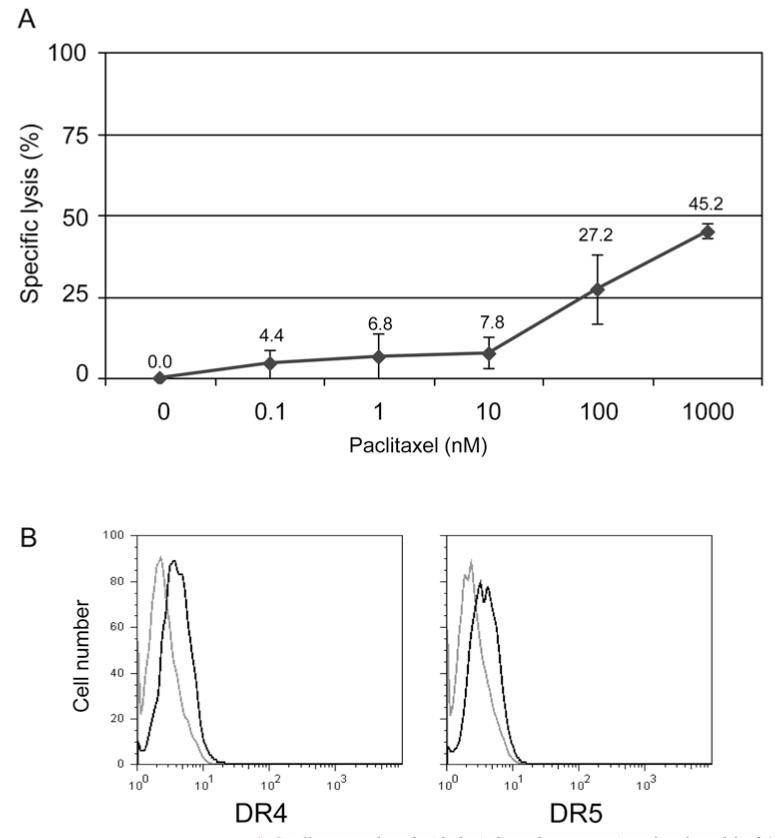
We determined the effect of paclitaxel monotherapy on SCLC cell line 86M1. The cells were not sensitive to biologically relevant doses (100-200 nM) of paclitaxel in vitro (Figure 1A). In fact, the lethal dose, 50% (LD50) was not achieved with 5-10 fold times this dose. For all remaining experiments, a biologically relevant concentration of 100 nM (which resulted in the killing of approximately one quarter of the cells) was used.
Figure 1.
Effect of paclitaxel on SCLC 86M1cells. A: SCLC cells were cultured with the indicated concentration of paclitaxel for 24 hours. Following the incubation, specific lysis was assessed by LDH release. Percentage specific lysis was calculated as: (sample release minus spontaneous release) divided by (maximum release minus spontaneous release) ×100. LD50 was not achieved even at 1000 nM paclitaxel. B: Death receptor 4 and 5 expression is up-regulated on SCLC 86M1 cells following treatment with paclitaxel. Cells were treated with paclitaxel (100 nM) for 20 hours. Cells were harvested and labelled with PE-conjugated anti-DR4 or -DR5 antibodies. Live cells were gated using exclusion of 7AAD labelling. The light grey histogram shows untreated cells and the black histogram corresponds to SCLC treated with paclitaxel.
DR expression on SCLC is increased following culture with paclitaxel
Paclitaxel has been shown to induce apoptosis of a cancer cells, however, the exact mechanism of this activity has not been fully elucidated, although paclitaxel was first described as disrupting microtubules and inhibiting mitosis. With a recent report suggesting that chemotherapy can promote the CTL-dependent induction of tumor cell apoptosis (42), it was a logical next step to determine if paclitaxel enhances the expression of DR that CTL bind to initiate apoptosis, namely DR4 and DR5. Flow cytometry was employed to show that DR4 and DR5 expression is upregulated on the surface of SCLC cells following 20 hours of treatment with 100 nM paclitaxel (Figure 1B).
PA and TRAIL synergize to kill SCLC
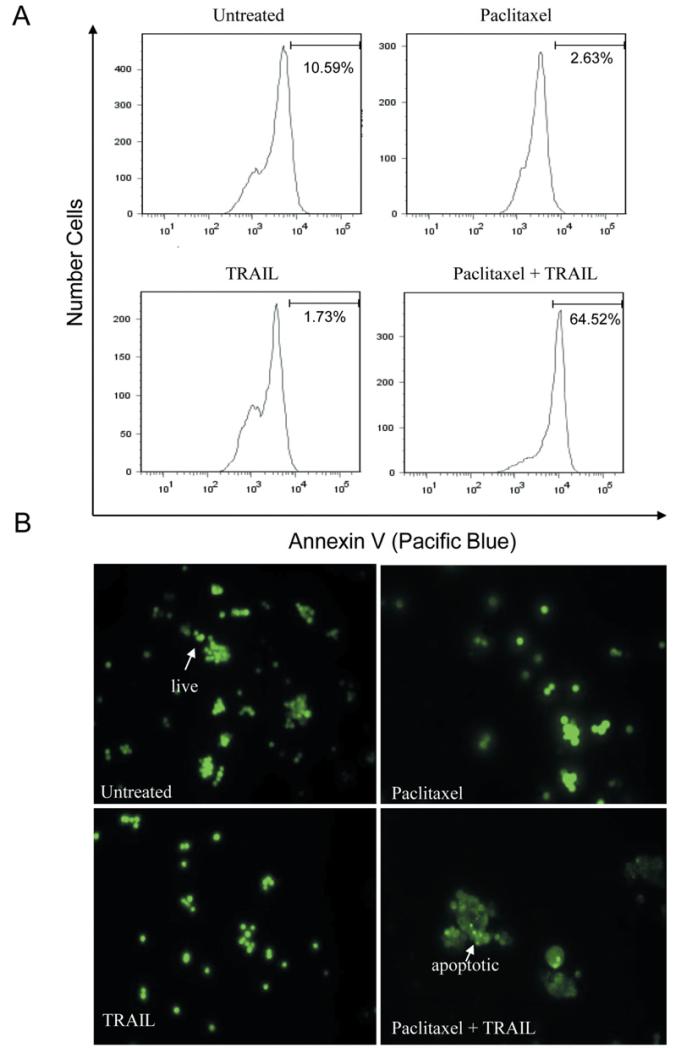
CD8+ T-cells are thought to be the principle mechanism by which the immune system recognizes and kills tumor cells, and the goal of a large proportion of proposed immunotherapies for cancer are based on promoting anti-tumor T-cell responses (43). The two major modes by which T-cells kill tumor cells are through the release of granzymes that enter the cells and set off a cascade of caspase activation and by the ligation of DR on the target cells. The most characterized death receptor ligands used by cytotoxic T-cells to induce apoptosis of target cells are FAS Ligand (FAS L), tumor necrosis factor (TNF), and TRAIL (44). Since the receptors for TRAIL (DR4 and DR5) were upregulated upon treatment with paclitaxel (Figure 1B), we next determined if paclitaxel would sensitize SCLC to killing via TRAIL. Cells were cultured alone or with paclitaxel for 20 hours. Following this incubation, the cells were then treated with TRAIL for 1-4 hours. We then evaluated apoptosis in two different experiments. Annexin staining was used to identify membrane instability (exposure of phosphotidyl serine). Paclitaxel alone induced only modest apoptosis, however when SCLC cells were treated with paclitaxel and TRAIL in combination, the number of apoptotic cells dramatically increased (Figure 2A). Concordant with the Annexin staining results, the visualization of chromatin condensation was also seen only in doubly treated cells. Either treatment alone only resulted in a minimal number of cells displaying condensed chromatin upon microscopic examination (Figure 2B).
Figure 2.
Paclitaxel and TRAIL synergize to kill SCLC cells. A: Cells were treated in the presence or absence of paclitaxel (100 nM) for 20 hours. Where indicated, 50 ng/ml TRAIL was added for the final 1-4 hours of culture. Apoptosis was also analyzed using Annexin V staining. A significant increase in Annexin staining was observed, as indicated by the histogram shift to the right. B: Cells were cultured as above, harvested, and then stained with acridine orange to visualize chromatin. Live cells have intact nuclei, whereas apoptotic cells have fragmented and condensed nuclear content.
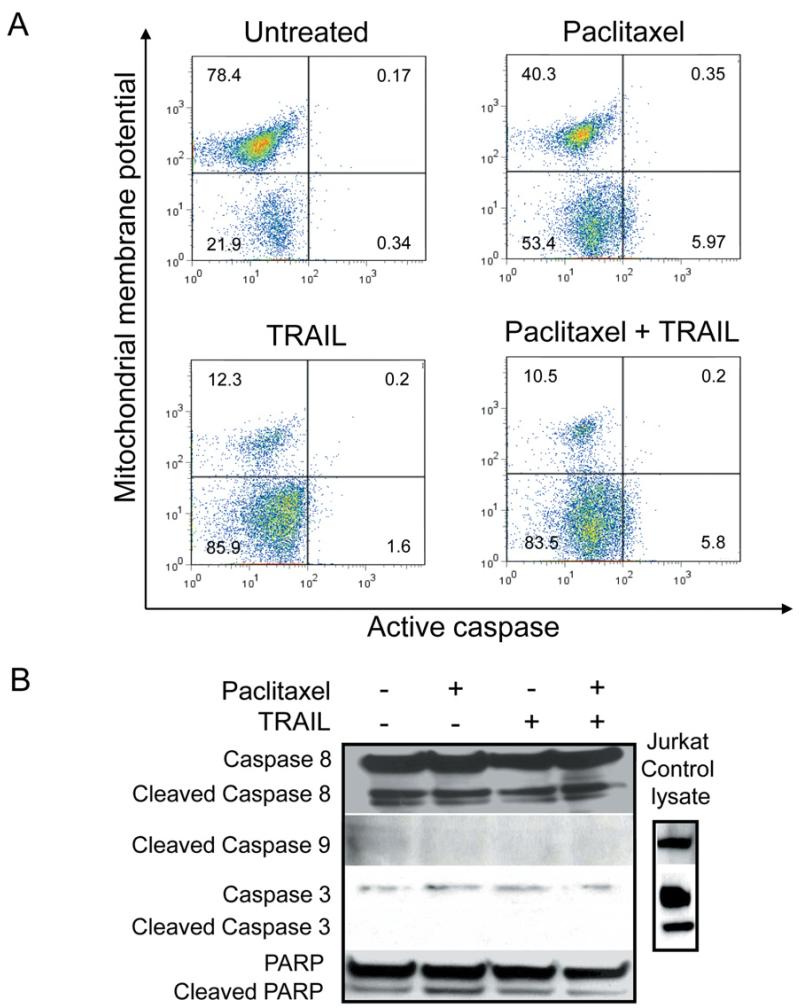
Paclitaxel/TRAIL combination induces apoptosis of SCLC through an apparent caspase-3 independent mechanism
The roles of mitochondria and caspases in the paclitaxel and TRAIL initiated-apoptosis was examined using a MitoCasp assay kit, as well as examining caspase activation. Mitochondrial membrane potential and caspase activation was determined using flow cytometry. Cells were treated with a fluorescently labeled cationic dye to detect membrane potential, or loss thereof. In healthy cells, the cationic dye is accumulated by the mitochondria in proportion to the membrane potential. In apoptotic cells, where the mitochondrial membrane potential is compromised, the cationic dye does not accumulate in the mitochondria and these cells exhibit a lower fluorescence signal. Cells were also treated with carboxyfluorescein (FAM) labeled fluoromethyl ketone (FMK)-peptide inhibitors of caspases that specifically bind active caspases. Using this assay we found that there was a loss of membrane potential in cells treated with paclitaxel, TRAIL and paclitaxel with TRAIL. There is no binding of the FAM-FMK molecules, indicating no caspase activation (Figure 3A). The loss of membrane potential in the paclitaxel and TRAIL singly treated cells was curious, as use of the two agents combined resulted in increased apoptosis as compared to either agent alone.
Figure 3.
SCLC apoptosis induced by paclitaxel/TRAIL. SCLC apoptosis induced by paclitaxel/TRAIL combination is concomitant with loss of mitochondrial membrane potential and independent of caspase activation. A: Cells were treated with or without paclitaxel (100 nM) for 20 hours. Where indicated, 50 ng/ml TRAIL was added for the final 1-4 hours of culture. Cells were harvested and stained using MitoCasp reagents. Following paclitaxel, TRAIL, or combined treatment there was a loss ofmitochondrial membrane potential. However, active caspases were not identified. Jurkat cells treated with cycloheximide and TRAIL were used as a positive control (data not shown). B: Western blot analysis was performed to verify the flow cytometric anlaysis of caspase activation: 100 μg of protein was loaded per well of the SCLC lysates, 50 μg of control Jurkat lysate (cycloheximide and TRAIL treated) was loaded. SCLC cells have lower levels of caspase-3 and -9 expression than do JurkaT-cells and no changes in cleaved caspases were observed in extracts from SCLC cells left untreated or treated with either paclitaxel, TRAIL, or the combined treatment. A representative GAPDH blot is shown to demonstrate equal protein loading.
To further confirm these results and understand the mechanism involved in the increased apoptosis induced by the paclitaxel/TRAIL combination, Western blot analysis was performed. We confirmed that paclitaxel and TRAIL (both as single agents and in combination) do not induce activation of caspases 3, 8, or 9 in 86M1 SCLC cells. We also demonstrated that the induced apoptosis, as described above, does not appear to be mediated through Poly (ADP-ribose) polymerase (PARP) cleavage (Figure 3B).
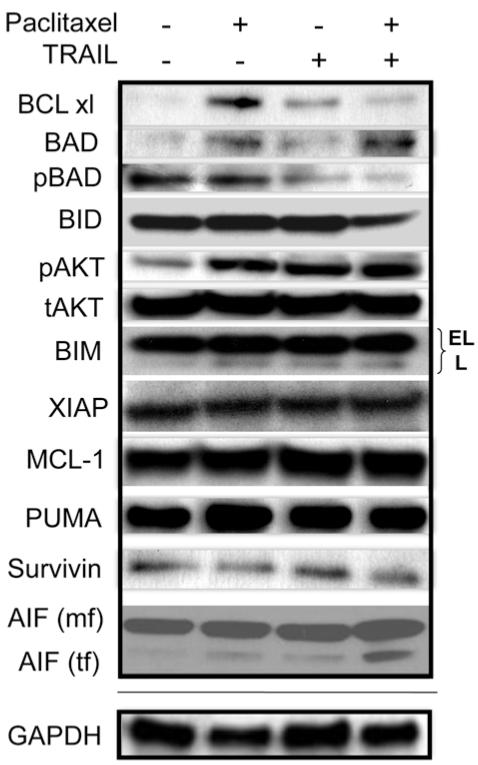
Paclitaxel actively induces resistance in SCLC tumor cells by up-regulating pro-survival BCL-xl expression
Huisman, et al. demonsrated that paclitaxel treatment of non-small cell lung cancer (NSCLC) cells triggers cell death through a caspase-independent, mitochondrial-dependent route (30). Therefore, we examined proteins that have been demonstrated to play significant roles in mitochondrial-dependent apoptosis. Most notably we observed that paclitaxel up-regulates the expression of the pro-survival protein BCL-xl (Figure 4). This increased expression was abrogated when TRAIL was combined with paclitaxel. In cells treated with both agents, another pro-survival protein, pBAD, was down-regulated as compared to paclitaxel treatment alone (Figure 4). Interestingly, there was alsoa decrease in BID expression following treatment with the paclitaxel/TRAIL combination (Figure 4). This was in contrast to a recently published report in which Huisman et al. demonstrated that paclitaxel killed non-small cell lung cancer (NSCLC) in a caspase-independent manner in which the cleavage of BID appears to play an important role in paclitaxel-specific killing (45). However, we did not observe BID cleavage with paclitaxel alone, but rather saw reduced expression of full length BID with only the doubly treated cells (Figure 4). However, as outlined below, upon mitochondrial and cytosolic fractionation, a role for BID becomes evident (Figure 5A). No changes in expression were observed for XIAP, MCL-1, Survivin, PUMA, and BIM. pAKT expression did increase upon treatment with either paclitaxel or TRAIL, as well as the combined treatment as compared to untreated cells (Figure 4). The significance of this is unknown, however Zhou et al. have demonstrated that active AKT (pAKT) plays an important role in suppressing apoptosis at the post-mitochondrial stage, i.e. downstream of cytochrome c release, and before activating caspase-9 (46). This may explain the lack of caspase activation in SCLC following treatment with paclitaxel and/or TRAIL. There was, however, low level endogenous caspase-8 activity in the cell lysates, but there was not a detectable difference in the levels of cleaved caspase-8 in untreated or singly/doubly-treated SCLC cells.
Figure 4.
Paclitaxel induces the upregulation of the expression of pro-survival BCL-xl in SCLC. SCLC cells were treated with single agent or paclitaxel/TRAIL combination (100 nM and 50 ng/ml, respectively) for 24 hours. Proteins from cell lysates were resolved on a gel and transferred to PVDF. Immunoblotting was performed using the indicated primary antibodies. Paclitaxel treatment resulted in the up-regulation of BCL-xl. BCL-xl expression decreased in SCLC treated with both paclitaxel and TRAIL. The expression of full length BID was modestly decreased in doubly treated cells. Truncated, active AIF (tf) can be seen only in doubly treated cells (mf – mitochondrial form). Apoptosis mediated by the paclitaxel/TRIAL combination occurs, at least in part, through AIF activation. A representative GAPDH blot is shown to demonstrate equal loading of protein.
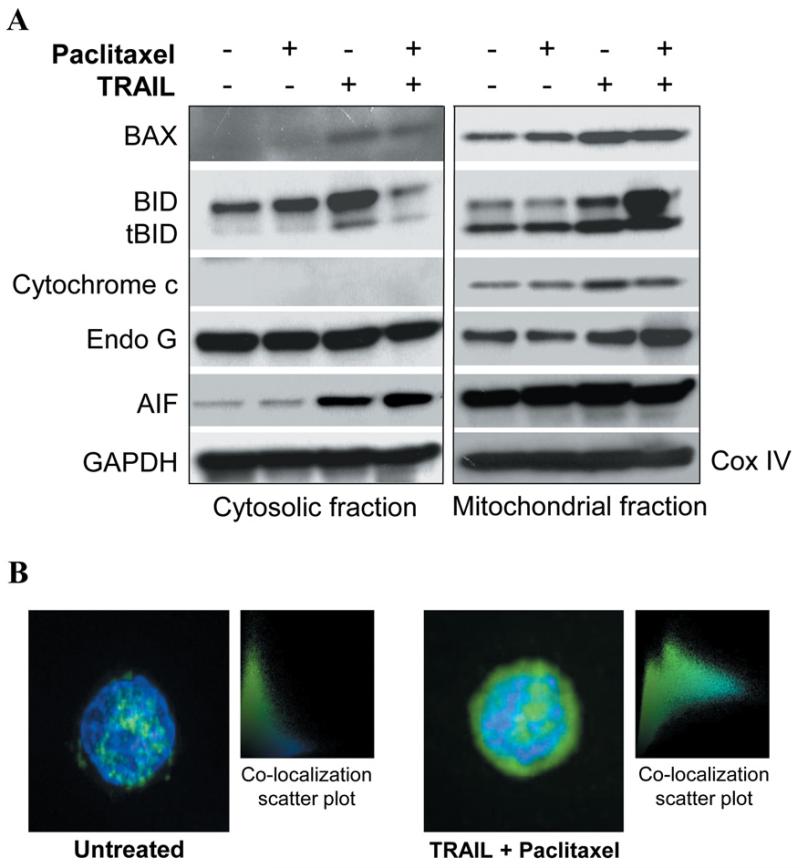
Figure 5.
Paclitaxel and TRAIL synergize to kill SCLC cells. Killing of SCLC cells by paclitaxel with TRAIL is independent of cytochrome c release and appears to be mediated through the translocation and ultimate nuclear localization of AIF. A: Mitochondrial and cytosolic fractions of SCLC treated as indicated were resolved on polyacrylamide gels, transferred and then subjected to immunoblotting with the indicated antibodies. Most notably, pro-apoptotic BID was demonstrated to leave the cytosol and enter the mitochondria, cytochrome c release from the mitochondria into cytosol was not observed, and AIF translocated into the cytosol following treatment with paclitaxel and TRAIL. GAPDH and CoxIV were used as loading controls for the cytosolic and mitochondrial fractions, respectively. B. AIF was also determined to localize in the nucleus of doubly-treated cells. Briefly, treated cells were stained with FITC-labeled AIF and DAPI. In untreated as well as singly treated cells (data not shown) AIF did not co-localize in the nucleus, however in doubly treated cells, AIF was found in the nucleus.
Paclitaxel//TRAIL combination induces AIF-dependent apoptosis in paclitaxel-resistant SCLC cells
Since PARP cleavage did not appear to be crucial in the apoptotic effect mediated by the paclitaxel/TRAIL combination, we determined which molecule was likely mediating the ultimate DNA fragmentation necessary to complete the cell death process. After separating mitochondrial and cytosolic proteins and performing immunoblotting, it was observed that although the mechanism of killing of SCLC by the paclitaxel/TRAIL combination is mitochondrial dependent, cytochrome c release is not part of the death pathway induced by this combination treatment (Figure 5A). Interestingly, the influx of BID, and to a much lesser extent of BAX, into the mitochondria was observed with doubly treated cells as compared to either paclitaxel or TRAIL treatment alone. We also found that apoptosis inducing factor (AIF) was cleaved into a mature form following co-treatment of SCLC cells with paclitaxel and TRAIL (Figure 4). Further investigation demonstrated that in PA and TRAIL doubly-treated SCLC cells, AIF does indeed leave the mitochondria, translocate through the cytosol, and localize to the nucleus of the cell where it is able to initiate DNA degradation (Figure 5A-B). No changes in Endo G expression or cellular localization were observed (Figure 5A).
Discussion
SCLC is frequently responsive to first-line chemotherapy. However, the response is often short-lived and response rates to second-line therapies are poor. We sought to determine the mechanism of resistance to paclitaxel and how this resistance can be overcome. Western blot analysis demonstrated that BCL-xl expression increases in response to paclitaxel, suggesting that this is a mechanism by which SCLC cells become resistant to this common second-line chemotherapy agent. Additionally, it was determined that biologically relevant doses of paclitaxel induced the surface expression of DR4 and DR5. Although there was not a robust increase in surface expression of the DR, in the case of DR5, the expression went from none to dim as detected by fluorescently labelled antibodies. Addition of the DR4/DR5 ligand, TRAIL, lead to the abrogation of paclitaxel-induced BCL-xl expression, and also resulted in a decrease in the expression of the pro-survival protein pBAD (47) and activation of pro-apoptotic BID. Cleaved BID translocates to mitochondria and induces cytochrome c release and mitochondrial damage (48). The combination also resulted in increased apoptosis of SCLC cells as determined by both Annexin V staining and chromatin condensation. With this treatment combination, the induced apoptosis was mediated, at least in part, through AIF and was independent of significant caspase activation and increases in PARP (Poly (ADP-ribose) polymerase) cleavage. The lack of caspase involvement in killing mediated by paclitaxel has been reported elsewhere for other cancer types, such as NSCLC (30). However, we now report that the addition of TRAIL to paclitaxel can potentiate apoptosis in a paclitaxel-resistant SCLC cell line, as well as the novel observation that the mechanism of this potentiation is through a reversal of paclitaxel-induced BCL-xl expression. Also involved in this synergistic killing, as determined by Western blot, is an up-regulation of the expression of pro-apoptotic pAKT and pBAD, concomitant with a decrease in pro-survival BAD (Figure 4), in the lysates from cells treated with the combination of paclitaxel and TRAIL. It is likely that the regulation of these apoptosis-associated proteins also contribute to the synergistic killing observed with paclitaxel and TRAIL.
Our research group recently conducted a clinical trial with a therapeutic cancer vaccine, Ad.p53-DC, for SCLC (49). Although there were some clinical responses, the majority of patients, 23 out of 27, had disease progression and were treated with additional chemotherapy. Interestingly, the patients who received additional chemotherapy subsequent to the vaccine responded at an unusually high rate and clinical response to salvage chemotherapy was closely associated with the induction of an immunological response to vaccination. This synergy has been observed by others as well (50-52). The findings presented here offer an explanation not only for the resistance of SCLC to second-line treatment with paclitaxel, but also provide a possible explanation for the increased sensitivity to second-line treatment in patients vaccinated in our Ad.p53 trial. We hypothesize that TRAIL-mediated CTL killing of SCLC cells is inadequate, however with the addition of paclitaxel, AIF-mediated killing can occur. The converse also occurs. Paclitaxel is inadequate in the absence of TRAIL, due to the induced resistance mediated through BCL-xl up-regulation. It is only when TRAIL and paclitaxel are combined that BCL-xl expression is reduced and AIF is activated and translocated. When co-delivered via polymeric micelles, the two agents are further shown to have synergistic focused anticancer activity (53). This bivalent activity reflects the widespread potential of TRAIL in countering chemoresistance, such as in combination with PPARγ ligands against ovarian cancer (54).
There is an emerging paradigm in cancer immunotherapy in which immunotherapeutic treatments may be more effective when used in direct combination with chemotherapy (49-51), (55-57). Specifically, immunotherapy alone for the treatment of SCLC is likely inefficacious for a variety of reasons. For decades, studies have suggested that tumor cells develop mechanisms to avoid immune-mediated rejection. Over twenty years ago, Doyle et al. showed that SCLC can have decreased expression of MHC I at both the mRNA and protein level, which could dramatically limit the expression of tumor-associated antigens (TAA) on the surface of the tumor cells (58). More recent studies show that there is a decrease in FAS expression on SCLC cells (59, 60) and that some lung cancer cells produce a soluble decoy receptor, DcR3, that mimics FAS and binds FAS L (61). DcR3 could act to bind FAS L on CTL and obstruct the interaction between the CTL ligand and the tumor cell receptor, allowing the tumor to escape T-cell-mediated killing. Another mechanism of immuno-evasion involving a relevant receptor pathway utilized by CTL to kill tumor cells has been described and involves the silencing or loss of DR5 and/or caspase-8 expression in SCLC (60, 62).
Additionally, it has recently been shown that there is an up-regulation of BCL-2 in SCLC and that this increased expression potentiates chemotherapy resistance in SCLC cell lines (63), possibly by blocking TRAIL-induced apoptosis (64). Interestingly, each of these described mechanisms of tumor immuno-evasion relate directly to pathways utilized by CTL to recognize and induce apoptosis of tumor cells. Emerging literature, in concordance with our data represented herein, points to a need to consider combining chemotherapy with immunotherapy. Understanding the mode of actions and the pharmaco-immunologic effects of chemotherapeutic drugs will allow for thoughtful development of combination treatment strategies, specifically with immunotherapeutic regimens, to treat cancer.
Acknowledgements
We thank and acknowledge the Flow Cytometry and the Analytical Microscopy core facilities at the Moffitt Cancer Center. This work was supported by a Flight Attendant Medical Research Insitute (FAMRI) Young Clinical Scientist Award (TBH).
Abbreviations
- 7AAD
7-Aminoactinomycin D
- AIF
apoptosis inducing factor
- BAK
Bcl-2 homologous antagonist/killer
- BAX
Bcl-2-associated X
- BCL-2
B-cell leukemia/lymphoma 2
- BCL-xl
B-cell lymphoma-extra large
- BIM
Bcl-2 interacting mediator of cell death
- CHAPS
3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate
- CTL
cytotoxic T lymphocyte
- DR4
death receptor 4
- DR5
death receptor 5
- DTT
Dithiothreitol
- ES
extensive stage
- FADD
Fas-Associated protein with Death Domain
- FAS L
FAS Ligand
- FBS
fetal bovine serum
- FITC
Fluorescein isothiocyanate
- FLICE
FADD-like interleukin-1 beta-converting enzyme (caspase 8)
- GAPDH
Glyceraldehyde 3-phosphate dehydrogenase
- LDH
lactase dehydrogenase
- MCL-1
Induced myeloid leukemia cell differentiation protein
- MHC
major histocompatibility complex
- OD
optical density
- PARP
Poly (ADP-ribose polymerase)
- PMSF
phenylmethanesulphonylfluoride
- PUMA
p53 upregulated modulator or apoptosis
- PVDF
Polyvinylidene Fluoride
- SCLC
small cell lung cancer
- SDS
sodium dodecyl sulfate
- TNF
tumor necrosis factor
- TRAIL
tumor necrosis factor related apoptosis inducing ligand
- XIAP
X-linked Inhibitor of Apoptosis Protein
Footnotes
Competing Interests
The Authors declare that they have no competing interests.
Authors’ Contribution
TBH participated in conception of the experiments and project design, performed cell death assays, cell fractionation, and microscopy, and drafted the manuscript. NM, KL and TC performed Western blot analyses. SJA assisted in conception of the project and design of the experiments and assisted in drafting of the manuscript. All authors have read and approved the final manuscript.
References
- 1.Schiller JH, Adak S, Cella D, DeVore RF, 3rd, Johnson DH. Topotecan versus observation after cisplatin plus etoposide in extensive-stage small-cell lung cancer: E7593 – a phase III trial of the Eastern Cooperative Oncology Group. J Clin Oncol. 2001;19(8):2114–2122. doi: 10.1200/JCO.2001.19.8.2114. [DOI] [PubMed] [Google Scholar]
- 2.Masters GA, Declerck L, Blanke C, et al. Phase II trial of gemcitabine in refractory or relapsed small-cell lung cancer: Eastern Cooperative Oncology Group Trial 1597. J Clin Oncol. 2003;21(8):1550–1555. doi: 10.1200/JCO.2003.09.130. [DOI] [PubMed] [Google Scholar]
- 3.Pujol JL, Daures JP, Riviere A, et al. Etoposide plus cisplatin with or without the combination of 4′-epidoxorubicin plus cyclophosphamide in treatment of extensive small-cell lung cancer: a French Federation of Cancer Institutes multicenter phase III randomized study. J Natl Cancer Inst. 2001;93(4):300–308. doi: 10.1093/jnci/93.4.300. [DOI] [PubMed] [Google Scholar]
- 4.Urban T, Chastang C, Lebas FX, et al. The addition of cisplatin to cyclophosphamide-doxorubicin-etoposide combination chemotherapy in the treatment of patients with small cell lung carcinoma: A randomized study of 457 patients. “Petites Cellules” Group. Cancer. 1999;86(11):2238–2245. [PubMed] [Google Scholar]
- 5.Eckardt JR. Emerging role of weekly topotecan in recurrent small cell lung cancer. Oncologist. 2004;9(Suppl 6):25–32. doi: 10.1634/theoncologist.9-90006-25. [DOI] [PubMed] [Google Scholar]
- 6.Sandler AB. Irinotecan in small-cell lung cancer: the US experience. Oncology (Williston Park) 2001;15(1 Suppl 1):11–12. [PubMed] [Google Scholar]
- 7.Ardizzoni A, Manegold C, Debruyne C, et al. European organization for research and treatment of cancer (EORTC) 08957 phase II study of topotecan in combination with cisplatin as second-line treatment of refractory and sensitive small cell lung cancer. Clin Cancer Res. 2003;9(1):143–150. [PubMed] [Google Scholar]
- 8.Eckardt JR. Second-line treatment of small-cell lung cancer. The case for systemic chemotherapy. Oncology (Williston Park) 2003;17(2):181–188. 91; discussion 91-2, passim. [PubMed] [Google Scholar]
- 9.Amos LA, Lowe J. How Taxol stabilises microtubule structure. Chem Biol. 1999;6(3):R65–69. doi: 10.1016/s1074-5521(99)89002-4. [DOI] [PubMed] [Google Scholar]
- 10.Crown J, O’Leary M. The taxanes: an update. Lancet. 2000;355(9210):1176–1178. doi: 10.1016/S0140-6736(00)02074-2. [DOI] [PubMed] [Google Scholar]
- 11.Vikhanskaya F, Vignati S, Beccaglia P, et al. Inactivation of p53 in a human ovarian cancer cell line increases the sensitivity to paclitaxel by inducing G2/M arrest and apoptosis. Exp Cell Res. 1998;241(1):96–101. doi: 10.1006/excr.1998.4018. [DOI] [PubMed] [Google Scholar]
- 12.Yoo YD, Park JK, Choi JY, et al. CDK4 down-regulation induced by paclitaxel is associated with G1 arrest in gastric cancer cells. Clin Cancer Res. 1998;4(12):3063–3068. [PubMed] [Google Scholar]
- 13.Jordan MA, Toso RJ, Thrower D, Wilson L. Mechanism of mitotic block and inhibition of cell proliferation by taxol at low concentrations. Proc Natl Acad Sci USA. 1993;90(20):9552–9556. doi: 10.1073/pnas.90.20.9552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Foss M, Wilcox BWL, Alsop GB, Zhang D. Taxol Crystals Can Masquerade as Stabilized Microtubules. PLoS ONE. 2008;3(1):e1476. doi: 10.1371/journal.pone.0001476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rodi DJ, Janes RW, Sanganee HJ, Holton RA, Wallace BA, Makowski L. Screening of a library of phage-displayed peptides identifies human bcl-2 as a taxol-binding protein. J Mol Biol. 1999;285(1):197–203. doi: 10.1006/jmbi.1998.2303. [DOI] [PubMed] [Google Scholar]
- 16.Rodi DJ, Makowski L. Similarity between the sequences of taxol-selected peptides and the disordered loop of the anti- apoptotic protein, Bcl-2. Pac Symp Biocomput. 1999:532–541. doi: 10.1142/9789814447300_0053. [DOI] [PubMed] [Google Scholar]
- 17.Cao L, Sun D, Cruz T, Moscarello MA, Ludwin SK, Whitaker JN. Inhibition of experimental allergic encephalomyelitis in the Lewis rat by paclitaxel. J Neuroimmunol. 2000;108(1-2):103–111. doi: 10.1016/s0165-5728(00)00268-x. [DOI] [PubMed] [Google Scholar]
- 18.Tsavaris N, Kosmas C, Vadiaka M, Kanelopoulos P, Boulamatsis D. Immune changes in patients with advanced breast cancer undergoing chemotherapy with taxanes. Br J Cancer. 2002;87(1):21–27. doi: 10.1038/sj.bjc.6600347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Javeed A, Ashraf M, Ghafoor A, Mukhtar MM. Paclitaxel and immune system. Eur J Pharm Sci. 2009 doi: 10.1016/j.ejps.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 20.Earnshaw WC, Martins LM, Kaufmann SH. Mammalian caspases: structure, activation, substrates, and functions during apoptosis. Annu Rev Biochem. 1999;68:383–424. doi: 10.1146/annurev.biochem.68.1.383. [DOI] [PubMed] [Google Scholar]
- 21.Lamarca V, Scorrano L. When separation means death: killing through the mitochondria, but starting from the endoplasmic reticulum. EMBO J. 2009;28(12):1681–1683. doi: 10.1038/emboj.2009.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Adams JM, Cory S. The Bcl-2 protein family: arbiters of cell survival. Science. 1998;281(5381):1322–1326. doi: 10.1126/science.281.5381.1322. [DOI] [PubMed] [Google Scholar]
- 23.Antignani A, Youle RJ. How do Bax and Bak lead to permeabilization of the outer mitochondrial membrane? Curr Opin Cell Biol. 2006;18(6):685–9. doi: 10.1016/j.ceb.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 24.Kayagaki N, Yamaguchi N, Nakayama M, Eto H, Okumura K, Yagita H. Type I Interferons (IFNs) Regulate Tumor Necrosis Factor-related Apoptosis-inducing Ligand (TRAIL) Expression on Human T-cells: A Novel Mechanism for the Antitumor Effects of Type I IFNs. J Exp Med. 1999;189(9):1451–1460. doi: 10.1084/jem.189.9.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carrington PE, Sandu C, Wei Y, et al. The structure of FADD and its mode of interaction with procaspase-8. Mol Cell. 2006;22(5):599–610. doi: 10.1016/j.molcel.2006.04.018. [DOI] [PubMed] [Google Scholar]
- 26.Kaufmann M, Bozic D, Briand C, et al. Identification of a basic surface area of the FADD death effector domain critical for apoptotic signaling. FEBS Lett. 2002;527(1-3):250–254. doi: 10.1016/s0014-5793(02)03146-0. [DOI] [PubMed] [Google Scholar]
- 27.Boldin MP, Varfolomeev EE, Pancer Z, Mett IL, Camonis JH, Wallach D. A novel protein that interacts with the death domain of Fas/APO1 contains a sequence motif related to the death domain. J Biol Chem. 1995;270(14):7795–7798. doi: 10.1074/jbc.270.14.7795. [DOI] [PubMed] [Google Scholar]
- 28.Peter ME. The TRAIL DISCussion: It is FADD and caspase-8! Cell Death Differ. 2000;7(9):759–760. doi: 10.1038/sj.cdd.4400735. [DOI] [PubMed] [Google Scholar]
- 29.Huang Y, Sheikh MS, Fornace AJ, Jr., Holbrook NJ. Serine protease inhibitor TPCK prevents Taxol-induced cell death and blocks c-Raf-1 and Bcl-2 phosphorylation in human breast carcinoma cells. Oncogene. 1999;18(23):3431–3439. doi: 10.1038/sj.onc.1202685. [DOI] [PubMed] [Google Scholar]
- 30.Huisman C, Ferreira CG, Broker LE, et al. Paclitaxel triggers cell death primarily via caspase-independent routes in the non-small cell lung cancer cell line NCI-H460. Clin Cancer Res. 2002;8(2):596–606. [PubMed] [Google Scholar]
- 31.Ofir R, Seidman R, Rabinski T, et al. Taxol-induced apoptosis in human SKOV3 ovarian and MCF7 breast carcinoma cells is caspase-3 and caspase-9 independent. Cell Death Differ. 2002;9(6):636–642. doi: 10.1038/sj.cdd.4401012. [DOI] [PubMed] [Google Scholar]
- 32.Park SJ, Wu CH, Gordon JD, Zhong X, Emami A, Safa AR. Taxol induces caspase-10-dependent apoptosis. J Biol Chem. 2004;279(49):51057–51067. doi: 10.1074/jbc.M406543200. [DOI] [PubMed] [Google Scholar]
- 33.Wieder T, Essmann F, Prokop A, et al. Activation of caspase-8 in drug-induced apoptosis of B-lymphoid cells is independent of CD95/Fas receptor-ligand interaction and occurs downstream of caspase-3. Blood. 2001;97(5):1378–1387. doi: 10.1182/blood.v97.5.1378. [DOI] [PubMed] [Google Scholar]
- 34.Blagosklonny MV, Schulte T, Nguyen P, Trepel J, Neckers LM. Taxol-induced apoptosis and phosphorylation of Bcl-2 protein involves c-Raf-1 and represents a novel c-Raf-1 signal transduction pathway. Cancer Res. 1996;56(8):1851–1854. [PubMed] [Google Scholar]
- 35.Berndtsson M, Konishi Y, Bonni A, et al. Phosphorylation of BAD at Ser-128 during mitosis and paclitaxel-induced apoptosis. FEBS Letters. 2005;579(14):3090. doi: 10.1016/j.febslet.2005.04.067. [DOI] [PubMed] [Google Scholar]
- 36.Li R, Moudgil T, Ross HJ, Hu HM. Apoptosis of non-small-cell lung cancer cell lines after paclitaxel treatment involves the BH3-only proapoptotic protein Bim. Cell Death Differ. 2005;12(3):292–303. doi: 10.1038/sj.cdd.4401554. [DOI] [PubMed] [Google Scholar]
- 37.Nimmanapalli R, Perkins CL, Orlando M, O’Bryan E, Nguyen D, Bhalla KN. Pretreatment with Paclitaxel Enhances Apo-2 Ligand/Tumor Necrosis Factor-related Apoptosis-inducing Ligand-induced Apoptosis of Prostate Cancer Cells by Inducing Death Receptors 4 and 5 Protein Levels. Cancer Res. 2001;61(2):759–763. [PubMed] [Google Scholar]
- 38.Blagosklonny MV, Fojo T. Molecular effects of paclitaxel: myths and reality (a critical review) Int J Cancer. 1999;83(2):151–156. doi: 10.1002/(sici)1097-0215(19991008)83:2<151::aid-ijc1>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 39.Bepler G, Jaques G, Koehler A, Gropp C, Havemann K. Markers and characteristics of human SCLC cell lines. Neuroendocrine markers, classical tumor markers, and chromosomal characteristics of permanent human small cell lung cancer cell lines. J Cancer Res Clin Oncol. 1987;113(3):253–259. doi: 10.1007/BF00396382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bepler G, Jaques G, Neumann K, Aumuller G, Gropp C, Havemann K. Establishment, growth properties, and morphological characteristics of permanent human small cell lung cancer cell lines. J Cancer Res Clin Oncol. 1987;113(1):31–40. doi: 10.1007/BF00389964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ribble D, Goldstein N, Norris D, Shellman Y. A simple technique for quantifying apoptosis in 96-well plates. BMC Biotechnology. 2005;5(1):12. doi: 10.1186/1472-6750-5-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Casares N, Pequignot MO, Tesniere A, et al. Caspase-dependent immunogenicity of doxorubicin-induced tumor cell death. J Exp Med. 2005;202(12):1691–701. doi: 10.1084/jem.20050915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rosenberg SA. Progress in human tumour immunology and immunotherapy. Nature. 2001;411(6835):380–384. doi: 10.1038/35077246. [DOI] [PubMed] [Google Scholar]
- 44.Park S-M, Schickel R, Peter ME. Nonapoptotic functions of FADD-binding death receptors and their signaling molecules. Current Opinion in Cell Biology. 2005;17(6):610. doi: 10.1016/j.ceb.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 45.Huisman C, Ferreira CG, Broker LE, et al. Paclitaxel Triggers Cell Death Primarily via Caspase-independent Routes in the Non-Small Cell Lung Cancer Cell Line NCI-H460. Clin Cancer Res. 2002;8(2):596–606. [PubMed] [Google Scholar]
- 46.Zhou H, Li X-M, Meinkoth J, Pittman RN. Akt Regulates Cell Survival and Apoptosis at a Postmitochondrial Level. J Cell Biol. 2000;151(3):483–494. doi: 10.1083/jcb.151.3.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zha J, Harada H, Yang E, Jockel J, Korsmeyer SJ. Serine phosphorylation of death agonist BAD in response to survival factor results in binding to 14-3-3 not BCL-X(L) Cell. 1996;87(4):619–628. doi: 10.1016/s0092-8674(00)81382-3. [DOI] [PubMed] [Google Scholar]
- 48.Luo X, Budihardjo I, Zou H, Slaughter C, Wang X. Bid, a Bcl2 interacting protein, mediates cytochrome c release from mitochondria in response to activation of cell surface death receptors. Cell. 1998;94(4):481–490. doi: 10.1016/s0092-8674(00)81589-5. [DOI] [PubMed] [Google Scholar]
- 49.Antonia SJ, Mirza N, Fricke I, et al. Combination of p53 cancer vaccine with chemotherapy in patients with extensive stage small cell lung cancer. Clin Cancer Res. 2006;12(3 Pt 1):878–887. doi: 10.1158/1078-0432.CCR-05-2013. [DOI] [PubMed] [Google Scholar]
- 50.Wheeler CJ, Das A, Liu G, Yu JS, Black KL. Clinical responsiveness of glioblastoma multiforme to chemotherapy after vaccination. Clin Cancer Res. 2004;10(16):5316–5326. doi: 10.1158/1078-0432.CCR-04-0497. [DOI] [PubMed] [Google Scholar]
- 51.Gribben JG, Ryan DP, Boyajian R, et al. Unexpected association between induction of immunity to the universal tumor antigen CYP1B1 and response to next therapy. Clin Cancer Res. 2005;11(12):4430–4436. doi: 10.1158/1078-0432.CCR-04-2111. [DOI] [PubMed] [Google Scholar]
- 52.Arlen PM, Gulley JL, Parker C, et al. A randomized phase II study of concurrent docetaxel plus vaccine versus vaccine alone in metastatic androgen-independent prostate cancer. Clin Cancer Res. 2006;12(4):1260–1269. doi: 10.1158/1078-0432.CCR-05-2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lee ALWY, Pervaiz S, Fan W, Yang YY. Synergistic anticancer effects achieved by co-delivery of TRAIL and paclitaxel using cationic polymeric micelles. Macromol Biosci. 2011;11(2):296–307. doi: 10.1002/mabi.201000332. [DOI] [PubMed] [Google Scholar]
- 54.Bräutigam KB-WJ, Bauerschlag DO, von Kaisenberg CS, Jonat W, Maass N, Arnold N, Meinhold-Heerlein I. Combined treatment with TRAIL and PPARγ ligands overcomes chemoresistance of ovarian cancer cell lines. J Cancer Res Clin Oncol. 2010:1–12. doi: 10.1007/s00432-010-0952-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lake RA, Robinson BW. Immunotherapy and chemotherapy – a practical partnership. Nat Rev Cancer. 2005;5(5):397–405. doi: 10.1038/nrc1613. [DOI] [PubMed] [Google Scholar]
- 56.Gabrilovich DI. Combination of chemotherapy and immunotherapy for cancer: a paradigm revisited. Lancet Oncol. 2007;8(1):2–3. doi: 10.1016/S1470-2045(06)70985-8. [DOI] [PubMed] [Google Scholar]
- 57.Andersen MH, Sorensen RB, Schrama D, Svane IM, Becker JC, Thor Straten P. Cancer treatment: the combination of vaccination with other therapies. Cancer Immunol Immunother. 2008;57(11):1735–1743. doi: 10.1007/s00262-008-0480-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Doyle A, Martin WJ, Funa K, et al. Markedly decreased expression of class I histocompatibility antigens, protein, and mRNA in human small-cell lung cancer. J Exp Med. 1985;161(5):1135–1151. doi: 10.1084/jem.161.5.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Viard-Leveugle I, Veyrenc S, French LE, Brambilla C, Brambilla E. Frequent loss of Fas expression and function in human lung tumours with overexpression of FasL in small cell lung carcinoma. J Pathol. 2003;201(2):268–277. doi: 10.1002/path.1428. [DOI] [PubMed] [Google Scholar]
- 60.Shivapurkar N, Reddy J, Matta H, et al. Loss of expression of death-inducing signaling complex (DISC) components in lung cancer cell lines and the influence of MYC amplification. Oncogene. 2002;21(55):8510–8514. doi: 10.1038/sj.onc.1205941. [DOI] [PubMed] [Google Scholar]
- 61.Pitti RM, Marsters SA, Lawrence DA, et al. Genomic amplification of a decoy receptor for FAS Ligand in lung and colon cancer. Nature. 1998;396(6712):699–703. doi: 10.1038/25387. [DOI] [PubMed] [Google Scholar]
- 62.Hopkins-Donaldson S, Ziegler A, Kurtz S, et al. Silencing of death receptor and caspase-8 expression in small cell lung carcinoma cell lines and tumors by DNA methylation. Cell Death Differ. 2003;10(3):356–364. doi: 10.1038/sj.cdd.4401157. [DOI] [PubMed] [Google Scholar]
- 63.Sartorius UA, Krammer PH. Up-regulation of Bcl-2 is involved in the mediation of chemotherapy resistance in human small cell lung cancer cell lines. Int J Cancer. 2002;97(5):584–592. doi: 10.1002/ijc.10096. [DOI] [PubMed] [Google Scholar]
- 64.Sun S-Y, Yue P, Zhou J-Y, et al. Overexpression of Bcl2 Blocks TNF-Related Apoptosis-Inducing Ligand (TRAIL)-Induced Apoptosis in Human Lung Cancer Cells. Biochemical and Biophysical Research Communications. 2001;280(3):788. doi: 10.1006/bbrc.2000.4218. [DOI] [PubMed] [Google Scholar]