Abstract
Introduction
Neuroblastoma (NB) is the most common and deadly solid tumor in children. Despite recent improvements, the long-term outlook for high-risk NB is still < 50%. Further, there is considerable short- and long-term toxicity. More effective, less toxic therapy is needed, and the development of targeted therapies offers great promise.
Areas covered
Relevant literature was reviewed to identify current and future therapeutic targets that are critical to malignant transformation and progression of NB. The potential or actual NB therapeutic targets are classified into four categories: i) genes activated by amplification, mutation, translocation or autocrine overexpression; ii) genes inactivated by deletion, mutation or epigenetic silencing; iii) membrane-associated genes expressed on most NBs but few other tissues; or iv) common target genes relevant to NB as well as other tumors.
Expert opinion
Therapeutic approaches have been developed to some of these targets, but many remain untargeted at the present time. It is unlikely that single targeted agents will be sufficient for long-term cure, at least for high-risk NBs. The challenge will be how to integrate targeted agents with each other and with conventional therapy to enhance their efficacy, while simultaneously reducing systemic toxicity.
Keywords: ARID1A/B, ATRX, AURKA, BCL2, CAMTA1, CASZ1, CHD5, CHEK1/CHK1, FOXR1, GD2, HDAC, KIF1Bβ, MCL1, MYCN, NCAM, NET, neuroblastoma, NTRK1/TrkA, NTRK2/TrkB, ODC, PTPN11, RAS, TP53/P53
1. Introduction
Neuroblastoma (NB) is the most common and deadly solid tumor in children. NB, a tumor of the sympathetic nervous system, accounts for 8 - 10% of all childhood cancers and 15% of deaths from pediatric cancer [1]. Some infants experience spontaneous regression, whereas older patients have maturation of their tumor into benign ganglioneuromas. Unfortunately, the majority of patients have metastatic disease, and many progress relentlessly despite intensive multimodality treatment. So, despite dramatic improvements in the cure rate for other pediatric neoplasms, the survival rate for NB patients has lagged behind. Further, most agents are being used at maximally tolerated doses, and there are serious short- and long-term toxicities in many patients. Nevertheless, recent advances in understanding the molecular pathogenesis of NB have provided considerable insight into the genetic and biochemical mechanisms underlying the more favorable or more aggressive clinical behaviors of NBs [2,3]. These, in turn, have identified the genes, proteins and pathways that should be effective targets for biologically based therapy.
Favorable NBs are usually hyperdiploid or near triploid with whole chromosome gains, but with few structural rearrangements. In contrast, high-risk NBs are usually near diploid with gross chromosomal aberrations, including cytogenetic evidence of gene amplification, trisomy of 1q and 17q, as well as deletions of 1p (1p36), 3p, 4p, 11q (11q23) and 14q [4,5]. These sites of recurring rearrangement were first identified using cytogenetic approaches, and all have been confirmed by higher resolution molecular approaches, but very few novel rearrangements have been identified. Next-generation sequencing has been applied to a small number of NBs. Overall, mutations are infrequent compared to adult tumors, but a few additional genes have been identified that have recurring mutations. Surprisingly, genes that are commonly mutated in adult cancers, such as TP53 and RAS genes, are rarely mutated in NBs, at least at diagnosis. Nevertheless, a number of novel therapeutic targets have been identified as a result of these efforts.
The potential or actual NB therapeutic targets have been classified into four categories: i) genes activated by amplification, mutation, translocation or autocrine overexpression; ii) genes inactivated by deletion, mutation or epigenetic silencing; iii) membrane-associated genes expressed on most NBs but few other tissues; or iv) common target genes relevant to NB as well as other tumors. Because the prevalence of most mutations or recurring genomic rearrangements found in NBs is < 10%, a threshold prevalence of 1% was used for inclusion. Constitutional genetic changes are not considered targets unless they also occur as somatically acquired gene mutations in sporadic tumors.
2. Genes activated by amplification, mutation, translocation or autocrine overexpression
2.1 Oncogenic drivers of NB
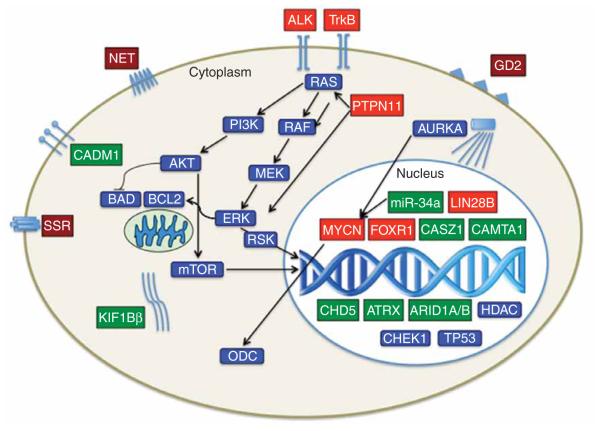
A major focus of attention has been on the identification of oncogenic drivers of NB. The first and best example of this is amplification of the MYCN proto-oncogene. However, there are now several additional examples of oncogenic drivers that result from amplification, mutation, translocation or autocrine overexpression. Given their critical role in driving malignant transformation or progression, as well as promoting survival and proliferation, they are particularly attractive as therapeutic targets (see Figure 1 and Table 1, for further details).
Figure 1. Diagrammatic representation and intracellular location of therapeutic targets of NB.
Activated genes and their encoded proteins are shown in red; inactivated genes/proteins are shown in green; selectively expressed membrane proteins (e.g., NET, GD2) are shown in burgundy; and common targets relevant to NBs and other cancers are shown in blue.
Table 1.
Genes activated by amplification, mutation, translocation or autocrine overexpression.
| Gene | Alteration | Frequency | Function | Ref. |
|---|---|---|---|---|
| ALK | Activating mutation/amplification | 9%/2 – 3% | Tyrosine kinase receptor | [16,22-28] |
| FOXR1 | Translocation/fusion | Unknown | FOXO transcription factor | [63] |
| LIN28B | Amplification/overexpression | Unknown | Regulates let-7 miRNAs | [64] |
| MYCN | Amplification/activating mutation | 22%/2% | BHLH-LZ transcription factor | [6-16] |
| ODC | Amplification/overexpression | Unknown | Rate-limiting enzyme in polyamine biosynthesis |
[18-20] |
| PTPN11 | Activating mutation | 2.9% | Protein tyrosine phosphatase | [16] |
| NTRK2/TrkB | Autocrine activation | 36% | Tyrosine kinase receptor | [35-45,47-57] |
2.1.1 MYCN transcription factor
MYCN was first identified as a MYC-related oncogene amplified in NBs and the presumed target of this gene amplification [6]. MYCN maps to 2p23 and encodes a nuclear transcription factor in the basic helix-loop-helix leucine zipper family [7]. MYCN protein forms a heterodimer with MAX in order to bind to E-boxes (canonically CACGTG) in promoter or other regulatory regions of target genes. MYCN presumably facilitates transcriptional activation (or suppression) of genes related to cell cycle progression. Although MYC is expressed ubiquitously, MYCN is more selectively expressed, especially in the nervous system, but it clearly plays a role in non-neural tissues and tumors as well.
The overall prevalence of MYCN amplification in NBs is about 22%, and amplification is strongly associated with advanced stages of disease, unfavorable biological features and a poor outcome [8,9]. MYCN amplification is also associated with poor outcome in otherwise favorable patient groups (e.g., infants and patients with lower stages of disease), underscoring its biological importance [9-13]. Because of the dramatic degree of MYCN amplification and consequent overexpression in a subset of aggressive NB, it should be an attractive therapeutic target [14,15]. Next-generation sequencing has identified a small percentage of MYCN mutations (1.7%) [16], which are presumably activating, but mutations in other MYCN-interacting genes or target genes are found rarely. As a nuclear oncoprotein, MYCN is difficult to target therapeutically. However, one group conducted a cell-based screen of cancer cell lines and identified JQ1, an inhibitor of the bromodomain and extra-terminal class of proteins, as a potent inhibitor of MYCN [17]. JQ1 displaces BRD4 from the MYCN promoter, leading to inhibition of MYCN transcription, cell cycle arrest and apoptosis.
2.1.2 Ornithine decarboxylase
Ornithine decarboxylase (ODC1) encodes the rate-limiting enzyme involved in polyamine synthesis. Polyamines are organic cations that enhance transcription, translation and replication and support many cellular processes that are governed by MYC genes, including MYCN. Indeed, ODC1 is a target of MYCN transcriptional control. Deregulated ODC1 can lead to polyamine accumulation and enhanced proliferation. Further, ODC1 maps to 2p23, and it is sometimes co-amplified with MYCN in high-risk NBs [18-20]. Inhibiting ODC1 activity with α-difluoromethylornithine impairs tumor growth in both MYCN amplified tumors and MYCN non-amplified tumors [18-20].
2.1.3 Anaplastic lymphoma kinase
Anaplastic lymphoma kinase (ALK) encodes a receptor tyrosine kinase (RTK) that is prevalently expressed in the nervous system. The cognate ligand(s) for ALK are unknown, but it is postulated that midkine and/or pleiotrophin may serve this role. ALK was identified based on its involvement in a t(2;5) translocation in anaplastic large cell lymphoma, resulting in a chimeric gene (and protein) encoded by the fusion of the ALK and NPM genes [21]. However, ALK is involved in translocations with other partners in a subset of small-cell lung cancer and in inflammatory myoblastic tumors [22].
ALK was identified as the major NB predisposition gene by several independent groups as a result of linkage studies in families carrying this preposition [23-26]. However, unlike other tumors, ALK was activated by mutation in the kinase domain. Subsequently, it was shown that ALK activation occurs by mutation (9%) or by gene amplification (2 - 3%) in sporadic NBs [16]. ALK-amplified tumors generally represent a subset of MYCN-amplified tumors, and ALK amplification seldom occurs independently. Crizotinib was developed as an inhibitor of ALK activation in adult cancers, and this drug is effective against most (but not all) NBs with ALK activation [27-29]. Like many RTK inhibitors, resistance develops quickly in many cases, especially if it is used as a single agent. Crizotinib resistance may be due to intrinsic resistance (conferred by the F1174L mutation), upregulating other RTKs or other mechanisms [30-32]. Newer inhibitors are being developed to address intrinsic resistance.
2.1.4 TRK family RTKs (especially TrkB)
The TRK family neurotrophin receptors play critical roles in development and maintenance of the central and peripheral nervous system [33,34]. TrkA, TrkB and TrkC are the cognate receptors for nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF) and neurotrophin-3 (NT3), respectively. TrkB also binds NT4, and all three receptors can bind and be activated by NT3. TrkA (and TrkC) is important for the development of sensory and sympathetic neurons, whereas TrkB is more important for motor neuron development.
TrkA and TrkB have also been implicated in the clinical and biological behavior of favorable and unfavorable NBs, respectively [35-45]. TrkA is expressed in biologically favorable tumors, and the TrkA/NGF pathway may play a role in the spontaneous regression or differentiation seen, depending on the absence or presence of NGF in the microenvironment. TrkC is expressed in a subset of favorable NBs, but TrkA is likely the most important driver of normal neural development [46]. Indeed, given its probable role in neural differentiation and cessation of proliferation, TrkA more appropriately belongs in the tumor suppressor category, but there is no evidence to date of selective inactivation of TrkA in NBs. However, TrkAIII, an isoform of TrkA, is constitutively active, induced by hypoxia, and may contribute to more aggressive behavior in some NBs with high TrkA expression [47,48].
TrkB is co-expressed with its ligand, BDNF, and this autocrine pathway leads to invasion, metastasis, angiogenesis and drug resistance but not differentiation [49-52]. TrkB expression and activation also suppresses anoikis, favoring the development of metastasis. TrkB and BDNF is overexpressed in 36% of NBs. Mutations in TRK genes have not been found, but there is autocrine activation of the TrkB/BDNF pathway in 50 - 60% of high-risk NBs, especially those with MYCN amplification [40], making this one of the most attractive targets for NB biological therapy. TrkB inhibition in vitro and in animal models leads to apoptosis and sensitivity to chemotherapy [50,53-56]. Indeed, in a Phase I clinical trial of the TRK inhibitor lestaurtinib, durable responses were seen in 50% of patients who received biologically significant doses [57].
2.1.5 Protein tyrosine phosphatase non-receptor 11
Activating mutations in protein tyrosine phosphatase non-receptor 11 (PTPN11) are found in about 50% of patients with Noonan syndrome, and these lead to unregulated activity [58]. PTPN11 encodes a protein tyrosine phosphatase SHP2 that regulates the activity of the RAS/MAPK pathway, which in turn controls a variety of cellular processes, including cell growth, differentiation, mitotic cycle and oncogenic transformation. Noonan syndrome patients are at increased risk for various types of cancer, including leukemias, sarcomas and epithelial cancers [59,60]. A comprehensive genomic analysis of high-risk NBs identified somatic mutations (presumably activating mutations) in 2.9% of cases [16]. These mutations may mimic the effects of an activated RTK, leading to constitutive signaling through the RAS/MAPK signaling pathway.
2.1.6 Forkhead box protein R1
FOXO family genes (FOXO1, FOXO3 and FOXO4) have been implicated in the genesis of several pediatric and adult cancers [61]. FOXO genes are generally thought to be tumor suppressor genes (TSGs), but there are notable exceptions. FOXM1 and FOXP1 are oncogenic when overexpressed, and FOXO1 is fused to either PAX3 or PAX7 by a translocation event in alveolar rhabdomyosarcoma [62]. Forkhead box protein R1 (FOXR1) is located at 11q23, a site of frequent deletion or rearrangement in NBs. An extensive analysis of this region used single-nucleotide polymorphism arrays, expression arrays and comparative genomic hybridization, leading to the identification of intrachromosomal deletion or fusion events that involved fusing myeloid lymphoid leukemia (MLL) or platelet-activating factor acetylhydrolase 1b, catalytic subunit 2 (PAFAH1B2) to FOXR1 [63]. To date, this is the only recurring ‘translocation’ that has been identified in NBs that involves a specific gene. Functional data indicated that these fusions were oncogenic drivers, because RNAi silencing of FOXR1 strongly inhibited proliferation of cells with this gene fusion [63]. Although several NBs were identified with a FOXR1 fusion event, the prevalence of this rearrangement is not known yet, but given the prevalence of 11q23 deletions or rearrangements, it is likely an important target.
2.1.7 LIN28B
LIN28B regulates developmental processes by modulating expression of microRNAs (miRNAs) of the let-7 family. LIN28B is selectively overexpressed in high-risk NBs, and high LIN28B expression is associated with adverse risk factors and poor long-term survival [64]. Further, overexpression of LIN28B in adrenergic neuronal cells in a mouse model was associated with lower let-7 levels and induced the development of NB. Thus, the LIN28B/let-7 pathway may be an attractive target for NBs.
2.1.8 Survivin/BIRC5 and NME1
Unbalanced gain of 17q may be the most common cytogenetic change in high-risk NBs. Although the region of gain is variable, the region around 17q21 - 25 is usually involved. At least two genes mapping to this region have been implicated as having a survival advantage or contributing to the aggressive phenotype associated with 17q gain: survivin/BIRC5 and nm23/NME1. Survivin/BIRC5 is an anti-apoptotic, pro-survival protein, and its expression is associated with adverse prognostic variables and poor prognosis [65,66]. Nm23/NME1 encodes a nucleoside diphosphate kinases involved in cell proliferation and differentiation. Mutation, amplification and overexpression of NME1 are associated with aggressive features and unfavorable outcome [67-69]. However, it is unknown if these two genes or other genes are the target of 17q gain.
3. Genes inactivated by deletion, mutation or epigenetic silencing
3.1 TSGs deleted from 1p36 or 11q23, or identified by mutation
Deletion of the distal short arm of chromosome 1 occurs in about 35% of NBs, and it is strongly correlated with MYCN amplification. Indeed, almost all tumors and cell lines with MYCN amplification have 1p deletion. The region of consistent deletion is limited to < 2 Mb of 1p36.3, although other groups have found similar regions of overlapping deletion. Most 1p deletions are large and encompass a number of candidate TSGs. For a gene to be included as a 1p36 TSG here, there needed to be decreased expression in tumors with 1p deletion, as well as structural or functional evidence that it functions as a TSG in NB. These include: CHD5, CAMTA1, miR-34a, KIF1Bβ, CASZ1 and AT-rich interactive domain-containing proteins 1A and 1B (ARID1A/B). Deletions or rearrangements of 11q23 occur in 20 - 25% of NBs, and so far only a single gene (cell adhesion molecule-1 [CADM1]) has evidence supporting its role as a TSG in NB. Finally, integrated genomic analyses and high-resolution DNA sequencing has identified additional genes with inactivating mutations that are likely involved in NB pathogenesis (ARID1A/B and α-thalassemia/mental retardation syndrome [ATRX]).
3.1.1 Chromodomain-helicase-DNA binding gene 5
Chromodomain-helicase-DNA binding gene 5 (CHD5) is the fifth member of the nine member CHD family of chromatin remodeling proteins (Table 2, Figure 1). CHD5 was first identified as a TSG that was consistently deleted in a large series of NBs [70]. CHD5 is most homologous to CHD4 and CHD3, which form nucleosome remodeling and deacetylation (NuRD) complexes [71,72]. NuRD-type chromatin remodeling complexes are generally considered to be transcriptionally repressive. They contain histone deacetylases (HDACs), methyl-DNA binding proteins and other components that facilitate binding to DNA and modulate the expression of particular genes or transcriptional domains. CHD4 is expressed ubiquitously, whereas CHD5 is preferentially expressed in the nervous system and testis.
Table 2.
Genes inactivated by deletion, mutation or epigenetic silencing.
| Gene | Location | Start site | Frequency | Function | Ref. |
|---|---|---|---|---|---|
| ARID1A/B | 1p36.1 | 27,022 kb | Del ~ 35% | SWI/SNF chromatin remodeling protein | [103] |
| ATRX | Xq21 | 76,760 kb | Mut 2.5% | Chromatin remodeling protein | [16,108,109] |
| CADM1 | 11q23 | 115,039 kb | Del ~ 20% | Cell adhesion molecule | [104-107] |
| CAMTA1 | 1p36.31 | 6,845 kb | Del ~ 35%% | Calmodulin-binding transcription factor | [83,84] |
| CASZ1 | 1p36.22 | 10,696 kb | Del ~ 35% | Zinc finger transcription factor | [97-99] |
| CHD5 | 1p36.31 | 6,161 kb | Del ~ 35% | CHD chromatin remodeling protein | [70,73-75] |
| KIF1B β | 1p36.22 | 10,270 kb | Del ~ 35% | Kinesin 3 cytoplasmic transport protein | [92,93,95,96] |
| miR-34a | 1p36.22 | 9,211 kb | Del ~ 35% | Noncoding miRNA, targets MYCN | [87-91] |
Del: Deletion; Mut: Mutation.
CHD5 was first identified as a candidate TSG that is frequently deleted from 1p36.31 in NBs [70]. CHD5 clearly functions as a TSG in NBs, since transfection of CHD5 into NB lines with 1p deletion and low/absent CHD5 expression causes suppression of clonogenicity and tumorigenicity [73]. In addition, high CHD5 is strongly correlated with favorable clinical and biological features as well as outcome, whereas low/absent expression is associated with higher stage, older age, MYCN amplification and a poor outcome [67,73-75]. Although one copy of CHD5 is deleted, the remaining copy is not mutated or inactivated by DNA rearrangement. However, the CHD5 promoter is heavily methylated, suggesting that there is epigenetic silencing. Further, CHD5 can be reexpressed by exposure to the demethylating agent 5-aza-2′-deoxycytidine [73]. Finally, CHD5 is apparently involved in a variety of other cancer types, thus suggesting that it is an important TSG [76-82]. Thus, CHD5 is a bona fide TSG in NBs, but the remaining allele is transcriptionally silenced by an epigenetic mechanism, rather than mutation.
3.1.2 Calmodulin binding transcription activator 1
CAMTA1 is a calmodulin-binding transcription activator that is frequently deleted from 1p36 in NBs. Low CAMTA1 expression is associated with unfavorable features (MYCN amplification, advanced stage) and unfavorable outcome [83]. Further, ectopic expression of CAMTA1 in NB cells with low endogenous expression resulted in decreased proliferation in vitro, inhibited colony formation, neural differentiation and suppressed growth of xenografts in a mouse model [84]. A study using a chromosome engineering mouse model of 1p deletion (orthologous region on mouse chromosome 4) found that shRNAs designed to inhibit CHD5 could mimic the proliferative advantage of deleting a larger region, but inhibition of CAMTA1 had no effect [76]. Nevertheless, based on its effects in vitro on NB cell growth and in vivo on NB xenografts, CAMTA1 qualifies as a bona fide TSG in NB.
3.1.3 MicroRNA-34a
The miRNAs are endogenously expressed small noncoding RNAs that regulate the genome in a post-transcriptional manner [85]. The miRNA-34a (miR-34a) is a putative tumor suppressor that maps to 1p36.23. The miRNA-34 family has two additional members, miR-34b and miR-34c, which map to 11q23, and both can function as TSGs in other systems [86]. Studies have shown that miR-34a is expressed at lower levels in unfavorable primary NB tumors and cell lines relative to normal adrenal tissue [86]. Overexpression of miR-34a in 1p36 deleted NB cell lines led to caspase-mediated growth inhibition, which was not seen in NB lines with normal 1p [87-89]. In vivo studies found that overexpression of miR-34a in a murine model of NB led to a significant reduction in tumor volume [90]. At least three targets of miR-34a have been identified - MYCN, BCL2 and YY1 - thus further strengthening its possible role in NB pathogenesis [88,89,91]. There is no evidence of homozygous deletion or mutational inactivation, thus suggesting that either the remaining allele is epigenetically silenced or that haploinsufficiency of miR-34a is sufficient to promote tumorigenesis.
3.1.4 Kinesin 3 superfamily intracellular motor transport protein
A NB cell line was identified with a 500 kb region of homozygous deletion at 1p36.2, and this region harbored at least six genes, including kinesin 3 superfamily intracellular motor transport protein (KIF1Bβ) [92,93]. KIF1Bβ is a microtubule-dependent intracellular motor protein involved in the transport of organelles (including mitochondria), vesicles and protein complexes. A point mutation in the ATP-binding site of the motor domain of KIF1Bβ is associated with Charcot-Marie-Tooth disease type 2A [94]. In NBs, expression of KIF1Bβ was low in advanced stage tumors, although the promoter was not hypermethylated [95]. Further, enforced expression of KIF1Bβ induced apoptotic cell death in NB cells, whereas knockdown of KIF1Bβ accelerated NB cell proliferation in vitro and NB xenograft growth in nude mice [96]. Thus, KIF1Bβ may function as a haploinsufficient TSG in NBs.
3.1.5 Zinc finger transcription factor, Castor 1 (CASZ1)
The Drosophila castor gene encodes a zinc finger transcription factor that is important for neural development and differentiation [97-99]. The human gene maps to 1p36 and encodes transcripts with either 5 or 11 zinc fingers. Low castor zinc finger 1 (CASZ1) expression is significantly correlated with older age, MYCN amplification and poor survival [97-99]. Further, ectopic expression of CASZ1 suppressed tumorigenicity in NB xenografts and induced neural differentiation in NB cells. Even though CASZ1 is slightly proximal to the smallest region of overlap defined by others, it is still deleted in the vast majority of NBs with 1p deletion. Thus, more than one NB TSG may be deleted from 1p36, and there is compelling functional evidence that CASZ1 is also a bona fide TSG in NBs.
3.1.6 AT-rich interactive domain-containing proteins 1A and 1B
ARID1 family genes are essential components of the SWI/SNF neural progenitor-specific chromatin-remodeling BAF complex, which is important for the self-renewal of multipotent neural stem cells [100]. Deletions or mutations of ARID1A/B have been reported in other cancers [101,102]. An integrated genomic analysis of a series of high-risk NBs identified inactivating mutations or deletions of ARID1A or ARID1B in two or more cases [103]. The predicted overall prevalence of inactivation is 2 - 3% of unselected NBs. Mutation was associated with a very poor overall survival among the cases studied. Although there was not functional evidence of tumor suppression in vitro or in animal models, the evidence of recurrent, somatically acquired inactivating mutations or rearrangements, as well as the association with a worse overall outcome, support ARID1A and ARID1B as TSGs in NB.
3.1.7 Cell adhesion molecule-1
CADM1 belongs to the family of immunoglobulin cell adhesion molecules and encodes a transmembrane glycoprotein involved in cell-cell interactions. Deletion of 11q23 occurs in ~ 20% of sporadic NBs, and deletion is associated with a poor outcome, especially in patients lacking MYCN amplification [104,105]. An integrated genomic analysis identified CADM1 as a candidate TSG from this region [106,107]. A disruption of cell-cell interactions could lead to invasion and metastasis. Analysis indicates that the remaining allele in cases with CADM1 deletion is not mutated or methylated, so haploinsufficiency may be sufficient to contribute to NB tumorigenesis.
3.1.8 Alpha thalassemia/mental retardation syndrome, X-linked
ATRX is part of a multiprotein complex that regulates ATP-dependent chromatin remodeling, nucleosome assembly and telomere maintenance. ATRX contributes to the a-thalassemia/mental retardation X-linked syndrome, and it has been identified recently as a gene with recurring mutations in NBs [108,109]. Whole genome sequencing was performed on stage 4 NB patients in three different age ranges: infants < 18 months of age, children from 1.5 to 11 years old and adolescents/young adults ≥ 12 years old [108]. Mutations in the ATRX gene were identified in 44% of the oldest age group and 0% in the youngest age group [108]. The overall prevalence of ATRX mutations in an unselected population is 2 - 3% [16]. Mutations in ATRX patients retain partial activity, whereas ATRX gene mutations identified in pancreatic neuroendocrine tumors and NBs appear to be loss-of-function mutations, with no protein detected. Thus, inactivation of ATRX contributes to the pathogenesis of NB, but especially in patients ≥ 12 years old [108].
4. Selective membrane molecules expressed by most NBs
4.1 Selectively expressed membrane molecules as NB targets
Antigens that are exclusively or selectively expressed on the surface of tumor cells provide an excellent opportunity for targeting. Approaches that take advantage of these molecules include targeted radiotherapy, targeted immunotherapy and affinity targeted nanoparticles. NBs have a few such molecules that have already been exploited for therapeutic approaches. The selectively expressed molecules include: the norepinephrine transporter (NET), the disialoganglioside GD2, the neural cell adhesion molecule (NCAM/CD56) and the somatostatin receptor (SSR). Both the NET and GD2 are expressed on ≥ 90% of NBs, making them the most attractive targets. NCAM/CD56 is also expressed on ≥ 90% of NBs, but it is also expressed on several normal tissues, and the SSR is expressed in 60 - 75% of NBs (Table 3, Figure 1).
Table 3.
Membrane-associated genes expressed on most NBs but few other tissues.
| Gene | Alteration | Frequency | Function | Ref. |
|---|---|---|---|---|
| CD56 | Endogenous expression | > 90% | Cell adhesion molecule | [120-123] |
| GD2 | Endogenous expression | > 90% | Disialoganglioside | [114-116] |
| NET | Endogenous expression | > 90% | Norepinephrine transporter | [111-113] |
| SSR | Endogenous expression | 60 – 75% | Somatostatin receptor | [124-126] |
4.1.1 NET
The NET, also known as solute carrier family 6 member 2 (SLC6A2), is a monoamine transporter responsible for the reuptake of extracellular norepinephrine and dopamine, which is important in regulating the concentrations of these two neurotransmitters in the synaptic cleft [110]. NBs are adrenergic neurons that synthesize these neurotransmitters and commonly express the NET on their surface. Metaiodobenzylguanidine (MIBG) is an iodinated monoamine that mimics norepinephrine that is taken up by the NET, and radiolabeled MIBG can be used for imaging or NB therapy [111-113]. This is one of the best and most effective examples for targeted delivery of a radionuclide.
4.1.2 GD2
GD2 is a disialoganglioside that is commonly expressed on cells and tumors of neuroectodermal origin, especially NB and melanoma [114-116]. Expression in humans is mainly in the cerebellum and on peripheral nerves. GD2 is shed in the blood, and circulating GD2 has been suggested as a crude means of following disease activity. However, its main utility is that it is a highly selective target for immunotherapy with monoclonal antibodies [114-116]. Several groups have made antibodies that target GD2, and they have been used in clinical trials for the treatment of NBs [114-116]. Further, a recent clinical trial showed a significant survival advantage of immunotherapy using an anti-GD2 antibody combined with granulocyte-macrophage colony-stimulating factor (GM-CSF) or interleukin-2 in high-risk NB patients [117]. Finally, cellular immunotherapy approaches using chimeric antigen receptors have been successful in treating recurrent/refractory leukemias and lymphomas expressing CD19 [118,119]; therefore, similar approaches targeting GD2 could be used to treat high-risk NBs.
4.1.3 NCAM/CD56
NCAM is a cell adhesion glycoprotein that is preferentially expressed on neurons, but it is also expressed on some glia, skeletal muscle and natural killer cells. NCAM appears to play a role in cell-cell adhesion, neurite outgrowth, synaptic plasticity and learning/memory. NCAM antibodies have been used to identify NB cells in marrow (in combination with other antibodies to additional antigens, such as GD2, synaptophysin and chromogranin) [120-123]. Although NCAM is not as exclusively expressed on neural cells (and NBs), it is relatively selective and could be an alternative surface target.
4.1.4 SSR
Experience with the SSR is more limited, in part due to the lower prevalence of SSR expression but also because it is expressed at higher levels in lower stage, more favorable NBs and at lower levels in high-risk NBs [124-126]. The SSR can be targeted with pentetreotide or octreotide, usually radiolabeled with 111I. These agents mimic somatostatin, bind to the receptor and are taken up by SSR-expressing cells, in a manner similar to MIBG in NET-expressing cells [124-126]. SSR may be a useful target for imaging and therapy for some neuroendocrine tumors, but overall its use in NBs has been limited.
5. Common targets relevant to NB and other cancers
5.1 TP53/p53
TP53, which encodes the P53 tumor suppressor protein is one of the most commonly mutated genes in human neoplasia (Table 4, Figure 1) [127,128]. However, unlike many adult tumors, the TP53 gene is rarely mutated or inactivated in NBs, at least at diagnosis [129,130]. There is controversy over whether or not TP53 is functionally inactivated, as there is sometimes cytoplasmic localization [131,132]. Nevertheless, mutations or functional inactivation (by MDM2 amplification or other mechanisms) are more common in recurrent/refractory NBs, contributing to enhanced survival and drug resistance [133]. Although there are currently no agents that target P53, Nutlin-3 inhibits MDM2, which stabilizes P53, and this renders cells more sensitive to chemotherapy-induced apoptotic cell death [134].
Table 4.
Common target genes relevant to NB as well as other tumors.
| Gene | Alteration | Function | Ref. |
|---|---|---|---|
| AURKA | Activated | Serine-threonine kinase/spindle assembly, stabilizes MYCN protein |
[146-149] |
| BAX, BAK, BID, BAD | Inactivated | Pro-apoptosis proteins | [135-138] |
| BCL2, BCLxL, MCL1 | Activated | Pro-survival proteins | [135-138] |
| CHEK1/CHK1 | Activated | Cell cycle checkpoint kinase | [150-155] |
| HDAC | Activated | Regulation of gene transcription | [156-160] |
|
RAS (MEK, ERK, AKT,
PI3K, mTOR) |
Activated | Signaling | [16,139-143] |
| TP53 | Inactivated | Regulator of cell cycle, genome stability | [129-133] |
5.2 BCL2, MCL1 and related mitochondrial proteins
BCL2 family proteins play important roles in regulating cell survival, and perturbations of the balance between prosurvival and proapoptotic proteins are common in many adult and pediatric cancers [135,136]. Although mutations are rare, BCL2 family pro-survival proteins play an important role in NBs, especially MCL1 [137,138]. Thus, deregulation of the cell death machinery clearly contributes to NB pathogenesis; therefore, efforts aimed at abrogating this survival advantage should be effective therapeutic approaches. Besides these mitochondrial proteins, upstream effectors, such as death receptors (e.g., TRAIL, DR3), caspases (e.g., CASP8) and inhibitors of apoptosis (XIAP, BIRC5) should be considered [137,138].
5.3 RAS and other signaling proteins of RTKs
RAS genes are also seldom activated by mutation in NBs at diagnosis, even though mutated NRAS was initially identified from a NB [139-143]. A comprehensive genomic analysis of NBs identified NRAS mutations in only 0.8% [16]. Interestingly, mutations appear to involve only NRAS, not other RAS family members, HRAS or KRAS [144]. Nevertheless, inhibiting RAS activation or activity could be a useful approach to NB therapy (see also Section 2.1.5 PTPN11, above).
A number of other common RTK signaling proteins, such as RAF, MEK, MAPK, PI3K, AKT, and mTOR, represent effective targets for therapy [145], although to date mutations have not been identified in NBs. These proteins are useful to target because they convey signals from a variety of upstream receptors or sources, and these signals regulate survival, invasion, metastasis, angiogenesis and drug resistance. Signaling proteins can be activated directly by mutation, overexpression or other mechanisms (e.g., inactivation of phosphatases), but they may also be induced by activation of upstream RTKs. Selective inhibitors have already been developed against several of these signaling proteins, such as AKT (e.g., perifosine), MEK (e.g., GSK1120212), mTOR (e.g., temsirolimus) and others.
5.4 Aurora kinase A
Aurora kinase A (AURKA) is a cell cycle regulated serine-threonine kinase involved in microtubule formation and spindle assembly [146]. AURKA also plays an important role in MYCN stability, independent of its kinase function [147]. Thus, AURKA is a rational target for the treatment of NBs, and other tumors, and this is supported by both preclinical and clinical evidence demonstrating the efficacy of an AURKA inhibitor, MLN8237 [148,149].
5.5 Checkpoint kinase 1
Checkpoint kinase 1 (CHK1) is a cell cycle checkpoint kinase that is a key component of the DNA damage response, which is important to maintain genome integrity [150]. An RNAi screen of RTKs in NBs [151], and other studies of CHK1 in MYC-expressing cells [152], identified CHK1 as a potential therapeutic target. Therapies aimed at inhibiting CHK1, alone or in combination with other agents, have also shown efficacy, supporting CHK1 as a therapeutic target for NBs [153-155].
5.6 HDAC and other epigenetic targets
The mutation rate in NBs is very low compared to most adult cancers [16,103,108,109], so it is likely that genes are activated (or inactivated) by copy number variation and/or by epigenetic modification of gene expression. Mechanisms of epigenetic modification include: i) promoter methylation, ii) histone methylation or acetylation, iii) miRNA regulation of transcript stability and translation, iv) regulation by chromatin remodeling proteins and others. At the present time, HDACs appear to be the most tractable target for therapy, but currently available agents lack specificity. Nevertheless, preclinical and clinical data of HDAC inhibitors, alone or in combination with other agents, have demonstrated efficacy and warrant further investigation [156-160].
6. Conclusion
Current multimodality therapy for high-risk NB has achieved some improvement in outcome, but at the cost of considerable short- and long-term toxicities. Further, conventional chemotherapeutic agents are being used at maximally tolerated doses, so it is unlikely that further escalation of dose or intensity will be tolerated. Thus, in order to improve outcome and reduce toxicities, new agents and approaches are needed. Fortunately, a number of therapeutic targets have been recently identified that could lead to novel approaches to therapy.
The first category of NB therapeutic targets is genes activated by amplification, mutation, translocation or autocrine overexpression. The major genes identified to date include: MYCN, ODC, ALK, NTRK2/TrkB, FOXR1 and PTPN11, of which two are transcription factors, MYCN and FOXR1. MYCN is amplified in 22% of NBs and is well established as an important oncogenic driver for high-risk NBs. FOXR1 is activated by translocation in an unknown percentage of cases, and it is the only recurring translocation identified in NBs. ALK and NTRK2/TrkB are RTKs. Activating mutations in ALK are responsible for most cases of hereditary NB, but somatically acquired activation occurs in about 9% of NBs. Most ALK-activated tumors respond to the ALK inhibitor, crizotinib, but some mutations confer intrinsic resistance. There is autocrine activation of TrkB by upregulated BDNF in 36% of all NBs and 50 - 60% of high-risk NBs. About 50% of patients treated with a pan-TRK inhibitor had durable responses to an inhibitor, thus suggesting that this is an attractive target. Finally, PTPN11 regulates the phosphorylation status of intracellular signaling proteins, and ODC modulates the polyamine status.
The second category of NB therapeutic targets is genes inactivated by deletion, mutation or epigenetic silencing. The major genes identified to date include: CHD5, CAMTA1, miR-34a, KIF1Bβ, CASZ1, ARID1A/B, CADM1 and ATRX. The first five were identified based on consistent deletion from 1p36 in NBs, and CADM1 is deleted from 11q23. ARID1A/B and ATRX inactivating mutations were identified through comprehensive genomic sequencing. Most of the TSGs encode proteins that reside in the nucleus (Figure 1). Three are involved in chromatin remodeling (CHD5, ATRX, ARID1A/B), two are transcription factors (CASZ1, CAMTA1) and one is a miRNA that targets MYCN. The remaining two are cytoplasmic transport proteins (KIF1Bβ) and CADM1). Because most of the TSGs are nuclear proteins, targeting them therapeutically will be challenging.
The third category of NB therapeutic targets is membrane-associated genes expressed on most NBs and few other tissues. The most prevalently and selectively expressed are the NET and GD2. There are targeted therapeutic approaches using radiolabeled MIBG that target the NET, and anti-GD2 antibodies that are in clinical use. NCAM (CD56) is also expressed on the vast majority of NBs, but it is also expressed by other cells and tissues, such as glia, skeletal muscle and natural killer cells. To date no therapeutic approaches have targeted NCAM, but it has utility in terms of identifying NB cells in tissues like the blood and bone marrow. The SSR is expressed on most but not all NBs, and there is lower expression in high-risk tumors. Nevertheless, radiolabeled somatostatin analogs, such as octreotide, bind to the SSR and have been used in treating neuroendocrine tumors, including NB, pheochromocytoma and paraganglioma. It is unclear if antagonists to this pathway would be of any utility.
The fourth category of NB therapeutic targets is common target genes relevant to NB as well as other tumors. TP53 is the most commonly mutated gene in human cancers, but it is rarely mutated in NBs at diagnosis, However, TP53 mutations may be more common at relapse. Pro-survival proteins, such as BCL2 and MCL1, are not mutated, but patterns of expression and the balance between pro-and anti-apoptotic proteins can dramatically affect NB behavior. NRAS is rarely mutated, but targeted therapy could be useful for this subset. Mutations are also rare or absent in other signaling genes that encode PI3K, AKT, MAPK, mTOR and others [161,162]. AURKA and CHK1 appear to be plausible targets for NB therapy, although current data are very preliminary. Indeed, there are other common target genes that may be relevant to NB that could also be included here. Finally, given the relative paucity of mutations in NBs, epigenetic modifications likely play an important role in regulating gene expression. Therefore, drugs targeting these epigenetic changes (such as HDAC inhibitors) could be useful in altering patterns of gene expression to reverse the changes that promoted malignant transformation.
7. Expert opinion
A substantial number of NBs have been analyzed by next-generation approaches to achieve whole exome or whole genome sequencing. Although a few novel mutated genes have been discovered, no new genomic lesions occurred in > 10% of cases, and most were in the 1 - 3% range. It is likely that a few additional abnormalities will be identified, but it is unlikely that any discovery will be as prevalent as RB1 mutations in retinoblastoma, or translocations involving the EWS gene in Ewing family tumors. There may be additional noncoding sequences like miRNAs that play an important role, but the focus should probably be on the genes that have been identified, the pathways that they represent and the targets of these genes. Further, there are likely genes that exert their pathogenetic influence through copy number variation or epigenetic modification of gene expression, so therapeutic approaches will benefit from a better understanding of epigenetic regulation of these genes.
The genes that are activated in NB are perhaps the most attractive therapeutic targets, as it is generally easier to turn off a gene than to turn it on. MYCN is probably the most important oncogenic driver, given its prevalence and strong association with aggressive behavior. The challenge is that MYCN encodes a nuclear oncoprotein, making it difficult to approach therapeutically. Further, the MYCN gene is amplified on extrachromosomal double minutes (DMs), and DMs accumulate in cells by random assortment until a steady state is reached. A weak inhibitory approach would likely result in resetting the copy number to a higher level, so a potent attack will be required. A better understanding of MYCN targets and understanding the ones that are critical to the MYCN amplification phenotype may provide more attractive targets. In addition, there may be genetic or biological approaches that would target MYCN directly in the nucleus, or selectively kill cells with marked overexpression of MYCN. It is worth noting that several of the other therapeutic targets identified (e.g., ODC, miR-34a, CHD5 and AURKA) appear to interact with, regulate or be regulated by MYCN, further supporting its role as an important target.
ALK and NTRK2/TrkB also represent very attractive targets. Transmembrane RTKs are more accessible to therapeutic interventions, and small molecule inhibitors have already been developed that selectively target these receptors. ALK is activated by mutations in the TK domain in ~ 9% of NBs, and genomic screening for these alterations would help identify patients whose tumors would be susceptible to ALK-targeted therapy. ALK is activated by amplification or overexpression in another 2 - 3% of NBs, which should also be susceptible to ALK inhibitors. TrkB is not mutated, but it is markedly overexpressed in 36% of all NBs and 50 - 60% of high-risk NBs. TrkB expression leads to increased expression of its ligand, BDNF, and this autocrine activation is an attractive and under-utilized target in NBs, as well as a variety of other tumors. Responses to the TRK-selective inhibitor lestaurtinib were durable, but more specific and potent inhibitors of TRK receptors are needed. Further, combining TRK-targeted therapy with conventional chemotherapy will likely be more effective than either therapy alone.
Most of the TSGs identified to date map to 1p36, and most of them are nuclear proteins, making them difficult to target. CHD5, ATRX and ARID1A/B are chromatin remodeling proteins or members of chromatin remodeling complexes, whereas CASZ1 and CAMTA1 are transcription factors. As with MYCN, a better understanding of the targets of these proteins and the key pathways they affect may provide insights and suggest alternative therapeutic approaches. Interestingly, miR-34a and possibly other NB TSGs target MYCN, so this connection may be exploited therapeutically.
Therapy that targets membrane molecules that are selectively expressed on NB cells has already proven to be effective. Clearly, targeted radiotherapy with radiolabeled MIBG takes advantage of preferential NET expression and substrate uptake, but this molecule may be exploited by other therapeutic approaches, such as nanoparticle drug delivery. Although entrance into the tumor microenvironment must take advantage of the enhanced permeability and retention effect, affinity targeting of the NET or other selectively expressed targets would be appealing. Anti-GD2 antibodies have been around for decades, and they have found a place in the post-stem-cell transplant setting to prevent recurrence of high-risk NB. Recently, cell-based immunotherapy using chimeric antigen receptors have shown great promise in treating recurrent/refractory hematopoietic malignancies expressing CD19, and similar approaches may be effective in targeting GD2. Affinity targeting of nanoparticles to NBs with anti-GD2 antibodies or ligands may be useful for drug delivery [163].
There are a number of proteins and pathways that are common to many types of pediatric and adult cancers but are also relevant to NB pathogenesis. Major genes and pathways include: i) TP53 TSG, ii) the pro- and anti-mitochondrial proteins that regulate apoptosis or survival (especially BCL2, MCL1, BCLxL, BAX and BAD), iii) the signaling pathway network common to many RTKs and other intracellular kinases (represented by RAS, RAF, MEK, MAPK, PI3K, AKT and mTOR) and iv) other miscellaneous target genes, represented by AURKA and CHEK1. Although none of these gene targets are unique to NBs, there is evidence for targeting all of these genes, proteins or pathways, and there are other common target genes that could be considered as well. Finally, it is likely that copy number variation and/or epigenetic modification are likely to play an important role in NB pathogenesis and behavior, so it will be critical to understand the relative importance of these changes.
In summary, the future treatment of NB is likely to change dramatically in the next 10 years or so. Although the number of specifically mutated (activated or inactivated) genes is low compared to most adult tumors, there is still a number of very compelling therapeutic targets, and there are already target-selective therapies (especially small molecule inhibitors, targeted radiation therapy and antibody-mediated immunotherapy) that have been developed. Further, targeted delivery of conventional agents using nanoparticles or other approaches holds promise. There is still a lot of work to be done, not only to better understand the genes, proteins and pathways represented by these targets, but also in developing selective therapeutic approaches to restore cellular control or induce cell death. Nevertheless, there is considerable optimism that the goal of developing novel NB therapies that are more effective and less toxic for NB patients will be realized in the coming years.
Article highlights.
Comprehensive genomic analyses have identified a limited number of genes that are potential or actual therapeutic targets.
Genes activated by amplification, mutation, translocation or autocrine overexpression include: ALK, FOXR1, MYCN, ODC, PTPN11, and NTRK2/TrkB.
Genes inactivated by deletion, mutation or epigenetic silencing include: ARID1A/B, ATRX, CADM1, CAMTA1, CASZ1, CHD5, KIF1Bβ and miR-34a.
Membrane-associated molecules expressed on most NBs but few other tissues are attractive targets, especially NET and GD2.
Common targets relevant to NB as well as other tumors include: TP53, cytoplasmic signaling proteins, pro- and anti-apoptotic proteins, epigenetic modifiers and other proteins.
Targeted approaches to NB treatment, with or without conventional agents, represent the future of NB therapy.
Acknowledgments
Declaration of interest
This work was supported in part by National Institutes of Health Grants CA039771 and CA094194, by the V Foundation, Alex’s Lemonade Stand Foundation and the Audrey E. Evans Endowed Chair (GMB). The authors have no financial interests in any agents or products mentioned in the review.
Bibliography
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1.Brodeur GM, Hogarty MD, Mosse YP, Maris JM. Neuroblastoma. In: Pizzo PA, Poplack DG, editors. Principles and practice of pediatric oncology. 6th Lippincott, Williams and Wilkins; Philadelphia: 2011. pp. 886–922. [Google Scholar]
- 2.Brodeur GM. Neuroblastoma: biological insights into a clinical enigma. Nat Rev Cancer. 2003;3(3):203–16. doi: 10.1038/nrc1014. [DOI] [PubMed] [Google Scholar]
- 3.Maris JM, Hogarty MD, Bagatell R, Cohn SL. Neuroblastoma. Lancet. 2007;369(9579):2106–20. doi: 10.1016/S0140-6736(07)60983-0. [DOI] [PubMed] [Google Scholar]
- 4.Mosse YP, Greshock J, Margolin A, et al. High-resolution detection and mapping of genomic DNA alterations in neuroblastoma. Genes Chromosomes Cancer. 2005;43(4):390–403. doi: 10.1002/gcc.20198. [DOI] [PubMed] [Google Scholar]
- 5.Tomioka N, Oba S, Ohira M, et al. Novel risk stratification of patients with neuroblastoma by genomic signature, which is independent of molecular signature. Oncogene. 2008;27(4):441–9. doi: 10.1038/sj.onc.1210661. [DOI] [PubMed] [Google Scholar]
- 6.Schwab M, Alitalo K, Klempnauer KH, et al. Amplified DNA with limited homology to myc cellular oncogene is shared by human neuroblastoma cell lines and a neuroblastoma tumour. Nature. 1983;305(5931):245–8. doi: 10.1038/305245a0. [DOI] [PubMed] [Google Scholar]
- 7.Schwab M, Varmus HE, Bishop JM, et al. Chromosome localization in normal human cells and neuroblastomas of a gene related to c-myc. Nature. 1984;308(5956):288–91. doi: 10.1038/308288a0. [DOI] [PubMed] [Google Scholar]
- •• These two papers by Schwab et al. were the first descriptions of the MYCN protooncogene.
- 8.Brodeur GM, Seeger RC, Schwab M, et al. Amplification of N-myc in untreated human neuroblastomas correlates with advanced disease stage. Science. 1984;224(4653):1121–4. doi: 10.1126/science.6719137. [DOI] [PubMed] [Google Scholar]
- •• This was the first desription of the clinical importance of MYCN oncogene activation by amplification in a human tumor.
- 9.Seeger RC, Brodeur GM, Sather H, et al. Association of multiple copies of the N-myc oncogene with rapid progression of neuroblastomas. N Engl J Med. 1985;313(18):1111–16. doi: 10.1056/NEJM198510313131802. [DOI] [PubMed] [Google Scholar]
- •• This paper first described the correlation of MYCN amplification with survival in NB patients.
- 10.Bagatell R, Rumcheva P, London WB, et al. Outcomes of children with intermediate-risk neuroblastoma after treatment stratified by MYCN status and tumor cell ploidy. J Clin Oncol. 2005;23(34):8819–27. doi: 10.1200/JCO.2004.00.2931. [DOI] [PubMed] [Google Scholar]
- 11.George RE, London WB, Cohn SL, et al. Hyperdiploidy plus nonamplified MYCN confers a favorable prognosis in children 12 to 18 months old with disseminated neuroblastoma: a Pediatric Oncology Group study. J Clin Oncol. 2005;23(27):6466–73. doi: 10.1200/JCO.2005.05.582. [DOI] [PubMed] [Google Scholar]
- 12.Look AT, Hayes FA, Shuster JJ, et al. Clinical relevance of tumor cell ploidy and N-myc gene amplification in childhood neuroblastoma: a Pediatric Oncology Group study. J Clin Oncol. 1991;9(4):581–91. doi: 10.1200/JCO.1991.9.4.581. [DOI] [PubMed] [Google Scholar]
- 13.Schneiderman J, London WB, Brodeur GM, et al. Clinical significance of MYCN amplification and ploidy in favorable-stage neuroblastoma: a report from the Children’s Oncology Group. J Clin Oncol. 2008;26(6):913–18. doi: 10.1200/JCO.2007.13.9493. [DOI] [PubMed] [Google Scholar]
- 14.Pession A, Tonelli R. The MYCN oncogene as a specific and selective drug target for peripheral and central nervous system tumors. Curr Cancer Drug Targets. 2005;5(4):273–83. doi: 10.2174/1568009054064606. [DOI] [PubMed] [Google Scholar]
- 15.Bell E, Chen L, Liu T, et al. MYCN oncoprotein targets and their therapeutic potential. Cancer Lett. 2010;293(2):144–57. doi: 10.1016/j.canlet.2010.01.015. [DOI] [PubMed] [Google Scholar]
- 16.Pugh TJ, Morozova O, Attiyeh EF, et al. The genetic landscape of high-risk neuroblastoma. Nat Genet. 2013;45(3):279–84. doi: 10.1038/ng.2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •• This paper described the prevalence of specific gene mutations in high-risk NBs.
- 17.Puissant A, Frumm SM, Alexe G, et al. Targeting MYCN in neuroblastoma by BET bromodomain inhibition. Cancer Discov. 2013;3(3):308–23. doi: 10.1158/2159-8290.CD-12-0418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •• This was the first paper to describe an inhibitor of MYCN expression to treat NBs.
- 18.Hogarty MD, Norris MD, Davis K, et al. ODC1 is a critical determinant of MYCN oncogenesis and a therapeutic target in neuroblastoma. Cancer Res. 2008;68(23):9735–45. doi: 10.1158/0008-5472.CAN-07-6866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koomoa DL, Geerts D, Lange I, et al. DFMO/eflornithine inhibits migration and invasion downstream of MYCN and involves p27Kip1 activity in neuroblastoma. Int J Oncol. 2013;42(4):1219–28. doi: 10.3892/ijo.2013.1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rounbehler RJ, Li W, Hall MA, et al. Targeting ornithine decarboxylase impairs development of MYCN-amplified neuroblastoma. Cancer Res. 2009;69(2):547–53. doi: 10.1158/0008-5472.CAN-08-2968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morris SW, Kirstein MN, Valentine MB, et al. Fusion of a kinase gene, ALK, to a nucleolar protein gene, NPM, in non-Hodgkin’s lymphoma. Science. 1994;263(5151):1281–4. doi: 10.1126/science.8122112. [DOI] [PubMed] [Google Scholar]
- 22.Webb TR, Slavish J, George RE, et al. Anaplastic lymphoma kinase: role in cancer pathogenesis and small-molecule inhibitor development for therapy. Expert Rev Anticancer Ther. 2009;9(3):331–56. doi: 10.1586/14737140.9.3.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen Y, Takita J, Choi YL, et al. Oncogenic mutations of ALK kinase in neuroblastoma. Nature. 2008;455(7215):971–4. doi: 10.1038/nature07399. [DOI] [PubMed] [Google Scholar]
- 24.George RE, Sanda T, Hanna M, et al. Activating mutations in ALK provide a therapeutic target in neuroblastoma. Nature. 2008;455(7215):975–8. doi: 10.1038/nature07397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Janoueix-Lerosey I, Lequin D, Brugieres L, et al. Somatic and germline activating mutations of the ALK kinase receptor in neuroblastoma. Nature. 2008;455(7215):967–70. doi: 10.1038/nature07398. [DOI] [PubMed] [Google Scholar]
- 26.Mosse YP, Laudenslager M, Longo L, et al. Identification of ALK as a major familial neuroblastoma predisposition gene. Nature. 2008;455(7215):930–5. doi: 10.1038/nature07261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •• The above four papers (Chen et al., George et al., Janoueix-Lerosey et al., Mosse et al.) were the simultaneous first description of ALK as the major hereditary NB predisposition gene.
- 27.Azarova AM, Gautam G, George RE. Emerging importance of ALK in neuroblastoma. Semin Cancer Biol. 2011;21(4):267–75. doi: 10.1016/j.semcancer.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carpenter EL, Mosse YP. Targeting ALK in neuroblastoma–preclinical and clinical advancements. Nat Rev Clin Oncol. 2012;9(7):391–9. doi: 10.1038/nrclinonc.2012.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mosse YP, Lim MS, Voss SD, et al. Safety and activity of crizotinib for paediatric patients with refractory solid tumours or anaplastic large-cell lymphoma: a Children’s Oncology Group phase 1 consortium study. Lancet Oncol. 2013;14(6):472–80. doi: 10.1016/S1470-2045(13)70095-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •• This was the first report of a clinical trial using the ALK inhibitor, crizotinib.
- 30.Berry T, Luther W, Bhatnagar N, et al. The ALK(F1174L) mutation potentiates the oncogenic activity of MYCN in neuroblastoma. Cancer Cell. 2012;22(1):117–30. doi: 10.1016/j.ccr.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bresler SC, Wood AC, Haglund EA, et al. Differential inhibitor sensitivity of anaplastic lymphoma kinase variants found in neuroblastoma. Sci Transl Med. 2011;3(108):108ra14. doi: 10.1126/scitranslmed.3002950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heuckmann JM, Holzel M, Sos ML, et al. ALK mutations conferring differential resistance to structurally diverse ALK inhibitors. Clin Cancer Res. 2011;17(23):7394–401. doi: 10.1158/1078-0432.CCR-11-1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang EJ, Reichardt LF. Neurotrophins: roles in neuronal development and function. Annu Rev Neurosci. 2001;24:677–736. doi: 10.1146/annurev.neuro.24.1.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Skaper SD. The neurotrophin family of neurotrophic factors: an overview. Methods Mol Biol. 2012;846:1–12. doi: 10.1007/978-1-61779-536-7_1. [DOI] [PubMed] [Google Scholar]
- 35.Brodeur GM, Minturn JE, Ho R, et al. Trk receptor expression and inhibition in neuroblastomas. Clin Cancer Res. 2009;15(10):3244–50. doi: 10.1158/1078-0432.CCR-08-1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brodeur GM, Nakagawara A, Yamashiro DJ, et al. Expression of TrkA, TrkB and TrkC in human neuroblastomas. J Neurooncol. 1997;31(1-2):49–55. doi: 10.1023/a:1005729329526. [DOI] [PubMed] [Google Scholar]
- 37.Kogner P, Barbany G, Dominici C, et al. Coexpression of messenger RNA for TRK protooncogene and low affinity nerve growth factor receptor in neuroblastoma with favorable prognosis. Cancer Res. 1993;53(9):2044–50. [PubMed] [Google Scholar]
- 38.Nakagawara A, Arima M, Azar CG, et al. Inverse relationship between trk expression and N-myc amplification in human neuroblastomas. Cancer Res. 1992;52(5):1364–8. [PubMed] [Google Scholar]
- • This paper first reported the expression of TrkA/NTRK1 in NBs, and its inverse relationship with MYCN amplification.
- 39.Nakagawara A, Arima-Nakagawara M, Scavarda NJ, et al. Association between high levels of expression of the TRK gene and favorable outcome in human neuroblastoma. N Engl J Med. 1993;328(12):847–54. doi: 10.1056/NEJM199303253281205. [DOI] [PubMed] [Google Scholar]
- •• This paper, and papers by Suzuki et al. (1993) and Kogner et al. (1993) were the first description of the favorable prognostic impact of high TrkA/NTRK1 expression.
- 40.Nakagawara A, Azar CG, Scavarda NJ, Brodeur GM. Expression and function of TRK-B and BDNF in human neuroblastomas. Mol Cell Biol. 1994;14(1):759–67. doi: 10.1128/mcb.14.1.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •• This paper was the first to describe the association of high TrkB/NTRK2 expression with MYCN amplification and with poor out come in NBs.
- 41.Ryden M, Sehgal R, Dominici C, et al. Expression of mRNA for the neurotrophin receptor trkC in neuroblastomas with favourable tumour stage and good prognosis. Br J Cancer. 1996;74(5):773–9. doi: 10.1038/bjc.1996.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Suzuki T, Bogenmann E, Shimada H, et al. Lack of high-affinity nerve growth factor receptors in aggressive neuroblastomas. J Natl Cancer Inst. 1993;85(5):377–84. doi: 10.1093/jnci/85.5.377. [DOI] [PubMed] [Google Scholar]
- 43.Thiele CJ, Li Z, McKee AE. On Trk-the TrkB signal transduction pathway is an increasingly important target in cancer biology. Clin Cancer Res. 2009;15(19):5962–7. doi: 10.1158/1078-0432.CCR-08-0651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yamashiro DJ, Liu XG, Lee CP, et al. Expression and function of Trk-C in favourable human neuroblastomas. Eur J Cancer. 1997;33(12):2054–7. doi: 10.1016/s0959-8049(97)00309-2. [DOI] [PubMed] [Google Scholar]
- 45.Yamashiro DJ, Nakagawara A, Ikegaki N, et al. Expression of TrkC in favorable human neuroblastomas. Oncogene. 1996;12(1):37–41. [PubMed] [Google Scholar]
- 46.Fagan AM, Zhang H, Landis S, et al. TrkA, but not TrkC, receptors are essential for survival of sympathetic neurons in vivo. J Neurosci. 1996;16(19):6208–18. doi: 10.1523/JNEUROSCI.16-19-06208.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tacconelli A, Farina AR, Cappabianca L, et al. TrkA alternative splicing: a regulated tumor-promoting switch in human neuroblastoma. Cancer Cell. 2004;6(4):347–60. doi: 10.1016/j.ccr.2004.09.011. [DOI] [PubMed] [Google Scholar]
- • This was the first description of TrkAIII isoform expression in NBs.
- 48.Tacconelli A, Farina AR, Cappabianca L, et al. Alternative TrkAIII splicing: a potential regulated tumor-promoting switch and therapeutic target in neuroblastoma. Future Oncol. 2005;1(5):689–98. doi: 10.2217/14796694.1.5.689. [DOI] [PubMed] [Google Scholar]
- 49.Acheson A, Conover JC, Fandl JP, et al. A BDNF autocrine loop in adult sensory neurons prevents cell death. Nature. 1995;374(6521):450–3. doi: 10.1038/374450a0. [DOI] [PubMed] [Google Scholar]
- 50.Ho R, Eggert A, Hishiki T, et al. Resistance to chemotherapy mediated by TrkB in neuroblastomas. Cancer Res. 2002;62(22):6462–6. [PubMed] [Google Scholar]
- 51.Matsumoto K, Wada RK, Yamashiro JM, et al. Expression of brain-derived neurotrophic factor and p145TrkB affects survival, differentiation, and invasiveness of human neuroblastoma cells. Cancer Res. 1995;55(8):1798–806. [PubMed] [Google Scholar]
- 52.Nakamura K, Martin KC, Jackson JK, et al. Brain-derived neurotrophic factor activation of TrkB induces vascular endothelial growth factor expression via hypoxia-inducible factor-1alpha in neuroblastoma cells. Cancer Res. 2006;66(8):4249–55. doi: 10.1158/0008-5472.CAN-05-2789. [DOI] [PubMed] [Google Scholar]
- • The above 4 papers by Acheson et al., Ho et al., Matsumoto et al., and Nakamura et al., describe the assocation of TrkB expression with aggressive behavior, such as invasion, metastasis, angiogenesis and drug resistance in NBs.
- 53.Evans AE, Kisselbach KD, Liu X, et al. Effect of CEP-751 (KT-6587) on neuroblastoma xenografts expressing TrkB. Med Pediatr Oncol. 2001;36(1):181–4. doi: 10.1002/1096-911X(20010101)36:1<181::AID-MPO1043>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 54.Evans AE, Kisselbach KD, Yamashiro DJ, et al. Antitumor activity of CEP-751 (KT-6587) on human neuroblastoma and medulloblastoma xenografts. Clin Cancer Res. 1999;5(11):3594–602. [PubMed] [Google Scholar]
- • These two papers by Evans et al., were the first preclinical description of the potential utility of a Trk inhibitor to treat NBs.
- 55.Iyer R, Evans AE, Qi X, et al. Lestaurtinib enhances the antitumor efficacy of chemotherapy in murine xenograft models of neuroblastoma. Clin Cancer Res. 2010;16(5):1478–85. doi: 10.1158/1078-0432.CCR-09-1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Iyer R, Varela CR, Minturn JE, et al. AZ64 inhibits TrkB and enhances the efficacy of chemotherapy and local radiation in neuroblastoma xenografts. Cancer Chemother Pharmacol. 2012;70(3):477–86. doi: 10.1007/s00280-012-1879-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Minturn JE, Evans AE, Villablanca JG, et al. Phase I trial of lestaurtinib for children with refractory neuroblastoma: a new approaches to neuroblastoma therapy consortium study. Cancer Chemother Pharmacol. 2011;68(4):1057–65. doi: 10.1007/s00280-011-1581-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •• This Phase I clinical trial describes the efficacy of a TRK inhibitor to treat NB patients with recurrent/refractory NBs.
- 58.Zenker M. Genetic and pathogenetic aspects of Noonan syndrome and related disorders. Horm Res. 2009;72(Suppl 2):57–63. doi: 10.1159/000243782. [DOI] [PubMed] [Google Scholar]
- 59.Grossmann KS, Rosario M, Birchmeier C, Birchmeier W. The tyrosine phosphatase Shp2 in development and cancer. Adv Cancer Res. 2010;106:53–89. doi: 10.1016/S0065-230X(10)06002-1. [DOI] [PubMed] [Google Scholar]
- 60.Hasle H. Malignant diseases in Noonan syndrome and related disorders. Horm Res. 2009;72(Suppl 2):8–14. doi: 10.1159/000243773. [DOI] [PubMed] [Google Scholar]
- 61.Myatt SS, Lam EW. The emerging roles of forkhead box (Fox) proteins in cancer. Nat Rev Cancer. 2007;7(11):847–59. doi: 10.1038/nrc2223. [DOI] [PubMed] [Google Scholar]
- 62.Olanich ME, Barr FG. A call to ARMS: targeting the PAX3-FOXO1 gene in alveolar rhabdomyosarcoma. Expert Opin Ther Targets. 2013;17(5):607–23. doi: 10.1517/14728222.2013.772136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Santo EE, Ebus ME, Koster J, et al. Oncogenic activation of FOXR1 by 11q23 intrachromosomal deletion-fusions in neuroblastoma. Oncogene. 2012;31(12):1571–81. doi: 10.1038/onc.2011.344. [DOI] [PubMed] [Google Scholar]
- • This was the first description of a recurring translocation in NBs, involving the FOXR1 gene.
- 64.Molenaar JJ, Domingo-Fernandez R, Ebus ME, et al. LIN28B induces neuroblastoma and enhances MYCN levels via let-7 suppression. Nat Genet. 2012;44(11):1199–206. doi: 10.1038/ng.2436. [DOI] [PubMed] [Google Scholar]
- •• This paper described LIN28B as a potential oncogenic driver in NBs.
- 65.Islam A, Kageyama H, Takada N, et al. High expression of Survivin, mapped to 17q25, is significantly associated with poor prognostic factors and promotes cell survival in human neuroblastoma. Oncogene. 2000;19(5):617–23. doi: 10.1038/sj.onc.1203358. [DOI] [PubMed] [Google Scholar]
- 66.Lamers F, van der Ploeg I, Schild L, et al. Knockdown of survivin (BIRC5) causes apoptosis in neuroblastoma via mitotic catastrophe. Endocr Relat Cancer. 2011;18(6):657–68. doi: 10.1530/ERC-11-0207. [DOI] [PubMed] [Google Scholar]
- 67.Garcia I, Mayol G, Rios J, et al. A three-gene expression signature model for risk stratification of patients with neuroblastoma. Clin Cancer Res. 2012;18(7):2012–23. doi: 10.1158/1078-0432.CCR-11-2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hailat N, Keim DR, Melhem RF, et al. High levels of p19/nm23 protein in neuroblastoma are associated with advanced stage disease and with N-myc gene amplification. J Clin Invest. 1991;88(1):341–5. doi: 10.1172/JCI115299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Leone A, Seeger RC, Hong CM, et al. Evidence for nm23 RNA overexpression, DNA amplification and mutation in aggressive childhood neuroblastomas. Oncogene. 1993;8(4):855–65. [PubMed] [Google Scholar]
- 70.Thompson PM, Gotoh T, Kok M, et al. CHD5, a new member of the chromodomain gene family, is preferentially expressed in the nervous system. Oncogene. 2003;22(7):1002–11. doi: 10.1038/sj.onc.1206211. [DOI] [PubMed] [Google Scholar]
- •• This paper first described the cloning and characterization of CHD5 as a potential TSG in NBs.
- 71.Hall JA, Georgel PT. CHD proteins: a diverse family with strong ties. Biochem Cell Biol. 2007;85(4):463–76. doi: 10.1139/O07-063. [DOI] [PubMed] [Google Scholar]
- 72.Murawska M, Brehm A. CHD chromatin remodelers and the transcription cycle. Transcription. 2011;2(6):244–53. doi: 10.4161/trns.2.6.17840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fujita T, Igarashi J, Okawa ER, et al. CHD5, a tumor suppressor gene deleted from 1p36.31 in neuroblastomas. J Natl Cancer Inst. 2008;100(13):940–9. doi: 10.1093/jnci/djn176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •• This report showed that CHD5 functions as a TSG in NBs.
- 74.Garcia I, Mayol G, Rodriguez E, et al. Expression of the neuron-specific protein CHD5 is an independent marker of outcome in neuroblastoma. Mol Cancer. 2010;9:277. doi: 10.1186/1476-4598-9-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Koyama H, Zhuang T, Light JE, et al. Mechanisms of CHD5 Inactivation in neuroblastomas. Clin Cancer Res. 2012;18(6):1588–97. doi: 10.1158/1078-0432.CCR-11-2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- • These two papers by Garcia et al. (2010) and Koyama et al. (2012) show that CHD5 expression has prognostic significance in NBs.
- 76.Bagchi A, Papazoglu C, Wu Y, et al. CHD5 is a tumor suppressor at human 1p36. Cell. 2007;128(3):459–75. doi: 10.1016/j.cell.2006.11.052. [DOI] [PubMed] [Google Scholar]
- • This paper used chromosome engineering to show that CHD5 functioned as a TSG in a mouse model and potentially in other tumor types, like gliomas.
- 77.Mulero-Navarro S, Esteller M. Chromatin remodeling factor CHD5 is silenced by promoter CpG island hypermethylation in human cancer. Epigenetics. 2008;3(4):210–15. doi: 10.4161/epi.3.4.6610. [DOI] [PubMed] [Google Scholar]
- 78.Wang J, Chen H, Fu S, et al. The involvement of CHD5 hypermethylation in laryngeal squamous cell carcinoma. Oral Oncol. 2011;47(7):601–8. doi: 10.1016/j.oraloncology.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 79.Wang L, He S, Tu Y, et al. Downregulation of chromatin remodeling factor CHD5 is associated with a poor prognosis in human glioma. J Clin Neurosci. 2013;20(7):958–63. doi: 10.1016/j.jocn.2012.07.021. [DOI] [PubMed] [Google Scholar]
- 80.Wang X, Lau KK, So LK, Lam YW. CHD5 is down-regulated through promoter hypermethylation in gastric cancer. J Biomed Sci. 2009;16:95. doi: 10.1186/1423-0127-16-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wong RR, Chan LK, Tsang TP, et al. CHD5 Downregulation Associated with Poor Prognosis in Epithelial Ovarian Cancer. Gynecol Obstet Invest. 2011;72(3):203–7. doi: 10.1159/000323883. [DOI] [PubMed] [Google Scholar]
- 82.Zhao R, Yan Q, Lv J, et al. CHD5, a tumor suppressor that is epigenetically silenced in lung cancer. Lung Cancer. 2012;76(3):324–31. doi: 10.1016/j.lungcan.2011.11.019. [DOI] [PubMed] [Google Scholar]
- 83.Henrich KO, Fischer M, Mertens D, et al. Reduced expression of CAMTA1 correlates with adverse outcome in neuroblastoma patients. Clin Cancer Res. 2006;12(1):131–8. doi: 10.1158/1078-0432.CCR-05-1431. [DOI] [PubMed] [Google Scholar]
- 84.Henrich KO, Bauer T, Schulte J, et al. CAMTA1, a 1p36 tumor suppressor candidate, inhibits growth and activates differentiation programs in neuroblastoma cells. Cancer Res. 2011;71(8):3142–51. doi: 10.1158/0008-5472.CAN-10-3014. [DOI] [PubMed] [Google Scholar]
- • These two papers by Henrich et al. show that CAMTA1 is a potential TSG in NBs.
- 85.Cho WC. OncomiRs: the discovery and progress of microRNAs in cancers. Mol Cancer. 2007;6:60. doi: 10.1186/1476-4598-6-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wang R, Ma J, Wu Q, et al. Functional role of miR-34 family in human cancer. Curr Drug Targets. 2013;14(10):1185–91. doi: 10.2174/13894501113149990191. [DOI] [PubMed] [Google Scholar]
- 87.Cole KA, Attiyeh EF, Mosse YP, et al. A functional screen identifies miR-34a as a candidate neuroblastoma tumor suppressor gene. Mol Cancer Res. 2008;6(5):735–42. doi: 10.1158/1541-7786.MCR-07-2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wei JS, Song YK, Durinck S, et al. The MYCN oncogene is a direct target of miR-34a. Oncogene. 2008;27(39):5204–13. doi: 10.1038/onc.2008.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Welch C, Chen Y, Stallings RL. MicroRNA-34a functions as a potential tumor suppressor by inducing apoptosis in neuroblastoma cells. Oncogene. 2007;26(34):5017–22. doi: 10.1038/sj.onc.1210293. [DOI] [PubMed] [Google Scholar]
- • The above three papers by Cole et al., Wei et al., and Welch et al., were the first description of miR-34a as a potential TSG in NBs.
- 90.Tivnan A, Tracey L, Buckley PG, et al. MicroRNA-34a is a potent tumor suppressor molecule in vivo in neuroblastoma. BMC Cancer. 2011;11:33. doi: 10.1186/1471-2407-11-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chen QR, Yu LR, Tsang P, et al. Systematic proteome analysis identifies transcription factor YY1 as a direct target of miR-34a. J Proteome Res. 2011;10(2):479–87. doi: 10.1021/pr1006697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ohira M, Kageyama H, Mihara M, et al. Identification and characterization of a 500-kb homozygously deleted region at 1p36.2-p36.3 in a neuroblastoma cell line. Oncogene. 2000;19(37):4302–7. doi: 10.1038/sj.onc.1203786. [DOI] [PubMed] [Google Scholar]
- 93.Chen YZ, Soeda E, Yang HW, et al. Homozygous deletion in a neuroblastoma cell line defined by a high-density STS map spanning human chromosome band 1p36. Genes Chromosomes Cancer. 2001;31(4):326–32. doi: 10.1002/gcc.1151. [DOI] [PubMed] [Google Scholar]
- 94.Zhao C, Takita J, Tanaka Y, et al. Charcot-Marie-Tooth disease type 2A caused by mutation in a microtubule motor KIF1Bbeta. Cell. 2001;105(5):587–97. doi: 10.1016/s0092-8674(01)00363-4. [DOI] [PubMed] [Google Scholar]
- 95.Schlisio S, Kenchappa RS, Vredeveld LC, et al. The kinesin KIF1Bbeta acts downstream from EglN3 to induce apoptosis and is a potential 1p36 tumor suppressor. Genes Dev. 2008;22(7):884–93. doi: 10.1101/gad.1648608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- • This paper first described KIF1Bb as a potential TSG in NBs.
- 96.Munirajan AK, Ando K, Mukai A, et al. KIF1Bbeta functions as a haploinsufficient tumor suppressor gene mapped to chromosome 1p36.2 by inducing apoptotic cell death. J Biol Chem. 2008;283(36):24426–34. doi: 10.1074/jbc.M802316200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Liu Z, Yang X, Tan F, et al. Molecular cloning and characterization of human Castor, a novel human gene upregulated during cell differentiation. Biochem Biophys Res Commun. 2006;344(3):834–44. doi: 10.1016/j.bbrc.2006.03.207. [DOI] [PubMed] [Google Scholar]
- •• This paper first described CASZ1 as a potential TSG in NBs.
- 98.Liu Z, Naranjo A, Thiele CJ. CASZ1b, the short isoform of CASZ1 gene, coexpresses with CASZ1a during neurogenesis and suppresses neuroblastoma cell growth. PLoS One. 2011;6(4):e18557. doi: 10.1371/journal.pone.0018557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Liu Z, Yang X, Li Z, et al. CASZ1, a candidate tumor-suppressor gene, suppresses neuroblastoma tumor growth through reprogramming gene expression. Cell Death Differ. 2011;18(7):1174–83. doi: 10.1038/cdd.2010.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ho L, Crabtree GR. Chromatin remodelling during development. Nature. 2010;463(7280):474–84. doi: 10.1038/nature08911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Jones S, Wang TL, Shih Ie M, et al. Frequent mutations of chromatin remodeling gene ARID1A in ovarian clear cell carcinoma. Science. 2010;330(6001):228–31. doi: 10.1126/science.1196333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Khursheed M, Kolla JN, Kotapalli V, et al. ARID1B, a member of the human SWI/SNF chromatin remodeling complex, exhibits tumour-suppressor activities in pancreatic cancer cell lines. Br J Cancer. 2013;108(10):2056–62. doi: 10.1038/bjc.2013.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sausen M, Leary RJ, Jones S, et al. Integrated genomic analyses identify ARID1A and ARID1B alterations in the childhood cancer neuroblastoma. Nat Genet. 2013;45(1):12–17. doi: 10.1038/ng.2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •• This paper was the first description of ARID1A and ARID1B mutations in NBs.
- 104.Attiyeh EF, London WB, Mosse YP, et al. Chromosome 1p and 11q deletions description of mutations in the ATRX gene in older NB patients. J Med. 2005;353(21):2243–53. doi: 10.1056/NEJMoa052399. [DOI] [PubMed] [Google Scholar]
- 105.Guo C, White PS, Weiss MJ, et al. Allelic deletion at 11q23 is common in MYCN single copy neuroblastomas. Oncogene. 1999;18(35):4948–57. doi: 10.1038/sj.onc.1202887. [DOI] [PubMed] [Google Scholar]
- 106.Michels E, Hoebeeck J, De Preter K, et al. CADM1 is a strong neuroblastoma candidate gene that maps within a 3.72 Mb critical region of loss on 11q23. BMC Cancer. 2008;8:173. doi: 10.1186/1471-2407-8-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Nowacki S, Skowron M, Oberthuer A, et al. Expression of the tumour suppressor gene CADM1 is associated with favourable outcome and inhibits cell survival in neuroblastoma. Oncogene. 2008;27(23):3329–38. doi: 10.1038/sj.onc.1210996. [DOI] [PubMed] [Google Scholar]
- 108.Cheung NK, Zhang J, Lu C, et al. Association of age at diagnosis and genetic mutations in patients with neuroblastoma. JAMA. 2012;307(10):1062–71. doi: 10.1001/jama.2012.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Molenaar JJ, Koster J, Zwijnenburg DA, et al. Sequencing of neuroblastoma identifies chromothripsis and defects in neuritogenesis genes. Nature. 2012;483(7391):589–93. doi: 10.1038/nature10910. [DOI] [PubMed] [Google Scholar]
- •• These papers by Cheung et al., and Molenaar et al., are the first description of mutations in the ATRX gene in older NB patients.
- 110.Sieber-Blum M, Ren Z. Norepinephrine transporter expression and function in noradrenergic cell differentiation. Mol Cell Biochem. 2000;212(1-2):61–70. [PubMed] [Google Scholar]
- 111.Dubois SG, Geier E, Batra V, et al. Evaluation of norepinephrine transporter expression and metaiodobenzylguanidine avidity in neuroblastoma: a report from the Children’s Oncology Group. Int J Mol Imaging. 2012;2012:250834. doi: 10.1155/2012/250834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Howard JP, Maris JM, Kersun LS, et al. Tumor response and toxicity with multiple infusions of high dose 131I-MIBG for refractory neuroblastoma. Pediatr Blood Cancer. 2005;44(3):232–9. doi: 10.1002/pbc.20240. [DOI] [PubMed] [Google Scholar]
- •• This report by Howard et al. shows that targeting the NET with radiolabeled MIBG is an important modality for the targeted radiotherapy of NBs. [Google Scholar]
- 113.Taggart D, Dubois S, Matthay KK. Radiolabeled metaiodobenzylguanidine for imaging and therapy of neuroblastoma. Q J Nucl Med Mol Imaging. 2008;52(4):403–18. [PubMed] [Google Scholar]
- 114.Navid F, Santana VM, Barfield RC. Anti-GD2 antibody therapy for GD2- expressing tumors. Curr Cancer Drug Targets. 2010;10(2):200–9. doi: 10.2174/156800910791054167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Modak S, Cheung NK. Disialoganglioside directed immunotherapy of neuroblastoma. Cancer Invest. 2007;25(1):67–77. doi: 10.1080/07357900601130763. [DOI] [PubMed] [Google Scholar]
- 116.Yu AL, Uttenreuther-Fischer MM, Huang CS, et al. Phase I trial of a human-mouse chimeric anti-disialoganglioside monoclonal antibody ch14.18 in patients with refractory neuroblastoma and osteosarcoma. J Clin Oncol. 1998;16(6):2169–80. doi: 10.1200/JCO.1998.16.6.2169. [DOI] [PubMed] [Google Scholar]
- 117.Yu AL, Gilman AL, Ozkaynak MF, et al. Anti-GD2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. N Engl J Med. 2010;363(14):1324–34. doi: 10.1056/NEJMoa0911123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •• This report demonstrated a significant prognostic advantage of immunotherapy with anti-GD2 antibody combined with GM-CSF and IL2 in patients with high-risk NB.
- 118.Porter DL, Levine BL, Kalos M, et al. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med. 2011;365(8):725–33. doi: 10.1056/NEJMoa1103849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Grupp SA, Kalos M, Barrett D, et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med. 2013;368(16):1509–18. doi: 10.1056/NEJMoa1215134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •• The above two papers by Porter et al., and Grupp et al., show great promise for using modified T-cells for the targeted cellular immunotherapy of selected cancers.
- 120.Bozzi F, Gambirasio F, Luksch R, et al. Detecting CD56+/NB84+/CD45- immunophenotype in the bone marrow of patients with metastatic neuroblastoma using flow cytometry. Anticancer Res. 2006;26(5A):3281–7. [PubMed] [Google Scholar]
- 121.Ferreira-Facio CS, Milito C, Botafogo V, et al. Contribution of multiparameter flow cytometry immunophenotyping to the diagnostic screening and classification of pediatric cancer. PLoS One. 2013;8(3):e55534. doi: 10.1371/journal.pone.0055534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Nagai J, Ishida Y, Koga N, et al. A new sensitive and specific combination of CD81/CD56/CD45 monoclonal antibodies for detecting circulating neuroblastoma cells in peripheral blood using flow cytometry. J Pediatr Hematol Oncol. 2000;22(1):20–6. doi: 10.1097/00043426-200001000-00004. [DOI] [PubMed] [Google Scholar]
- 123.Park SJ, Park CJ, Kim S, et al. Detection of bone marrow metastases of neuroblastoma with immunohistochemical staining of CD56, chromogranin A, and synaptophysin. Appl Immunohistochem Mol Morphol. 2010;18(4):348–52. doi: 10.1097/PAI.0b013e3181d2ed4c. [DOI] [PubMed] [Google Scholar]
- 124.Pashankar FD, O’Dorisio MS, Menda Y. MIBG and somatostatin receptor analogs in children: current concepts on diagnostic and therapeutic use. J Nucl Med. 2005;46(Suppl 1):55S–61S. [PubMed] [Google Scholar]
- 125.O’Dorisio MS, Hauger M, Cecalupo AJ. Somatostatin receptors in neuroblastoma: diagnostic and therapeutic implications. Semin Oncol. 1994;21(5):33–7. Suppl 13. [PubMed] [Google Scholar]
- • This report summarizes the potential utility of targeting SSR’s for the diagnosis and treatment of NBs.
- 126.O’Dorisio MS, Chen F, O’Dorisio TM, et al. Characterization of somatostatin receptors on human neuroblastoma tumors. Cell Growth Differ. 1994;5(1):1–8. [PubMed] [Google Scholar]
- 127.Muller PA, Vousden KH. p53 mutations in cancer. Nat Cell Biol. 2013;15(1):2–8. doi: 10.1038/ncb2641. [DOI] [PubMed] [Google Scholar]
- 128.Prabhu VV, Allen JE, Hong B, et al. Therapeutic targeting of the p53 pathway in cancer stem cells. Expert Opin Ther Targets. 2012;16(12):1161–74. doi: 10.1517/14728222.2012.726985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Vogan K, Bernstein M, Leclerc JM, et al. Absence of p53 gene mutations in primary neuroblastomas. Cancer Res. 1993;53(21):5269–73. [PubMed] [Google Scholar]
- 130.Hosoi G, Hara J, Okamura T, et al. Low frequency of the p53 gene mutations in neuroblastoma. Cancer. 1994;73(12):3087–93. doi: 10.1002/1097-0142(19940615)73:12<3087::aid-cncr2820731230>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 131.Goldman SC, Chen CY, Lansing TJ, et al. The p53 signal transduction pathway is intact in human neuroblastoma despite cytoplasmic localization. Am J Pathol. 1996;148(5):1381–5. [PMC free article] [PubMed] [Google Scholar]
- 132.Tweddle DA, Pearson AD, Haber M, et al. The p53 pathway and its inactivation in neuroblastoma. Cancer Lett. 2003;197(1-2):93–8. doi: 10.1016/s0304-3835(03)00088-0. [DOI] [PubMed] [Google Scholar]
- 133.Keshelava N, Zuo JJ, Chen P, et al. Loss of p53 function confers high-level multidrug resistance in neuroblastoma cell lines. Cancer Res. 2001;61(16):6185–93. [PubMed] [Google Scholar]
- • This resport suggests that loss of p53 function at relapse may be associated with a multidrug resistance phenotype in NBs.
- 134.Barbieri E, Mehta P, Chen Z, et al. MDM2 inhibition sensitizes neuroblastoma to chemotherapy-induced apoptotic cell death. Mol Cancer Ther. 2006;5(9):2358–65. doi: 10.1158/1535-7163.MCT-06-0305. [DOI] [PubMed] [Google Scholar]
- 135.Cory S, Adams JM. The Bcl2 family: regulators of the cellular life-or-death switch. Nat Rev Cancer. 2002;2(9):647–56. doi: 10.1038/nrc883. [DOI] [PubMed] [Google Scholar]
- 136.Barille-Nion S, Bah N, Vequaud E, Juin P. Regulation of cancer cell survival by BCL2 family members upon prolonged mitotic arrest: opportunities for anticancer therapy. Anticancer Res. 2012;32(10):4225–33. [PubMed] [Google Scholar]
- 137.Goldsmith KC, Hogarty MD. Targeting programmed cell death pathways with experimental therapeutics: opportunities in high-risk neuroblastoma. Cancer Lett. 2005;228(1-2):133–41. doi: 10.1016/j.canlet.2005.01.048. [DOI] [PubMed] [Google Scholar]
- 138.Lestini BJ, Goldsmith KC, Fluchel MN, et al. Mcl1 downregulation sensitizes neuroblastoma to cytotoxic chemotherapy and small molecule Bcl2-family antagonists. Cancer Biol Ther. 2009;8(16):1587–95. doi: 10.4161/cbt.8.16.8964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- • These reports by Goldsmith et al., and Lestini et al., provide evidence that targeting apoptotic pathways may be a useful therapeutic strategy in NBs.
- 139.Ballas K, Lyons J, Janssen JW, Bartram CR. Incidence of ras gene mutations in neuroblastoma. Eur J Pediatr. 1988;147(3):313–14. doi: 10.1007/BF00442704. [DOI] [PubMed] [Google Scholar]
- 140.Ireland CM. Activated N-ras oncogenes in human neuroblastoma. Cancer Res. 1989;49(20):5530–3. [PubMed] [Google Scholar]
- 141.Moley JF, Brother MB, Wells SA, et al. Low frequency of ras gene mutations in neuroblastomas, pheochromocytomas, and medullary thyroid cancers. Cancer Res. 1991;51(6):1596–9. [PubMed] [Google Scholar]
- 142.Shimizu K, Goldfarb M, Perucho M, Wigler M. Isolation and preliminary characterization of the transforming gene of a human neuroblastoma cell line. Proc Natl Acad Sci USA. 1983;80(2):383–7. doi: 10.1073/pnas.80.2.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Tanaka T. Ha-ras p21 in neuroblastoma: a new marker in prediction of patient outcome. Prog Clin Biol Res. 1994;385:275–80. [PubMed] [Google Scholar]
- 144.Shimizu K, Goldfarb M, Suard Y, et al. Three human transforming genes are related to the viral ras oncogenes. Proc Natl Acad Sci USA. 1983;80(8):2112–16. doi: 10.1073/pnas.80.8.2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Lemmon MA, Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell. 2010;141(7):1117–34. doi: 10.1016/j.cell.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- • This is an excellent review of RTKs, as well as their activation, signaling and inhibition.
- 146.Nikonova AS, Astsaturov I, Serebriiskii IG, et al. Aurora A kinase (AURKA) in normal and pathological cell division. Cell Mol Life Sci. 2013;70(4):661–87. doi: 10.1007/s00018-012-1073-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Otto T, Horn S, Brockmann M, et al. Stabilization of N-Myc is a critical function of Aurora A in human neuroblastoma. Cancer Cell. 2009;15(1):67–78. doi: 10.1016/j.ccr.2008.12.005. [DOI] [PubMed] [Google Scholar]
- • This paper describes a new function of AURKA in stablizing the MYCN protein, which makes it an attractive target, especially for high-risk NBs with MYCN amplification.
- 148.Carol H, Boehm I, Reynolds CP, et al. Efficacy and pharmacokinetic/ pharmacodynamic evaluation of the Aurora kinase A inhibitor MLN8237 against preclinical models of pediatric cancer. Cancer Chemother Pharmacol. 2011;68(5):1291–304. doi: 10.1007/s00280-011-1618-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Maris JM, Morton CL, Gorlick R, et al. Initial testing of the aurora kinase A inhibitor MLN8237 by the Pediatric Preclinical Testing Program (PPTP) Pediatr Blood Cancer. 2010;55(1):26–34. doi: 10.1002/pbc.22430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Maugeri-Sacca M, Bartucci M, De Maria R. Checkpoint kinase 1 inhibitors for potentiating systemic anticancer therapy. Cancer Treat Rev. 2013;39(5):525–33. doi: 10.1016/j.ctrv.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 151.Cole KA, Huggins J, Laquaglia M, et al. RNAi screen of the protein kinome identifies checkpoint kinase 1 (CHK1) as a therapeutic target in neuroblastoma. Proc Natl Acad Sci USA. 2011;108(8):3336–41. doi: 10.1073/pnas.1012351108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Hoglund A, Nilsson LM, Muralidharan SV, et al. Therapeutic implications for the induced levels of Chk1 in Mycexpressing cancer cells. Clin Cancer Res. 2011;17(22):7067–79. doi: 10.1158/1078-0432.CCR-11-1198. [DOI] [PubMed] [Google Scholar]
- • These reports by Cole et al., and Hoglund et al., suggest CHK1 is a therapeutic target for a subset of high-risk NBs.
- 153.Russell MR, Levin K, Rader J, et al. Combination therapy targeting the Chk1 and Wee1 kinases shows therapeutic efficacy in neuroblastoma. Cancer Res. 2013;73(2):776–84. doi: 10.1158/0008-5472.CAN-12-2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Walton MI, Eve PD, Hayes A, et al. CCT244747 is a novel potent and selective CHK1 inhibitor with oral efficacy alone and in combination with genotoxic anticancer drugs. Clin Cancer Res. 2012;18(20):5650–61. doi: 10.1158/1078-0432.CCR-12-1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Xu H, Cheung IY, Wei XX, et al. Checkpoint kinase inhibitor synergizes with DNA-damaging agents in G1 checkpoint-defective neuroblastoma. Int J Cancer. 2011;129(8):1953–62. doi: 10.1002/ijc.25842. [DOI] [PubMed] [Google Scholar]
- 156.Condorelli F, Gnemmi I, Vallario A, et al. Inhibitors of histone deacetylase (HDAC) restore the p53 pathway in neuroblastoma cells. Br J Pharmacol. 2008;153(4):657–68. doi: 10.1038/sj.bjp.0707608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Fouladi M, Park JR, Stewart CF, et al. Pediatric phase I trial and pharmacokinetic study of vorinostat: a Children’s Oncology Group phase I consortium report. J Clin Oncol. 2010;28(22):3623–9. doi: 10.1200/JCO.2009.25.9119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.George RE, Lahti JM, Adamson PC, et al. Phase I study of decitabine with doxorubicin and cyclophosphamide in children with neuroblastoma and other solid tumors: a Children’s Oncology Group study. Pediatr Blood Cancer. 2010;55(4):629–38. doi: 10.1002/pbc.22607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Mueller S, Yang X, Sottero TL, et al. Cooperation of the HDAC inhibitor vorinostat and radiation in metastatic neuroblastoma: efficacy and underlying mechanisms. Cancer Lett. 2011;306(2):223–9. doi: 10.1016/j.canlet.2011.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Witt O, Milde T, Deubzer HE, et al. Phase I/II intra-patient dose escalation study of vorinostat in children with relapsed solid tumor, lymphoma or leukemia. Klin Padiatr. 2012;224(6):398–403. doi: 10.1055/s-0032-1323692. [DOI] [PubMed] [Google Scholar]
- 161.Chesler L, Schlieve C, Goldenberg DD, et al. Inhibition of phosphatidylinositol 3-kinase destabilizes Mycn protein and blocks malignant progression in neuroblastoma. Cancer Res. 2006;66(16):8139–46. doi: 10.1158/0008-5472.CAN-05-2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Dam V, Morgan BT, Mazanek P, Hogarty MD. Mutations in PIK3CA are infrequent in neuroblastoma. BMC Cancer. 2006;6:177. doi: 10.1186/1471-2407-6-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Tivnan A, Orr WS, Gubala V, et al. Inhibition of neuroblastoma tumor growth by targeted delivery of microRNA-34a using anti-disialoganglioside GD2 coated nanoparticles. PLoS One. 2012;7(5):e38129. doi: 10.1371/journal.pone.0038129. [DOI] [PMC free article] [PubMed] [Google Scholar]